Figure 3.
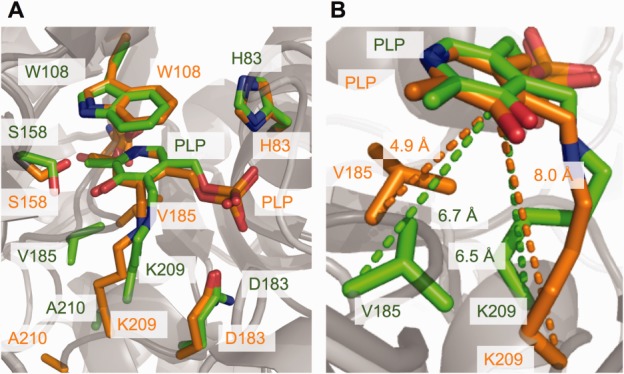
(a) Stick representation of the active site in the S187F variant (green) and normal AGT (orange). Because of the mutation, the main chain atoms of Lys209 in the mutant enzyme move closer to the PLP, which results in a shortened side chain conformation of Lys209. Other active site residues are only slightly affected by the mutation-induced structural changes. (b) Changes in the PLP-Lys209 and PLP-Val185 distances are represented by dashed lines in the respective colors. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
