Abstract
Intrauterine inflammation impairs fetal pulmonary vascular development and increases cerebral metabolic rate in fetal sheep. We hypothesized that these structural and metabolic effects of intrauterine inflammation would be accompanied by reduced fetal pulmonary blood flow and increased cerebral perfusion. Fetal sheep were instrumented at 112 days of gestation (term is 147 days) for measurement of cardiopulmonary and cerebral haemodynamics. At 118 days ewes were randomly assigned to receive intra-amniotic lipopolysaccharide (LPS, 20 mg from Escherichia coli; n= 7) or saline (control, 4 ml; n= 6). Fetal haemodynamic data were recorded continually from 1 h before intra-amniotic LPS or saline, until 144 h after. Fetal arterial blood was sampled before, and periodically after, intra-amniotic LPS or saline. End-diastolic and mean pulmonary blood flows were significantly lower than control from 48 and 96 h after LPS exposure, respectively, until the end of the experiment. Carotid blood flow was transiently increased at 96 and 120 h after LPS exposure. Carotid arterial oxygen content was lower than control from 48 h after intra-amniotic LPS. Fetal arterial lactate concentration was higher than control between 4 and 12 h after intra-amniotic LPS. Experimental intrauterine inflammation reduces pulmonary blood flow in fetal sheep, over a time course consistent with impaired pulmonary vascular development. Increased carotid blood flow after LPS administration may reflect an inflammation-induced increase in cerebral metabolic demand.
Key points
Intrauterine inflammation impairs fetal pulmonary vascular development and increases cerebral metabolism in fetal sheep. Whether these structural and metabolic changes have functional consequences for fetal cardiopulmonary and cerebral haemodynamics is presently unknown.
We demonstrated that intra-amniotic administration of lipopolysaccharide increased fetal pulmonary vascular resistance, and reduced pulmonary blood flow and carotid arterial oxygen content in the fetus and caused a transient increase in fetal cerebral blood flow. These effects occurred over a time course consistent with previously observed effects on pulmonary vascular development and cerebral metabolism.
Our data suggest that pathophysiological changes in cardiopulmonary and cerebral haemodynamics observed in fetuses exposed to intrauterine inflammation may be present and detectable in human pregnancies, offering potential for detecting fetuses affected by intrauterine inflammation.
Introduction
Histological chorioamnionitis is a common antecedent of human preterm birth, present in up to 75% of deliveries ≤30 weeks (Lahra & Jeffery, 2004; Lahra et al. 2009). In many cases histological chorioamnionitis results in a fetal inflammatory response defined by funisitis, fetal vasculitis and increased concentrations of pro-inflammatory cytokines within the fetal blood and amniotic fluid (Gotsch et al. 2007).
Clinical and experimental studies demonstrate that the fetal inflammatory response is associated with altered development of many organ systems including the lungs and brain (Watterberg et al. 1996; Grether & Nelson, 1997; Hannaford et al. 1999; Moss et al. 2002; Nitsos et al. 2006; Hansen-Pupp et al. 2008; Gavilanes et al. 2009; Westover et al. 2012). These developmental effects may directly increase or decrease the risk of neonatal disease, or they may interact with postnatal events to contribute to altered risk of neonatal morbidity. For example, histological chorioamnionitis decreases the risk of respiratory distress syndrome (Hannaford et al. 1999), probably as a result of precocious stimulation of surfactant production (Bachurski et al. 2001; Moss et al. 2002), but chorioamnionitis appears to interact with postnatal events such as oxygen exposure and mechanical ventilation to increase the risk of bronchopulmonary dysplasia (BPD) and pulmonary hypertension (Jobe & Ikegami, 2001; Woldesenbet & Perlman, 2005). Chorioamnionitis has also been implicated in the pathogenesis of intraventricular haemorrhage (IVH) (De Felice et al. 2001; Yanowitz et al. 2002, 2004a), periventricular leukomalacia (PVL) (Zupan et al. 1996; Yanowitz et al. 2002, 2004b) and cerebral palsy (de Vries et al. 1988; Zupan et al. 1996; Yanowitz et al. 2006), but the mechanistic basis for these associations is not completely understood.
Experimental studies investigating fetal inflammatory response syndrome in preterm sheep have demonstrated abnormal pulmonary vascular development (Kallapur et al. 2003, 2004), cerebral inflammation (Nitsos et al. 2006; Gavilanes et al. 2009) and increased cerebral oxygen consumption prior to preterm birth (Gavilanes et al. 2009; Andersen et al. 2011). Whether these structural and metabolic changes have functional consequences for cardiopulmonary and cerebral haemodynamics in the fetus in utero is presently unknown.
The aim of our experiment was to examine the effect of intrauterine inflammation on fetal cardiopulmonary and cerebral haemodynamics. We hypothesized that intrauterine inflammation would reduce pulmonary blood flow but increase cerebral perfusion in preterm fetal sheep.
Methods
Fetal surgery
All procedures were approved by the relevant Animal Ethics Committee. At 112 ± 1 (mean ± standard deviation) days of gestation (term is ∼147 days), pregnant ewes bearing singleton or twin fetuses underwent aseptic surgery under general anaesthesia (2% isoflurane in oxygen: Bomac Animal Health, Hornsby, NSW, Australia) for instrumentation as described in detail previously (Polglase et al. 2005). For twin pregnancies only one fetus underwent instrumentation and experimentation. Local anaesthetic (Marcaine, 12.5 mg; Astra Zeneca, North Ryde, NSW, Australia) was injected s.c. prior to making a paramedial incision in the maternal abdominal wall to access the pregnant uterus. The fetal head was delivered through a uterine incision and polyvinyl catheters containing heparinized saline were inserted into the fetal brachiocephalic artery (BCA), via the right brachial artery, and main pulmonary artery (MPA) for subsequent measurement of arterial pressures and periodic collection of arterial blood samples (from the BCA). A catheter was inserted into the amniotic cavity for later administration of lipolysaccharide (LPS) or saline, and to measure amniotic fluid pressure for adjustment of fetal arterial pressure. Flow probes (Transonic systems, Ithaca, NY, USA) were placed around the left MPA (size: 4 mm) and left carotid artery (size: 3 mm) for measurement of pulmonary and carotid arterial blood flows (PBF and CaBF), respectively. Ewes received post-operative analgesia (transdermal fentanyl patch; 75 μg h−1: Janssen Cilag, North Ryde, NSW, USA) for 3 days and i.v. antibiotics (Engemycin; 500 mg; Schering-Plough, Upper Hutt, New Zealand) once daily for 3 days. Ewes were allowed 6 days to recover after surgery before experiments began. At the completion of the experiment, ewes were killed via a pentobarbital sodium overdose.
Experimental protocol
At ∼118 days of gestation fetal arterial and amniotic catheters were attached to pressure transducers (ADIns-truments, Castle Hill, NSW, Australia) and flow probes were attached to a flow meter (Transonic systems) for digital recording of cardiopulmonary and cerebral haemodynamics using Powerlab 8000 hardware and Labchart Pro v7.3.1 software (ADInstruments). A fetal arterial blood sample was collected for blood gas measurement (ABL30; Radiometer, Copenhagen, Denmark). Ewes were randomized to receive intra-amniotic LPS (Escherichia coli 055:B5, 20 mg; Sigma Aldrich, NSW, Australia; n= 7) or saline (controls; 4 ml; n= 6) via the amniotic catheter. It has been demonstrated previously that in twin pregnant sheep the response to intra-amniotic LPS exposure is confined to the LPS-exposed fetus (Gantert et al. 2012). Fetal blood gas measurements were taken at 2, 4, 8, 12 and 24 h, and then at daily intervals for 6 days after intra-amniotic LPS or saline.
Fetal cardiopulmonary and cerebral haemodynamic data analysis
Fetal cardiopulmonary and cerebral haemodynamic recordings began ∼1 h prior to intra-amniotic administration of LPS or saline and continued uninterrupted for 6 days. During the first 24 h after intra-amniotic LPS or saline, blood flows and pressures were determined by averaging three 60 s epochs taken 20 min apart at 0, 4, 8, 12 and 24 h. Data from 2 to 6 days were analysed by averaging three 60 s epochs taken 20 min apart at 6 h intervals, beginning at 02:00 h. Due to the difficulty associated with maintaining brachiocephalic arterial catheter patency, pressure in the brachiocephalic artery (PBCA) and cerebral vascular resistance (CVR) were recorded in 4 of 7 LPS-exposed fetuses and 5 of 6 controls.
Calculations
All blood flow data are expressed relative to estimated fetal body weight at the time of measurement, calculated using an established method (Alexander, 1978; Lumbers et al. 1985). Carotid arterial oxygen content (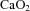 ) was calculated based on measurements made from blood sampled at the base of the carotid artery (the site of the tip of the BCA catheter) according to the equation:
) was calculated based on measurements made from blood sampled at the base of the carotid artery (the site of the tip of the BCA catheter) according to the equation:
Cerebral oxygen delivery was calculated as the product of carotid arterial blood oxygen content ×CaBF. CVR was calculated according to the equation:
Carotid arterial pulse amplitude was calculated by subtracting peak carotid arterial blood flow during ventricular systole from carotid arterial blood flow at the beginning of ventricular systole. Left pulmonary arterial acceleration time/ejection time ratio (At/Et) was calculated from the left pulmonary artery flow waveform by dividing the time interval from the beginning of ventricular systole and the achievement of peak flow (At) by the time interval from the beginning of ventricular systole to the end of ventricular systole, as previously described (Subhedar et al. 1998; Azpurua et al. 2010). All calculations were performed in Labchart Pro v7.3.1.
Statistical analysis
Data are presented as mean ± SEM. Statistical analyses were undertaken using Sigmaplot (v12.0; Systat software, Washington, DC, USA). Serial data were compared between groups by two-way ANOVA with repeated measures, using treatment (LPS vs. saline) and time as factors. The Holm–Sidak post hoc test was used for time-point and group comparisons where effects were identified by ANOVA. Fetal body weights and relative organ weights were compared using an unpaired t test. Statistical significance was defined as P < 0.05.
Results
Fetal weights and sex at autopsy
Fetal body weights and relative organ weights were not different between control and LPS-exposed fetuses (Table 1). The ratio of males to females was 1:1 in the control group and 5:2 in the LPS group. The ratio of singletons to twins was 2:1 in the control group and 4:3 in the LPS group.
Table 1.
Fetal body weights and relative organ weights of control and LPS-exposed fetal sheep at 125 days of gestation
| Control | LPS | |
|---|---|---|
| Body weight (kg) | 2.87 ± 0.16 | 2.99 ± 0.27 |
| Lung (g kg−1) | 34.0 ± 3.8 | 32.0 ± 2.5 |
| Brain (g kg−1) | 15.1 ± 0.6 | 14.5 ± 0.4 |
| Heart (g kg−1) | 6.7 ± 0.4 | 7.7 ± 0.3 |
| Liver (g kg−1) | 37.4 ± 1.8 | 35.0 ± 3.8 |
| Spleen (g kg−1) | 2.0 ± 0.4 | 2.1 ± 0.4 |
| Thymus (g kg−1) | 4.6 ± 0.3 | 3.6 ± 0.6 |
Values are means ± SEM.
Fetal blood gas and acid base status
Fetal 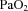 and
and 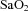 were not different between control and LPS-exposed fetuses (Fig. 1A and B, respectively).
were not different between control and LPS-exposed fetuses (Fig. 1A and B, respectively). 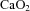 was lower in LPS-exposed fetuses from 48 h than in control (P= 0.01; Fig. 1C). Fetal haemoglobin concentration did not differ statistically between groups.
was lower in LPS-exposed fetuses from 48 h than in control (P= 0.01; Fig. 1C). Fetal haemoglobin concentration did not differ statistically between groups.
Figure 1. Fetal blood gas and acid-base status.
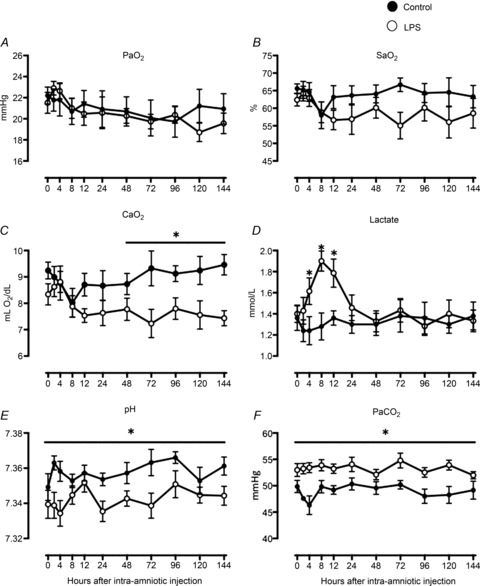
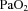 (A),
(A), 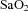 (B),
(B), 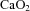 (C), arterial lactate concentration (D), pH (E) and
(C), arterial lactate concentration (D), pH (E) and 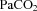 (F) in control (filled circles) and LPS-exposed fetal sheep (open circles) after intra-amniotic injection. Data are mean ± SEM. *P < 0.05 control vs. LPS.
(F) in control (filled circles) and LPS-exposed fetal sheep (open circles) after intra-amniotic injection. Data are mean ± SEM. *P < 0.05 control vs. LPS.
Fetal arterial lactate concentrations were increased at 4, 8 and 12 h after LPS administration but returned to control levels by 24 h (P < 0.05; Fig. 1D). Arterial pH was lower and 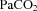 was higher in LPS-exposed fetuses (P < 0.05; Fig. 1E and F), but differences were present prior to intra-amniotic injection.
was higher in LPS-exposed fetuses (P < 0.05; Fig. 1E and F), but differences were present prior to intra-amniotic injection.
Fetal cardiopulmonary and cerebral haemodynamics
Fetal pulmonary blood flow was lower than control from 96 h after LPS administration (P= 0.04; Fig. 2B) until the end of the recording period. End-diastolic PBF was lower in LPS-exposed fetuses than in control (P < 0.05; Fig. 2C) from 48 h until the end of the recording period. Heart rate and At/Et did not differ between groups (P= 0.6 and 0.9, respectively; Fig. 2C and D). Main pulmonary arterial pressure across all time points was 39 ± 1 mmHg in the LPS group and 40 ± 4 mmHg in controls (P= 0.80).
Figure 2. Fetal heart rate and pulmonary haemodynmaics.
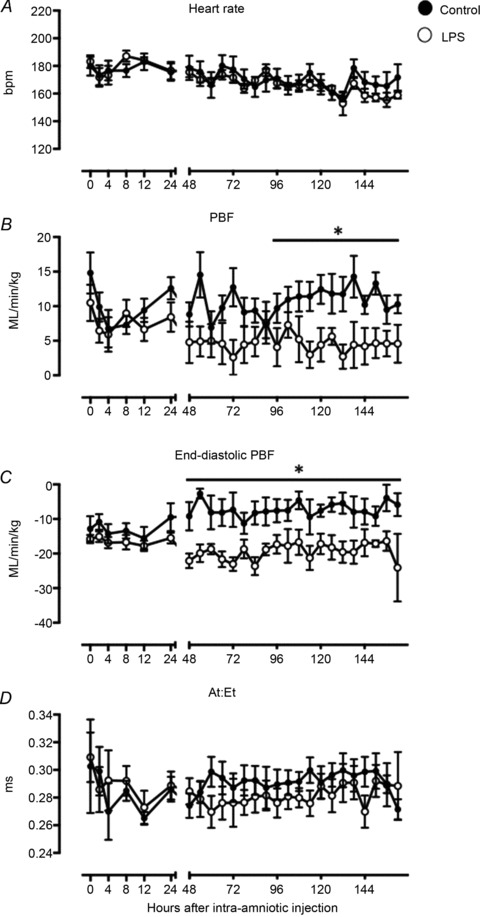
Heart rate (A), left pulmonary arterial blood flow (PBF) (B), end-diastolic PBF (C) and left pulmonary arterial acceleration-to-ejection time ratio (At/Et) (D) in control (filled circles) and LPS-exposed fetal sheep (open circles) after intra-amniotic injection. Data are mean ± SEM. *P < 0.05 control vs. LPS.
CaBF tended to be higher in the LPS group than control at 48, 72 and 144 h (P < 0.08; Fig. 3A) and was significantly increased at 96 and 120 h after LPS administration (P < 0.05; Fig. 3A). Cerebral vascular resistance was lower in LPS-exposed fetuses between 48 and 96 h after LPS administration (P < 0.05, Fig. 3B). Carotid arterial pulse amplitude tended higher in LPS-exposed fetuses than controls, from 48 h after LPS exposure (P= 0.06; Fig. 3C) until the end of the experiment. Cerebral oxygen delivery did not differ between groups (P= 0.85; Fig. 3D). PBCA across all time points was 35 ± 3 mmHg in the LPS group vs. 36 ± 1 mmHg in controls (P= 0.50, data not shown).
Figure 3. Fetal cerebral haemodynamics.
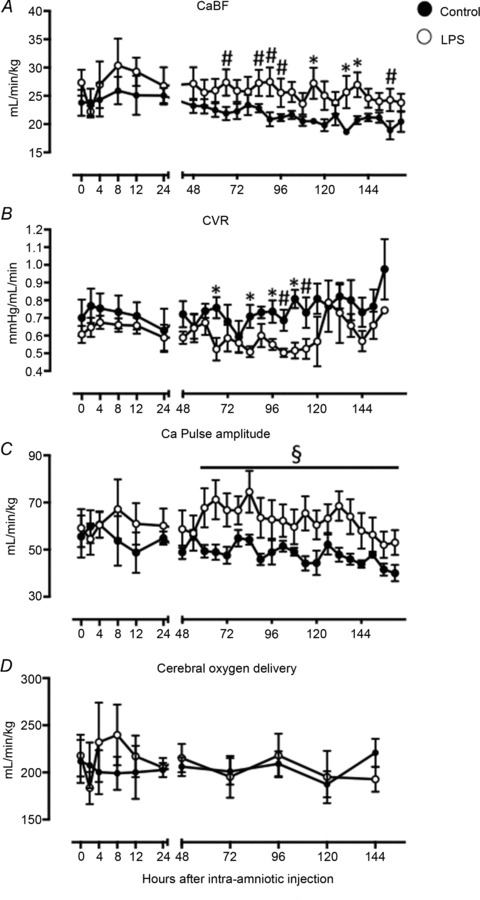
Carotid blood flow (CaBF) (A), cerebral vascular resistance (CVR) (B), carotid arterial (Ca) pulse amplitude (C) and cerebral oxygen delivery (D) in control (filled circles) and LPS-exposed fetal sheep (open circles) after intra-amniotic injection. Data are mean ± SEM. *P < 0.05 control vs. LPS, §P= 0.06 control vs. LPS, #P < 0.08 control vs. LPS.
Discussion
Intrauterine inflammation increased fetal pulmonary vascular resistance, reduced PBF and increased cerebral perfusion. Our data demonstrate that the previously identified structural changes to the pulmonary vascular bed (Kallapur et al. 2003, 2004; Polglase et al. 2010) and increased cerebral metabolism (Andersen et al. 2011) following exposure to intrauterine inflammation have functional consequences for fetal cardiopulmonary and cerebral haemodynamics that occur over a similar time course.
Preterm infants exposed to histological chorio-amnionitis are at increased risk of persistent pulmonary hypertension, a condition characterized by increased resistance to PBF (Woldesenbet & Perlman, 2005; Woldesenbet et al. 2008). We have previously shown that pulmonary vascular resistance and main pulmonary arterial pressure (PMPA) are increased, and PBF and left ventricular output are reduced during the immediate neonatal period, in preterm lambs exposed to LPS in utero (Polglase et al. 2010; Galinsky et al. 2013). In the fetus, PBF was reduced 4 days after LPS exposure. This was caused by an increase in pulmonary vascular resistance 2 days after LPS exposure, as indicated by a reduction in end-diastolic PBF, which is an indicator of downstream resistance to PBF (Polglase et al. 2005). These data support previous observations that intra-amniotic LPS exposure at a similar time in gestation impairs pulmonary vascular development. Following intra-amniotic LPS exposure at 119 days of gestation, fetal sheep display reduced expression of pulmonary vascular growth factors and endothelial nitric oxide synthase from 2 days (Kallapur et al. 2003, 2004). Adventitial fibrosis and smooth muscle hypertrophy of pulmonary resistance arterioles is evident from 4 days (Kallapur et al. 2004). These molecular and structural changes to the pulmonary vascular bed probably underlie the physiological changes to PBF in LPS-exposed fetal sheep that occur over a similar time course. Taken collectively, our previous studies (Galinsky et al. 2013) and the results presented here confirm that intrauterine inflammation induces persistent changes in cardiopulmonary haemodynamics in the fetus and preterm neonate, which are probably attributable to vascular remodeling, as previously demonstrated (Kallapur et al. 2003, 2004).
In humans, fetal pulmonary artery Doppler waveform assessment has been used to assess fetal pulmonary perfusion (Subhedar et al. 1998; Azpurua et al. 2010). Specifically, fetal pulmonary arterial At/Et increases with gestational age (Chaoui et al. 1998). This is due to an increase in the At interval and a progressive reduction in pulmonary vascular resistance (Rasanen et al. 1996; Chaoui et al. 1998). Since PBF was lower and pulmonary vascular resistance was higher in LPS-exposed fetuses than controls we expected to observe a lower At/Et in the LPS-exposed group, but there was no difference between groups. This suggests that assessment of mean and end-diastolic PBF may provide a more accurate assessment of pulmonary perfusion and vascular resistance in the fetus than At/Et.
We have previously demonstrated that CaBF is increased in LPS-exposed preterm lambs immediately after delivery (Polglase et al. 2012b; Galinsky et al. 2013). In LPS-exposed fetuses, increased carotid blood flow was observed at 4 and 5 days after intra-amniotic LPS-exposure. This was caused by a reduction in cerebral vascular resistance, indicating that inflammation-induced changes in cerebral haemodynamics can be detected before birth. The increase in CaBF was associated with a reduction in 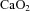 . A reduction in
. A reduction in 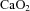 is known to increase CaBF in the fetus (Jones & Traystman, 1984; Peebles et al. 2003; Feng et al. 2009): maintaining adequate oxygen delivery by increasing perfusion is a protective mechanism to ensure metabolic demand by the fetal brain is met (Jones & Traystman, 1984). Thus, fetuses in our study compensated for reduced
is known to increase CaBF in the fetus (Jones & Traystman, 1984; Peebles et al. 2003; Feng et al. 2009): maintaining adequate oxygen delivery by increasing perfusion is a protective mechanism to ensure metabolic demand by the fetal brain is met (Jones & Traystman, 1984). Thus, fetuses in our study compensated for reduced 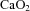 by increasing carotid blood flow to maintain cerebral oxygen delivery at the level observed in controls.
by increasing carotid blood flow to maintain cerebral oxygen delivery at the level observed in controls.
We have demonstrated previously that fetal cerebral metabolic demand is increased at 2 and 4 days after intra-amniotic LPS injection (Andersen et al. 2011). The alterations in CaBF observed in this study follow a consistent time course; however, the increased CaBF did not result in a net increase in cerebral oxygen delivery. This raises the possibility that the elevated oxygen demand by the brain of a fetus exposed to intrauterine inflammation may be unmet despite the increased cerebral blood flow, potentially contributing to brain injury, which is common in preterm infants exposed to intrauterine inflammation (Ellison et al. 2005; Inder et al. 2005).
In this study, histological chorioamnionitis was modelled using a single injection of LPS into the amniotic cavity of pregnant sheep (Jobe et al. 2000; Newnham et al. 2002; Nitsos et al. 2002). This causes a fetal inflammatory response that is tolerated without fetal demise or clinical symptoms in the ewe (Kramer et al. 2001; Newnham et al. 2002; Nitsos et al. 2002). This model of intrauterine inflammation causes alterations in fetal pulmonary and cerebral development that are commonly observed in infants exposed to histological chorioamnionitis (Willet et al. 2000; Moss et al. 2002; Nitsos et al. 2006; Gavilanes et al. 2009). The transient increase in arterial lactate concentration observed between 4 and 12 h after LPS exposure supports previous observations (Nitsos et al. 2002) and may be a consequence of altered placental and/or fetal metabolism resulting in increased release of lactate into the fetal circulation. The reduction in 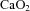 resulted from a slight reduction in oxygen saturation and haemoglobin levels after LPS exposure and is consistent with previous studies (Nitsos et al. 2002; Feng et al. 2009).
resulted from a slight reduction in oxygen saturation and haemoglobin levels after LPS exposure and is consistent with previous studies (Nitsos et al. 2002; Feng et al. 2009).
Although the sexes of our fetuses were not exactly balanced between groups this is unlikely to be a major confounder of our study. We have recently shown that the physiological transition at birth is not different between males and females (Polglase et al. 2012a). Furthermore, a recent analysis of data from a series of studies in sheep (Kramer et al. 2001, 2005, 2007, 2009; Kallapur et al. 2007, 2009; Kunzmann et al. 2007; Sweet et al. 2008; Kunzmann et al. 2010) showed no differences in lung structural effects or inflammatory responses between male and female fetal sheep (Lambermont et al. 2012).
Alterations in pulmonary and cerebral haemodynamics observed in our study may contribute to the increased incidence of chronic lung disease and neurodevelopmental abnormalities observed in preterm infants exposed to intrauterine inflammation. Our data suggest that pathophysiological changes in cardiopulmonary and cerebral haemodynamics observed in fetuses exposed to intrauterine inflammation may be present, and detectable, in human pregnancies, offering potential for detecting fetuses affected by intrauterine inflammation and thus predisposed to an increased risk of postnatal disease.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Alex Satragno, Alison Moxham, Karyn Rodgers and Valerie Zahra.
Glossary
- At/Et
pulmonary arterial acceleration/ejection time ratio
- BCA
brachiocephalic artery
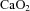
carotid arterial oxygen content
- CaBF
carotid blood flow
- CVR
cerebral vascular resistance
- LPS
lipopolysaccharide
- MPA
main pulmonary artery
- PBCA
brachiocephalic arterial pressure
- PMPA
main pulmonary arterial pressure
- PaBF
pulmonary arterial blood flow
Additional information
Competing interests
None.
Author contributions
R.G., S.B.H., G.R.P. and T.J.M.M. conceived and designed the experiments; R.G., S.B.H., G.R.P. and T.J.M.M. collected, analysed and interpreted the data; R.G., S.B.H., G.R.P. and T.J.M.M. drafted the article or revised it critically for important intellectual content. All authors approved the final version of the manuscript.
Funding
This study was supported by NHMRC Program grants (384100 and 606789), an Australian Postgraduate Award (R.G.), a National Heart Foundation of Australia (NHFA) grant in aid (G.R.P.), National Health and Medical Research Council of Australia (NHMRC) Career Development Fellowships (T.J.M.M.; 303261: G.R. P.: 1026890), NHMRC Fellowship (S.B.H.; 545921), a Rebecca L. Cooper Medical Research Foundation Fellowship (G.R.P.) and the Victorian Government's Operational Infrastructure Support Program.
The authors report no conflict of interest in accordance with journal policy.
References
- Alexander G. Quantitative development of adipose-tissue in fetal sheep. Aust J Biol Sci. 1978;31:489–503. doi: 10.1071/bi9780489. [DOI] [PubMed] [Google Scholar]
- Andersen CC, Pillow JJ, Gill AW, Allison BJ, Moss TJ, Hooper SB, Nitsos I, Kluckow M, Polglase GR. The cerebral critical oxygen threshold of ventilated preterm lambs and the influence of antenatal inflammation. J Appl Physiol. 2011;111:775–781. doi: 10.1152/japplphysiol.00214.2011. [DOI] [PubMed] [Google Scholar]
- Azpurua H, Norwitz ER, Campbell KH, Funai EF, Pettker CM, Kleine M, Bahtiyar MO, Malkus H, Copel JA, Thung SF. Acceleration/ejection time ratio in the fetal pulmonary artery predicts fetal lung maturity. Am J Obstet Gynecol. 2010;203:40.e41–48. doi: 10.1016/j.ajog.2010.01.075. [DOI] [PubMed] [Google Scholar]
- Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280:L279–285. doi: 10.1152/ajplung.2001.280.2.L279. [DOI] [PubMed] [Google Scholar]
- Chaoui R, Taddei F, Rizzo G, Bast C, Lenz F, Bollmann R. Doppler echocardiography of the main stems of the pulmonary arteries in the normal human fetus. Ultrasound Obstet Gynecol. 1998;11:173–179. doi: 10.1046/j.1469-0705.1998.11030173.x. [DOI] [PubMed] [Google Scholar]
- De Felice C, Toti P, Laurini RN, Stumpo M, Picciolini E, Todros T, Tanganelli P, Buonocore G, Bracci R. Early neonatal brain injury in histologic chorioamnionitis. J Pediatr. 2001;138:101–104. doi: 10.1067/mpd.2001.109605. [DOI] [PubMed] [Google Scholar]
- de Vries LS, Regev R, Dubowitz LM, Whitelaw A, Aber VR. Perinatal risk factors for the development of extensive cystic leukomalacia. Am J Dis Child. 1988;142:732–735. doi: 10.1001/archpedi.1988.02150070046023. [DOI] [PubMed] [Google Scholar]
- Ellison VJ, Mocatta TJ, Winterbourn CC, Darlow BA, Volpe JJ, Inder TE. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005;57:282–286. doi: 10.1203/01.PDR.0000148286.53572.95. [DOI] [PubMed] [Google Scholar]
- Feng SY, Phillips DJ, Stockx EM, Yu VY, Walker AM. Endotoxin has acute and chronic effects on the cerebral circulation of fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2009;296:R640–650. doi: 10.1152/ajpregu.00087.2008. [DOI] [PubMed] [Google Scholar]
- Galinsky R, Hooper SB, Wallace MJ, Westover AJ, Black MJ, Moss TJ, Polglase GR. Intrauterine inflammation alters cardiopulmonary and cerebral haemodynamics at birth in preterm lambs. J Physiol. 2013;591:2127–2137. doi: 10.1113/jphysiol.2012.249680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantert M, Jellema RK, Heineman H, Gantert J, Collins JJ, Seehase M, Lambermont VA, Keck A, Garnier Y, Zimmermann LJ, Kadyrov M, Gavilanes AW, Kramer BW. Lipopolysaccharide-induced chorioamnionitis is confined to one amniotic compartment in twin pregnant sheep. Neonatology. 2012;102:81–88. doi: 10.1159/000338015. [DOI] [PubMed] [Google Scholar]
- Gavilanes AW, Strackx E, Kramer BW, Gantert M, Van den Hove D, Steinbusch H, Garnier Y, Cornips E, Zimmermann L, Vles J. Chorioamnionitis induced by intraamniotic lipopolysaccharide resulted in an interval-dependent increase in central nervous system injury in the fetal sheep. Am J Obstet Gynecol. 2009;200:437.e431–438. doi: 10.1016/j.ajog.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. [Erratum appears in JAMA 1998 Jan 14;279(2):118] JAMA. 1997;278:207–211. [PubMed] [Google Scholar]
- Hannaford K, Todd DA, Jeffery H, John E, Blyth K, Gilbert GL. Role of ureaplasma urealyticum in lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 1999;81:F162–167. doi: 10.1136/fn.81.3.f162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Pupp I, Hallin AL, Hellstrom-Westas L, Cilio C, Berg AC, Stjernqvist K, Fellman V, Ley D, Hansen-Pupp I, Hallin A-L, Hellstrom-Westas L, Cilio C, Berg A-C, Stjernqvist K, Fellman V, Ley D. Inflammation at birth is associated with subnormal development in very preterm infants. Pediatr Res. 2008;64:183–188. doi: 10.1203/PDR.0b013e318176144d. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Jobe AH, Ikegami M. Prevention of bronchopul-monary dysplasia. Curr Opin Pediatr. 2001;13:124–129. doi: 10.1097/00008480-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Jobe AH, Newnham JP, Willet KE, Moss TJ, Gore Ervin M, Padbury JF, Sly P, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med. 2000;162:1656–1661. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- Jones MD, Traystman RJ. Cerebral oxygenation of the fetus, newborn, and adult. Semin Perinatol. 1984;8:205–216. [PubMed] [Google Scholar]
- Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1178–1185. doi: 10.1152/ajplung.00049.2004. [DOI] [PubMed] [Google Scholar]
- Kallapur SG, Jobe AH, Ball MK, Nitsos I, Moss TJM, Hillman NH, Newnham JP, Kramer BW. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179:8491–8499. doi: 10.4049/jimmunol.179.12.8491. [DOI] [PubMed] [Google Scholar]
- Kallapur SG, Jobe AH, Ikegami M, Bachurski CJ, Kallapur SG, Jobe AH, Ikegami M, Bachurski CJ. Increased IP-10 and MIG expression after intra-amniotic endotoxin in preterm lamb lung. Am J Respir Crit Care Med. 2003;167:779–786. doi: 10.1164/rccm.2203030. [DOI] [PubMed] [Google Scholar]
- Kallapur SG, Moss TJ, Auten RL, Jr, Nitsos I, Pillow JJ, Kramer BW, Maeda DY, Newnham JP, Ikegami M, Jobe AH. IL-8 signalling does not mediate intra-amniotic LPS-induced inflammation and maturation in preterm fetal lamb lung. Am J Physiol Lung Cell Mol Physiol. 2009;297:L512–519. doi: 10.1152/ajplung.00105.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171:73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, Kallapur SG. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L345–353. doi: 10.1152/ajplung.00003.2007. [DOI] [PubMed] [Google Scholar]
- Kramer BW, Kallapur SG, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Intra-amniotic LPS modulation of TLR signalling in lung and blood monocytes of fetal sheep. Innate Immun. 2009;15:101–107. doi: 10.1177/1753425908100455. [DOI] [PubMed] [Google Scholar]
- Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med. 2001;164:982–988. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]
- Kunzmann S, Glogger K, Been JV, Kallapur SG, Nitsos I, Moss TJ, Speer CP, Newnham JP, Jobe AH, Kramer BW. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. Am J Obstet Gynecol. 2010;202:476.e471–479. doi: 10.1016/j.ajog.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann S, Speer CP, Jobe AH, Kramer BW. Antenatal inflammation induced TGF-β1 but suppressed CTGF in preterm lungs. Am J Physiol Lung Cell Mol Physiol. 2007;292:L223–231. doi: 10.1152/ajplung.00159.2006. [DOI] [PubMed] [Google Scholar]
- Lahra MM, Beeby PJ, Jeffery HE. Maternal versus fetal inflammation and respiratory distress syndrome: a 10-year hospital cohort study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F13–16. doi: 10.1136/adc.2007.135889. [DOI] [PubMed] [Google Scholar]
- Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol. 2004;190:147–151. doi: 10.1016/j.ajog.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Lambermont VA, Been JV, Kunzmann S, Vanterpool SF, Newnham JP, Kallapur SG, Jobe AH, Kramer BW. Sex differences in lung gas volumes after lipopolysaccharide-induced chorioamnionitis in fetal sheep. Gend Med. 2012;9:278–286. doi: 10.1016/j.genm.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Lumbers ER, Smith FG, Stevens AD. Measurement of net transplacental transfer of fluid to the fetal sheep. J Physiol. 1985;364:289–299. doi: 10.1113/jphysiol.1985.sp015745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss TJ, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med. 2002;165:805–811. doi: 10.1164/ajrccm.165.6.2108053. [DOI] [PubMed] [Google Scholar]
- Newnham JP, Moss TJ, Kramer BW, Nitsos I, Ikegami M, Jobe AH. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol. 2002;186:1062–1068. doi: 10.1067/mob.2002.122293. [DOI] [PubMed] [Google Scholar]
- Nitsos I, Moss TJ, Cock ML, Harding R, Newnham JP. Fetal responses to intra-amniotic endotoxin in sheep. J Soc Gynecol Investig. 2002;9:80–85. doi: 10.1016/s1071-5576(01)00155-1. [DOI] [PubMed] [Google Scholar]
- Nitsos I, Rees SM, Duncan J, Kramer BW, Harding R, Newnham JP, Moss TJ. Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J Soc Gynecol Investig. 2006;13:239–247. doi: 10.1016/j.jsgi.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Peebles DM, Miller S, Newman JP, Scott R, Hanson MA. The effect of systemic administration of lipopolysa-ccharide on cerebral haemodynamics and oxygenation in the 0.65 gestation ovine fetus in utero. BJOG. 2003;110:735–743. [PubMed] [Google Scholar]
- Polglase GR, Hooper SB, Gill AW, Allison BJ, Crossley KJ, Moss TJ, Nitsos I, Pillow JJ, Kluckow M. Intrauterine inflammation causes pulmonary hypertension and cardiovascular sequelae in preterm lambs. J Appl Physiol. 2010;108:1757–1765. doi: 10.1152/japplphysiol.01336.2009. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Hooper SB, Kluckow M, Gill AW, Harding R, Moss TJ. The cardiopulmonary haemodynamic transition at birth is not different between male and female preterm lambs. Reprod Fertil Dev. 2012a;24:510–516. doi: 10.1071/RD11121. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Morley CJ, Crossley KJ, Dargaville P, Harding R, Morgan DL, Hooper SB. Positive end-expiratory pressure differentially alters pulmonary hemodynamics and oxygenation in ventilated, very premature lambs. J Appl Physiol. 2005;99:1453–1461. doi: 10.1152/japplphysiol.00055.2005. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Nitsos I, Baburamani AA, Crossley KJ, Slater MK, Gill AW, Allison BJ, Moss TJ, Pillow JJ, Hooper SB, Kluckow M. Inflammation in utero exacerbates ventilation-induced brain injury in preterm lambs. J Appl Physiol. 2012b;112:481–489. doi: 10.1152/japplphysiol.00995.2011. [DOI] [PubMed] [Google Scholar]
- Rasanen J, Huhta JC, Weiner S, Wood DC, Ludomirski A. Fetal branch pulmonary arterial vascular impedance during the second half of pregnancy. Am J Obstet Gynecol. 1996;174:1441–1449. doi: 10.1016/s0002-9378(96)70586-0. [DOI] [PubMed] [Google Scholar]
- Subhedar NV, Hamdan AH, Ryan SW, Shaw NJ. Pulmonary artery pressure: early predictor of chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;78:F20–24. doi: 10.1136/fn.78.1.f20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DG, Huggett MT, Warner JA, Moss TJ, Kloosterboer N, Halliday HL, Newnham JP, Kallapur SG, Jobe AH, Kramer BW. Maternal betamethasone and chorioamnionitis induce different collagenases during lung maturation in fetal sheep. Neonatology. 2008;94:79–86. doi: 10.1159/000115949. [DOI] [PubMed] [Google Scholar]
- Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- Westover AJ, Hooper SB, Wallace MJ, Moss TJ. Prostaglandins mediate the fetal pulmonary response to intrauterine inflammation. Am J Physiol Lung Cell Mol Physiol. 2012;302:L664–678. doi: 10.1152/ajplung.00297.2011. [DOI] [PubMed] [Google Scholar]
- Willet KE, Jobe AH, Ikegami M, Newnham J, Brennan S, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res. 2000;48:782–788. doi: 10.1203/00006450-200012000-00014. [DOI] [PubMed] [Google Scholar]
- Woldesenbet M, Perlman JM. Histologic chorioamnionitis: an occult marker of severe pulmonary hypertension in the term newborn. J Perinatol. 2005;25:189–192. doi: 10.1038/sj.jp.7211240. [DOI] [PubMed] [Google Scholar]
- Woldesenbet M, Rosenfeld CR, Ramilo O, Johnson-Welch S, Perlman JM. Severe neonatal hypoxic respiratory failure correlates with histological chorioamnionitis and raised concentrations of interleukin 6 (IL6), IL8 and C-reactive protein. Arch Dis Child Fetal Neonatal Ed. 2008;93:F413–417. doi: 10.1136/adc.2007.124503. [DOI] [PubMed] [Google Scholar]
- Yanowitz TD, Baker RW, Roberts JM. Hemodynamic changes in premature infants exposed to chorioamnionitis. Pediatr Res. 2004a;55:524A. [Google Scholar]
- Yanowitz TD, Baker RW, Roberts JM, Brozanski BS. Low blood pressure among very-low-birth-weight infants with fetal vessel inflammation. J Perinatol. 2004b;24:299–304. doi: 10.1038/sj.jp.7211091. [DOI] [PubMed] [Google Scholar]
- Yanowitz TD, Jordan JA, Gilmour CH, Towbin R, Bowen A, Roberts JM, Brozanski BS. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr Res. 2002;51:310–316. doi: 10.1203/00006450-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Yanowitz TD, Potter DM, Bowen A, Baker RW, Roberts JM. Variability in cerebral oxygen delivery is reduced in premature neonates exposed to chorioamnionitis. Pediatr Res. 2006;59:299–304. doi: 10.1203/01.pdr.0000196738.03171.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan V, Gonzalez P, Lacaze-Masmonteil T, Boithias C, d’Allest AM, Dehan M, Gabilan JC. Periventricular leukomalacia: risk factors revisited. Dev Med Child Neurol. 1996;38:1061–1067. doi: 10.1111/j.1469-8749.1996.tb15068.x. [DOI] [PubMed] [Google Scholar]


