Abstract
The morphogenesis of the composite epicuticular wax coverage and regeneration ability of the upper wax layer in Nepenthes alata pitchers were studied using a cryo-scanning electron microscopy. Examination of pitchers of different ages revealed six stages in the wax coverage development. In the first stage, wax crystals resemble those found recently in mature pitches of N. dicksoniana and N. ventricosa. Platelets of the upper wax layer originate from broadened tips of stalks during the last developmental stage. Contrary to previous hypotheses, we found that wax crystals of both layers as well as the stalks connecting them are oriented perpendicularly to the pitcher wall. No changes in the height of the wax coverage were detected in 4–8 weeks after mechanical removal of the upper wax layer from mature pitchers on plants. This indicates that the wax coverage in N. alata pitchers is unable to regenerate.
Growing in nutrient poor habitats, carnivorous plants from the genus Nepenthes rely on the capturing success of their trapping organs, highly modified pitcher-like leaves1. Thus captured prey, mostly insects2,3, serve as an additional source of essential nutrients lacking in these habitats1,4,5,6.
Nepenthes pitchers are separated into several functional zones such as (1) the lid and peristome, (2) the slippery zone, (3) the transitional zone, and (4) the digestive zone, showing different macro-morphologies and surface architectures that fulfil different functions1,7 (Figure 1i). Recently, not only different aspects of Nepenthes biology, among them the structure and functions of pitchers, especially with respect to their trapping efficiency, were the focus of numerous studies (see review by Moran and Clarke6). Trapping organs of these pitcher plants are also of great interest as biological models for the development of bio-inspired materials with specific anti-adhesive surface properties8,9,10,11,12,13.
Figure 1. Pitcher development in N. alata.
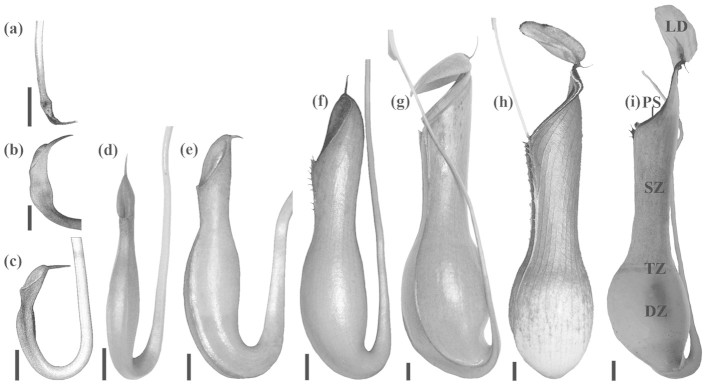
(a) Billowed tip of the tendril. (b), (c) Tendril tip with an enlarged swell, the lid is evident, the terminal spur is formed next to the lid. (d) Pitcher gets its typical shape, the spur is fully developed. (e) Pitcher is inflating, the digestive fluid is present. (f) Pitcher begins to elongate, wings start to grow, the spur decreases in size, the peristome is forming. (g) Lower half of the pitcher gets its bulbous shape, the lid has just opened, the peristome is directed vertically. (h) Mature pitcher, the lid has a long neck region, the peristome is bulged. (i) Longitudinally dissected mature pitcher of N. alata (lateral aspect, view from inside) with different functional zones: the lid (LD), the peristome (PS), the slippery zone (SZ), the transitional zone (TZ) and the digestive zone (DZ). Scale bars: 1 cm.
The upper inner surface of the pitcher situated just below the peristome, the slippery zone, serves for prey trapping and retention; it bears numerous downward-pointing lunate cells in almost all Nepenthes taxa7,14,15,16,17,18,19. In many Nepenthes plants, this zone is additionally covered with microscopic epicuticular wax platelets1,4,16,19,20,21,22,23,24 that create a surface slippery for arthropods. Microstructure and arrangement of wax crystals within this three-dimensional wax coverage were the focus of several microscopy studies performed in the last decade7,21,23,24. Our recent studies showed that in N. alata, the epicuticular wax coverage consists of two well distinguishable superimposed wax layers, differing in ultrastructure, chemical composition and mechanical properties16,19,22. The observed decrease in insect attachment ability on the wax-covered slippery zone was explained by the prevention of insect adhesion due to the contaminating effect of wax crystals on insects adhesive pads1,4,22,25 and/or reduction of real contact area between the pitcher surface and adhesive pads7,17,22,24.
Development of pitchers, starting from their initiation as small flattened structures at the tip of tendrils up to mature trapping organs was previously studied for N. alata by Owen and Lennon26. The authors indicated a uniform rate of pitcher growth from initiation to the point of lid opening and described six developmental phases (see Figure 1a,b/c,d,e,g,h), with particular focus on nectaries and glands. Whereas appearance and formation of the lid bearing a characteristic pattern of trichomes, the peristome with specific arrangements of nectaries, lunate cells in the slippery zone and digestive glands were well documented by these authors, the morphogenesis of the wax coverage was not investigated. The only publication about the wax coverage development in Nepenthes20 presented the hypothesis based on electron microscopy data on two wax layers obtained by Juniper and Burras4 on mature pitchers of N. rufescens. According to this hypothesis, the formation of wax crystals, which starts in young immature pitchers and ends just before the lid opens, is a two-step secretion process: during the first step, crystals of the lower layer are produced, and the second step with another secretion pattern forms the upper layer structures. Several successive pulses of wax formation result in a dense two-layered wax coverage. Up until now, no experimental study has been performed to test the proposed hypothesis about wax formation in Nepenthes pitchers, whereas there are numerous data on the development, movement, self-assembly and recrystallization of epicuticular waxes and effects of environmental factors on these processes in other plants27,28,29,30,31,32,33. Also, the recent experimental work on the ontogeny of the waxy zone in successively produced pitchers during a plant's juvenile stage in N. rafflesiana (types typica and elongata)34 provided no data on the wax morphogenesis.
Our experimental data22,25 as well as the observations of previous authors4 have shown the ability of platelets from the upper wax layer to contaminate insects' feet during their contact with the slippery zone. Similar contaminating effects have also been reported for a number of other plant waxes35,36. The question appears of whether the upper wax layer in Nepenthes pitchers is able to regenerate after its damage or complete removal, in order to fully re-establish anti-adhesive properties of the slippery zone. Regeneration ability of epicuticular plant waxes was studied by many authors37,38,39. Based on a scanning electron microscopy (SEM) study on leaves of 24 plant species, Neinhuis et al.31 distinguished four groups according to their regeneration behaviour: from species where regeneration occurs in all stages of organ development up to those which are not able to regenerate their waxes. However, until now the regeneration ability of the Nepenthes wax has not been studied.
Here, by applying a high-resolution cryo-SEM technique combined with freeze-fracturing, we aimed at direct visualisation of (1) morphogenesis of the complex two-layered wax coverage and (2) regeneration ability of the upper wax layer in the slippery zone of N. alata pitchers. The rise and development of wax layers were scrutinized in pitchers having different ages, starting from young small ones up to fully developed mature ones. The regeneration of the upper layer crystals was examined at four and eight weeks after they were removed mechanically from mature living pitchers that continued growing on plants. The results of the present study demonstrate how the complex hierarchically organized wax coverage is developed on the native plant surface.
Results
Development of the wax coverage
The pitcher morphology varied strongly during development (Figure 1). Usually in each plant, many more than only one tendril bore a bud at the tip, but only one or two of them completed their development to mature pitchers. We did not observe a strong correlation between pitcher age/size and development of inner pitcher zones, especially in regard to the rise and morphogenesis of the wax coverage in the slippery zone. The emergence of the first wax crystals on the pitcher's inner surface usually happened in fully inflated pitchers (Figure 1e). During this developmental phase of the pitcher, the difference between the digestive and slippery zones became obvious and a small pool of the digestive fluid was already present.
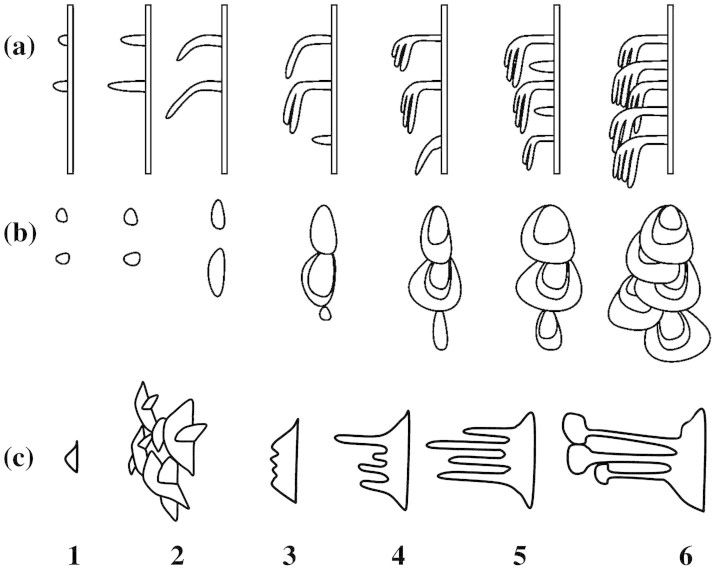
We distinguished six stages in the wax coverage morphogenesis. (1) On the hoods of lunate cells, single separate scale-like crystals, varying greatly in size, protrude perpendicular from the pitcher surface (Figure 2a). They are of irregular shape, however, often almost semicircular, and have regular margins. The appearance of these crystals marks the beginning of the formation of the lower wax layer. (2) The above crystals form more or less radial clusters and grow in size, gradually changing into platelets. Subsequently, crystal clusters become interconnected and finally form the solid foam-like lower wax layer (Figure 2b). (3) Numerous small outgrowths appear regularly on distal margins of interconnected crystals (Figure 2c). (4) Platelets of the lower wax layer reach their final size. Outgrowths elongate and turn into filiform extensions (stalks) (Figure 2d,e). (5) By the end of this developmental stage, the elongation process is finished and stalks are fully formed (Figure 2f,g). (6) The tips of stalks grow and broaden until rhomboid flat structures, platelets, are developed (Figure 2h,i). Toward the end of this stage, the upper platelets consist of multiple wax layers orientated parallel to each other and to the crystal plane (see Supplementary Information and Supplementary Figure S1). These separate platelets conjointly represent the upper wax layer. The completely developed wax coverage consists of the two superimposed wax layers; platelets of the upper layer are connected to the lower ones through thin long stalks, which are oriented parallel to the crystal plane (Supplementary Figure S1). By the time the lid opens, the pitcher bears the two-layered wax coverage in the slippery zone and is fully functional.
Figure 2. Cryo-SEM micrographs of the slippery zone wax coverage in developing pitchers of N. alata.
(a) Developmental stage 1: protruding of crystals of the lower wax layer from the pitcher wall surface. (b) Developmental stage 2: crystals start to arrange into radial clusters and interconnect with each other. (c) Developmental stage 3: small outgrowths appear on distal edges of semicircular crystals. (d), (e) Developmental stage 4: outgrowths elongate turning into filiform structures (stalks). (f), (g) Developmental stage 5: elongation of stalks is finished. (h), (i) Developmental stage 6: at distal ends of stalks, flat platelets of the upper wax layer develop. Insets in (a)–(d), (f) and (h) are not to scale. Insets schematically indicate the wax structure at corresponding developmental stages: wax coverage (a,b) and single crystals (c,d,f,h). Arrows in (a)–(d), (f) and (h) show the direction to the pitcher bottom. (a)–(d), (f), (h): view from above; (e), (g), (i): fracture. Scale bars: 1 μm.
Morphometrical variables of the developing wax coverage are presented in Table 1. Comparison of the height of the wax coverage showed significant differences between successive developmental stages (Table 2). The length of the base of lower crystals enlarged significantly during developmental stages 1 – 4 and showed no differences between stages 4 – 6 (Table 2). Whereas the length of filiform outgrowths/stalks increased significantly starting from their appearance at stage 3 up to stage 5, their diameter did not change during wax morphogenesis (Table 3).
Table 1. Morphometrical variables of the wax coverage measured at different stages of the wax morphogenesis. Data are presented as a mean value ± standard deviation. n, number of measurements; N, number of tested pitchers. Stalks were not present during the developmental stages 1 and 2.
| Developmental stage | Height of wax coverage [μm] | Length of base of lower crystals [μm] | Length of stalks [μm] | Diameter of stalks [μm] |
|---|---|---|---|---|
| 1 | 0.16 ± 0.03 n = 13, N = 1 | 0.16 ± 0.03 n = 13, N = 1 | - | - |
| 2 | 0.44 ± 0.04 n = 12, N = 1 | 0.44 ± 0.04 n = 12, N = 1 | - | - |
| 3 | 1.12 ± 0.11 n = 17, N = 1 | 0.68 ± 0.20 n = 26, N = 1 | 0.08 ± 0.02 n = 27, N = 1 | 0.11 ± 0.01 n = 32, N = 1 |
| 4 | 1.95 ± 0.26 n = 19, N = 1 | 0.83 ± 0.16 n = 10, N = 1 | 0.44 ± 0.15 n = 27, N = 1 | 0.11 ± 0.01 n = 4, N = 1 |
| 5 | 2.07 ± 0.19 n = 21, N = 2 | 0.82 ± 0.17 n = 17, N = 2 | 0.80 ± 0.26 n = 16, N = 1 | 0.12 ± 0.01 n = 23, N = 2 |
| 6 | 3.03 ± 0.24 n = 36, N = 4 | 0.81 ± 0.14 n = 26, N = 4 | 0.80 ± 0.30 n = 10, N = 1 | 0.12 ± 0.01 n = 46, N = 4 |
Table 2. Results of statistical analyses (t-test or Mann-Whitney rank sum test) of the height of the wax coverage and the length of the base of lower crystals between successive developmental stages. d. f., degrees of freedom; No, no significant difference; P, probability value; t, t-test statistics; T, Mann-Whitney rank sum test statistics; Yes, significantly different.
| Comparison | Normality test | Equal variance test | d. f. | Test statistics | P | Significantly different |
|---|---|---|---|---|---|---|
| height of the wax coverage | ||||||
| 1 vs. 2 | Passed P = 0.545 | Passed P = 0.350 | 23 | t = −23.170 | < 0.001 | Yes |
| 2 vs. 3 | Passed P = 0.187 | Failed P < 0.050 | - | T = 78.000 | < 0.001 | Yes |
| 3 vs. 4 | Passed P = 0.447 | Failed P < 0.050 | - | T = 153.000 | < 0.001 | Yes |
| 4 vs. 5 | Passed P = 0.306 | Failed P < 0.050 | - | T = 336.000 | = 0.151 | No |
| 5 vs. 6 | Passed P = 0.725 | Passed P = 0.293 | 55 | t = −15.607 | < 0.001 | Yes |
| length of the base of lower crystals | ||||||
| 1 vs. 2 | Passed P = 0.545 | Passed P = 0.350 | 23 | t = −23.170 | < 0.001 | Yes |
| 2 vs. 3 | Passed P = 0.724 | Failed P < 0.050 | - | T = 125.000 | < 0.001 | Yes |
| 3 vs. 4 | Passed P = 0.300 | Passed P = 0.124 | 34 | t = −2.071 | = 0.046 | Yes |
| 4 vs. 5 | Passed P = 0.649 | Passed P = 0.580 | 25 | t = 0.095 | = 0.925 | No |
| 5 vs. 6 | Passed P = 0.258 | Passed P = 0.283 | 55 | t = 0.233 | = 0.817 | No |
Table 3. Results of statistical analyses (t-test or Mann-Whitney rank sum test) of the length and diameter of stalks between successive developmental stages. Stalks were not present during the developmental stages 1 and 2. d. f., degrees of freedom; No, no significant difference; P, probability value; t, t-test statistics; T, Mann-Whitney rank sum test statistics; Yes, significantly different.
| Comparison | Normality test | Equal variance test | d. f. | Test statistics | P | Significantly different |
|---|---|---|---|---|---|---|
| stalks length | ||||||
| 3 vs. 4 | Failed P < 0.050 | - | - | T = 729.000 | < 0.001 | Yes |
| 4 vs. 5 | Passed P = 0.677 | Passed P = 0.292 | 41 | t = −5.765 | < 0.001 | Yes |
| 5 vs. 6 | Passed P = 0.072 | passed P = 0.573 | 24 | t = 0.043 | = 0.966 | No |
| stalks diameter | ||||||
| 3 vs. 4 | Passed P = 0.118 | Passed P = 0.934 | 46 | t = 0.268 | 0.790 | No |
| 4 vs. 5 | Passed P > 0.200 | Passed P = 0.861 | 37 | t = −1.648 | 0.108 | No |
| 5 vs. 6 | Passed P > 0.200 | passed P = 0.895 | 55 | t = −1.228 | 0.224 | No |
Wax regeneration ability
By mechanical treatment of the intact slippery zone in mature living pitchers on plants, we removed the upper wax layer and exposed the lower wax layer22. The height of the remaining wax coverage, measured on pitcher samples prepared immediately after mechanical treatment, amounted 0.82 ± 0.07 μm (Table 4) and was similar in all five pitchers studied (normality test passes: P = 0.343; equal variance test passed: P = 0.124; one way ANOVA: F4,71 = 0.736, P = 0.570, non-significant). Data collections were repeated at four and eight weeks after mechanical treatment. Comparisons between freshly manipulated pitchers and those after four weeks of recovery or between four and eight weeks of recovery showed no increase in the height of the wax coverage (Tables 4 and 5). This was observed in most pitchers tested. Thus, results obtained in experiments on pitchers with mechanically removed wax demonstrated its disability to regenerate.
Table 4. Results of regeneration experiments. Data are presented as a mean value ± standard deviation (n = 15 measurements). HW, height of the wax coverage; t, time after manipulation (mechanical removal of the upper wax layer).
| Examined pitchers | HW [μm], t = 0 | HW [μm], t = 4 weeks | HW [μm], t = 8 weeks |
|---|---|---|---|
| 1 | 0.80 ± 0.07 | 0.80 ± 0.08 | 0.89 ± 0.12 |
| 2 | 0.82 ± 0.10 | 0.76 ± 0.11 | 0.80 ± 0.09 |
| 3 | 0.82 ± 0.06 | 0.80 ± 0.08 | 0.81 ± 0.08 |
| 4 | 0.81 ± 0.09 | 0.79 ± 0.10 | 0.83 ± 0.06 |
| 5 | 0.84 ± 0.05 | 0.84 ± 0.05 | 0.84 ± 0.06 |
Table 5. Results of statistical analyses (Mann-Whitney rank sum test for the pitcher 1, 0 vs. 4 weeks; t-test for all other comparisons) of the wax coverage height measurements on the mechanically manipulated pitcher surface. The normality and equal variance tests passed for all data sets except pitcher 1, comparison 0 vs. 4 weeks (normality test failed, P < 0.050). d. f., degrees of freedom; No, no significant difference; P, probability value; t, t-test statistics; T, Mann-Whitney rank sum test statistics; Yes, significantly different.
| Comparison | Pitcher 1 | Pitcher 2 | Pitcher 3 | Pitcher 4 | Pitcher 5 |
|---|---|---|---|---|---|
| 0 vs. 4 weeks | T = 232.000 P = 1.000 No | t = 1.521 d. f. = 28 P = 0.139 No | t = 0.782 d. f. = 28 P = 0.441 No | t = 0.492 d. f. = 28 P = 0.626 No | t = −0.004 d. f. = 28 P = 0.844 No |
| 4 vs. 8 weeks | t = −2.388 d. f. = 28 P = 0.024 Yes | t = −0.912 d. f. = 28 P = 0.370 No | t = −0.438 d. f. = 28 P = 0.665 No | t = −1.430 d. f. = 28 P = 0.164 No | t = 0.134 d. f. = 28 P = 0.894 No |
Discussion
Our cryo-SEM data on the surface structure of the slippery zone in N. alata pitchers having different ages showed the morphogenesis of the wax coverage from its rise as separate small crystals in the fully inflated pitcher stage up to a composite three-dimensional structure in fully functional pitchers with the lid just opened. Based on these data, we divided the wax development process into six stages (Figure 3c,d). During all of these stages, a continuous growth in the height of the wax coverage was observed.
Figure 3. Diagrams illustrating the wax coverage morphogenesis in Nepenthes.
(a), (b) Hypothesis proposed by Martin and Juniper20. (c) Six developmental stages (1–6) according to our original data on N. alata. (a), (c): side view; (b): in surface view (view from above).
The formation of the lower wax layer occurred in the course of the first four stages. An increase in the length of the base of the lower crystals was detected only during these stages. This length did not change in later stages. Interestingly, the very first crystals, appearing sporadically during developmental stage 1, resembled those found recently in mature pitches of N. dicksoniana and N. ventricosa19. It has been hypothesized that this so called reduced wax coverage in pitchers of some Nepenthes species is a result of either hybridization (N. dicksoniana) or an evolutionary process with a most suitable compromise between a costly wax coverage and the benefits of a possible increase in catching efficiency, providing additional nutrients for the plant. The reduced wax coverage may present an intermediate step (between complex wax coverage and minimal amount of wax) within the process of the plants' adaptation to a changing environment, e.g. to the most common prey available. Our results suggested that in plants bearing reduced wax coverage, the wax development process stops at the first stage and this results in the presence of irregularly distributed small wax crystals on the surface of the slippery zone.
During the next (fifth) stage, growth and development of the stalks, which started at the third stage, was completed: the length of the stalks increased considerably from stage 3 to stage 5. However, the stalks did not grow in diameter, even later, during the sixth developmental stage, by the end of which they bear relatively large platelets of the upper wax layer. This geometry of the wax is responsible for the strong breaking ability of slim long stalks resulting in contamination of insect feet by upper wax platelets4,22,25. The formation of the crystals of the upper layer, showing the layered wax structure, was observed on the tips of stalks during the last (6th) developmental stage.
According to the previous hypothesis about wax development in Nepenthes pitchers20 (Figure 3a,b), the lower layer (projections and ridges) is produced first, long before the pitcher lid opens, and then the second layer (scales) is formed either by flattening of the projections' tips or by secretion of a wax having different properties. After the second layer structures bend and spread, the next successive pulses of wax formation take place, resulting in a dense coverage composed by flat crystals arranged like roof tiles. Thus, the projections and scales, although representing one unit, are oriented in different planes: perpendicular or parallel to the pitcher wall surface, respectively. Our results verified the successive development of the first and then the second wax layers, however, as a single, non-recurring process. Contrary to the previously proposed hypothesis20, we have found that (1) during all developmental stages, formation of wax crystals in both layers as well as stalks, connecting them, occurred in an upright orientation and (2) also in mature pitchers, these structures of the two-layered wax coverage were oriented more or less perpendicular to the pitcher wall surface (Figure 3).
The ability of plant wax coverage to regenerate was first reported more than half a century ago37,40. Since that time, wax regeneration has been studied in a number of plant species by different authors38,39,41,42. The experimental study on leaves of 24 species has shown that the ability to regenerate waxes and the amount of regeneration depended on the plant species and developmental stage of the organ31. Our regeneration experiments performed on mature pitchers with mechanically removed wax showed that the upper wax layer in N. alata is not able to regenerate. From the perspective of prey capturing by pitchers, this means that after the upper platelets are removed, e.g. by crawling insects, the exposed lower wax layer should compensate for anti-adhesive surface properties of the slippery zone responsible for trapping and retention functions. Previously performed traction tests with beetles Adalia bipunctata on the intact two-layered wax and lower wax layer (upper platelets were stripped off by applying polyvinylsiloxane) showed similar anti-adhesive effects of both wax layers on insect attachment force22. It has been hypothesized that contrary to the contamination of insect feet by wax crystals detached from the upper layer1,4,22,25, the lower wax layer creates a surface micro-roughness that reduces the real contact area between the pitcher surface and the adhesive pads hence impairing insect adhesion7,17,22,24.
Thus, using the cryo-SEM technique allowing high-resolution imaging of frozen and fractured samples under native conditions, we obtained a new insight into the ultrastructure and development of the complex wax coverage on the slippery zone in N. alata pitchers. Based on results obtained and literature data, a detailed model describing different stages in the morphogenesis of the two-layered Nepenthes wax is proposed. We experimentally showed that mature N. alata pitchers are not able to regenerate the upper wax layer after its mechanical removal. Both morphogenesis and regeneration studies were performed in Nepenthes for the first time.
Methods
Plant material
All examined pitchers were obtained from two N. alata plants grown in the greenhouse of the Botanical Garden at the University of Stuttgart (Stuttgart, Germany). Both plants were genetically identical (derived from a single plant via asexual propagation) and were cultivated under similar conditions. Only upper (aerial) pitchers were used in this study. Pitcher macro-morphology is described using the terminology after Juniper et al.1.
Wax morphogenesis
To investigate the development of the wax coverage, pitchers having different ages, from young (small) ones to fully developed (mature) ones, were harvested. Pieces of ~ 5 × 5 mm, cut out of the central part of the slippery zone using a razor blade, were examined in a cryo-SEM. In the wax coverage description, the terminology proposed by Barthlott et al.43 for plant epicuticular waxes was used.
Four morphometrical variables (total height of the wax coverage, length of the base of lower crystals, length and diameter of stalks connecting upper and lower platelets) were measured from digital images. Pairwise comparisons of morphometrical variables between successive developmental stages were performed using t-test (normally distributed data) or Mann-Whitney rank sum test (non-normally distributed data).
Wax regeneration
Regeneration experiments were performed with mature living pitchers on plants. The upper wax layer was mechanically removed using a two-component polyvinylsiloxane polymer (Coltène® President light body, Coltène Whaledent Dentalvertriebs Ltd., Constance, Germany). First, the fluid polyvinylsiloxane was applied to a central part of the slippery zone (~2 cm2). After polymerising for about five minutes, the polymer with the upper wax crystals attached to it was peeled off. Through this treatment, the upper layer was removed and the lower layer was exposed22. Immediately after this procedure, a small piece (~5 mm × 5 mm) of the treated plant surface was cut out for cryo-SEM examination. The same sample collection and cryo-SEM examination procedures were repeated at four and eight weeks after mechanical treatment. The height of the wax coverage was evaluated from digital images. Data were statistically analysed with one way ANOVA (differences between individual pitchers) and t-test or Mann-Whitney rank sum test (differences between time periods).
Microscopy
The inner surface of the slippery pitcher zone was studied in a scanning electron microscope Hitachi S-4800 (Hitachi High-Technologies Corp., Tokyo, Japan) equipped with a Gatan ALTO 2500 cryo-preparation system (Gatan Inc., Abingdon, UK). For details of sample preparation and mounting, see Gorb and Gorb16 and Benz et al.19. Both total mount specimens and fractured samples were examined at 2 kV acceleration voltage and temperature −120°C.
All measurements of structures from digital images were performed by means of the image analysis software SigmaScan Pro 5.0.0 (SPSS Inc., Chicago, USA). Data obtained were statistically analysed with the SigmaStat 3.5 software (SPSS Inc., Chicago, USA).
Author Contributions
E.V.G. and S.N.G. designed experiments. M.J.B. performed SEM examination, measured and statistically analysed some variables. E.V.G. completed measurements and analyses. E.V.G. and M.J.B. wrote the manuscript. S.N.G. provided corrections of the manuscript.
Supplementary Material
Visualization of isolated wax crystals of the upper wax layer in transmission electron microscope (TEM)
Acknowledgments
Plant material was kindly provided by D. Gotthardt (Botanical Garden at the University of Stuttgart, Stuttgart, Germany). We thank the employees of the greenhouse for taking care of plants and V. Kastner (Tübingen, Germany) for linguistic corrections of the manuscript. This study was partly supported by the SPP 1420 priority program of the German Science Foundation (DFG) ‘Biomimetic Materials Research: Functionality by Hierarchical Structuring of Materials' (project GO 995/9-2) to S.N.G.
References
- Juniper B. E., Robins R. J. & Joel D. M. The Carnivorous Plants (Academic Press, London, 1989). [Google Scholar]
- Moran J. A. Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. J. Ecol. 84, 515–525 (1996). [Google Scholar]
- Moran J. A., Booth W. E. & Charles J. K. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: implications for prey capture. Ann. Bot. 83, 521–528 (1999). [Google Scholar]
- Juniper B. E. & Burras J. K. How pitcher plant trap insects. New Sci. 269, 75–77 (1962). [Google Scholar]
- Ellison A. M. & Gotelli N. J. Evolutionary ecology of carnivorous plants. TREE 16, 623–629 (2001). [Google Scholar]
- Moran J. A. & Clarke C. M. The carnivorous syndrome in Nepenthes pitcher plants: current state of knowledge and potential future directions. Plant Signal. Behav. 5, 644–648 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L., Gorb S. & Rowe N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytol. 156, 479–489 (2002). [DOI] [PubMed] [Google Scholar]
- Koch K. & Barthlott W. Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Phil. Trans. R. Soc. A 367, 1487–1509 (2009). [DOI] [PubMed] [Google Scholar]
- Koch K., Bhushan B. & Barthlott W. Multifunctional surface structures of plants: an inspiration for biomimetics. Prog. Mater. Sci. 54, 137–178 (2009). [Google Scholar]
- Koch K. [Design of Hierarchically Sculptured Biological Surfaces with Anti-adhesive Properties] Proceedings of the Beilstein Bozen Symposium on Functional Nanoscience (http://www.beilstein-institut.de/Bozen2010/Proceedings/Koch/Koch.pdf) 167–178 (October132010). [Google Scholar]
- Wang L. & Zhou Q. Numerical characterization of surface structures of slippery zone in Nepenthes alata pitchers and its mechanism of reducing locust's attachment. Adv. Nat. Sci. 3, 152–160 (2010). [Google Scholar]
- Wong T.-S. et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 477, 443–447 (2011). [DOI] [PubMed] [Google Scholar]
- Gorb E. V. & Gorb S. N. [Anti-adhesive surfaces in plants and their biomimetic potential] Materials Design Inspired by Nature: Function Through Inner Architecture [Frazl, P., Dunlop, J. W. C. & Weinkamer, R. (eds)] 282–309 (RSC Publishing, Cambridge, 2013). [Google Scholar]
- Lloyd F. E. The Carnivorous Plants (Chronica Botanica Company, Waltham, 1942). [Google Scholar]
- Pant D. D. & Bhatnagar S. Morphological studies in Nepenthes (Nepenthaceae). Phytomorphology 27, 13–34 (1977). [Google Scholar]
- Gorb E. & Gorb S. [Functional surfaces in the pitcher of the carnivorous plant Nepenthes alata: a cryo-SEM approach] Functional Surfaces in Biology: Adhesion Related Effects [Gorb, S. N. (ed.)] 205–238 (Springer, Dordrecht, 2009). [Google Scholar]
- Wang L. & Zhou Q. Numerical characterization of surface structures of slippery zone in Nepenthes alata pitchers and its mechanism of reducing locust's attachment. Adv. Nat. Sci. 3, 152–160 (2010). [Google Scholar]
- Gorb E. V. & Gorb S. N. The effect of surface anisotropy in the slippery zone of Nepenthes alata pitchers on beetle attachment. Beilstein J. Nanotechnol. 2, 302–310 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz M. J., Gorb E. V. & Gorb S. N. Diversity of the slippery zone microstructure in pitchers of nine carnivorous Nepenthes taxa. Arthropod-Plant Interactions 6, 147–158 (2012). [Google Scholar]
- Martin J. T. & Juniper B. E. The Cuticle of Plants (Edward Arnold, London, 1970). [Google Scholar]
- Riedel M., Eichner A. & Jetter R. Slippery surfaces of carnivorous plants: composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta 218, 87–97 (2003). [DOI] [PubMed] [Google Scholar]
- Gorb E. et al. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. J. Exp. Biol. 208, 4651–4662 (2005). [DOI] [PubMed] [Google Scholar]
- Riedel M., Eichner A., Meimberg H. & Jetter R. Chemical composition of epicuticular wax crystals on the slippery zone in pitchers of five Nepenthes species and hybrids. Planta 225, 1517–1534 (2007). [DOI] [PubMed] [Google Scholar]
- Scholz I. et al. Slippery surfaces of pitcher plants: Nepenthes wax crystals minimize insect attachment via microscopic surface roughness. J. Exp. Biol. 213, 1115–1125 (2010). [DOI] [PubMed] [Google Scholar]
- Gaume L. et al. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Athropod Struct. Dev. 33, 103–111 (2004). [DOI] [PubMed] [Google Scholar]
- Owen Jr T. P. & Lennon K. A. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae). Am. J. Bot. 86, 1382–1390 (1999). [PubMed] [Google Scholar]
- Jetter R. & Riederer M. Epicuticular crystals of nonacosan-10-ol: in-vitro reconstitution and factors influencing crystal habits. Planta 195, 257–270 (1994). [Google Scholar]
- Jetter R. & Riederer M. In vitro reconstitution of epicuticular wax crystals: formation of tubular aggregates by long chain secondary alkanediols. Bot. Acta 108, 111–120 (1995). [Google Scholar]
- Meusel I., Neinhuis C., Markstädter C. & Barthlott W. Ultrastructure, chemical composition and recrystallisation of epicuticular waxes: transversely ridged rodlets. Can. J. Bot. 77, 706–720 (1999). [Google Scholar]
- Meusel I., Neinhuis C., Markstädter C. & Barthlott W. Chemical composition and recrystallization of epicuticular waxes: coiled rodlets and tubules. Plant Biol. 2, 462–470 (2000). [Google Scholar]
- Neinhuis C., Koch K. & Barthlott W. Movement and regeneration of epicuticular waxes through plant cuticles. Planta 213, 427–434 (2001). [DOI] [PubMed] [Google Scholar]
- Koch K., Neinhuis C., Ensikat H. J. & Barthlott W. Self assembly of epicuticular waxes on living plant surfaces imaged by atomic force microscopy (AFM). J. Exp. Bot. 55, 711–718 (2004). [DOI] [PubMed] [Google Scholar]
- Koch K. et al. Structural analysis of wheat wax (Triticum aestivum cv Naturastar L.): from the molecular level to three dimensional crystals. Planta 223, 258–270 (2006). [DOI] [PubMed] [Google Scholar]
- Gaume L. & Di Giusto B. Adaptive significance and ontogenetic variability of the waxy zone in Nepenthes rafflesiana. Ann. Bot. 104, 1281–1291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork N. E. Role of waxbloom in preventing attachment to brassicas by the mustard beetle Phaedon cochleariae. Ent. Exp. Appl. 28, 100–107 (1980). [Google Scholar]
- Gorb E. & Gorb S. Do plant waxes make insect attachment structures dirty? Experimental evidences for the contamination-hypothesis] Ecology and Biomechanics: a Mechanical Approach to the Ecology of Animals and Plants [Herrel, A., Speck, T. & Rowe, N. (eds)] 147–162 (CRC Press, Boca Raton, 2006). [Google Scholar]
- Juniper B. E. Growth, development, and effect of the environment on ultra-structure of plant surfaces. J. Linn. Soc. Bot. 56, 413–419 (1960). [Google Scholar]
- Baker E. A. [Chemistry and morphology of plant epicuticular waxes] The Plant Cuticle [Cutler, D. F., Alvin, K. L. & Price, C. E. (eds)] 139–166 (Academic Press, London, 1982). [Google Scholar]
- Schwab M., Noga G. & Barthlott W. Einfluβ eines Wasser- und Nährstoffmangels auf die epicuticulären Wachse von Kohlrabi. Angew. Bot. 67, 186–191 (1993). [Google Scholar]
- Juniper B. E. Die Oberflächen von Pflanzen. Endeavour 18, 20–25 (1959). [Google Scholar]
- Hallam N. D. Growth and regeneration of waxes on the leaves of Eucalyptus. Planta 93, 257–268 (1970). [DOI] [PubMed] [Google Scholar]
- Wolter M., Barthlott W., Knoche M. & Noga G. J. Concentration effects and regeneration of epicuticular waxes after treatment with Triton X-100 surfactant. Angew. Bot. 62, 53–62 (1988). [Google Scholar]
- Barthlott W. et al. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 126, 237–260 (1998). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visualization of isolated wax crystals of the upper wax layer in transmission electron microscope (TEM)