Abstract
Ageing is thought to be associated with decreased vascular function partly due to oxidative stress. Resveratrol is a polyphenol, which in animal studies has been shown to decrease atherosclerosis, and improve cardiovascular health and physical capacity, in part through its effects on Sirtuin 1 signalling and through an improved antioxidant capacity. We tested the hypothesis that resveratrol supplementation enhances training-induced improvements in cardiovascular health parameters in aged men. Twenty-seven healthy physically inactive aged men (age: 65 ± 1 years; body mass index: 25.4 ± 0.7 kg m−2; mean arterial pressure (MAP): 95.8 ± 2.2 mmHg; maximal oxygen uptake: 2488 ± 72 ml O2 min−1) were randomized into 8 weeks of either daily intake of either 250 mg trans-resveratrol (n= 14) or of placebo (n= 13) concomitant with high-intensity exercise training. Exercise training led to a 45% greater (P < 0.05) increase in maximal oxygen uptake in the placebo group than in the resveratrol group and to a decrease in MAP in the placebo group only (−4.8 ± 1.7 mmHg; P < 0.05). The interstitial level of vasodilator prostacyclin was lower in the resveratrol than in the placebo group after training (980 ± 90 vs. 1174 ± 121 pg ml−1; P < 0.02) and muscle thromboxane synthase was higher in the resveratrol group after training (P < 0.05). Resveratrol administration also abolished the positive effects of exercise on low-density lipoprotein, total cholesterol/high-density lipoprotein ratio and triglyceride concentrations in blood (P < 0.05). Resveratrol did not alter the effect of exercise training on the atherosclerosis marker vascular cell adhesion molecule 1 (VCAM-1). Sirtuin 1 protein levels were not affected by resveratrol supplementation. These findings indicate that, whereas exercise training effectively improves several cardiovascular health parameters in aged men, concomitant resveratrol supplementation can blunt these effects.
Key points
In rodents, resveratrol has been shown to enhance training-induced changes in cardiovascular function, exercise performance and the retardation of atherosclerosis. We examined the effect of 8 weeks of exercise training with and without concomitant resveratrol supplementation in aged men.
Exercise training potently improved blood pressure, blood cholesterol, maximal oxygen uptake and the plasma lipid profile.
Resveratrol supplementation reduced the positive effect of exercise training on blood pressure, blood cholesterol and maximal oxygen uptake and did not affect the retardation of atherosclerosis.
Whereas exercise training improved formation of the vasodilator prostacyclin, concomitant resveratrol supplementation caused a shift in vasoactive systems favouring vasoconstriction.
The present study is the first to demonstrate negative effects of resveratrol on training-induced improvements in cardiovascular health parameters in humans and adds to the growing body of evidence questioning the positive effects of resveratrol supplementation in humans.
Introduction
A sedentary lifestyle with a subsequent poor physical fitness level is one of the major cardiovascular risk factors in otherwise healthy individuals (Perk et al. 2012). Moreover, atherosclerosis, and its clinical sequelae, are well-described consequences of a physically inactive lifestyle and progressing age (Wang & Bennett, 2012). Lowering of blood lipids other than high-density lipoprotein (HDL) has a major impact on decreasing the atherosclerotic process (Perk et al. 2012) and exercise training has been recognized as an intervention for improving the balance of blood lipids (Lopez-S et al. 1974). The plasma vascular cell adhesion molecule 1 (VCAM-1) level is a good indicator of atherosclerosis progression (Peter et al. 1997) and a positive effect of exercise training on plasma VCAM-1 levels has previously been shown (Schumacher et al. 2006; Rankovićet al. 2009).
In young healthy subjects, skeletal muscle blood flow and oxygen supply are tightly regulated to match oxygen demand of the muscle (Andersen & Saltin, 1985). The precise vascular control is the result of a complex interplay between sympathetic nervous activity, mechanical factors and vasoactive substances including nitric oxide (NO), prostacyclin (PGI2) and endothelin-1 (ET-1) (Maeda et al. 1997; Clifford & Hellsten, 2004; Hellsten et al. 2012b). With age, vascular function is impaired (Nyberg et al. 2012), resulting in increased resting blood pressure and decreased limb blood flow for a given submaximal workload (Wahren et al. 1974; Proctor et al. 1998; Lawrenson et al. 2003; Kirby et al. 2009; Nyberg et al. 2012; Mortensen et al. 2012b). These age-related vascular changes have been shown to be associated with alterations within the NO, PGI2 and ET-1 systems (Taddei et al. 1995, 2001; Stauffer et al. 2008; Kirby et al. 2009). Specifically, reactive oxygen species (ROS) have been shown to have adverse effects on these systems by interfering with PGI2 and NO synthesis and by scavenging of NO (Higashi et al. 2002; Schulz et al. 2008; Schildknecht & Ullrich, 2009). ROS formation occurs continuously and, although deleterious to cell vitality when excessive, ROS also exert beneficial functions such as in molecular signalling (Gomes et al. 2012). This may be why ROS production and removal is well regulated in young healthy subjects (Gomes et al. 2012). In aged subjects, however, ROS levels appear to be less well regulated as production increases and the endogenous antioxidant defence decreases leading to adverse oxidative stress and decreased vascular function (Wei et al. 1998; Finkel & Holbrook, 2000). Known sources of ROS include NAD(P)H oxidase (NOX), uncoupled endothelial NO synthase (eNOS) and mitochondria (Ungvari et al. 2007a; Durrant et al. 2009; Yang et al. 2009). NOX is expressed in endothelial, smooth muscle and skeletal muscle cells and is the main source of ROS implicated in vascular dysfunction (Schulz et al. 2008) and exercise-induced oxidative stress (Cave et al. 2006). ROS removal is accomplished by antioxidants and antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) (Powers & Lennon, 1999). The endogenous antioxidant defence system is highly important in the prevention of excessive ROS levels, tissue inflammation and consequent vascular dysfunction (Förstermann, 2008). Exercise training effectively enhances blood antioxidant capacity (Miyazaki et al. 2001) and improves vascular function by reducing oxidative stress (Taddei et al. 2000; Eskurza et al. 2004) but the antioxidant defence may also be boosted by exogenous antioxidant supplementation (Anderson et al. 1997; Cao et al. 1998; Hellsten et al. 2007). Thus, as exercise is associated with enhanced production of ROS (Davies et al. 1982; Hellsten et al. 2007), antioxidant supplementation in combination with physical training may be particularly relevant for aged sedentary individuals in whom ROS levels are likely to be less well regulated (Bailey et al. 2010).
The antioxidant resveratrol is a naturally occurring polyphenol (Baur & Sinclair, 2006). In rodents resveratrol supplementation has been shown to decrease cardiovascular risk factors, including blood lipids (Murase et al. 2009) and VCAM-1 (Matos et al. 2012), to improve cardiovascular function and physical capacity (Taubert & Berkels, 2003; Lagouge et al. 2006), and to decrease inflammation in the vasculature of aged animals leading to improved vascular function (Pearson et al. 2008). Specifically, the positive effect of resveratrol on training response and aerobic capacity in rats has been shown to be mediated via sirtuin 1 (SIRT1; Lagouge et al. 2006; Price et al. 2012; Hart et al. 2013). In humans resveratrol has been shown to improve metabolic function in obese men mediated via SIRT1 (Timmers et al. 2011) but recent reports contradict this finding (Skrobuk et al. 2012; Yoshino et al. 2012). Although the effects of resveratrol on cardiovascular health have been examined in rats, few studies have been conducted on humans and to date no study has examined the combined effect of exercise training and resveratrol on vascular function in aged humans.
Based on evidence that (1) ageing is associated with increased oxidative stress, (2) exercise is associated with an increased ROS production and (3) resveratrol improves cardiovascular parameters by decreasing ROS- and via SIRT1-mediated signalling, the following hypothesis was tested in the present study: oral resveratrol supplementation enhances the positive cardiovascular adaptations to exercise training in aged subjects by increasing SIRT1-mediated signalling and by promoting the endogenous antioxidant system. We conducted a double blind exercise training study including 27 aged healthy men randomized into two groups. Both groups underwent 8 weeks of high-intensity exercise training and one group received resveratrol and the other group placebo supplementation.
Methods
Ethical approval
The study was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (H-2-2011-079) and was conducted in accordance with the latest guidelines of the Declaration of Helsinki. Written informed consent was obtained from all subjects before enrollment in the study.
Subjects
Twenty-seven healthy aged (60–72 years) physically inactive (less than 2 h of moderate intensity physical activity per week) men were recruited. All subjects were non-smokers and underwent a medical examination. None had been diagnosed with cardiovascular disease, hypertension, renal dysfunction, insulin resistance or type 2 diabetes and all subjects had normal electrocardiogram. Two subjects were diagnosed with hypercholesterolaemia regulated by their own physician (medication was maintained during the experimental period) whereas the other participants had normal cholesterol levels.
Randomization
The study was of a randomized double-blind placebo-controlled design. Subjects were allocated to either a combination of exercise training and placebo (n= 13) or exercise training and 250 mg day−1 trans-resveratrol (Fluxome Inc., Stenlose, Denmark; n= 14) based on maximal oxygen consumption, body mass index (BMI), blood glucose and cholesterol. The interventions lasted for 8 weeks. The subjects received tablets every 2 weeks and were instructed to take their daily tablet at the same time every morning. Subjects noted time of consumption for each tablet and any discomforts that might appear throughout the intervention period.
Exercise training regime
All subjects performed 8 weeks of supervised high-intensity interval training (cycle ergometer) twice a week and full body circuit training (Crossfit) once a week. Intensity of the training sessions was controlled with TEAM2 WearLink+ (Polar, Kempele, Finland) heart rate monitors. In addition subjects conducted a timed 5 km walk once a week.
Pre-testing
Before the two main experimental days the subjects visited the laboratory where body composition was determined with whole-body dual-energy X-ray absorptiometry scanning (DEXA; Prodigy, GE Healthcare, Chalfont St. Giles, UK). In addition femoral arterial blood flow was measured at rest, after 30 s of passive leg movement and during one-leg knee-extensor exercise (at 10 and 30 W) with ultrasound Doppler (Logic E9, GE Healthcare, Pittsburgh, PA, USA) equipped with a linear probe operating at an imaging frequency of 9 MHz and Doppler frequency of 4.2–5.0 MHz (Nyberg et al. 2012). Due to technical issues passive flow was only measured after training in the two groups. After 30 min rest, subjects performed an incremental bicycle ergometer test to determine maximal pulmonary oxygen uptake (l min−1; Oxycon Pro, Viasys Healthcare, Hoechberg, Germany). For determination of performance related to functional performance, subjects conducted the following series of tests on a separate day: (1) a timed ‘Up & Go’ test (Podsiadlo & Richardson, 1991), as a test of functional mobility, (2) a 30 s ‘Chari-stand’ test (Jones et al. 1999), as a test of lower body strength, (3) ‘The New Danish Steptest’ (Isaksen & Pedersen, 2006), as a test of maximal functional capacity, and (4) ‘Unipedal stance test’ (Springer et al. 2007), as a test of balance.
Experimental days
On the first experimental day, subjects arrived at the laboratory after an overnight fast. Blood samples were collected and a muscle biopsy was obtained from m. vastus lateralis with percutaneous needle biopsy technique (Bergstrom, 1975).
On the second experimental day, subjects were seated in a one-leg knee-extensor ergometer and performed a 10 min exercise bout (10 W) to become accustomed to the movement. After local anaesthesia (lidocaine, 20 mg ml−1), four custom-made microdialysis probes with a 4 cm membrane (960 kDa cut-off) were inserted into the m. vastus lateralis of the experimental leg. After insertion of the probes, the subjects performed another 10 min knee-extensor exercise bout (10 W) to minimize the tissue response to insertion trauma (Nordsborg et al. 2003). The probes were perfused with Ringer acetate buffer at a rate of 5 μl min−1 and to determine the relative exchange over the membrane, a small amount (2.7 nm) of [2-3H] adenosine (<0.1 μCi ml−1) was added to the perfusate, to allow for calculation of probe recovery. After 45 min of rest microdialysate was collected for 3 × 20 min, while subjects were resting. After 1.5 h of supine rest blood pressure was measured three consecutive times with an automatic sphygmomanometer (M7, OMRON, Vernon Hills, IL, USA) on the left and right upper arm. Subjects then performed 45 min of knee-extensor exercise (10 W) and dialysate was collected for 2 × 20 min, excluding the first 5 min to account for probe delay. Immediately after collection, samples were weighed, 5 μl of the dialysate was allocated into 3 ml Ultima Gold (Perkin Elmer, Waltham, MA, USA) scintillation liquid and the remaining dialysate was frozen at −80°C. Probe recovery (PR) was calculated as [PR = (dpminfusate– dpmdialysate/dpminfusate)], where dpm denotes disintegrations per minute (Scheller & Kolb, 1991; Jansson et al. 1994). The [2-3H] ATP activity of the dialysate was measured on a liquid scintillation counter (Tri-Carb 2910 TR; Perkin Elmer). Probes with abnormal perfusion rate (>±10%) were excluded.
All tests and experimental days were performed before and after the 8 week intervention period.
Quantification of protein expression by Western blot
Freeze-dried tissue samples were dissected free of connective tissue, visible fat and blood under a stereomicroscope with an ambient temperature of ∼18°C and relative humidity below 30%. The tissue samples were homogenized in lysis buffer and Western blot analysis was preformed as previously described (Høier et al. 2011) with the exception that the membrane image was digitalized on a ChemiDoc MP system (Bio-Rad, Hercules, CA, USA). Equal amounts of total protein were loaded for each sample in accordance with the antibody optimization (detailed antibody information is available in supplemental Table S1). Samples from each group were distributed evenly across the gel and all samples from one subject were loaded on the same gel. To control for loading differences, the blots were also analysed for GAPDH.
Analysis of NOx, ET-1 and VCAM-1 in plasma
The stable metabolites of NO in plasma, nitrite and nitrate, were measured using a fluorometric EIA kit (Cayman Chemical Co., Ann Harbor, MI, USA). ET-1 and VCAM-1 in plasma were measured with a Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA).
Analysis of 6-keto prostaglandin F1α in microdialysate
The stable metabolite of PGI2, 6-keto prostaglandin F1α, in microdialysate was measured using a fluorometric assay kit (Cayman Chemical Co.).
Statistical analysis
To test the effect of training and training plus resveratrol, a two-way repeated-measures ANOVA was conducted. After a significant F-test, pairwise differences were identified using the Student–Newman–Keuls post hoc procedure. The significance level was set at P < 0.05. Data are means ± SEM and n= 13/14 for the placebo/resveratrol groups, respectively, unless otherwise stated. The occasional missing data were either due to sample limitations or technical issues.
Results
Compliance with the interventions
Both groups exhibited high training compliance and completed an average 1.9 ± 0.1 and 1.0 ± 0.1 spinning and Crossfit sessions per week. Training intensity was equally high in both groups (67% of training time above 70% maximum heart rate (HRmax) and 14% of training time above 90% HRmax). Based on self-reports all subjects took their daily tablet and the ingested doses of resveratrol were well tolerated by the subjects.
Functional capacity
Maximal oxygen uptake was not different between the groups before training but 8 weeks of exercise training increased maximal pulmonary oxygen uptake more (P < 0.05) in the placebo group than in the resveratrol group (443 ± 38 and 308 ± 46 ml O2 min−1 increase in the placebo and resveratrol group, respectively; P= 0.03; Table 1). After training, maximal oxygen uptake was higher in the placebo group than in the resveratrol group (P < 0.05; Fig. 1). Performances in the timed Up & Go test, 30 s Chair-stand test, Step test and Unipedal stance test were not different between groups before training. After training, performance in the Up & Go test was increased in the placebo group only (P < 0.001) while performances in the Chair-stand test, Step test and 5k walk test were increased in both groups (P < 0.001). Balance was unaffected by the interventions in both groups (Table S2).
Table 1.
Subject characteristics
| Placebo, n= 13 | Resveratrol, n= 14 | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age (years) | 65 ± 1 | – | 65 ± 1 | – |
| Weight (kg) | 84.2 ± 2.2 | 83.0 ± 2.0* | 79.8 ± 3.8 | 79.5 ± 3.7 |
| Body mass index (kg m−2) | 26.0 ± 0.5 | 25.7 ± 0.5 | 25.4 ± 0.7 | 25.3 ± 0.7 |
| Body fat (%) | 25.8 ± 1.5 | 24.4 ± 1.4* | 27.6 ± 1.9 | 26.1 ± 1.8* |
| MAP (mmHg) | 95.3 ± 2.2 | 90.8 ± 1.8* | 96.3 ± 3.0 | 93.7 ± 2.7 |
| HRrest (b.p.m.) | 63 ± 2 | 58 ± 2* | 63 ± 1 | 61 ± 2 |
| HRmax (b.p.m.) | 168 ± 4 | 168 ± 3 | 167 ± 5 | 164 ± 4 |
 (ml O2 min−1) (ml O2 min−1) |
2582 ± 84 | 3026 ± 88* | 2402 ± 113 | 2710 ± 112*# |
 (ml O2 min−1) (ml O2 min−1) |
– | 444 ± 38 | – | 308 ± 46 # |
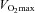 kg−1 (ml O2 min−1 kg−1) kg−1 (ml O2 min−1 kg−1) |
30.8 ± 1.2 | 36.6 ± 1.1* | 30.1 ± 1.0 | 34.1 ± 0.9*# |
| Glucose (mmol l−1) | 5.3 ± 0.1 | 5.2 ± 0.1 | 5.4 ± 0.2 | 5.4 ± 0.2 |
| Total cholesterol (TC, mmol l−1) | 5.1 ± 0.2 | 4.9 ± 0.3 | 5.6 ± 0.2 | 5.6 ± 0.3 |
| HDL (mmol l−1) | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 |
| LDL (mmol l−1) | 3.3 ± 0.2 | 3.0 ± 0.2* | 3.6 ± 0.2 | 3.4 ± 0.2 |
| TC/HDL ratio | 3.9 ± 0.3 | 3.6 ± 0.3* | 4.3 ± 0.3 | 4.1 ± 0.3 |
| Triglycerides (mmol l−1) | 1.3 ± 0.2 | 1.0 ± 0.2* | 1.4 ± 0.2 | 1.5 ± 0.2 |
| VCAM-1 (ng ml−1) | 519 ± 45 | 450 ± 21* | 554 ± 29 | 456 ± 26* |
Data are presented as mean ± SEM. *Significantly different from pre; #significantly different from placebo (P < 0.05). MAP, mean arterial pressure; HR, heart rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VCAM-1, vascular cell adhesion molecule 1.
Figure 1. Absolute changes in maximal oxygen uptake from baseline to after a period of exercise training with or without resveratrol supplementation in aged men.
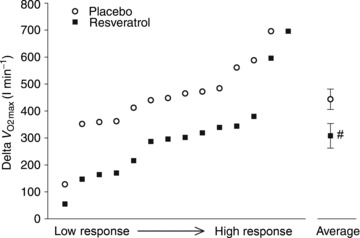
Left, individual delta values in maximal oxygen uptake ( ) arranged from low change to high change. Right, group mean delta values in maximal oxygen uptake. Data are presented as mean ± SEM. #Significantly different from placebo (P < 0.05).
) arranged from low change to high change. Right, group mean delta values in maximal oxygen uptake. Data are presented as mean ± SEM. #Significantly different from placebo (P < 0.05).
Body composition
Training did not change BMI but total body fat was decreased similarly in both groups after training relative to baseline (P < 0.05; Table 1).
Cardiovascular parameters
Mean arterial blood pressure was not different between the placebo group and resveratrol group before training but was 5 mmHg lower after than before training in the placebo group only (P < 0.005, n= 13/13, Table 1). Blood glucose, total cholesterol (TC) and HDL concentrations were not different between groups and were not changed with training. The TC/HDL ratio and concentrations of low-density lipoprotein (LDL) and triglycerides were not different between groups before training, but were lower in the placebo group only after training (P < 0.05, n= 13/13, Table 1). VCAM-1 levels were similar between groups before training and decreased significantly in both groups with training (P < 0.05; Table 1).
Training did not change exercise hyperemia in either group and there was no difference in blood flow between groups at rest, after 30 s passive work (data were collected only for rest and passive work post intervention), or at either 10 or 30 W workloads before or after the training period (n= 13/12, Fig. 2).
Figure 2. Leg blood flow in response to exercise and passive movement in aged men after 8 weeks of either a placebo or resveratrol supplementation in combination with endurance training.
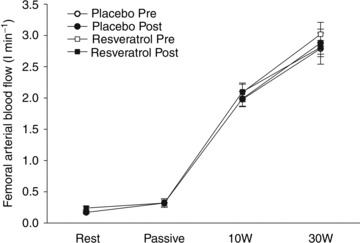
Femoral arterial blood flow was measured at rest and after 30 s of passive leg extension movement and during active leg extensions performed at a work load of 10 and 30 W. Blood flow during exercise was determined both before and after training. Rest and passive leg movement flows were only measured post intervention. Data are presented as mean ± SEM.
Protein expression in skeletal muscle
eNOS levels were not different between groups before training and were not affected by training. Phosphorylation of eNOS at serine residue 1177 (eNOS-PSer1177; phosphorylation at this residue increases enzyme activity) was not different between groups before training, but was decreased by 11% in the placebo group with training (P < 0.01, n= 11/13; Fig. 3A). Neural NOS (nNOS) protein amount was not different between groups before or after training with no effect of resveratrol. There was a significant overall increase in nNOS protein expression with training (P < 0.02; Fig. 3A) independent of groups. Muscle PGI2-synthase protein expression was similar in the two groups before and after training and the PGI2-synthase protein levels increased similarly, 31 and 43% (P < 0.05, n= 12/14; Fig. 3B), with training in the placebo and resveratrol group, respectively. COX-1 and COX-2 protein expression in muscle homogenates were not different between groups before or after training. There was a significant overall increase in COX-1 protein expression with training (P < 0.05; Fig. 3B) with no difference between groups. COX-2 increased similarly in both groups with training (P < 0.001, n= 12/14; Fig. 3B). Thromboxane (TBX) synthase was not different between the groups before training but increased 61% with training in the resveratrol group only (P < 0.01; Fig. 3B).
Figure 3. Protein expression and phosphorylation status of nitric oxide synthase before and after a period of exercise training and either placebo or resveratrol supplementation in aged men.
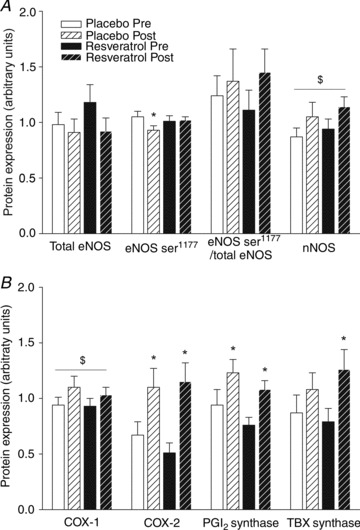
A, protein expression of total endothelial nitric oxide synthase (eNOS), phosphorylated eNOS (eNOSSer1177), ratio of eNOSSer1177 to total eNOS, and neural NOS (nNOS) in homogenates from m. vastus lateralis. Data are presented as mean ± SEM. *Significantly different from pre training; $significant effect of time (P < 0.05). B, protein expression of the prostaglandin system enzymes. Protein expression of cyclooxygenase 1 (COX-1), cyclooxygenase 2 (COX-2), prostacyclin synthase (PGI2) and thromboxane synthase (TBX) in homogenates from m. vastus lateralis. Data are presented as mean ± SEM. *Significantly different from pre training; $significant effect of time (P < 0.05).
ET-1 levels in muscle lysate were similar in the two groups prior to training and did not change with training (Fig. 4). Muscle endothelin receptor type A (ET-A) levels were not different between the placebo and the resveratrol group before training and were increased similarly with training (P < 0.02, n= 12/13; Fig. 4). Endothelin receptor type B (ET-B) protein content was not different between groups before training but increased significantly in the resveratrol group with training (P < 0.05, n= 12/13; Fig. 4). Muscle SIRT1 protein content was similar in the two groups before the training intervention and did not change with training in either group (Fig. 5). Content of NOX in muscle lysate was similar in the placebo and resveratrol groups before training and increased 55 and 71% in the placebo and resveratrol group, respectively (P < 0.001, n= 12/14; Fig. 6) with training. CAT, GPX-1 and SOD1 protein amounts were not different between groups either before or after training. GPX-1 protein content showed a significant overall increase with training (P < 0.05; Fig. 6). SOD2 protein expression was not different between groups before training and increased by 30 and 50% in the placebo and the resveratrol group, respectively, with training (P < 0.05; Fig. 6).
Figure 4. Muscle endothelin-1 protein expression.
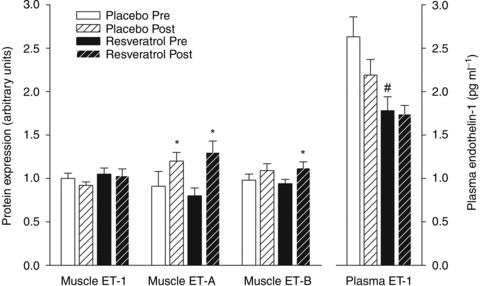
Protein expression of endothelin 1 (ET-1), endothelin receptor A (ET-A) and endothelin receptor B (ET-B) in homogenates from m. vastus lateralis and fasting plasma levels of ET-1 before and after a period of exercise training and either placebo or resveratrol supplementation in aged men. Data are presented as mean ± SEM. *Significantly different from pre training, #significantly different from placebo (P < 0.05).
Figure 5. Protein expression of SIRT1 before and after a period of exercise training and either placebo or resveratrol supplementation in aged men.

Protein expression of sirtuin 1 (SIRT1) in homogenates from m. vastus lateralis. Data are presented as mean ± SEM.
Figure 6. Protein expression of endogenous antioxidant enzymes before and after a period of exercise training and either placebo or resveratrol supplementation in aged men.
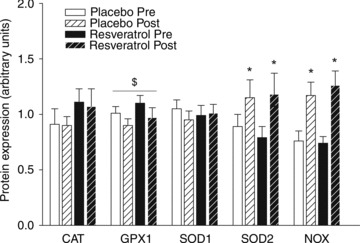
Protein expression of catalase (CAT), gluthatione peroxidase 1 (GPX1), superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2) and NAD(P)H oxidase (NOX) in homogenates from m. vastus lateralis. Data are presented as mean ± SEM. *Significantly different from pre training; $significant effect of time (P < 0.05).
Interstitial prostacyclin
PGI2 in dialysate from m. vastus lateralis at rest was not different between the placebo group and the resveratrol group before training (1143 ± 122 vs. 1233 ± 198 pg ml−1), but was higher in the placebo group than in the resveratrol group after training (1174 ± 121 vs. 980 ± 90 pg ml−1; P < 0.02, n= 13/12). Before training PGI2 was unchanged during exercise in the placebo group (1397 ± 172 pg ml−1) and increased significantly in the resveratrol group (2242 ± 525 pg ml−1; P < 0.02). After training PGI2 increased during exercise in the placebo group (1921 ± 323 pg ml−1; P < 0.02; Fig. 7) but not significantly in the resveratrol group (1510 ± 179 pg ml−1)
Figure 7. Changes in skeletal muscle interstitial prostacyclin levels before and after a period of exercise training and either placebo or resveratrol supplementation in aged men.
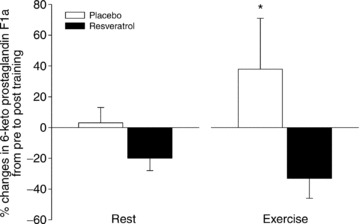
Relative changes from pre to post in interstitial 6-keto prostaglandin F1α in m. vastus lateralis at rest and during exercise. Muscle interstitial fluid was collected through microdialysis probes positioned in the m.v. lateralis muscle. Data are presented as mean ± SEM. *Significant changes from pre to post (P < 0.05).
Plasma NOx and ET-1
Plasma NOx levels were similar in the placebo and the resveratrol group before (34.5 ± 3.0 vs. 34.7 ± 2.7 μmol min−1; n= 12/14) and after training (33.9 ± 2.3 vs. 34.5 ± 2.7 μmol min−1). The placebo group had significantly higher plasma ET-1 levels than the resveratrol group before training (P < 0.01; Fig. 4), but not after training.
Discussion
The major findings of the present study were that 8 weeks of exercise training induced a number of beneficial cardiovascular effects in aged men, but in contrast to our hypothesis, parallel supplementation with resveratrol reduced several of these improvements. Specifically, resveratrol supplementation combined with training abolished the reduction in blood pressure and in blood lipids, altered the balance between prostanoid vasodilators and vasoconstrictors and led to a significantly lower increase in the training-induced increase in maximal oxygen uptake. The observed effects were not related to a change in SIRT1 protein expression as the SIRT1 level was not altered with either exercise training or training combined with resveratrol supplementation.
Based on previous animal studies (Murase et al. 2009; Dolinsky et al. 2012; Menzies et al. 2013; Hart et al. 2013), we hypothesized that supplementation with resveratrol would potentiate the positive effects of exercise training on cardiovascular risk factors. However, although exercise training alone was effective in decreasing the plasma LDL cholesterol level, TC/HDL ratio, triglyceride concentration and VCAM-1 level, resveratrol supplementation did not potentiate the effect of exercise training on these cardiovascular risk factors. Moreover, resveratrol supplementation abolished the effect of exercise training on blood lipids and had no effect on plasma VCAM-1 levels. Regulation of lipoproteins has recently been shown to be mediated by AMP-activated protein kinase (AMPK) and PGC-1α (Greene et al. 2012) and recent evidence suggest that resveratrol inhibits, rather than activates, AMPK and PGC-1α in humans (Skrobuk et al. 2012; Yoshino et al. 2012), which could explain the negative effect of resveratrol on the training-induced improvements in blood lipids.
In the present study exercise training alone lowered mean arterial blood pressure whereas the group receiving resveratrol in the training period experienced no reduction in blood pressure. Decreased vascular function and increased blood pressure in ageing and various cardiovascular diseases are associated with alterations in several factors including an imbalance in vasodilator and vasoconstrictor systems (Taddei et al. 1993; Spratt et al. 2001; Kirby et al. 2009). One of the vasodilator systems that has been proposed to be a major factor in improvements in cardiovascular function with exercise training is NO and eNOS (Hambrecht et al. 2003). In this study, eNOS content and the concentration of NO metabolites in plasma remained unaltered with training. However, in accordance with our previous observations (Hansen et al. 2011; Hellsten et al. 2012a) the prostanoid system was improved by training as evidenced by an increase in the protein content of COX and PGI2 synthase in the muscle tissue and the muscle interstitial concentration of PGI2. The mechanism underlying the lack of blood pressure reduction in the group receiving resveratrol supplementation in parallel with training is not clear, but the training-induced increase in interstitial PGI2, observed in the placebo group, was absent in the resveratrol group and there was a parallel increase in the protein content of TBX synthase in muscle in the resveratrol group that was not present in the placebo group. As TBX synthase and PGI2 synthase both compete for substrates in the arachidonic acid pathway, the increase in TBX synthase may suggest a shift in the balance between prostanoid vasodilator versus vasoconstrictor formation in the resveratrol group. This shift in balance could be one explanation for the abolished training-induced reduction in blood pressure in the resveratrol group.
Another factor that could have affected blood pressure changes was the level of ET-1. Plasma ET-1 levels tended (P= 0.06) to be reduced after training in the placebo group, and may, combined with the apparent increase in PGI2 formation, have contributed to the reduced blood pressure after training. Furthermore, the observed training-induced increase in ET-A in the present study could be associated with the ameliorations in glucose handling generally seen after a training period (Goodyear & Kahn, 1998; Shemyakin et al. 2011). Resveratrol supplementation had no further effect on ET-1 levels in plasma, ET-1 or ET-A concentration in the skeletal muscle but it was found that the ET-B protein content in the muscle increased only in the resveratrol group. This increase could promote either vasodilation or constriction depending of the cellular site of the receptor (Miyauchi & Masaki, 1999).
As an indicator of vascular function, we measured femoral arterial blood flow in response to exercise. The magnitude of flow during exercise was similar to that observed previously in our group for aged individuals (Nyberg et al. 2012), but no differences in flow between groups or with exercise training were observed. Vascular function was also assessed in the two groups by measurements of leg blood flow in response to passive movement of the lower leg. Passive movement leads to an increase in leg blood flow which has been shown to be highly dependent on the formation of NO (Mortensen et al. 2012a). In both the placebo and the resveratrol groups, the increase in flow with passive movement was limited relative to what has been observed in young healthy individuals (Mortensen et al. 2012a) and there was no difference between groups. This finding is in accordance with our previous observation of an age-related decline in passive flow response (Mortensen et al. 2012a). Due to technical issues passive flow was only measured after training in the two groups, and thus a training effect was not possible to assess.
The current intervention with high-intensity cycling training in combination with intense cross-fit training was shown to be highly effective in improving aerobic power, as evidenced by an extensive increase in maximal oxygen uptake (19%) in the placebo group. However, the observation that resveratrol supplementation combined with exercise training induced a 45% lower increase in maximal oxygen uptake than training with placebo was unexpected. Recent studies on rodents have shown that resveratrol supplementation during a period of exercise training potentiates the increase in maximal oxygen uptake compared with exercise training alone (Dolinsky et al. 2012; Menzies et al. 2013; Hart et al. 2013). The effect of resveratrol has been related to increased mitochondrial biogenesis mediated via SIRT1 activation of AMPK and PGC-1α. The opposite finding in the current study may be explained by species differences in the effect of resveratrol on these pathways. Accordingly, resveratrol has recently been shown to blunt (Yoshino et al. 2012) or even inhibit (Skrobuk et al. 2012) AMPK and PGC-1α in human tissue. Discrepancies between animal and human responses have previously been shown and when investigating models of inflammatory diseases the translational potential of murine models to the human species have been shown to be particularly poor (Seok et al. 2013). In the present study we did not observe any increase in protein expression of SIRT1 in either the placebo or the resveratrol group, supporting the findings that neither exercise nor resveratrol are stimulators of SIRT1 protein content in aged humans. In comparing the current negative effect of resveratrol on maximal oxygen uptake to previous rodent studies it is also important to emphasize that, in contrast to what is suggested in rodents (Lagouge et al. 2006; Menzies et al. 2013; Hart et al. 2013), in humans, aerobic power is generally not thought to be associated with limitations in mitochondrial respiration capacity (Boushel et al. 2011) but rather limited by central cardiovascular components such as ventricular size, myocardial contractility and blood volume (Saltin et al. 1976; Ehsani et al. 1991). Thus, the lower increase in maximal oxygen uptake with training in the resveratrol group was unlikely to be related to mitochondrial biogenesis. Future studies should address whether the negative effect of resveratrol is due to adverse effects on central cardiovascular aspects such as blood volume and/or myocardial adaptations in humans.
Another possible explanation for the lower increase in maximal oxygen uptake in the resveratrol group may be related to the described role of ROS in signalling processes and in mediating part of the response and adaptations to exercise training (Jackson, 2011). The increase in maximal oxygen uptake with endurance training has been shown to be mediated by ROS (Baar, 2004; Radak et al. 2008) and thus abrogating ROS by increasing the antioxidant defence level may retard exercise-induced increase in maximal oxygen uptake. This theory has in part been shown in training studies with oral administration of antioxidant vitamin C + E in young healthy men (Ristow et al. 2009) and with oral administration of statins in subjects with metabolic syndrome (Mikus et al. 2013). Thus, it could be speculated that at least some of the negative effects of resveratrol supplementation on training-induced adaptations in the current study may have been related to the antioxidant properties of this compound.
An additional finding in the present study was that exercise training increases the ROS synthesizing NOX protein content in aged men without an effect of resveratrol supplementation. This up-regulation could also be related to ROS-mediated adaptive responses to exercise training (Silveira et al. 2003; Gomes et al. 2012). NOX has also been proposed to stimulate Ca2+ release and uptake, respectively, potentially increasing muscle contractility (Xia et al. 2003; Hidalgo et al. 2006). The observation that resveratrol supplementation had no effect on the training-induced increase in NOX is in contrast to the observation that incubation of endothelial cells with resveratrol downregulates NOX (Orallo et al. 2002). The discrepancy may suggest that the response in NOX in cultured endothelial cells differs from an in vivo situation.
Based on the available literature, one of the hypotheses of the present study was that resveratrol supplementation combined with exercise training would increase the antioxidant defense and thereby lower excessive ROS and improve bioavailability of the potent vasodilator NO in aged individuals. However, we found that high-intensity exercise training increased the antioxidant enzyme SOD2 and decreased GPX-1 protein content whereas CAT and SOD1 protein content were unaltered in skeletal muscle and that resveratrol supplementation had no additional effect. The present observation that resveratrol supplementation with concomitant exercise training was unable to induce any changes in the antioxidant system is in contrast to the previous findings from animal models where resveratrol has been shown to upregulate CAT, GPX-1 and SOD1 protein expression in skeletal muscle (Ungvari et al. 2007b; Spanier et al. 2009). To our knowledge, no previous study has measured antioxidant enzymes in skeletal muscle of aged subjects in response to exercise training and data from young healthy subjects are scarce and inconclusive (Hellsten et al. 1996; Tiidus et al. 1996; Devries et al. 2008). The present results suggest that resveratrol supplementation has no additive effect on endogenous antioxidant systems.
In the present study a daily dose of 250 mg of trans-resveratrol was used. Previous studies on physically inactive humans using varying concentrations of resveratrol (10–2000 mg day−1) show a diverging effect of resveratrol supplementation (Brasnyóet al. 2011; Timmers et al. 2011; Yoshino et al. 2012; Poulsen et al. 2012; Crandall et al. 2012). Given that several effects were observed in the present study, albeit mainly negative, the used dose appears to have been effective. The duration of the supplementation period was 8 weeks, which is substantially longer than previously used in most human studies.
In conclusion, we demonstrate that high-intensity exercise training potently improves a number of parameters related to vascular function and cardiovascular health in aged men, but that concomitant oral resveratrol supplementation blunts several of these positive effects of exercise training. Specifically, resveratrol had adverse effects on improvements in maximal oxygen uptake, on blood pressure reduction and on the lowering of blood lipids induced by exercise training. The finding rejects the hypothesis that resveratrol improves cardiovascular health by enhanced SIRT1-dependent signalling and improved antioxidant defence. Furthermore, our data suggest that training enhances the capacity for ROS formation via increased levels of NOX and that removal of ROS via resveratrol treatment may limit training-induced adaptations. It may therefore also be questioned whether in general the level of ROS formation in aged men indeed is detrimental to cardiovascular health as previously proposed.
Acknowledgments
We would like to thank Fluxome Inc. (Stenlose, Denmark) for providing us with trans-resveratrol and placebo tablets. Special thanks to Jens Jung Nielsen for skilled execution of microdialysis and Thorbjørn Åkerstrøm, Ninna Iversen, Marie Henriksen, Line Nielsen, Louise Seier and Kamilla Winding for technical assistance.
Glossary
- BMI
body mass index
- CAT
catalase
- COX
cyclooxygenase
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin 1
- ET-A
endothelin receptor A
- ET-B
endothelin receptor B
- GPX
glutathione persoxidase
- HDL
high-density lipoprotein
- HR
heart rate
- LDL
low-senity lipoprotein
- MAP
mean arterial pressure
- nNOS
neural nitric oxide synthase
- NOx
nitrite and nitrate
- PGI2
prostacyclin
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- SOD
superoxide dismutase
- TBX
thromboxane
- TC
total cholesterol
- VCAM-1
vascular cell adhesion molecule 1
Additional information
Competing interests
None.
Author contributions
The contributions of the authors were as follows. Conception and design of the study: all authors; collection of data: all authors; analysis and interpretation of data: L.G, J.O, M.N and Y.H.; drafting the article or revising it critically for important intellectual content: L.G, H.P and Y.H. All authors approved the final version.
Funding
This study was supported by The Danish Ministry of Culture, The Danish Council for Independent Research – Medical Sciences.
Supplementary material
Supplemental Table S1
Supplemental Table S2
References
- Andersen P, Saltin B. Maximal perfusion of skeletal-muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D, Phillips BJ, Yu TW. The effects of vitamin C supplementation on biomarkers of oxygen radical generated damage in human volunteers with ‘low’ or ‘high’ cholesterol levels. Environ Mol Mutagen. 1997;30:161–174. [PubMed] [Google Scholar]
- Baar K. Involvement of PPARγ co-activator-1, nuclear respiratory factors 1 and 2, and PPARα in the adaptive response to endurance exercise. Proc Nutr Soc. 2004;63:269–273. doi: 10.1079/PNS2004334. [DOI] [PubMed] [Google Scholar]
- Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, Pietri S, Young IS, Richardson RS. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol. 2010;109:449–456. doi: 10.1152/japplphysiol.00354.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature Reviews Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Inv. 1975;35:609–616. [PubMed] [Google Scholar]
- Boushel R, Gnaiger E, Calbet JAL, González-Alonso J, Wright-Paradis C, Sondergaard H, Ara I, Helge JW, Saltin B. Muscle mitochondrial capacity exceeds maximal oxygen delivery in humans. Mitochondrion. 2011;11:303–307. doi: 10.1016/j.mito.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Brasnyó P, Molnár GA, Mohás M, Markó L, Laczy B, Cseh J, Mikolás E, Szijártó IA, Mérei A, Halmai R, Mészáros LG, Sümegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Cao G, Russell RM, Lischner N, Prior RL. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J Nutr. 1998;128:2383–2390. doi: 10.1093/jn/128.12.2383. [DOI] [PubMed] [Google Scholar]
- Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antiox Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67:1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA, Tarnopolsky MA. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic Biol Med. 2008;45:503–511. doi: 10.1016/j.freeradbiomed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Jones KE, Sidhu RS, Haykowsky M, Czubryt MP, Gordon T, Dyck JRB. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590:2783–2799. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsani AA, Ogawa T, Miller TR, Spina RJ, Jilka SM. Exercise training improves left ventricular systolic function in older men. Circulation. 1991;83:96–103. doi: 10.1161/01.cir.83.1.96. [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- Gomes EC, Silva AN, de Oliveira MR. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid Med Cell Longev. 2012;2012:756132. doi: 10.1155/2012/756132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- Greene NP, Fluckey JD, Lambert BS, Greene ES, Riechman SE, Crouse SF. Regulators of blood lipids and lipoproteins? PPARδ and AMPK, induced by exercise, are correlated with lipids and lipoproteins in overweight/obese men and women. Am J Physiol Endocrinol Metab. 2012;303:E1212–1221. doi: 10.1152/ajpendo.00309.2012. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Erbs S, Linke N, Kränkel Y, Shu Y, Baither S, Gielen H, Thiele F. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise training alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension. 2011;58:943–949. doi: 10.1161/HYPERTENSIONAHA.111.176529. [DOI] [PubMed] [Google Scholar]
- Hart N, Sarga L, Csende Z, Koltai E, Koch LG, Britton SL, Davies KJA, Kouretas D, Wessner B, Radak Z. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem Toxicol. 2013 doi: 10.1016/j.fct.2013.01.051. doi: 10.1016/j.fct.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten Y, Apple FS, Sjödin B. Effect of sprint cycle training on activities of antioxidant enzymes in human skeletal muscle. J Appl Physiol. 1996;81:1484–1487. doi: 10.1152/jappl.1996.81.4.1484. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Jensen L, Thaning P, Nyberg M, Mortensen S. Impaired formation of vasodilators in peripheral tissue in essential hypertension is normalized by exercise training: role of adenosine and prostacyclin. J Hypertens. 2012a;30:2007–2014. doi: 10.1097/HJH.0b013e328356dd57. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Nielsen JJ, Lykkesfeldt J, Bruhn M, Silveira L, Pilegaard H, Bangsbo J. Antioxidant supplementation enhances the exercise-induced increase in mitochondrial uncoupling protein 3 and endothelial nitric oxide synthase mRNA content in human skeletal muscle. Free Radic Biol Med. 2007;43:353–361. doi: 10.1016/j.freeradbiomed.2007.02.029. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol. 2012b;590:6297–6305. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C, Sánchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J Biol Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, Chayama K. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15:326–332. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- Høier B, Nordsborg N, Andersen S, Jensen L, Nybo L, Bangsbo J, Hellsten Y. Pro- and anti-angiogenic factors in human skeletal muscle in response to acute exercise and training. J Physiol. 2011;590:595–606. doi: 10.1113/jphysiol.2011.216135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksen CB, Pedersen KL. 2006. ‘Den nye steptest’ og svært overvægtige. http://fysio.dk/fafo/Afhandlinger/Bachelor/2006/Nastved/Den-nye-Steptest/
- Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal. 2011;15:2477–2486. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson PA, Veneman T, Nurjhan N, Gerich J. An improved method to calculate adipose tissue interstitial substrate recovery for microdialysis studies. Life Sci. 1994;54:1621–1624. doi: 10.1016/0024-3205(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70:113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol. 2009;587:1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol. 2003;285:H1023–1031. doi: 10.1152/ajpheart.00135.2003. [DOI] [PubMed] [Google Scholar]
- Lopez-S A, Vial R, Balart L, Arroyave G. Effect of exercise and physical fitness on serum lipids and lipoproteins. Atherosclerosis. 1974;20:1–9. doi: 10.1016/0021-9150(74)90073-2. [DOI] [PubMed] [Google Scholar]
- Maeda S, Miyauchi T, Sakane M, Saito M, Maki S, Goto K, Matsuda M. Does endothelin-1 participate in the exercise-induced changes of blood flow distribution of muscles in humans. J Appl Physiol. 1997;82:1107–1111. doi: 10.1152/jappl.1997.82.4.1107. [DOI] [PubMed] [Google Scholar]
- Matos RS, Baroncini LAV, Précoma LB, Winter G, Lambach PH, Caron EY, Kaiber F, Précoma DB. Resveratrol causes antiatherogenic effects in an animal model of atherosclerosis. Arq Bras Cardiol. 2012;98:136–142. doi: 10.1590/s0066-782x2012005000006. [DOI] [PubMed] [Google Scholar]
- Menzies KJ, Singh K, Saleem A, Hood DA. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013;288:6968–6979. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.02.074. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annu Rev Physiol. 1999;61:391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- Miyazaki H, Oh-ishi S, Ookawara T, Kizaki T, Toshinai K, Ha S, Haga S, Ji LL, Ohno H. Strenuous endurance training in humans reduces oxidative stress following exhausting exercise. Eur J Appl Physiol. 2001;84:1–6. doi: 10.1007/s004210000342. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Askew CD, Walker M, Nyberg M, Hellsten Y. The hyperaemic response to passive leg movement is dependent on nitric oxide; a new tool to evaluate endothelial nitric oxide function. J Physiol. 2012a;590:4391–4400. doi: 10.1113/jphysiol.2012.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the human leg. J Physiol. 2012b;590:6227–6236. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase T, Haramizu S, Ota N, Hase T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. 2009;10:423–434. doi: 10.1007/s10522-008-9177-z. [DOI] [PubMed] [Google Scholar]
- Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003;285:R143–148. doi: 10.1152/ajpregu.00029.2003. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol. 2012;590:5361–5370. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orallo F, Alvarez E, Camiña M, Leiro JM, Gómez E, Fernández P. The possible implication of trans-Resveratrol in the cardioprotective effects of long-term moderate wine consumption. Mol Pharmacol. 2002;61:294–302. doi: 10.1124/mol.61.2.294. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perk J, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- Peter K, Nawroth P, Conradt C, Nordt T, Weiss T, Boehme M, Wunsch A, Allenberg J, Kübler W, Bode C. Circulating vascular cell adhesion molecule-1 correlates with the extent of human atherosclerosis in contrast to circulating intercellular adhesion molecule-1, E-selectin, P-selectin, and thrombomodulin. Arterioscler Thromb Vasc Biol. 1997;17:505–512. doi: 10.1161/01.atv.17.3.505. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stødkilde-Jørgensen H, Møller N, Jessen N, Pedersen SB, Jørgensen JOL. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2012;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proc Nutr Soc. 1999;58:1025–1033. doi: 10.1017/s0029665199001342. [DOI] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ranković G, Milicić B, Savić T, Dindić B, Mancev Z, Pesić G. Effects of physical exercise on inflammatory parameters and risk for repeated acute coronary syndrome in patients with ischemic heart disease. Vojnosanit Pregl. 2009;66:44–48. doi: 10.2298/vsp0901044r. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essén B, Gollnick D. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96:289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Ullrich V. Peroxynitrite as regulator of vascular prostanoid synthesis. Arch Biochem Biophys. 2009;484:183–189. doi: 10.1016/j.abb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Schulz E, Jansen T, Wenzel P, Daiber A, Münzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Peersen K, Sommervoll L, Seljeflot I, Arnesen H, Otterstad JE. Physical performance is associated with markers of vascular inflammation in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:356–362. doi: 10.1097/01.hjr.0000188244.54287.96. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemyakin A, Salehzadeh F, Esteves Duque-Guimaraes D, Böhm F, Rullman E, Gustafsson T, Pernow J, Krook A. Endothelin-1 reduces glucose uptake in human skeletal muscle in vivo and in vitro. Diabetes. 2011;60:2061–2067. doi: 10.2337/db10-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LR, Pereira-Da-Silva L, Juel C, Hellsten Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med. 2003;35:455–464. doi: 10.1016/s0891-5849(03)00271-5. [DOI] [PubMed] [Google Scholar]
- Skrobuk P, Kraemer von S, Semenova MM, Zitting A, Koistinen HA. Acute exposure to resveratrol inhibits AMPK activity in human skeletal muscle cells. Diabetologia. 2012;55:3051–3060. doi: 10.1007/s00125-012-2691-1. [DOI] [PubMed] [Google Scholar]
- Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):111–116. [PubMed] [Google Scholar]
- Spratt JC, Goddard J, Patel N, Strachan FE, Rankin AJ, Webb DJ. Systemic ETA receptor antagonism with BQ-123 blocks ET-1 induced forearm vasoconstriction and decreases peripheral vascular resistance in healthy men. Br J Pharmacol. 2001;134:648–654. doi: 10.1038/sj.bjp.0704304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther. 2007;30:8. doi: 10.1519/00139143-200704000-00003. [DOI] [PubMed] [Google Scholar]
- Stauffer BL, Westby CM, DeSouza CA. Endothelin-1, aging and hypertension. Curr Opin Cardiol. 2008;23:350–355. doi: 10.1097/HCO.0b013e328302f3c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Salvetti A. Vasodilation to acetylcholine in primary and secondary forms of human hypertension. Hypertension. 1993;21:929–933. doi: 10.1161/01.hyp.21.6.929. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- Taubert D, Berkels R. Upregulation and activation of eNOS by resveratrol. Circulation. 2003;107:e78–e79. doi: 10.1161/01.cir.0000060819.46705.ee. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Pushkarenko J, Houston ME. Lack of antioxidant adaptation to short-term aerobic training in human muscle. Am J Physiol. 1996;271:R832–836. doi: 10.1152/ajpregu.1996.271.4.R832. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MKC, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007a;293:H37–47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007b;292:H2417–2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest. 1974;33:79–86. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
- Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- Wei YH, Lu CY, Lee HC, Pang CY, Ma YS. Oxidative damage and mutation to mitochondrial DNA and age-dependent decline of mitochondrial respiratory function. Ann N Y Acad Sci. 1998;854:155–170. doi: 10.1111/j.1749-6632.1998.tb09899.x. [DOI] [PubMed] [Google Scholar]
- Xia R, Webb JA, Gnall LLM, Cutler K, Abramson JJ. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am J Physiol Cell Physiol. 2003;285:C215–221. doi: 10.1152/ajpcell.00034.2002. [DOI] [PubMed] [Google Scholar]
- Yang Y-M, Huang A, Kaley G, Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol Heart Circ Physiol. 2009;297:H1829–1836. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S-I, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


