Abstract
Nanoparticles are increasingly used for biomedical purposes. Many different diagnostic and therapeutic applications are envisioned for nanoparticles, but there are often also serious concerns regarding their safety. Given the fact that numerous new nanomaterials are being developed every day, and that not much is known about the long-term toxicological impact of exposure to nanoparticles, there is an urgent need to establish efficient methods for nanotoxicity testing. The zebrafish (Danio rerio) embryo assay has recently emerged as an interesting ‘intermediate’ method for in vivo nanotoxicity screening, enabling (semi-) high-throughput analyses in a system significantly more complex than cultured cells, but at the same time also less ‘invasive’ and less expensive than large-scale biocompatibility studies in mice or rats. The zebrafish embryo assay is relatively well-established in the environmental sciences, but it has not yet gained wide notice in the nanomedicine field. Using prototypic polymeric drug carriers, gold-based nanodiagnostics and nanotherapeutics, and iron oxide-based nanodiagnostics, we here show that toxicity testing using zebrafish embryos is easy, efficient and informative, and faithfully reflects, yet significantly extends, cell-based toxicity testing. We therefore expect that the zebrafish embryo assay will become a popular future tool for in vivo nanotoxicity screening.
Introduction
Nanomaterials hold significant potential for biomedical applications.1,2 Proper assessment of the toxicological aspects of nanomaterials is extremely important, especially if intending to administer them to patients.3,4 In vitro cytotoxicity testing in cell lines and/or isolated primary human cells (e.g. macrophages), as well as comparative analyses versus established or clinically approved reference materials, are generally implemented to obtain initial information on nanoparticle toxicity. As opposed to large scale in vivo analyses, which are laborious, expensive and to some extent also unethical, in vitro nanotoxicity testing is rapid, simple and cheap. It does suffer, however, from several obvious shortcomings, such as over-simplified systems and setups, moderately informative and conclusive results, and limited translational value. In addition to this, the in vitro-in vivo correlation (IVIVC) of nanoparticle toxicity in cells vs. animals (and patients) is known to be at best moderate, since the in vivo situation is known to be much more complex. Factors such as route of administration, biodistribution, biodegradability, long-term disposition, induction of developmental defects, and activation of the compliment and/or immune system, all are major issues in determining in vivo nanotoxicity, and cannot be properly addressed using in vitro experimental setups.
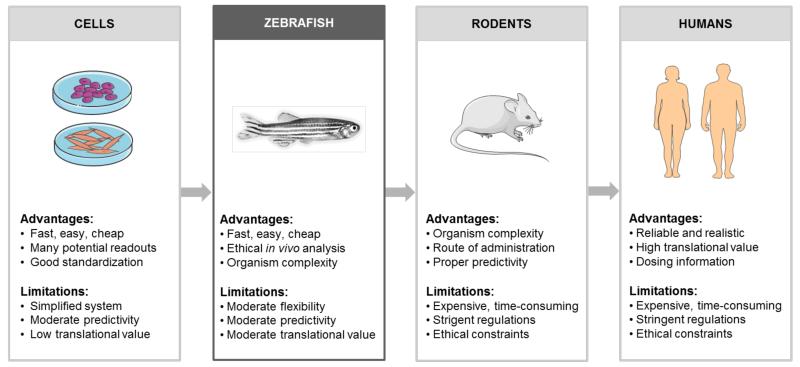
To enable simple, efficient and high-throughput toxicity testing in systems significantly more complex than cultured cells, and to at the same time reduce the potential suffering of ‘higher’ organisms, such as mice or rats, in vivo experiments involving Danio rerio zebrafish (and their embryos) are becoming increasingly popular.5,6 The zebrafish embryo assay is relatively well-established in the environmental sciences, to assess both the acute toxic effects and the ‘long-term’ developmental defects resulting from exposure to environmental chemicals and (nano-) particles. In the nanomedicine and drug delivery field, however, in which toxicity is a highly important issue, this assay has not yet gained much attention. As outlined in Figure 1, there are several reasons why this assay might be highly useful for ‘intermediate’ nanomedicine toxicity testing, after completing initial experiments in cells, and before turning to in vivo experiments in rodents.
Figure 1.
Suggested workflow for translational toxicity testing of nanomedicine formulations. In general, toxicity studies commence with in vitro experiments in several different cells lines, at several different nanoparticle concentrations. Before going into patients, the material is then tested in several animal models, in particular in mice and rats. Before going into such ‘higher’ animal models, acute and chronic toxicity testing can be performed - at relatively high-throughput - using the zebrafish embryo assay. Assessing both acute and chronic effects in fish embryos which might be somewhat less informative than standard in vivo nanotoxicity screening, but it is relatively easy, more economic and arguably also more ethical. Consequently, we expect this in vivo nanotoxicity testing tool to become increasingly popular in the nanomedicine field. Figures are partially based on Servier Medical Art (www.servier.com).
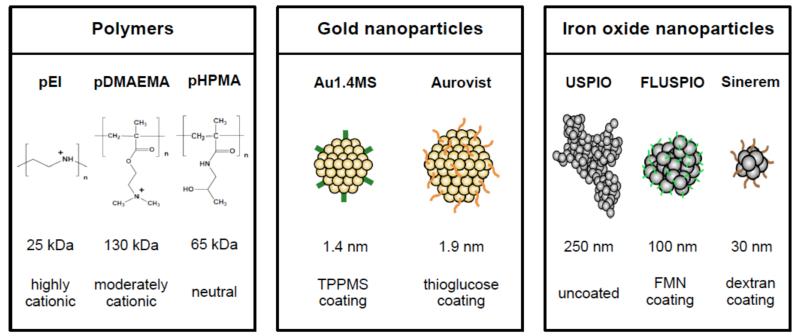
In the present study, the toxicity of three different classes of commercially available and/or previously very extensively characterized nanomedicine materials was evaluated in cells and zebrafish embryos. Charged and neutral polymers, routinely employed for gene and drug delivery, were used in the initial set of experiments, followed by experiments involving gold and iron oxide nanoparticles (Figure 2). Polymers included the well-known cationic transfection agents pEI (i.e. poly(ethylene imine)) and pDMAEMA (i.e. poly(2-(dimethylamino) ethylmethacrylate)),7-9 as well as the uncharged macromolecular drug carrier material pHPMA (i.e. poly(N-2-hydroxypropyl) methacrylamide), which has been extensively used for improving the delivery of chemotherapeutic agents to tumors.10-12 Gold nanoparticles encompassed the well-tolerated computed tomography (CT) contrast agent Aurovist (1.9 nm), as well as slightly smaller triphenylphosphane monosulfonate (TPPMS) -capped gold nanoparticles (Au1.4MS; 1.4 nm), previously shown to be highly efficient in killing (cancer) cells.13,14 Finally, three different ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles were evaluated, including standard uncoated USPIO, flavin mononucleotide-coated fluorescent USPIO (i.e. FLUSPIO), and the dextran- and sodium-citrate-coated formulation Sinerem, which is clinically used for iron replacement therapy in anemia.15-18 USPIO are widely used as nanodiagnostics, and can be used for liver and macrophage imaging, as well as for lymph node and (stem) cell tracking. In addition, they have also been more and more used for drug delivery and theranostic purposes.19,20
Figure 2.
Schematic depiction of the prototypic nanomaterials evaluated. Three different polymeric carrier materials and iron oxide nanoparticles, as well as two different gold nanoparticles, were used. Polymers differed in size and charge, and gold and iron oxide nanoparticles in size and surface coating. Routinely used, commercially available and/or previously very extensively characterized materials were used. More details on the synthesis, properties and physicochemical characteristics of these materials can be found in refs 7-22.
Due to the ever-increasing interest in using such polymer- and particle-based nanomaterials for biomedical purposes, tools to properly evaluate their in vitro and in vivo biocompatibility are highly needed. We here show that in vivo nanotoxicity testing using the zebrafish embryo assay is easy, efficient and informative, and we therefore propose to use this assay as an intermediate between cell-based toxicity screening and nanotoxicity testing in rodents.
Experimental
Polymeric carrier materials
Poly(ethylenimine) (pEI; 25 kDa) was purchased from Polysciences Inc. Poly(N-2-hydroxypropyl) methacrylamide (pHPMA; 65 kDa) and poly(2-(dimethylamino) ethyl-methacrylate) (pDMAEMA; 130 kDa) were synthesized as described in Ref. 7-9 and 21-22. All polymers had a PDI ≤2. The transfection agents pEI and pDMAEMA contain positively charged side chains, while pHPMA is a neutrally charged macromolecular drug carrier.
Gold nanoparticles
Au1.4MS gold particles (overall chemical formula: Au55[(C6H5)2P(C6H4SO3Na)]12Cl6 ; core size: 1.4 nm) were synthesized as described by Pan et al.13, using the Schmid method.23 Their synthesis was based on a ligand exchange reaction of Au1.4TPP nanoparticles (Au55[(C6H5)3P]12Cl6) with TPPMS, in which Au1.4TPP (the non-sulfonate ligand derivative P(C6H5)3), dissolved in methylene chloride, was transferred into the aqueous phase. During phase transfer, TPP was replaced TPPMS present in the water phase. The two-phase system was stirred for 3 days, solvent was removed and the precipitate was washed, before being filtered and stored in solid form. Aurovist®, a 1.9 nm-sized commercially available gold nanoparticle formulation with a thioglucose coating, was purchased from Nanoprobes Inc.
Iron oxide nanoparticles
Three different ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles were used. Uncoated USPIO nanoparticles (~5 nm core size in TEM; ~252 nm aggregates in PBS) and FLUSPIO (~100 nm hydrodynamic diameter in DLS) were prepared as described in Ref. 17. FLUSPIO particles were synthesized using USPIO cores and coating them with flavin mononucleotide (FMN) and guanosine monophosphate (GMP). Sinerem (Combidex®; ~30 nm hydrodynamic diameter in DLS) was kindly provided by Guerbet SA (France).
Cell lines
HeLa (human cervical carcinoma), HUVEC (human umbilical vein endothelial) and SMC (ovine smooth muscle) cells were obtained from ATCC, and were used for in vitro cytotoxicity analysis. HUVEC were cultivated in Endopan 3 Medium (Pan Biotech, GmbH, Germany), and HeLa and SMC cells in DMEM (Gibco, Invitrogen, Germany), supplemented with 1% Pen/Strep and 10% FBS. Cell lines were maintained under defined conditions of 95% relative humidity, 5% CO2 and 37°C.
Cytotoxicity assays
HeLa cells, HUVEC and SMC were exposed to different concentrations of the polymers and nanoparticles for 3-48 h. Upon incubation, cells were washed, MTT solution (Roche, Switzerland) was added, and plates were incubated with MTT for 4 h. After this, the MTT solution was discarded and DMSO was added to dissolve the formed formazan crystals. Solubilization of the crystals was performed overnight. Supernatant absorbance was measured in a TECAN reader at 570 nm, with a reference wavelength of 690 nm. Based on this, IC50 values (representing the concentration of a drug or (nano-) chemical material inhibiting in vitro cellular proliferation by 50% as compared to untreated controls) were calculated and compared.
Zebrafish assay
The in vivo toxicity of the polymers, gold and iron oxide nanoparticles was also evaluated using the zebrafish embryo assay. Fertilized eggs were transferred into 96-well plates (16-cell stadium, 1 egg/well), and different polymer and nanoparticle concentrations were added. Polymers were tested at 0.0001-1.0 mg/ml, and gold- and iron oxide nanoparticles at concentrations of 0.01-10 mg/ml. Gold concentrations correspond to × (mg/ml) / 55000 (MW of cluster in gram) * 55 (number of Au atoms per cluster) mM Au. Concentrations were chosen on the basis of in vitro toxicity profiles, and were diluted in E3 zebrafish embryo medium (i.e. NaCl, KCl, CaCl2.2H2O and MgCl2.6H2O; pH 7.2). The development of the zebrafish embryos was evaluated using a Leica DMI 6000B inverted microscope from the moment of nanoparticle addition onwards, and was longitudinally monitored at 1, 3 and 7 days regarding toxicity and potential developmental defects.
Results and discussion
Polymeric drug carriers
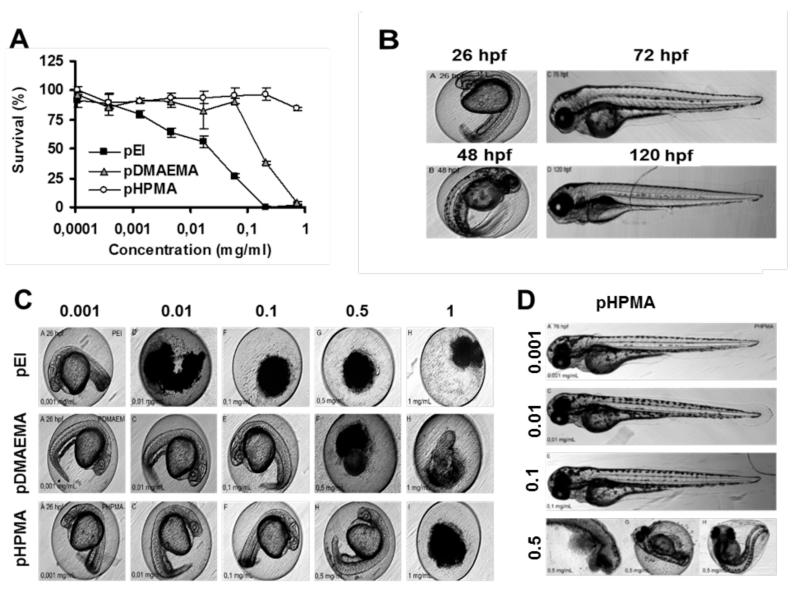
Assessment of the toxicity profile of the three polymeric carrier materials demonstrated that cationic pEI (25 kDa; and pDMAEMA showed a much higher degree of in vitro cell killing than did neutral pHPMA. In line with previous experiments, the latter was found to be non-toxic up to a concentration of 1 mg/ml, whereas the former two had IC50 values of ~0.03 and 0.3 mg/ml, respectively (Figure 3A). These in vitro toxicological profiles correlated very well with the observed in vivo toxicity in zebrafish embryos. As shown in Figure 3C, at 24 h after adding the polymers to the embryos, pEI had prevented them from properly developing already at concentrations as low as 0.01 mg/ml, while pDMAEMA prevented proper embryo development from concentrations of 0.5 mg/ml onwards. For pHPMA, embryo development was shown to be affected only at a concentration of 1 mg/ml. For pEI and pDMAEMA, similar trends were observed over time. At later time points, also for pHPMA, abnormalities regarding embryo development were observed, and also upon exposure to 0.5 mg/ml for 76 h, some indications for teratogenicity could be obtained (Figure 3D).
Figure 3.
Toxicity induced by cationic and neutral polymeric carrier materials. A: In vitro survival of HeLa cells, as determined by MTT, upon exposure to highly cationic pEI, moderately cationic pDMAEMA, and neutral pHPMA. B: Zebrafish embryo development under standard/healthy conditions (hpf = hours post fertilization). C: Zebrafish embryo development after 24 h of incubation with the indicated polymeric carrier materials (in mg/ml), showing good correlation with the effects observed in vitro. For pHPMA, only at the highest dose, significant mortality could be observed. D: Upon prolonged exposure (76 h) to 0.5 mg/ml of pHPMA, intermediate developmental defects (i.e. delayed hatching) were observed.
Overall, in vitro and in vivo toxicity correlated very well for these three polymer-based carrier materials. The findings in Figure 3D, however, indicate that also the prolonged exposure of zebrafish embryos to relatively high concentrations of polymers believed to be non-toxic on the basis of in vitro results, i.e. pHPMA, might lead to - at least some - growth retardation and/or developmental defects. In line with previous studies, these findings therefore suggest that there might be a discrepancy between the results obtained purely on the basis of in vitro findings and those observed in vivo. With regard to this, the in vivo zebrafish embryo assay seems to be more sensitive for picking up potential adverse effects than in vitro experiments in cells. Consequently, the zebrafish embryo assay might prove to be an interesting and important ‘intermediate’ tool for facilitating efficient and ethical in vivo nanotoxicity testing.
Gold nanoparticles
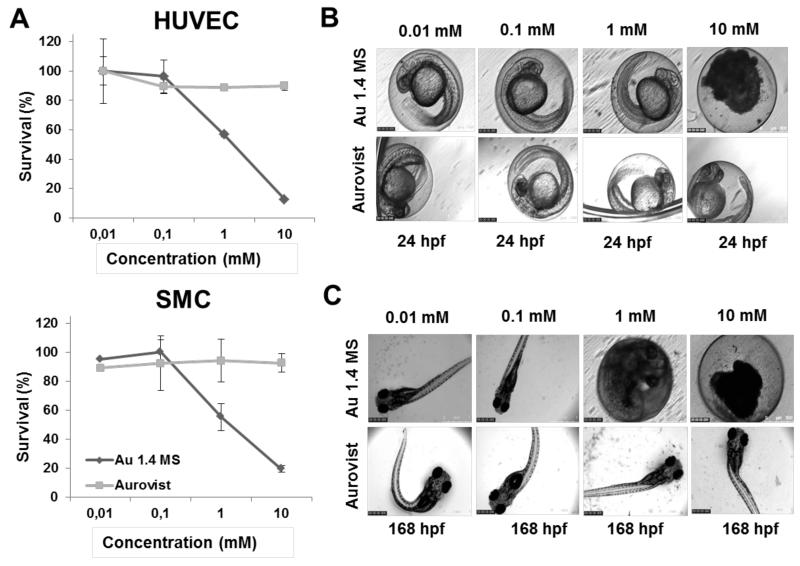
In vitro cytotoxicity analyses clearly demonstrated that ‘therapeutic’ Au1.4Ms nanoparticles were much more toxic than ‘diagnostic’ Aurovist nanoparticles (Figure 4A). HUVEC and SMC showed an evident decrease in viability at a dose of 1 and 10 mg/ml of Au1.4MS, while no toxicity was observed for Aurovist particles, at all of the tested concentrations. These results are largely in line with the results observed using the in vivo zebrafish embryo assay, which also clearly demonstrated that at 24 h after particle addition, the toxicity of Au1.4MS nanoparticles was much higher than that of Aurovist nanoparticles (Figure 4B). These results were well-reflected at later time points, though with somewhat higher sensitivity, showing also toxicity and developmental defects at lower concentrations. As exemplified by Figure 3C, longer exposure to lower doses (i.e. 0.1 and 1 mg/ml) of Au1.4MS particles also had effects on the embryos, revealing delayed embryonic development (embryos still in the egg at 72 and 168 h post fertilization (hpf)).
Figure 4.
Toxicity induced by gold nanoparticles. A: In vitro survival of HUVEC and SMC exposed to increasing amounts of ‘therapeutic’ 1.4 nm (Au1.4MS) and ‘diagnostic’ 1.9 nm (Aurovist) gold nanoparticles. B-C: Short- and long-term impact of gold nanoparticle exposure on zebrafish embryo development, showing very good correlation with in vitro findings. While Au1.4MS particles induced both acute (24 h; at 10 mM) and delayed (168 h; at 1 mM) toxicity, Aurovist did not demonstrate any toxic effects, not even at high doses.
The observed higher toxicity of Au1.4MS nanoparticles in comparison to Aurovist are in line with previously published results,13,24,25 suggesting that gold nanoparticle toxicity depends both on size and surface chemistry26. The thioglucose coating, as present in the commercially available product Aurovist, is likely responsible for the significantly reduced toxicity profile of these particles. Furthermore, gold nanoparticles do not present aggregation patterns, which means that due to their small size (1.4 nm for Au1.4MS and 1.9 nm for Aurovist), they can be easily internalized by cells via endocytosis, and enter the embryos via chorion pores. As shown by Pan et al.,25 both Au1.4MS and Aurovist nanoparticles demonstrate similar internalization rates, providing further evidence that the thioglucose coating in Aurovist conveys protection towards both cells and zebrafish embryos.
Iron oxide nanoparticles
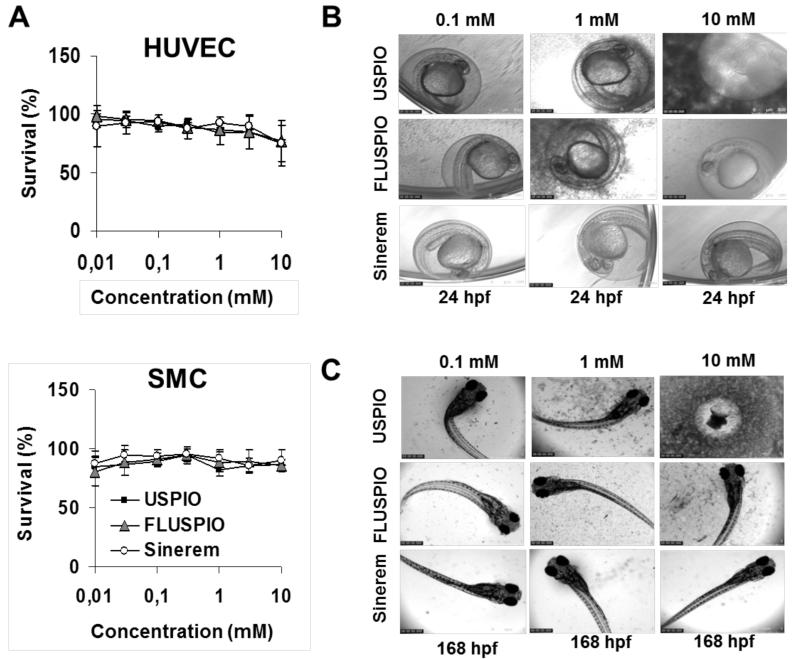
In vitro MTT analyses did not show any difference in cytotoxicity between standard USPIO, FMN-coated FLUSPIO and Sinerem. At concentrations up until 10 mg/ml, none of the three iron oxide nanoparticles caused a significant decrease in viability, neither in HUVEC, nor in SMC (Figure 5A). Conversely, however, in vivo toxicity analyses using zebrafish embryos rendered different results, hinting towards a higher level of toxicity for uncoated USPIO as compared to FLUSPIO and Sinerem (Figure 5B). For the former, clear evidence for embryonic lethality was obtained at a dose of 10 mg/ml, killing all embryos already at 24 hpf. For the latter two, no signs of toxicity or developmental abnormalities were observed at this dose, not even upon incubation for 7 days (Figure 5C).
Figure 5.
Toxicity induced by iron oxide nanoparticles. A: In vitro survival of HUVEC and SMC exposed to increasing amounts of uncoated USPIO, FMN-coated USPIO (FLUSPIO), and dextran- and sodium citrate-coated USPIO (Sinerem). B-C: Short- and long-term impact of iron oxide nanoparticle exposure on zebrafish embryo development, showing a partially very good, and a partially discordant correlation with in vitro findings. In vitro, neither of the three formulations demonstrated toxicity at doses up until 10 mM. In vivo, on the other hand, uncoated USPIO were found to be toxic at a dose of 10 mM.
These results can be partially explained by nanoparticle aggregation, which in zebrafish embryo medium (i.e. NaCl, KCl, CaCl2.2H2O and MgCl2.6H2O; pH 7.2) occurred predominantly for USPIO. As already demonstrated by Jayapaul et al.,17 USPIO particles are quite instable at pH=7 in both PBS and saline solution (0.9% NaCl), and tend to aggregate, while FLUSPIO and Sinerem tend to be much more stable. This is exemplified by the higher background darkness of the USPIO images in Figures 5B-C, in particular at higher concentrations, which is the result of nanoparticle aggregation and sedimentation. Properly dispersed (coated) iron oxide nanoparticles tend to have hydrodynamic diameters of 10-100 nm, and therefore are small enough to pass through the egg chorion pores (which are 500-700 nm).27 Aggregates, on the other hand, which can have sizes of up to several microns, might get trapped in the chorion pores, and as suggested by Cheng et al.28 and Bai et al.,29 they might thereby block oxygen transport from the medium into the embryo. This notion is expected to contribute, at least to some extent, to the higher toxicity caused by uncoated USPIO nanoparticles.
The fact that no toxicity was observed in vitro, but that contrasting results were found in zebrafish embryos, might be explained on the basis of uncoated USPIO aggregation in PBS and saline solutions, which tends to be avoided when uncoated USPIO are diluted in cell culture medium (most likely because of colloidal stabilization of iron oxide particles by serum proteins).17 Further and more detailed studies on nanoparticle aggregation, rate of particle internalization by both cells and embryos, and hypoxia levels in the embryos will likely provide more insights on the toxicity mechanism(s) of these iron oxide-based nanoparticles.
In vitro vs. in vivo toxicity screening
The present toxicity analyses with three different classes of representative nanomedicine materials highlight the importance of critically evaluating the correlation between in vitro vs. in vivo nanotoxicity testing. The assessment of polymers, gold and iron oxide nanoparticles towards both cells and zebrafish embryos clearly demonstrated that not always a correlation between the two techniques can be observed. Our study revealed an agreeable correlation between in vitro vs. in vivo findings for both polymeric drug carriers and gold nanoparticles. These nanosystems presented good correspondence between in vitro toxicity evaluations and its dose-dependent effects on embryonic development in zebrafish. Iron oxide nanoparticles, on the other hand, revealed contrasting results: although no toxicity was observed in cells (at least not in the tested concentrations; which were maximally concentrated), evident particle-related effects were observed in the zebrafish embryo assay. Particle aggregation of uncoated USPIO is thought to be, at least to some extent, involved in the toxicity observed in the embryos, as aggregates trapped in the chorion pores might obstruct oxygen transport between the medium and the embryos. Coating the particles, and thereby preventing them from aggregation, seems to have a beneficial impact on their biocompatibility, as neither FLUSPIO nor Sinerem turned out to be toxic in vivo.
For the particles that did show a toxic effect in the embryos (Au1.4MS and USPIO particles), this effect seemed to be more pronounced at 24 hpf, when the first impact on the embryos was already evident. This observation is in line with the findings reported by Tyl et al.,30 which point out that the vulnerability to teratogenic malformations is highly dependent on the embryonic stage. The most critical and sensitive stage of development is indeed suggested to be 1 day after fertilization (i.e. 24 hpf), when organogenesis is taking place. At this phase, rapid differentiation of organs occurs and the extensive rate of cell proliferation makes them particularly prone to teratogenic factors and leading to most of the structural malformations.
According to guidelines from the European Centre for Validation of Alternative Methods, studies on the toxicity of chemicals and (nano-) particles in zebrafish embryos reveals a fairly acceptable level of toxicity predictivity, ranging from “sufficient” (65-75%) to “good” (75-85%) predictivity. This discrepancy is mainly related to non-standardized methodologies among the different research institutions, as well as to a lack of systematic definition of parameters to allow head-to-head comparison and validation of results.31
Taken together, the zebrafish embryo assay is emerging as a rapid, easy and efficient method to assess nanoparticle toxicity. It provides the benefit of in vivo toxicity evaluation in a complex vertebrate organism, much more complex than (over-) simplified setups in cells. The assay is shown to be a simple way to assess overall fish viability towards different types of nanoparticles, longitudinal monitoring of embryonic fish development and also the potential of detecting potentially teratogenic effects. Although not a truly representative replacement for traditional in vivo analyses in mice and rats, the zebrafish embryo assay is shown to be highly suitable as an intermediate screening tool, between preliminary toxicity evaluation in cells, and more conclusive and more translationally relevant follow-up toxicity assessment in ‘higher’ organisms. Future efforts regarding this in vivo nanotoxicity screening tool will also encompass the possibility of analyzing nanoparticle-mediated stress induction, using HSP70-GFP-transgenic zebrafish embryos, and correlating in vitro stress induction (via ROS assays) with in vivo HSP70 expression (via the activation of the 70 kDa heat shock protein, which is coupled to GFP expression in transgenic fish embryos). Initial studies in this regard have already been undertaken for cytotoxic Au1.4MS gold nanoparticles,13,25 and will be extended to several other types of diagnostic, therapeutic and theranostic nanomaterials in the years to come.
Conclusions
We here demonstrate that in vivo nanotoxicity testing using the zebrafish embryo assay can be used to compliment and validate findings obtained in vitro. Polymeric carrier materials and gold nanoparticles presented with a good in vitro vs. in vivo correlation regarding toxicity parameters. It should be noted, however, that upon long-term incubation with high doses of neutral and in vitro untoxic pHPMA polymers, some developmental abnormalities were observed in the zebrafish embryo assay, illustrating its potential for more sensitive, more detailed and more informative nanotoxicity testing. In line with this, non-coated iron oxide-based nanoparticles were found to be non-toxic in vitro, but presented with significant toxicity towards zebrafish embryos in vivo. Based on these notions, the zebrafish embryo assay seems to be an easy, efficient and ethical method to assess both the acute effects of exposure to nanoparticles, as well as their long(er)-term impact on embryonic development, providing important information on whole organism teratogenicity, and a clear benefit in comparison to (over-) simplified in vitro toxicity parameters. This in vitro vs. in vivo toxicity correlation, as well as the elucidation of potential discrepancies, has important implications for facilitating the translation of nanomedicine materials into clinical trials.
Acknowledgements
This work was financially supported by the DAAD (290084/2011-3), by the ERC (StG-309495-NeoNano), by European Union Seventh Framework Programme (FP7 / 2007-2013; grant agreement n° NMP4-LA-2013-310451; and COST-TD1004), by the German Federal State of North Rhine Westfalia (HighTech.NRW / EU-Ziel2-Programm (EFRE; 2007-2013): “Entwicklung und Bildgebung patientenoptimierter Implantate” and “ForSaTum”), and by the DFG (LA 2937/1-1; Research Training Group “Biointerface” (No. 1035); and PAK 56).
References
- 1.Petros RA, DeSimone JM. Nature Reviews Drug Discovery. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 2.Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Curr Opin Biotech. 2013 doi: 10.1016/j.copbio.2013.02.020. in press (DOI: http://dx.doi.org/10/1016/j.copbio.2013.02.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nel A, Xia T, Mädler L, Li N. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 4.Nyström M, Fadeel B. Journal of Controlled Release. 2012;161:403–408. doi: 10.1016/j.jconrel.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 5.George S, Xia T, Rallo R, Zhao Y, Ji Z, Lin S, Wang X, Zhang H, France B, Schoenfeld D, Damoiseaux R, Liu R, Lin S, Bradley KA, Cohen Y, Nel AE. ACS Nano. 2011;5:1805–1817. doi: 10.1021/nn102734s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming A, Alderton WK. Drug Discovery Today: Disease Models. 2013 (in press) [Google Scholar]
- 7.Varkouhi AK, Lammers T, Schiffelers RM, Van Steenbergen MJ, Hennink WE, Storm G. European Journal of Pharmaceutics and Biopharmaceutics. 2011;77:450–457. doi: 10.1016/j.ejpb.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, Lok MC, Hennink WE. Bioconjugate Chem. 2007;18:2077–2084. doi: 10.1021/bc0701186. [DOI] [PubMed] [Google Scholar]
- 9.Luten J, van Nostrum CF, De Smedt SC, Hennink WE. Journal of Controlled Release. 2008;126:97–110. doi: 10.1016/j.jconrel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Kopecek J, Kopeckova P. Advanced Drug Delivery Reviews. 2010;62:122–149. doi: 10.1016/j.addr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicent MJ, Ringsdorf H, Duncan R. Advanced Drug Delivery Reviews. 2009;61:1117–1120. doi: 10.1016/j.addr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Lammers T, Ulbrich K. Advanced Drug Delivery Reviews. 2010;62:119–121. doi: 10.1016/j.addr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, Schmid G, Brandau W, Jahnen-Dechent W. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 14.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Angewandte Chemie International Edition. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta AK, Gupta M. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller R. Chemical Reviews. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 17.Jayapaul J, Hodenius M, Arns S, Lederle W, Lammers T, Comba P, Kiessling F, Gaetjens J. Biomaterials. 2011;32:5863–5871. doi: 10.1016/j.biomaterials.2011.04.056. [DOI] [PubMed] [Google Scholar]
- 18.Jayapaul J, Arns S, Lederle W, Lammers T, Comba P, Gätjens J, Kiessling F. Biomaterials. 2012;33:8822–8829. doi: 10.1016/j.biomaterials.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 19.Veiseh O, Gunn JW, Zhang M. Advanced Drug Delivery Reviews. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santra S, Kaittanis C, Grimm J, Perez JM. Small. 2009;5:1862–1868. doi: 10.1002/smll.200900389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varkouhi AK, Schiffelers RM, van Steenbergen MJ, Lammers T, Hennink WE, Storm G. J. Control. Release. 2010;148:e98–99. doi: 10.1016/j.jconrel.2010.07.072. [DOI] [PubMed] [Google Scholar]
- 22.Lammers T, Kühnlein R, Kissel M, Subr V, Etrych T, Pola R, Pechar M, Ulbrich K, Storm G, Huber P, Peschke P. Journal of Controlled Release. 2005;110:103–118. doi: 10.1016/j.jconrel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Schmid G, Klein N, Korste L. Polyhedron. 1988;7:605–608. [Google Scholar]
- 24.Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G, Brandau W, Simon U, Jahnen-Dechent W. Small. 2009;5:2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y, Leifert A, Graf M, Schiefer F, Thoröe-Boveleth S, Broda J, Halloran MC, Hollert H, Laaf D, Simon U, Jahnen-Dechent W. Small. 2013;9:863–869. doi: 10.1002/smll.201201173. [DOI] [PubMed] [Google Scholar]
- 26.Rawson DM, Zhang T, Kalicharan D, Jongebloed WL. Aquaculture Research. 2000;31:325–336. [Google Scholar]
- 27.Jahnen-Dechent W, Simon U. Nanomedicine (London) 2008;3:601–603. doi: 10.2217/17435889.3.5.601. [DOI] [PubMed] [Google Scholar]
- 28.Cheng JP, Flahaut E, Cheng SH. Environ. Toxicol. Chem. 2007;26:708–716. doi: 10.1897/06-272r.1. [DOI] [PubMed] [Google Scholar]
- 29.Bai W, Zhang Z, Tian W, He X, Ma Y, Zhao Y, Chai Z. Journal.of Nanoparticle Research. 2010;12:1645–1654. [Google Scholar]
- 30.Tyl RW. General and Applied Toxicology. 2000;2:1167–1201. [Google Scholar]
- 31.Eimon M, Rubinstein AL. Expert Opinion on Drug Metabolism and Toxicology. 2009;5:393–401. doi: 10.1517/17425250902882128. [DOI] [PubMed] [Google Scholar]