 |
Dr. Stanley Nattel received his MD from McGill University, then trained in Internal Medicine, Clinical Pharmacology, Cardiology and cardiac Physiology/Pharmacology. He is Paul-David Chair in Cardiovascular Electrophysiology at the University of Montreal and Montreal Heart Institute, Editor-in-Chief of Canadian Journal of Cardiology, Associate Editor of Heart Rhythm and Cardiovascular Research, and Reviewing/Distributing Editor of Journal of Physiology. His research focuses on cardiac bioelectricity and remodeling, particularly atrial fibrillation, ventricular proarrhythmia, ion-channel molecular physiology and mechanisms of disease-substrate development/drug action. His lab uses molecular, cellular, whole-animal and theoretical methods to gain insights into clinically-relevant basic mechanisms and novel therapeutic targets.
Physical activity is an efficient way to fight cardiovascular disease and prolong life expectancy. For many years the concept ‘the more, the better’ has prevailed in relation to physical activity. However, recent studies suggest that high-intensity, long-lasting exercise can have harmful effects. To approach exercise as a ‘drug’ with upper-limit safety thresholds for some individuals, we address three puzzling questions: (1) Why did these deleterious effects remain concealed until recently? (2) What precisely are the potentially deleterious consequences of excessive training? (3) Which mechanisms mediate these negative consequences?
Endurance exercise: the dose makes the poison
Recent American Heart Association (AHA) Guidelines propose thrice-weekly 20 min vigorous exercise sessions (Haskell et al. 2007). This recommendation stems from epidemiological studies showing graded benefit when exercise load is categorized into quantiles. However, categorization does not reveal outcomes for small groups that remain obscured within larger populations. Thus, exercise benefits might not apply to extreme forms, which are poorly represented in most epidemiological studies. AHA guidelines recognize that ‘[…] the shape of the dose–response curves, the possible points of maximal benefit, […] remain unclear.’ Accordingly, deleterious effects of exercise should be specifically sought beyond a threshold where exercise becomes potentially excessive.
In the absence of such knowledge, extreme exercise is gaining adherents. In the USA, more than 500,000 runners finished a marathon in 2012 (Fig. 1), this number having steadily increased for the last 20 years (Lamppa, 2013). Typically, preparing for a marathon requires 20–40 running-miles per week, along with cross-training. This exercise regime is several-fold greater than that recommended by the AHA (Haskell et al. 2007). Moreover, the prevalence of extreme training adherents will probably continue to rise, increasing the potential impact of negative cardiac effects of excess exercise.
Figure 1.
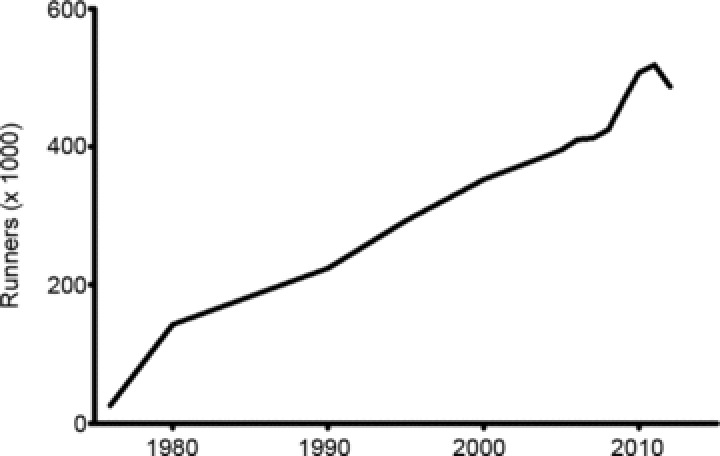
Temporal evolution of numbers of individuals participating in marathon runs in the USA
Is there evidence for exercise-induced cardiac dysfunction?
An increasing body of evidence points to increased arrhythmia incidence and a risk of accelerated atherosclerosis in highly trained athletes. Isolated ventricular premature beats and ventricular runs are frequent in athletes and reversible after detraining (Biffi et al. 2004). Complex arrhythmias usually arise from a dysfunctional and enlarged right ventricle (RV) (Ector et al. 2007). Symptomatic ventricular arrhythmias in predisposed individuals subjected to high-intensity training are associated with increased sudden death risk when accompanied by arrhythmia inducibility at electrophysiological study (Heidbuchel et al. 2003) or in the presence of overt cardiac disease (Biffi et al. 2002).
Studies in Finnish orienteering runners first suggested an increased incidence of atrial fibrillation (AF) in athletes (Karjalainen et al. 1998). These results were later confirmed in elite endurance sport practitioners such as marathon runners (Molina et al. 2008), cyclists (Baldesberger et al. 2008) and Nordic skiers (Grimsmo et al. 2010). Overall, AF risk is increased 5- to 10-fold in elite athletes. Furthermore, AF risk correlates with the number of finished marathons, indicating a dose–response relationship (Wilhelm et al. 2012). Most epidemiological studies failing to show increased AF risk with exercise training in general populations probably had an underrepresentation of highly trained athletes (Ofman et al. 2013). These results underline the fact that high-intensity training is needed to promote AF.
Recent analyses suggest reversal of the beneficial effects of exercise on coronary artery disease at high exercise levels in elite athletes. Increased coronary artery calcium score in veteran marathon runners indicates a higher atherosclerotic burden (Mohlenkamp et al. 2008). Moderate exercise-induced survival benefit is lost in individuals jogging at a fast pace more than 4 h week−1 (Schnohr et al. 2013). Further studies focusing on the atherosclerotic burden in high-intensity athletes are warranted.
Linking high-intensity exercise and pathology
Myocardial fibrosis is a hallmark of maladaptive cardiac remodelling associated with long-term endurance training. Recent experimental reports show myocardial fibrosis in the atria (Guasch et al. 2013) and RV (Benito et al. 2011) of high-intensity trained rats, providing a substrate for arrhythmias. Plasma fibrosis markers are increased in veteran endurance athletes (Lindsay & Dunn, 2007). In the atria, the electrocardiographic P-wave duration (reflecting atrial conduction time) is increased in marathon runners, a finding not explained by changes in atrial size and thus suggesting substrate modification (Wilhelm et al. 2011). RV tissue samples obtained from selected athletes with a high burden of ventricular arrhythmias show inflammatory infiltrates and fibrosis (Dello et al. 2011).
Magnetic resonance imaging has proven valuable for non-invasive assessment of left ventricular (LV) structural remodelling. Late gadolinium enhancement, an indicator of myocardial fibrosis, is detectable in the LV of roughly 10% of marathon runners (Mohlenkamp et al. 2008; Breuckmann et al. 2009; La Gerche et al. 2012). Among very extreme athletes (averaging four ironman triathlons, 65 ultra-marathons and 178 marathons), up to 50% presented with ischaemic or myocarditis-like LV myocardial fibrosis patterns (Wilson et al. 2011). Case reports showing diffuse LV fibrosis in autopsy specimens from highly trained athletes support these studies.
Mechanisms leading to myocardial fibrosis in endurance athletes remain elusive. Haemodynamic changes probably play an important role. Intense physical activity induces a 6-fold increase in cardiac output and doubles systemic and pulmonary systolic blood pressure, to which the RV is particularly sensitive because of its thin wall and particular geometry (La Gerche et al. 2011). Repetitive insults eventually lead to RV dilatation and dysfunction (La Gerche et al. 2012), particularly in genetically prone individuals (Kirchhof et al. 2006). The renin–angiotensin–aldosterone system probably contributes. Ultramarathon running transiently increases plasma concentrations of the profibrotic neurohormone aldosterone 5-fold (Burge et al. 2011), and the angiotensin-receptor blocker losartan prevents experimental exercise-induced myocardial fibrosis (Gay-Jordi et al. 2013). Marathon running induces transient immune deficiency after a race (Gleeson et al. 2011), which may facilitate subclinical myocarditis and ventricular scarring.
The cardiac chamber dilatation occurring in athletes might contribute to arrhythmogenesis (Guasch et al. 2013) by increasing vulnerability to re-entry. The incomplete reversal of left atrial and LV dilatation several years after the suspension of exercise training further supports a ‘non-physiological’ enlargement in highly trained athletes (Pelliccia et al. 2002).
Endurance training initiates a complex cascade of proinflammatory followed by anti-inflammatory cytokines. Exercise-induced inflammation develops earlier and more intensely (Kim et al. 2009), while persisting for longer periods (Scherr et al. 2011), as the exercise duration increases.
Other factors could play a dual role. Parasympathetic enhancement in athletes drives beneficial anti-inflammatory effects and those preventing sudden death, but is also a potentially central contributor to the AF substrate associated with repetitive high-intensity endurance exercise (Guasch et al. 2013).
Summary and conclusions
The health benefits of moderate exercise are well established, but there is growing evidence of the potentially deleterious cardiovascular effect of sustained, very high-intensity training. This evidence raises several important and unanswered questions: Does a deleterious-effect threshold exist? Is such a threshold present in all individuals, or only in those who are predisposed to high-level exercise risks? How can we identify the threshold for individual athletes? Are all sorts of exercise equivalent? The answers to these questions are important if we are to ensure that the beneficial cardiac effects of exercise are not compromised by overdosing the medicine.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word’. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;591/20/4939
Additional information
Competing interests
The authors declare no conflict of interest.
Funding
Supported by the Canadian Institutes of Health Research (MOP68929 and MGP6957), the Heart and Stroke Foundation of Canada and the Fondation Leducq.
References
- Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, Jenni R, Oechslin E, Luthi P, Scharf C, Marti B, Attenhofer Jost CH. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–78. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S, Mont L. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- Biffi A, Maron BJ, Verdile L, Fernando F, Spataro A, Marcello G, Ciardo R, Ammirati F, Colivicchi F, Pelliccia A. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2004;44:1053–1058. doi: 10.1016/j.jacc.2004.05.065. [DOI] [PubMed] [Google Scholar]
- Biffi A, Pelliccia A, Verdile L, Fernando F, Spataro A, Caselli S, Santini M, Maron BJ. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2002;40:446–452. doi: 10.1016/s0735-1097(02)01977-0. [DOI] [PubMed] [Google Scholar]
- Breuckmann F, Mohlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, Sievers B, Schlosser T, Jockel KH, Heusch G, Erbel R, Barkhausen J. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–57. doi: 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- Burge J, Knechtle B, Knechtle P, Gnadinger M, Rust CA, Rosemann T. Maintained serum sodium in male ultra-marathoners–the role of fluid intake, vasopressin, and aldosterone in fluid and electrolyte regulation. Horm Metab Res. 2011;43:646–652. doi: 10.1055/s-0031-1284352. [DOI] [PubMed] [Google Scholar]
- Dello RA, Pieroni M, Santangeli P, Bartoletti S, Casella M, Pelargonio G, Smaldone C, Bianco M, Di BL, Bellocci F, Zeppilli P, Fiorentini C, Natale A, Tondo C. Concealed cardiomyopathies in competitive athletes with ventricular arrhythmias and an apparently normal heart: role of cardiac electroanatomical mapping and biopsy. Heart Rhythm. 2011;8:1915–1922. doi: 10.1016/j.hrthm.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Ector J, Ganame J, van der Merwe N, Adriaenssens B, Pison L, Willems R, Gewillig M, Heidbuchel H. Reduced right ventricular ejection fraction in endurance athletes presenting with ventricular arrhythmias: a quantitative angiographic assessment. Eur Heart J. 2007;28:345–353. doi: 10.1093/eurheartj/ehl468. [DOI] [PubMed] [Google Scholar]
- Gay-Jordi G, Guash E, Benito Ba, Brugada J, Nattel S, Mont Ls, Serrano-Mollar A. Losartan prevents heart fibrosis induced by long-term intensive exercise in an animal model. PLoS ONE. 2013;8:e55427. doi: 10.1371/journal.pone.0055427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Grimsmo J, Grundvold I, Maehlum S, Arnesen H. High prevalence of atrial fibrillation in long-term endurance cross-country skiers: echocardiographic findings and possible predictors–a 28–30 years follow-up study. Eur J Cardiovasc Prev Rehabil. 2010;17:100–105. doi: 10.1097/HJR.0b013e32833226be. [DOI] [PubMed] [Google Scholar]
- Guasch E, Benito B, Qi XY, Cifelli C, Naud P, Shi Y, Mighiu A, Tardif JC, Tadevosyan A, Chen Y, Gillis MA, Iwasaki Y, Dobrev D, Mont L, Heximer S, Nattel S. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol. 2013;62:68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- Heidbuchel H, Hoogsteen J, Fagard R, Vanhees L, Ector H, Willems R, Van LJ. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J. 2003;24:1473–1480. doi: 10.1016/s0195-668x(03)00282-3. [DOI] [PubMed] [Google Scholar]
- Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case-control study. BMJ. 1998;316:1784–1785. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee Y, Kim C. Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42.195 km) and an ultra-marathon (200 km) race. Eur J Appl Physiol. 2009;105:765–770. doi: 10.1007/s00421-008-0961-x. [DOI] [PubMed] [Google Scholar]
- Kirchhof P, Fabritz L, Zwiener M, Witt H, Schafers M, Zellerhoff S, Paul M, Athai T, Hiller KH, Baba HA, Breithardt G, Ruiz P, Wichter T, Levkau B. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation. 2006;114:1799–1806. doi: 10.1161/CIRCULATIONAHA.106.624502. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbuchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- La Gerche A, Heidbuchel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI, Prior DL. Disproportionate exercise load and remodeling of the athlete's right ventricle. Med Sci Sports Exerc. 2011;43:974–981. doi: 10.1249/MSS.0b013e31820607a3. [DOI] [PubMed] [Google Scholar]
- Lamppa R. 2013. Running USA 2013 Annual Report [online]. Obtained from http://www.runningusa.org/index.cfm?fuseaction=news.details&ArticleId=332&returnTo=annual-reports. Retrieved 27 May 2013.
- Lindsay MM, Dunn FG. Biochemical evidence of myocardial fibrosis in veteran endurance athletes. Br J Sports Med. 2007;41:447–452. doi: 10.1136/bjsm.2006.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlenkamp S, Lehmann N, Breuckmann F, Brocker-Preuss M, Nassenstein K, Halle M, Budde T, Mann K, Barkhausen J, Heusch G, Jockel KH, Erbel R. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29:1903–1910. doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, Rebato C, Elosua R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008;10:618–623. doi: 10.1093/europace/eun071. [DOI] [PubMed] [Google Scholar]
- Ofman P, Khawaja O, Rahilly-Tierney CR, Peralta A, Hoffmeister P, Reynolds MR, Gaziano JM, Djousse L. Regular physical activity and risk of atrial fibrillation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2013;6:252–256. doi: 10.1161/CIRCEP.113.000147. [DOI] [PubMed] [Google Scholar]
- Pelliccia A, Maron BJ, De LR, Di Paolo FM, Spataro A, Culasso F. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation. 2002;105:944–949. doi: 10.1161/hc0802.104534. [DOI] [PubMed] [Google Scholar]
- Scherr J, Braun S, Schuster T, Hartmann C, Moehlenkamp S, Wolfarth B, Pressler A, Halle M. 72-h kinetics of high-sensitive troponin T and inflammatory markers after marathon. Med Sci Sports Exerc. 2011;43:1819–1827. doi: 10.1249/MSS.0b013e31821b12eb. [DOI] [PubMed] [Google Scholar]
- Schnohr P, Marott JL, Lange P, Jensen GB. Longevity in male and female joggers: the Copenhagen City Heart Study. Am J Epidemiol. 2013;177:683–689. doi: 10.1093/aje/kws301. [DOI] [PubMed] [Google Scholar]
- Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, Gaze D, Thompson PD. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol. 2010;56:169–176. doi: 10.1016/j.jacc.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Roten L, Tanner H, Schmid JP, Wilhelm I, Saner H. Long-term cardiac remodeling and arrhythmias in nonelite marathon runners. Am J Cardiol. 2012;110:129–135. doi: 10.1016/j.amjcard.2012.02.058. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Roten L, Tanner H, Wilhelm I, Schmid JP, Saner H. Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am J Cardiol. 2011;108:580–585. doi: 10.1016/j.amjcard.2011.03.086. [DOI] [PubMed] [Google Scholar]
- Wilson M, O’Hanlon R, Prasad S, Deighan A, Macmillan P, Oxborough D, Godfrey R, Smith G, Maceira A, Sharma S, George K, Whyte G. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol. 2011;110:1622–1626. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


