Abstract
Atrial and brain natriuretic peptides (ANP and BNP, respectively) are cardiac hormones released into the bloodstream in response to hypervolaemia or fluid shifts to the central circulation. The actions of both peptides include natriuresis and diuresis, a decrease in systemic blood pressure, and inhibition of the renin–angiotensin–aldosterone system. Further, ANP and BNP elicit increases in blood microvessel permeability sufficient to cause protein and fluid extravasation into the interstitium to reduce the vascular volume. Given the importance of the lymphatic vasculature in maintaining fluid balance, we tested the hypothesis that ANP or BNP (100 nm) would likewise elevate lymphatic permeability (Ps) to serum albumin. Using a microfluorometric technique adapted to in vivo lymphatic vessels, we determined that rat mesenteric collecting lymphatic Ps to rat serum albumin increased by 2.0 ± 0.4-fold (P= 0.01, n= 7) and 2.7 ± 0.8-fold (P= 0.07, n= 7) with ANP and BNP, respectively. In addition to measuring Ps responses, we observed changes in spontaneous contraction amplitude and frequency from the albumin flux tracings in vivo. Notably, ANP abolished spontaneous contraction amplitude (P= 0.005) and frequency (P= 0.006), while BNP augmented both parameters by ∼2-fold (P < 0.01 each). These effects of ANP and BNP on contractile function were examined further by using an in vitro assay. In aggregate, these data support the theory that an increase in collecting lymphatic permeability opposes the absorptive function of the lymphatic capillaries, and aids in the retention of protein and fluid in the interstitial space to counteract volume expansion.
Key points
Atrial and brain natriuretic peptides (ANP and BNP, respectively) are hormones released into the bloodstream when heart muscle is stretched (e.g. zero-gravity, hypertension, congestive heart failure) and serve to reduce the blood volume.
One way that these peptides relieve blood volume is to increase the permeability of the smallest blood vessels, facilitating fluid and protein distribution into the tissue spaces.
Whether these peptides target lymphatic vessel function to participate in fluid distribution is currently unknown.
ANP and BNP (100 nm) both elicited significant increases in lymphatic vessel permeability, but altered contractile function differentially in vivo.
A likely consequence is that more fluid leaks from the lymphatics into the tissues, which represents a novel compensation for volume overload. This work demonstrates for the first time that lymphatic vessel permeability can be regulated in vivo.
Introduction
Natriuretic peptides (NPs), a class of peptide hormones synthesized by the chambers of the heart, are released into the bloodstream upon cardiac myocyte stretch resulting from hypervolaemia or fluid shifts favouring the central circulation (as occurs in microgravity, heart failure, pulmonary hypertension). Renal excretion of sodium and diuresis along with increases in vascular permeability to water and protein serve to reduce the vascular fluid volume. As a result, NPs have been the focus of investigation for treatment of congestive heart failure (CHF) due to their numerous favourable physiological actions (McGrath et al. 2005). Likewise, the lymphatic vasculature has been shown to be a critical regulator of fluid distribution (Brookes et al. 2009; Machnik et al. 2009, 2010; Scallan & Huxley, 2010; Wiig et al. 2013), but whether lymphatic vessel function is modulated by NP signalling is unknown.
In healthy humans there is a constitutive low-level secretion of atrial (ANP) and brain natriuretic peptides (BNP), synthesized by the atria and ventricles of the heart, respectively (Clerico et al. 2002). During CHF, the circulating levels of ANP and BNP become elevated and range between 0.1 and 1 nm (Yoshimura et al. 1993, Maisel et al. 2002). Notably, the heart is the main source for these circulating peptides although other organs are capable of secreting NPs (e.g. brain, lung), albeit at lower levels (Gerbes et al. 1994). Elevation of circulating ANP or BNP stimulates the well-established physiological responses of marked natriuresis and diuresis, a decrease in systemic blood pressure, inhibition of the renin–angiotensin–aldosterone system, and at higher concentrations, arterial vasodilatation (Woods, 2004; McGrath et al. 2005; Rubattu et al. 2008). De Bold et al. (1981) observed an additional property of ANP infusion: a redistribution of vascular volume (manifest as an increase in haematocrit) later shown to occur in the absence of the kidneys (Almeida et al. 1986). It was then demonstrated that in selected tissues and portions of the vasculature, ANP elicited a rapid and sustained increase in microvascular permeability to both protein and water (Huxley et al. 1987b; Meyer & Huxley, 1990; McKay & Huxley, 1995; Curry et al. 2010). A response of this magnitude is sufficient to displace a significant portion of the circulating fluid and protein into the interstitium (Valentin et al. 1989; Tucker et al. 1992), thus alleviating volume overload. Another study investigating the permeability response of venular capillaries to BNP demonstrated a response mimicking that of ANP (McKay, 1994).
In a recent study, we demonstrated that collecting lymphatic vessel permeability to albumin does not differ from that of venules, resulting in a small amount of protein and fluid being lost to the interstitium under conditions of health (Scallan & Huxley, 2010). In CHF, we realized that displacement of the capillary filtrate into the interstitium only reduces the vascular volume if the lymphatic vasculature does not reabsorb and return this fluid to the vasculature. Therefore, we hypothesized that collecting lymphatic permeability to albumin would respond similarly to that of venular capillaries with a 2-fold increase upon exposure to either ANP or BNP (McKay, 1994; McKay & Huxley, 1995). Our rationale for hypothesizing equivalent lymphatic and venular responses arose from work demonstrating that lymphatic endothelium is derived from venous endothelium during embryological development (Srinivasan et al. 2007, Yang et al. 2012), which led us to reason that a possible consequence of their shared genetic profiles may be comparable phenotypic function.
Paired measures of albumin flux were made during perfusion of in situ rat mesenteric collecting lymphatics with control and natriuretic peptide solutions containing fluorescently labelled albumin, from which solute permeability was determined. We accepted our hypothesis that lymphatics would, like venules, undergo a 2-fold increase in permeability to albumin (Ps) during exposure to 100 nm ANP or BNP. Additionally, we observed that the contractile function of collecting lymphatic vessels seemed to be altered differentially in vivo by ANP versus BNP treatment. Investigating this further using isolated collecting lymphatics in vitro confirmed these effects, but only at this concentration. Thus, we conclude that the lymphatic vasculature represents an additional target of natriuretic peptide signalling and additional surface area from which protein and fluid is extravasated to relieve vascular volume expansion.
Methods
Ethical approval
Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Missouri-Columbia and conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. All animals were killed after the experiment by an overdose of anaesthetic followed by bilateral pneumothorax and aortic transection to conform to the protocol.
General surgical preparation
Both in situ and in vitro experiments were performed on juvenile male (35–55 days, 200–250 g) Sprague Dawley rats (Hilltop Lab Animals, PA, USA) housed three to a cage for a minimum 1 week acclimation period prior to the experiment. Juvenile males were chosen for their relative lack of perivascular fat, which facilitated location and subsequent cannulation of mesenteric collecting lymphatics. One collecting lymphatic (60–170 μm diameter) was cannulated in vivo per animal and up to two collecting lymphatics were isolated per animal.
Following induction of anaesthesia with Inactin (i.p., thiobutabarbital, Research Biochemicals Int., MA, USA, 128 mg (kg body weight)−1), the animal was placed on a custom, heated Plexiglas™ board that enabled transport of the animal from a dissection microscope to an intravital microscope. The mesentery was exteriorized and superfused continuously (2–3 ml min−1) with mammalian Krebs solution at 37 ± 0.5°C while a vessel was located under the Zeiss dissection microscope. For the in situ experiments, the animal and board were then transferred to a Leitz Diavert inverted microscope and the suffused preparation was allowed to equilibrate for approximately 30 min prior to vessel cannulation and data collection.
Collecting lymphatic vessels were identified by the presence of valves, spontaneous contractions, and their transparent contents (Zweifach & Prather, 1975). Additionally, leukocytes were often visualized in collecting lymphatics. Vessels shrouded by adipose tissue in vivo were not studied because the fat cells hindered cannulation.
Isolated vessel experiments
To perform dose–response experiments examining the effects of ANP and BNP on collecting lymphatic vessel contractile parameters, we necessarily turned to the isolated vessel approach, which enables several doses of drugs to be applied sequentially to a single vessel under tightly controlled conditions and where diameter can be measured with high resolution. Collecting lymphatic vessels were microdissected from rat mesentery, cleaned of surrounding tissue, and cannulated on two glass pipettes capable of fine pressure control as previously described in several publications (Scallan & Davis, 2013; Scallan et al. 2013; Davis et al. 2012). Collecting lymphatic vessels were held at a constant intraluminal pressure of 3 cmH2O, and superfused continuously with Krebs buffer. Small volumes (<300 μl) of either ANP or BNP stock solutions were added directly to the bath solution to achieve 0.1, 1, 10, or 100 nm concentrations. Doses were applied in ascending order, with each exposure lasting for 2 min. End-diastolic diameter, contraction amplitude, and contraction frequency were quantified for each dose as has been previously described (Davis et al. 2012; Scallan & Davis, 2013; Scallan et al. 2013). Passive diameters were obtained at the end of each experiment by superfusing the vessel with a similar Krebs buffer that contained 3 mm EGTA instead of calcium, and measuring the diameter at the same pressure used in the experiments (3 cmH2O).
Solutions and drugs
Mammalian Krebs
All superfusion and perfusion solutions were prepared fresh and used on the same day. Unless otherwise stated all materials were purchased from Sigma (MO, USA). The Krebs superfusion solution consisted of (in mmol): 141.4 NaCl, 4.7 KCl, 2.0 CaCl2·2H2O, 1.2 MgSO4, 1.2 NaH2PO4·H2O, 5.0 d-glucose, 3.0 NaHCO3, and 1.5 NaHepes. The pH of the solution was 7.4 ± 0.05 at 37°C.
Krebs–BSA
Dialysed bovine serum albumin (BSA, Sigma cat. no. A7906) was added to the Krebs superfusion solution to achieve a final concentration of 1 mg ml−1 on the day of the experiment. The osmolarity, measured by freezing point osmometry, was generally between 292–297 mosmol after the addition of BSA.
Labelled protein
The macromolecular probe used in this study, rat serum albumin (RSA, Sigma cat. no. A6272), was bound to the Alexa-488® fluorophore (Invitrogen, CA, USA) by modifying the manufacturer's protocol. Briefly, the Alexa-488® and RSA (3:1 molar ratio) were reacted for 30 min at room temperature. Free fluorescent dye was removed in two steps: first by centrifugation using Vivaspin™ 20 tubes (30 kDa nominal molecular weight limit, VivaScience, Hanover, Germany) to retain labelled RSA; second, any remaining free dye was removed by buffer exchange column chromatography. Protein concentration of the conjugate was determined by Micro BCA Protein Assay (Thermo Fisher Scientific, IL, USA) and stored at a final concentration of 10 mg ml−1 at −20°C. This approach removes unconjugated dye while preserving the primary structure of albumin (Bingaman et al. 2003).
The perfusate solution contained 10% (w/v) labelled RSA (1 mg) with 9 mg of unlabelled RSA and was brought to a volume of 1 ml with freshly made Krebs solution so that the final total protein concentration was 10 mg ml−1. A washout solution was prepared, with unlabelled protein, at an identical total protein concentration. Both solutions of 10 mg ml−1 RSA possessed an oncotic pressure of 4.1 cmH2O calculated from the Landis-Pappenheimer equation (Landis & Pappenheimer, 1963).
Dialysis procedure for BSA and RSA
Proteins (BSA or RSA) for use in the perfusate or suffusate solutions were ‘washed’ with saline to remove hydrophilic solutes carried on albumin prior to use. In this procedure dialysis is achieved by ultrafiltration (Amicon 30 kDa molecular weight cutoff, Millipore, MA, USA). The dialysed BSA and RSA were stored in 10 ml and 100 μl aliquots, respectively, at a concentration of 100 mg ml−1 at −20°C until the day of the experiment.
Perfusates containing natriuretic peptide
Rat atrial natriuretic peptide (ANP, Sigma cat. no. A8208) and brain natriuretic peptide (BNP, Sigma cat. no. B9901) were dissolved in saline to prepare 10 μm stock solutions. The 100× stock solutions were divided into 15 μl aliquots and stored at −20°C. On the day of the experiment, an ANP or BNP aliquot was thawed and 5 μl of stock solution were added to 500 μl (half) of the perfusion solution to a final concentration of 100 nm. The other half of the perfusion solution was used as the control. For natriuretic peptides the concentration of 100 nm was chosen to elicit a stable, maximal Ps response (Meyer & Huxley, 1990).
Microvascular solute flux measurements
The method we use for determining solute permeability (Ps) to proteins in lymphatic and blood microvessels and its limitations is described in several publications (Huxley et al. 1987a; McKay & Huxley, 1995; Rumbaut & Huxley, 2002; Sarelius et al. 2006; Scallan & Huxley, 2010). Briefly, collecting lymphatics studied here were cannulated in situ with single lumen pipettes and perfused with unlabelled RSA (washout) followed by fluorescently labelled RSA (dye) in Krebs solution. Vessel diameter (D) was measured under brightfield via an ocular ruler following all measures of Ps. To reduce variability, all Ps and contractile responses were recorded from the same vessel segment throughout the experiment.
Direct measures of albumin flux (Js, mmol s−1) were made over an area of vessel defined by a rectangular diaphragm (width = 4 vessel diameters, length = 8 vessel diameters) in front of a photometer (PTI, Canada). When dye-labelled albumin filled the vessel lumen there was an initial step increase in fluorescence intensity (Io), followed by a gradual, but linear, increase in intensity as fluorescent probe accumulated in the interstitial space over time (dIf/dt) in addition to the constant signal generated from the fluorophore flowing through the vessel lumen. Solute permeability (Ps, cm s−1) was calculated from the equation relating solute flux (Js, mmol s−1) to surface area (S, cm2) at a constant translymphatic concentration difference (ΔC, mmol ml−1) (Huxley et al. 1987a):
| (1) |
Experimental protocols
Collecting lymphatic vessels were entered into one of two protocols. In the first, designed to measure the permeability responses to ANP and BNP, a vessel was cannulated and perfused with the washout solution to measure baseline fluorescence, then recannulated and perfused with the dye solution so that both I0 and dIf/dt were obtained. This provided the control Ps measurement. The Ps response was obtained by recannulating the vessel with the washout solution for a period of time sufficient to return tissue fluorescence to baseline, and recannulating the vessel a final time with a pipette containing the dye and natriuretic peptide solution to repeat the measures of flux in the presence of the peptide. In the second protocol, designed to allow observation of the contractile responses to ANP and BNP, a vessel was cannulated and perfused with the dye solution at a hydrostatic pressure just above the native vessel pressure (to elicit perfusion without inhibiting contractions; Gashev et al. 2002; Scallan & Huxley, 2010). To obtain the contractile responses to NP, the vessel was recannulated and perfused at the same hydrostatic pressure with a pipette containing both the dye and natriuretic peptide. Contractile amplitude was measured as the height of the excursion of the fluorescence intensity during perfusion, while contraction frequency was measured as the number of excursions per minute.
Statistical analyses
Prism™ (GraphPad Software, CA, USA) software was used for all statistics. The mean ± standard error of the mean (SEM) was reported to facilitate comparison with published data. Student's paired t tests were performed for the permeability and contractile function experiments where only two treatments were used. The Student's unpaired t test was utilized to compare the Ps responses of ANP and BNP. To determine whether fitted curves differed between treatment groups, the extra-sum-of-squares F test was performed. For the dose–response tests, differences were determined by one-way ANOVA in conjunction with Dunnett's post hoc analysis for multiple pairwise comparisons.
Power analysis was performed beforehand using the mean and standard deviation from our previous study (Scallan & Huxley, 2010) as a first approximation. To detect a 40% change in collecting lymphatic Ps between groups (2.0 × 10−7 cm s−1) with 95% confidence (significance level P < 0.05), six animals were needed per group (Neter et al. 1990).
Results
Collecting lymphatic vessel permeability responses to two natriuretic peptides
Panels A–C in Fig. 1 each presents data from seven collecting lymphatic vessels. Solute permeability (Ps) was assessed during perfusion with a control (Krebs) solution followed by an identical solution containing 100 nm atrial (ANP; Fig. 1A and C) or brain natriuretic peptide (BNP; Fig. 1B and C). Both natriuretic peptide solutions induced increases in Ps to RSA (P= 0.01 for ANP and P= 0.07 for BNP) without eliciting significant changes in vessel diameter. Because the distributions of Ps during peptide exposure appeared to differ slightly, the ratios were graphed in Fig. 1C for further analysis. The fold changes in Ps elicited by ANP and BNP did not differ (P= 0.44) as the mean ± SEM Ps responses to ANP and to BNP were 2.0 ± 0.4-fold and 2.7 ± 0.8-fold increases, respectively. Further, when the one vessel that did not respond to BNP was excluded from the analysis, the permeability response reached statistical significance (P= 0.04).
Figure 1. Collecting lymphatic vessel permeability in vivo increases upon exposure to either atrial or brain natriuretic peptide.
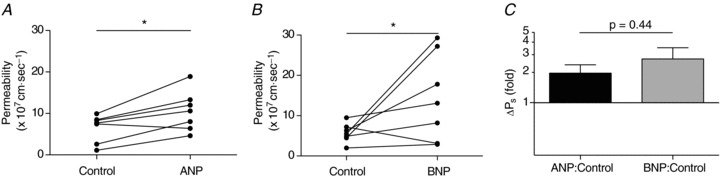
A and B, collecting lymphatic vessels underwent a significant increase in Ps to rat serum albumin versus control when perfused with either 100 nm ANP (P= 0.01) or BNP (P= 0.07). For each peptide there are n= 7 paired measures. One vessel from each data set did not respond to the natriuretic peptide. C, Ps responses are graphed as the ratio of Ps during natriuretic peptide infusion to that measured during control conditions. The mean ± SEM fold changes for ANP and BNP are 2.0 ± 0.4 and 2.7 ± 0.8, respectively. Note the logarithmic y-scale. At y= 1 there is no change from control, and lies where the x-axis is drawn. *P < 0.10.
Sensitivity of collecting lymphatic permeability responses to natriuretic peptides
Previous work from this laboratory with venular capillaries demonstrated that the response to ANP was more robust in vessels with a lower basal Ps (i.e. tighter barrier; McKay & Huxley, 1995). To determine whether collecting lymphatic vessels shared this feature, the fold increases in Ps to ANP or BNP, respectively, were graphed as a function of the control Ps in Fig. 2. Not only did lymphatic Ps responses to ANP recapitulate this characteristic of venular capillaries, but Ps responses to BNP also followed the same general trend. While the means of the responses did not differ between the two groups (Fig. 1C), the curve fitted to the BNP response data (r2= 0. 46, n= 6) was shifted to the right compared to the ANP response curve (r2= 0. 91, n= 7). An extra-sum-of-squares F test was performed to determine whether one curve was sufficient to fit all the data, and concluded that two curves were needed (P < 0.05), indicating that the right shift was statistically significant.
Figure 2. Sensitivity of the in vivo Ps response to natriuretic peptide infusion as a function of the control Ps.
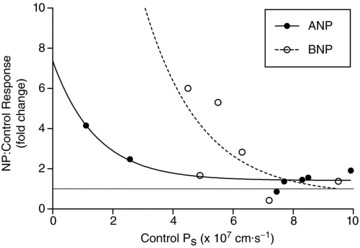
The fold increase in Ps is plotted on the y-axis for vessels exposed to either 100 nm ANP (filled circles, n= 7) or BNP (open circles, n= 6). The general trend is that vessels with a low basal Ps are more responsive to perfusion with natriuretic peptides. The gray line drawn at y= 1 marks where there is no change in Ps during perfusion with natriuretic peptides relative to control. The two curves are significantly different (P < 0.05).
Diffusion- and convective-mediated albumin flux are similarly affected by ANP
Solute moves across vascular walls chiefly by two mechanisms: by diffusion and/or by solvent (or convective) drag when solute becomes entrained by water movement. Figure 3 illustrates the contribution of each of these mechanisms to solute flux. In Fig. 3A the Ps responses to ANP (n= 6, excluding the single unresponsive vessel) were plotted as a function of the net pressure (difference between hydrostatic and oncotic pressures) at which they were assessed. The effective oncotic pressure of the perfusate, 3.85 cmH2O, was calculated according to the Landis–Pappenheimer equations (Landis & Pappenheimer, 1963) as described previously (Scallan & Huxley, 2010). The axis intercept of each flux curve at the translymphatic pressure of zero defines the diffusional permeability, Pd, and the limiting slope is equal to Lp(1 –σ), a measure of water permeability. While biological variation in the data render the two curves as indistinguishable statistically, physiologically relevant information may still be obtained (Fig. 3B), namely Pd and Lp(1 –σ). The estimated values of Pd and Lp(1 –σ) under control conditions were 3 × 10−7 cm s−1 and 0.3 × 10−7 cm s−1 cmH2O−1, respectively. During perfusion with ANP, Pd and Lp(1 –σ) rose to 5 × 10−7 cm s−1 and 0.5 × 10−7 cm s−1 cmH2O−1, respectively, almost doubling upon exposure to ANP, with ∼1.8-fold changes for each. The Péclet number (Pé), a unitless ratio describing the contributions of convective relative to diffusional solute flux, was calculated for the average collecting lymphatic hydrostatic pressure of 7 cmH2O (refer to: Scallan & Huxley, 2010) to yield remarkably similar values for control and ANP-modified Péclet numbers (0.66 vs. 0.70, respectively; Fig. 3B).
Figure 3. ANP increases the diffusion-mediated solute flux and convective (water-driven) coupling of solute flux to water flux.
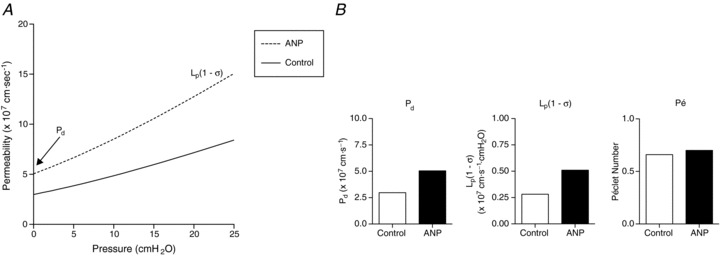
A, the continuous curve represents individual in vivo measures of control Ps, while the dashed curve represents the same vessels perfused with 100 nm ANP (n= 6 measures per group). Net pressure, on the x-axis, is defined as the difference between the hydrostatic and effective oncotic pressures. The y-intercept is equal to the diffusional permeability (Pd), and the limiting slope of each fitted line is equal to Lp(1 −σ). The fitted curves were not statistically different, but were used to obtain the information in panel B. B, values derived from the graph in A are shown and include estimates of the diffusive permeability to albumin (Pd), hydraulic conductivity (Lp), and the Péclet number (Pé) at the average in vivo collecting lymphatic hydrostatic pressure (7 cmH2O; Scallan & Huxley, 2010).
Effects of natriuretic peptide exposure on collecting lymphatic contractile parameters
During measurement of albumin flux from collecting lymphatic vessels, we observed that the fluorescence intensity tracing recorded the amplitude and frequency of lymphatic spontaneous contractions, and could be potentially used as a method to evaluate these parameters (Scallan & Huxley, 2010). Figure 4 depicts the lymphatic contraction amplitude and frequency responses to either ANP (Fig. 4A and B) or BNP (Fig. 4C and D). In every vessel perfused with ANP, spontaneous contractions appeared to be abolished completely (amplitude, P= 0.005; frequency P= 0.006). In marked contrast, perfusion with BNP resulted in apparently stronger (P= 0.01) and more frequent (P= 0.01) contractions.
Figure 4. Perfusion of 100 nm ANP and BNP appear to differentially regulate lymphatic spontaneous contractions in vivo.
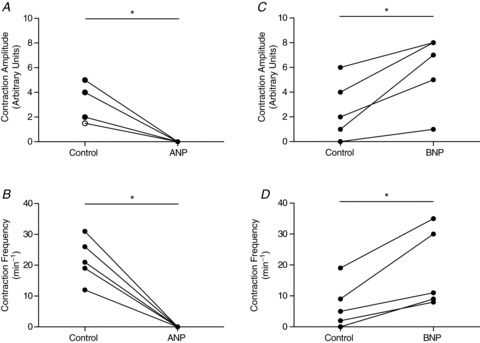
Luminal exposure to ANP (n= 6) apparently inhibits collecting lymphatic vessel contraction amplitude (A) and frequency (B). Exposure to BNP, however, enhances both contraction amplitude (C) and frequency (D) by approximately 2-fold each (n= 5). Amplitude and frequency were measured from the fluorescence intensity data tracing obtained prior to or during solute flux measurements made in vivo. All measures were paired, meaning that the same vessel was perfused with a control solution followed by an identical one containing ANP or BNP. Each pair was measured at one hydrostatic pressure and at the same site on the vessel. No significant changes in diameter were observed. The open circle in A indicates two overlapping data points. *P < 0.05.
To verify whether these effects on contractile function were dose dependent, similar vessels were isolated from rat mesentery and cannulated on glass pipettes in vitro. This approach enabled the application of several concentrations of peptides/drugs and was exquisitely sensitive to changes in contractile features. The effects of ANP application on end-diastolic diameter, contraction amplitude, and contraction frequency were plotted in Fig. 5 as a function of peptide concentration (n= 4). Only the highest concentration of 100 nm ANP significantly dilated the collecting lymphatics (Fig. 5A). Interestingly, no significant differences were found in contraction amplitude or frequency (Fig. 5B and C), although there was a tendency for ANP to inhibit the contraction frequency with increasing concentration. The effects of BNP on contractile function were also investigated and graphed in Fig. 6 for the same range of concentrations (n= 5). BNP did not significantly change the contraction amplitude at any dose tested (Fig. 6B). However, BNP did significantly cause a constriction of the collecting lymphatics (Fig. 6A) and accelerate the rate of contractions (Fig. 6C), but only at the highest concentration tested, 100 nm. Notably, these data indicate that the effects of natriuretic peptides on contractile function were attenuated compared to those observed using a less sensitive fluorometric approach in vivo.
Figure 5. In vitro dose responsiveness of collecting lymphatic vessel contractile function to ANP.
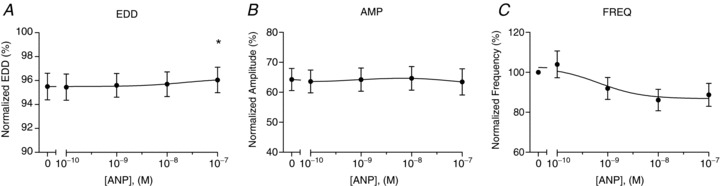
The normalized end-diastolic diameter (EDD; A), contraction amplitude (AMP; B), and contraction frequency (FREQ; C) were each plotted as a function of ANP concentration, ranging from 0.1 to 100 nm (n= 4). End-diastolic diameter and contraction amplitude were normalized to the maximal passive diameter at a pressure of 3 cmH2O, while contraction frequency was normalized to that of the control period. The first data point of each graph represents the mean of the control data. *Significantly different from the control data point (P < 0.05).
Figure 6. In vitro dose-responsiveness of isolated collecting lymphatic vessel contractile function to BNP.
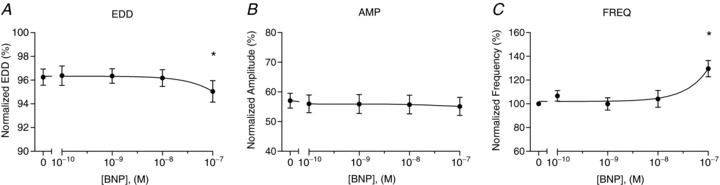
The normalized end-diastolic diameter (EDD; A), contraction amplitude (AMP; B), and contraction frequency (FREQ, C) were each plotted as a function of BNP concentration, ranging from 0.1 to 100 nm (n= 5). End-diastolic diameter and contraction amplitude were normalized to the maximal passive diameter at a pressure of 3 cmH2O, while contraction frequency was normalized to that of the control period. The first data point of each graph represents the mean of the control data. *Significantly different from the control data point (P < 0.05).
Discussion
The lymphatic vasculature is well documented to absorb protein-containing fluid from the interstitium (Schmid-Schönbein, 1990; Aukland & Reed, 1993). More recent reports, however, have shown that lymphatic vessels are capable of both absorption and extravasation of protein and fluid (Brookes et al. 2009; Machnik et al. 2009, 2010; Scallan & Huxley, 2010; Wiig et al. 2013) probably as a mechanism to control the fluid distribution between the tissue and vascular spaces. Therefore, the present study aimed to clarify whether, and to what extent, NPs, which are released into the circulation upon volume expansion, modulate collecting lymphatic vessel permeability. We accepted our hypothesis that in vivo collecting lymphatics respond to ANP and BNP through an increase in permeability to albumin (Ps) and that the response does not differ between peptides. Further, when the coupling of albumin flux to water flux was examined with respect to ANP treatment, pathways carrying albumin and water were affected similarly. Overall, our data support the conclusion that lymphatic vessels are capable of regulating their Ps in response to natriuretic peptides and the theory that lymphatic extravasation of protein and water is a compensatory response to volume overload.
Collecting lymphatic vessel permeability to albumin: responses to ANP and BNP
The lymphatic Ps responses to ANP and BNP were a 2-fold elevation above control and compare well with the 2- to 3-fold response reported previously for venular capillaries (McKay & Huxley, 1995). That collecting lymphatic vessels appear to mirror the venules with respect to regulation of microvascular permeability supports further the rationale that these two vessel types are likely to possess similar function as a result of their common embryological origin (Srinivasan et al. 2007, Yang et al. 2012). In addition, the responses to ANP and BNP do not differ statistically, although there is a tendency for a stronger mean Ps response to BNP (2.0 ± 0.4- versus 2.7 ± 0.8-fold). When the sensitivity of the lymphatic Ps response is viewed graphically (i.e. when the response is plotted against control Ps in Fig. 2), it becomes evident that on exposure to ANP, a lymphatic vessel with a lower basal Ps will respond more strongly than a vessel with a higher basal Ps, confirming the relationship of the venular hydraulic conductivity response to ANP observed previously (McKay & Huxley, 1995). Interestingly, when the Ps response to BNP is plotted on the same graph, the fitted curve is shifted to the right of the ANP data. From this relationship we conclude that although the mean lymphatic Ps responses to ANP and BNP do not differ, a single vessel with a low basal Ps (<4.0 × 10−7 cm s−1) will probably produce a stronger response to BNP than ANP (compare Fig. 1B with Fig. 1A). These results suggest that once lymphatic Ps is increased, then the capacity to respond to ANP will be lost before that to BNP.
If changes in lymphatic diameter occurred on exposure to either peptide, they were beyond the resolution of the ocular ruler, especially during lymphatic contractions. According to Brookes & Kaufman (2005), we should have expected a <5% increase in diameter, which would equate to <5 μm, much less than one eyepiece unit of 12 μm at the magnifications used in the in vivo studies. A previous study (Ji & Huxley, 2000) also failed to observe changes in diameter upon perfusion of rat skeletal muscle arterioles with 1 nm ANP (P= 0.3), under conditions where changes on the order of 5 μm could be resolved, suggesting that the lack of vasodilation in response to the natriuretic peptides at these concentrations is not limited to collecting lymphatics. Finally, no change in diameter was observed in isolated collecting lymphatic vessels exposed to pathophysiological concentrations (0.1 or 1 nm) of ANP or BNP (Figs 5 and 6).
The present study could be criticized for comparing Ps responses from rat mesentery to those obtained from frog mesentery (Meyer & Huxley, 1990; McKay & Huxley, 1995). Yet data from preceding studies demonstrated that 1 nm ANP elicited increases in Ps of rat skeletal muscle arterioles on the order of 1.6 ± 0.2-fold (n= 12, P < 0.01; Ji & Huxley, 2000) and of porcine coronary arterioles, 2.0 ± 0.6-fold (n= 7, P < 0.05; Huxley et al. 2007). Therefore, the microvascular permeability responses to ANP appear to be of similar magnitude among these three species, ranging from amphibians to large mammals. Another limitation of the present study is that we chose to use a natriuretic peptide concentration exceeding the pathophysiological range of 0.1 to 1 nm (Yoshimura et al. 1993, Maisel et al. 2002). However, we previously demonstrated that venular permeability is elevated 2-fold upon stimulation with 0.1, 1, 10, or 100 nm ANP (Meyer & Huxley, 1990). Thus, the dose of 100 nm used here tested whether or not collecting lymphatics possessed the capacity to respond to these peptides.
The data thus far illustrate that lymphatic permeability to the protein, albumin, can be elevated significantly and acutely in response to atrial and brain natriuretic peptides. However, during volume expansion a shift of fluid – in addition to protein – from the vascular space to the tissues would be necessary to relieve volume overload and stress on the heart. To address how water transport might be affected, we graphed the control and ANP treatment Ps measures as a function of net driving pressure (hydrostatic minus oncotic pressures) across the lymphatic vessel wall (Fig. 3A). While the two curves do not differ statistically, probably reflecting the non-normal and left skewed distribution in Ps data (Scallan & Huxley, 2010), the two curves still provide physiologically relevant data, namely, estimates of the diffusive permeability (Pd) and Lp(1 –σ). Apparent in Fig. 3B are that both Lp(1 –σ) (a measure of volume flux through the pathways conducting both fluid and protein) and Pd (a measure of diffusive protein permeability) are nearly doubled upon treatment of collecting lymphatics with ANP. Consistent with a study of venules (McKay & Huxley, 1995), Pé did not change with ANP perfusion since it is the ratio that describes water-driven (Lp(1 –σ)) to diffusion-mediated (Pd) solute flux. The Pé value (Fig. 3B) also suggests that if lymphatic Lp (i.e. hydraulic conductivity) undergoes an increase similar in magnitude to Pd during perfusion with ANP, then the reflection coefficient to albumin, σ, would not change. The latter scenario probably describes the effect of ANP on lymphatic Lp as the basal lymphatic Ps and its response mirrored that of the venules, for which σ has been demonstrated not to change during ANP infusion (Meyer & Huxley, 1990; McKay & Huxley, 1995) or, to provide another example, during hyperglycaemia (Perrin et al. 2007). Additionally, changes in pore size (σ) result in large, exponential increases in Lp (to the fourth power if the transendothelial pathways are cylindrical or the second power if they are described by slits; Curry, 1984). Instead, for changes to occur in Lp and Pd comparable to those measured here, either the number of ‘pores’ doubled or the thickness of the transendothelial channels was halved. While it is possible that a fraction of the permeability to albumin reflects a vesicular transport mechanism, the changes in Pd and Lp(1 –σ) are not consistent with changes occurring solely in this pressure-independent component (i.e. increases in vesicular turnover would not change Lp(1 –σ)). These data point to the need for follow-up studies to determine lymphatic vessel Lp and its response to NP, and whether the lymphatic σ for albumin is altered by NP.
Collecting lymphatic vessel contractile function: responses to ANP and BNP
While we were recording albumin flux of collecting lymphatics in vivo, we observed that the contraction amplitude and frequency were affected by ANP and BNP oppositely. The effects at this concentration were largely confirmed by studying collecting lymphatic contractile activity in vitro. Isolated collecting lymphatic vessels exposed to 100 nm ANP demonstrated a decreased spontaneous contraction frequency (Fig. 5), a finding that is supported by the literature (Ohhashi et al. 1990, Anderson et al. 1991). In contrast to the ANP response, 100 nm BNP increased the contraction frequency and evoked a constriction in vitro (Fig. 6). At lower concentrations of each peptide (0.1, 1, or 10 nm), significant contractile effects were not observed. Still, it remains to be determined whether or not exposure to pathophysiological concentrations of each peptide (0.1–1 nm) results in significant changes in lymphatic contractile function in vivo where other cell types are present, or whether lymphatic permeability is sensitive to these lower concentrations. Importantly, the pro-contractile effects of BNP demonstrated here would promote lymph flow and the consequent return of interstitial fluid to the bloodstream to increase, instead of decrease, the vascular volume.
Physiological significance of the lymphatic responses to natriuretic peptides
The data obtained from this study reveal that rat mesenteric collecting lymphatic vessels possess the capacity to respond to ANP and BNP by doubling their permeability to albumin and fluid acutely. Further, collecting lymphatic spontaneous contractions were inhibited by ANP and enhanced by BNP in vivo, although these effects were not observed at pathophysiological concentrations (0.1–1 nm) of either peptide when applied in vitro. Importantly, one must realize that increases in solute permeability, and probably hydraulic conductivity, serve to augment the rate of protein and fluid extravasation from the microvasculature into the interstitium as demonstrated by others (Valentin et al. 1989; Tucker et al. 1992; Brookes et al. 2009; Scallan & Huxley, 2010). An extravasation of fluid and protein of this magnitude from the lymphatic space would reduce the vascular volume by preventing the capillary filtrate from being absorbed and returned to the bloodstream by the lymphatic vessels.
Acknowledgments
The authors thank Susan Bingaman for her technical assistance with this project. This study represents a portion of the research by J. P. Scallan in partial fulfillment of the requirements for the PhD in Physiology, University of Missouri-Columbia.
Glossary
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- BSA
bovine serum albumin
- CHF
congestive heart failure
- NP
natriuretic peptide
- Pé
Péclet number
- RSA
rat serum albumin
Additional information
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
To test whether collecting lymphatic permeability is dynamic or static was decided by J.P.S. and V.H.H. J.P.S. designed the experiments from which he collected and analysed the data. Additionally, V.H.H. provided guidance not only with experimental design and data analysis, but also with concepts underlying the regulation of solute permeability. V.H.H. revised and approved each version of the manuscript that J.P.S. wrote. M.J.D. supported the isolated lymphatic vessel experiments and revised and approved the final version of the manuscript. Experiments were performed at the University of Missouri at Columbia in the Center for Diabetes and Cardiovascular Health, and the National Center for Gender Physiology.
Funding
This work was supported by NIH grant HL078816, NASA grant NNJ05HF37G, NIH grant HL-089784, and the Department of Medical Pharmacology and Physiology.
References
- Almeida FA, Suzuki M, Maack T. Atrial natriuretic factor increases hematocrit and decreases plasma volume in nephrectomized rats. Life Sci. 1986;39:R1193–R1199. doi: 10.1016/0024-3205(86)90351-6. [DOI] [PubMed] [Google Scholar]
- Anderson WD, Kulik TJ, Mayer JE. Inhibition of contraction of isolated lymphatic ducts by atrial natriuretic peptide. Am J Physiol Regul Integr Comp Physiol. 1991;260:R610–R614. doi: 10.1152/ajpregu.1991.260.3.R610. [DOI] [PubMed] [Google Scholar]
- Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- Bingaman S, Huxley VH, Rumbaut RE. Fluorescent dyes modify properties of proteins used in microvascular research. Microcirculation. 2003;10:221–231. doi: 10.1038/sj.mn.7800186. [DOI] [PubMed] [Google Scholar]
- Brookes ZL, Kaufman S. Effects of atrial natriuretic peptide on the extrasplenic microvasculature and lymphatics in the rat in vivo. J Physiol. 2005;565:269–277. doi: 10.1113/jphysiol.2005.083147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes ZL, Mansart A, McGown CC, Ross JJ, Reilly CS, Brown NJ. Macromolecular leak from extrasplenic lymphatics during endotoxemia. Lymphat Res Biol. 2009;7:131–137. doi: 10.1089/lrb.2008.1019. [DOI] [PubMed] [Google Scholar]
- Clerico A, Del Ry S, Maffei S, Prontera C, Emdin M, Giannessi D. The circulating levels of cardiac natriuretic hormones in healthy adults: effects of age and sex. Clin Chem Lab Med. 2002;40:371–377. doi: 10.1515/CCLM.2002.060. [DOI] [PubMed] [Google Scholar]
- Curry FE. Mechanics and thermodynamics of trancapillary exchange. In: Renkin EM, Michel CC, editors. Handbook of Physiology, section II, The Cardiovascular System, vol. IV, Microcirculation. Bethesda, MD, USA: American Physiological Society; 1984. pp. 309–374. [Google Scholar]
- Curry FR, Rygh CB, Karlsen T, Wiig H, Adamson RH, Clark JF, Lin YC, Gassner B, Thorsen F, Moen I, Tenstad O, Kuhn M, Reed RK. Atrial natriuretic peptide modulation of albumin clearance and contrast agent permeability in mouse skeletal muscle and skin: role in regulation of plasma volume. J Physiol. 2010;588:325–339. doi: 10.1113/jphysiol.2009.180463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Physiol Heart Circ Physiol. 2012;303:H795–H808. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbes AL, Dagnino L, Nguyen T, Nemer M. Transcription of brain natriuretic peptide and atrial natriuretic peptide genes in human tissues. J Clin Endocrinol Metab. 1994;78:1307–1311. doi: 10.1210/jcem.78.6.8200930. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: alpha-lactalbumin transport. Am J Physiol Heart Circ Physiol. 1987a;252:H188–H197. doi: 10.1152/ajpheart.1987.252.1.H188. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Tucker VL, Verburg KM, Freeman RH. Increased capillary hydraulic conductivity induced by atrial natriuretic peptide. Circ Res. 1987b;60:304–307. doi: 10.1161/01.res.60.2.304. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Wang JJ, Sarelius IH. Adaptation of coronary microvascular exchange in arterioles and venules to exercise training and a role for sex in determining permeability responses. Am J Physiol Heart Circ Physiol. 2007;293:H1196–H1205. doi: 10.1152/ajpheart.00069.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L-P, Huxley VH. Elevation of skeletal muscle arteriole permeability to protein by atrial natriuretic peptide. FASEB J. 2000;14:A25. [Google Scholar]
- Landis EM, Pappenheimer JR. Exchange of substances through the capillary walls. In: Hamilton WF, editor. Handbook of Physiology. Washington, DC, USA: American Physiological Society; 1963. pp. 961–1034. [Google Scholar]
- McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–477. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Machnik A, Dahlmann A, Kopp C, Goss J, Wagner H, van Rooijen N, Eckardt KU, Muller DN, Park JK, Luft FC, Kerjaschki D, Titze J. Mononuclear phagocyte system depletion blocks interstitial tonicity-responsive enhancer binding protein/vascular endothelial growth factor C expression and induces salt-sensitive hypertension in rats. Hypertension. 2010;55:755–761. doi: 10.1161/HYPERTENSIONAHA.109.143339. [DOI] [PubMed] [Google Scholar]
- Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–52. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- McKay MK. Department of Medical Pharmacology and Physiology. Columbia, MO, USA: University of Missouri at Columbia; 1994. Studies on the permeability of the capillary barrier: effects of perfusate proteins and natriuretic factors. [Google Scholar]
- McKay MK, Huxley VH. ANP increases capillary permeability to protein independent of perfusate protein composition. Am J Physiol Heart Circ Physiol. 1995;268:H1139–H1148. doi: 10.1152/ajpheart.1995.268.3.H1139. [DOI] [PubMed] [Google Scholar]
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA, Breathing Not Properly Multinational Study Investigators Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Eng J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- Meyer DJ, Jr, Huxley VH. Differential sensitivity of exchange vessel hydraulic conductivity to atrial natriuretic peptide. Am J Physiol Heart Circ Physiol. 1990;258:H521–H528. doi: 10.1152/ajpheart.1990.258.2.H521. [DOI] [PubMed] [Google Scholar]
- Neter J, Wasserman W, Kutner M. Applied Linear Statistical Models: Regression, Analysis of Variance, and Experimental Designs. Homewood, IL, USA: Irwin; 1990. [Google Scholar]
- Ohhashi T, Watanabe N, Kawai Y. Effects of atrial natriuretic peptide on isolated bovine mesenteric lymph vessels. Am J Physiol Heart Circ Physiol. 1990;259:H42–H47. doi: 10.1152/ajpheart.1990.259.1.H42. [DOI] [PubMed] [Google Scholar]
- Perrin RM, Harper SJ, Corrall R, Bates DO. Hyperglycemia stimulates a sustained increase in hydraulic conductivity in vivo without any change in reflection coefficient. Microcirculation. 2007;14:683–696. doi: 10.1080/10739680701436129. [DOI] [PubMed] [Google Scholar]
- Rubattu S, Sciarretta S, Valenti V, Stanzione R, Volpe M. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am J Hypertens. 2008;21:733–741. doi: 10.1038/ajh.2008.174. [DOI] [PubMed] [Google Scholar]
- Rumbaut RE, Huxley VH. Similar permeability responses to nitric oxide synthase inhibitors of venules from three animal species. Microvasc Res. 2002;64:21–31. doi: 10.1006/mvre.2002.2394. [DOI] [PubMed] [Google Scholar]
- Sarelius IH, Kuebel JM, Wang J, Huxley VH. Macromolecule permeability of in situ and excised rodent skeletal muscle arterioles and venules. Am J Physiol Heart Circ Physiol. 2006;290:H474–H480. doi: 10.1152/ajpheart.00655.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J Physiol. 2013;591:2139–56. doi: 10.1113/jphysiol.2012.250662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Huxley VH. in vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J Physiol. 2010;588:243–254. doi: 10.1113/jphysiol.2009.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan JP, Wolpers JH, Davis MJ. Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J Physiol. 2013;591:443–459. doi: 10.1113/jphysiol.2012.237909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Schönbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker VL, Simanonok KE, Renkin EM. Tissue-specific effects of physiological ANP infusion on blood-tissue albumin transport. Am J Physiol. 1992;263:R945–953. doi: 10.1152/ajpregu.1992.263.4.R945. [DOI] [PubMed] [Google Scholar]
- Valentin JP, Ribstein J, Mimran A. Effect of nicardipine and atriopeptin on transcapillary shift of fluid and proteins. Am J Physiol Regul Integr Comp Physiol. 1989;257:R174–R179. doi: 10.1152/ajpregu.1989.257.1.R174. [DOI] [PubMed] [Google Scholar]
- Wiig H, Schröder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, Dahlmann A, Brenner S, Tenstad O, Nurmi H, Mervaala E, Wagner H, Beck FX, Müller DN, Kerjaschki D, Luft FC, Harrison DG, Alitalo K, Titze J. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RL. Cardioprotective functions of atrial natriuretic peptide and B-type natriuretic peptide: a brief review. Clin Exp Pharmacol Physiol. 2004;31:791–794. doi: 10.1111/j.0305-1870.2004.04073.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340–2348. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Yasue H, Okumura K, Ogawa H, Jougasaki M, Mukoyama M, Nakao K, Imura H. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- Zweifach BW, Prather JW. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am J Physiol. 1975;228:1326–1335. doi: 10.1152/ajplegacy.1975.228.5.1326. [DOI] [PubMed] [Google Scholar]


