Abstract
Colonic transit and mucosal integrity are believed to be impaired in obesity. However, a comprehensive assessment of altered colonic functions, inflammatory changes and neuronal signalling of obese animals is missing. In mice, we studied the impact of diet-induced obesity (DIO) on: (i) in vivo colonic transit; (ii) signalling in the myenteric plexus by recording responses to nicotine and 2-methyl-5-hydroxytryptamine (2-methyl-5-HT), together with the expression of tryptophan hydroxylase (TPH) 1 and 2, serotonin reuptake transporter, choline acetyltransferase and the paired box gene 4; and (iii) expression of proinflammatory cytokines, epithelial permeability and density of macrophages, mast cells and enterochromaffin cells. Compared with controls, colon transit and neuronal sensitivity to nicotine and 2-methyl-5-HT were enhanced in DIO mice fed for 12 weeks. This was associated with increased tissue acetylcholine and 5-hydroxytryptamine (5-HT) content, and increased expression of TPH1 and TPH2. In DIO mice, upregulation of proinflammatory cytokines was found in fat tissue, but not in the gut wall. Accordingly, mucosal permeability or integrity was unaltered without signs of immune cell infiltration in the gut wall. Body weight showed positive correlations with adipocyte markers, tissue levels of 5-HT and acetylcholine, and the degree of neuronal sensitization. DIO mice fed for 4 weeks showed no neuronal sensitization, had no signs of gut wall inflammation and showed a smaller increase in leptin, interleukin-6 and monocyte chemoattractant protein 1 expression in fat tissue. DIO is associated with faster colonic transit and impacts on acetylcholine and 5-HT metabolism with enhanced responsiveness of enteric neurones to both mediators after 12 weeks of feeding. Our study demonstrates neuronal plasticity in DIO prior to the development of a pathological histology or abnormal mucosal functions. This questions the common assumption that increased mucosal inflammation and permeability initiate functional disorders in obesity.
Key points
We investigated altered colonic functions in high fat diet-induced obesity in pre-diabetic mice.
After feeding an adipogenic diet for 12 weeks, accelerated colonic transit was associated with upregulation and enhanced signalling of acetylcholine and serotonin, two key mediators in the enteric nervous system (ENS). Importantly, these changes occurred without signs of impaired mucosal integrity or immune cell infiltration in the gut wall. Neuronal sensitization was not observed in obese mice fed for 4 weeks.
Weight gain correlated positively with the level of adipocyte markers and the degree of neuronal sensitization.
We conclude that enhanced neural excitation in the colon by acetylcholine and serotonin is a key feature of a later phase of obesity and is involved in altered ENS functions and abnormal colonic transit.
Furthermore, the results suggest that the occurrence of altered gut functions in obesity is independent of inflammation in the gut wall.
Introduction
There is increasing evidence that obesity is linked to common functional gastrointestinal disorders (see Mayer, 2012; Raybould, 2012). These dysfunctions encompass changes in both gastrointestinal motor and epithelial barrier functions. In particular, the modulation of gastric emptying is directly involved in the control of satiety. Although the role of the colon in food intake remains largely unexplored, diarrhoea and faecal incontinence, which may result from accelerated colonic transit, have been described in obese patients (Gallagher et al. 2007; Delgado-Aros et al. 2008; Ho & Spiegel, 2008; Rodger et al. 2010; Poylin et al. 2011). However, the mechanisms responsible for colonic motor changes are unknown. Based on the critical role played by the enteric nervous system (ENS) in the control of gastrointestinal motility, one would postulate the existence of neuroplastic changes in the colonic ENS associated with obesity.
Consistently, altered functional innervation of the gut by the ENS has been shown in various animal models of obesity. However, these changes are not uniform and appear to vary between gut regions and among animal models. This might reflect the complex nature of obesity (nutritional versus genetic), the comorbidities in obese animals (metabolic syndrome, diabetes) as well as age-dependent responses to an adipogenic diet. For instance, in an obese diabetic mouse model, the volume density of nitric oxide synthase-positive nerve fibres was decreased in the antrum and duodenum, but not in the colon (Spångéus & El-Sahly, 2001). Conversely, in a non-diabetic mouse DIO model, an increase in the proportion of antral, but not duodenal, nitric oxide synthase neurones associated with enhanced gastric emptying was observed (Baudry et al. 2012). In an obese diabetic mouse model, galanin-positive nerve fibres were decreased in the duodenum in these mice, and neuropeptide Y and cholinergic nerve fibres were increased in density in the colon, but not in the antrum and duodenum (Spångéus & El-Sahly, 2001). The relevance of such regional changes remains unknown.
Obesity induced by genetic manipulation is also associated with functional gastrointestinal disorders. For example, in leptin-deficient obese mice, increased cholecystokinin responses, together with faster transit time in the proximal intestine, were observed (Kiely et al. 2005). In this mouse model, it must be considered that the leptin deficiency has a direct impact on ENS signalling, as leptin activates myenteric and submucous neurones (Reichardt et al. 2011). Changes in gastrointestinal functions may therefore be related to altered excitability of enteric neurones.
Diet-induced obesity (DIO) has become an accepted model to study changes in gut functions. Although several such studies have reported changes in gastric or small intestinal functions, there is only limited information available on altered colonic functions in obese mice. Most studies have focused on an individual aspect without providing a more comprehensive assessment of functional disorders. Data from different rodent models are often contradictory. For example, gastric emptying is slowed in DIO rats (Li J et al. 2011), but increased in DIO mice, where obesity seems to protect against neuronal loss in the antral myenteric plexus (Baudry et al. 2012). This neuroprotection was not observed in the jejunum of these mice, whereas altered nerve function occurred in obese rats (Hyland et al. 2010). It is therefore difficult to know whether these changes are common responses or species specific. Irrespective of the discrepant results on the type of gastrointestinal functional alterations, numerous studies have highlighted increased intestinal permeability and signs of intestinal inflammation in both DIO rats and mice (Lam et al. 2012; Raybould, 2012; Stenman et al. 2012). This suggests that tissue alterations and functional disorders develop as a consequence of a low grade inflammation which is often viewed as a common feature in obesity (Ding & Lund, 2011).
There are numerous animal models of obesity (see Lutz & Woods, 2012). Differences between the models include the type of diet, the species and the timing of dietary intervention. We used a mouse model of DIO that we have characterized and validated recently (Baudry et al. 2012) to determine the impact of DIO on key colonic functions and ENS phenotype. It differs from other models in two main aspects. First, the model reflects juvenile obesity, as the feeding regimen started in young, 5-week-old mice. Second, during the 12 weeks of feeding with the high fat diet, animals remained in a pre-diabetic state. They developed leptinaemia and insulinaemia, but had normal fasting glucose levels. Results of the oral glucose tolerance test suggested that the animals had glucose intolerance.
The aim of this study was to provide a comprehensive assessment of altered functions in the distal colon of DIO mice. We investigated the colonic transit time, neurobiology of myenteric neurones, tissue acetylcholine (ACh) and serotonin contents, and the expression of the enzymes involved in their synthesis. Moreover, adipose tissue mass, mucosal integrity, histological scores for inflammation and the upregulation of proinflammatory cytokines in adipose tissue and the gut wall were evaluated. Myenteric neurones were stimulated by the applications of nicotine (nicotinic ACh receptor agonist), which activates the majority of neurones, and 2-methyl-5-hydroxytryptamine (2-methyl-5-HT) (5-hydroxytryptamine type 3 receptor agonist). We also determined the proportion of recently characterized rapidly adapting mechanosensitive enteric neurones (RAMEN) in order to investigate altered sensory signalling in the myenteric plexus (Mazzuoli & Schemann, 2012). The results of this study support the concept that altered colonic functions are associated with neural plasticity in the ENS, independent of mucosal inflammation or impaired mucosal integrity.
Methods
Ethics statement
All work performed at TU München in Freising was conducted according to the German guidelines for animal care and welfare (Deutsches Tierschutzgesetz) and approved by the Bavarian state ethics committee (Regierung Oberbayern, which serves as the Institutional Care and Use Committee for the Technische Universität München) according to §4 and §11 Deutsches Tierschutzgesetz under the reference number 32-568-2. Experiments performed at INSERM in Nantes were in accordance with an experimental protocol for animal study approved by the INSERM Institutional Animal Care and Use Committee.
Animals
The model used in this work has been described in detail previously (Baudry et al. 2012). Male C57BL/6J Rj mice, aged 4 weeks (Charles-Rivers GmbH, Sulfzield, Germany or Janvier Laboratory, Le Genest-Saint-Isle, France), were housed (four to five mice per cage) in a conventional state under adequate temperature (22°C) control with a 12 h light/12 h dark cycle and free access to food and water. After 1 week of adaptation, animals were randomly assigned to receive 4 or 12 weeks of either normal chow diet [ND group, 23 kcal% protein, 54 kcal% complex carbohydrate, 13 kcal% fat; Purified diet 210, SAFE, Augy, France] or high fat diet [DIO group, 13 kcal% protein, 26 kcal% simple carbohydrate (mainly saccharose), 61 kcal% fat (primarily lard); Purified diet 230 HF, SAFE].
Colonic transit
Colonic transit was measured as described previously (Osinski et al. 1999). Briefly, mice were lightly anaesthetized with isoflurane to facilitate the placement of a spherical 2 mm glass bead at 2 cm proximal to the anal opening using a fire-polished glass rod. The animals were immediately placed into their cages with a white paper towel at its base to allow for easier detection of the bead. Distal colonic transit was determined to the nearest 6 s by monitoring the time required for the expulsion of the glass bead (bead latency).
Tissue sampling and histological observations
Mice were killed by cervical dislocation and the distal colon, colonic mesenteric adipose tissue (MAT) and epididymal adipose tissue (EAT) were quickly removed. Samples for adipocyte size measurement and histological scoring were fixed in 4% formaldehyde, embedded in paraffin, sectioned at 5 μm and stained with haematoxylin and eosin. Blind histology scoring was performed by an experienced scientist by assessing the degree of lamina propria mononuclear cell infiltration, crypt hyperplasia, goblet cell depletion and architectural distortion. It resulted in a score from zero (not inflamed) to 12 (inflamed), as described previously (Katakura et al. 2005). Colonic mucosal thickness was measured by the analysis of 10 cross-sectional areas per tissue and mouse using the software AxioVision 4.6 (Carl Zeiss AG, Jena, Germany). The adipocyte sizes (μm2) were determined on MAT and EAT sections at ×200 magnification.
Colonic permeability
The permeability was measured using full-thickness segments of the distal colon mounted in 2 mm diameter Ussing chambers (World Precision Instrument, Sarasota, FL, USA), as described previously (Tasseli et al. 2013). Tissues were bathed on each side with 2 ml of Krebs solution (NaCl, 117 mm; KCl, 4.7 mm; MgCl2, 1.2 mm; NaH2PO4, 1.2 mm; NaHCO3, 25 mm; CaCl2, 2.5 mm; glucose, 11 mm), continuously oxygenated and maintained at 37°C. After 30 min of equilibration, 200 μl of apical medium was replaced by 200 μl of solution containing fluorescein sulfonic acid (10 mg ml−1). After 1 h, the fluorescence level of a basolateral aliquot of 150 μl was determined using a fluorimeter.
Immunohistochemistry
Whole mount tissue was fixed for 4 h at room temperature in phosphate-buffered 4% paraformaldehyde and 0.2% picric acid, washed (3 × 10 min) in phosphate buffer and incubated overnight (4°C) in sucrose solution. Cryostat sections (20 μm) were incubated in a humidified chamber for 1 h at room temperature in phosphate-buffered saline containing 0.5% Triton X-100 and 4% horse serum to block non-specific binding. The tissues were then incubated for 16 h overnight at room temperature with the primary antibody rat anti F4/80 (1 : 2000, BMA Biomedicalis, Augst, Switzerland). This was followed by incubation for 1.5 h at room temperature with species-specific secondary antibody coupled to Cy3 (1 : 500, Dianova, Hamburg, Germany). Fluorescence was detected by an Olympus microscope (BX61 WI; Olympus, Hamburg, Germany) equipped with appropriate filter blocks, Fview II CCD camera and image software (CellP, Olympus). Macrophage density was measured in two regions of interest, which were the submucosa/mucosa and the muscle/myenteric plexus layers. The results were expressed as percentage of F4/80 immunoreactivity per area (mm2). The density of macrophages was based on the analysis of five consecutive sections per animal covering an area of 0.02–0.4 mm2.
Enterochromaffin (EC) cells were labelled with mouse anti-5-hydroxytryptamine (anti-5-HT) (1 : 1000, Merck Millipore, Billerica, MA, USA) and mast cells with rat anti-CD117 (c-kit; 1 : 50; eBioscience, San Diego, CA, USA). Rodent mast cells also contain 5-HT. However, they were easily identified by their distinct morphology and subepithelial localization. A c-kit staining after 5-HT labelling verified that epithelial cells identified as 5-HT-positive EC cells were not mast cells. Moreover, there are only very few mast cells in the mouse gut wall (Li et al. 2004), which correspond to the values reported in our study (see Results). The densities of EC cells and mast cells are expressed as cells per crypt. EC cell counts were based on the analysis of three consecutive sections per animal, each containing 13–79 crypt areas. Mast cell counts were based on the analysis of one section per animal, containing 84–171 crypt areas. As a result of the very dense macrophage network, it was not feasible to analyse macrophage number per crypt.
In preliminary experiments, we tested whether the number of EC cells per epithelial area and per crypt would yield different results. This was not the case, arguing that changes in tissue size were not a problem, probably because we fixed the tissue at maximal stretch before fixation.
mRNA expression
RNA from MAT, EAT and isolated epithelial cells was extracted using Trizol Reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's instructions. Extracted RNA was dissolved in 15–20 μl of water containing 0.1% diethyl-pyrocarbonate. The RNA concentration and purity (A260/A280 ratio) were determined by spectrophotometric analysis (ND-1000 spectrophotometer, NanoDrop Technologies, Wilmington, DE, USA). Reverse transcription was performed using 1 μg of total RNA employing the Promega M-MLV system (Promega, Madison, WI, USA). Real-time PCR was performed using 1 μl of cDNA in a Light Cycler system (Roche Diagnostics, Mannheim, Germany). Primers and probes were designed using the universal probe library (Roche) and purchased from Roche. Primer sequences were as follows: glyceraldehyde 3-phosphate dehydrogenase (GAPDH): sense, 5′-tccactcat ggcaaattcaa; reverse, 5′-tttgatgttagtggggtctcg; leptin: sense, 5′-caggatcaatgacatttcacaca; reverse, 5′-gctggtgaggacctg ttgat; interleukin-6 (IL-6): sense, 5′-gatggatgctaccaaact ggat; reverse, 5′-ccaggtagctatggtactccaga; monocyte chemoattractant protein 1 (MCP1): sense, 5′-catcca cgtgttggctca; reverse, 5′-gatcatcttgctggtgaatgagt; serum amyloid A 3 (SAA3): sense, 5′-atgctcgggggaactatgat; reverse, 5′-acagcctctctggcatcg; interferon-inducible protein-10 (IP-10): sense, 5′-gctgccgtcattttctgc; reverse, 5′-tctcactggcccgtcatc; B-cell lymphoma 2 (Bcl2): sense, 5′-gtacctgaaccggcatctg; reverse, 5′-ggggccatatagttccacaa.
Total RNA was extracted from colonic tissues using a Nucleospin RNA/protein kit (Macherey-Nagel, Düren, Germany), and cDNA was synthesized using standard procedures as described previously (Soret et al. 2010). Primer sequences were as follows: S6: sense, 5′-ccaagcttattcagcgtcttgttactcc-3′; reverse, 5′-ccctcgagtccttcattctcttggc-3′; tryptophan hydroxylase 1 (TPH1): sense, 5′-cacgagtgcaagccaaggttt; reverse, 5′-agtttccagccccgacatcag; tryptophan hydroxylase 2 (TPH2): sense, 5′-cgatctggcttcacagtgagac; reverse, 5′-tgggtgcagtggaatactgtag; serotonin reuptake transporter (SERT): sense, 5′-gacagccaccttcccttaca; reverse, 5′-ctagcaaacgccaggagaac; acetylcholine transferase (ChAT): sense, 5′-ctccagctggcttactacagg; reverse, 5′-ccccaggtaccttaaaccagt; paired box gene 4 (PAX4): sense, 5′-ccacaggaatcggactatcttctc; reverse, 5′-tgcccacgctaaactctttc; tumour necrosis factor-α (TNF-α): sense, 5′-ctgtagcccacgtctagc; reverse, 5′-ttgagatc catgccgttg; interleukin-1β (IL-1β): sense, 5′-gcctcgtgctg tcggacccata; reverse, 5′-ttgaggcccaaggccacaggt; IL-6: sense, 5′-gctggagtcacagaaggagtggc; reverse, 5′-ggcataacgcatagg tttgccg.
The amplified product was detected by the presence of a fluorescent signal by a probe reaction. The relative induction of gene mRNA expression was calculated according to the 2 –ΔΔCp method and normalized to the expression of GAPDH or S6. TPH1 and TPH2 gene expression is low in the gut wall; detection was based on 33–35 cycle numbers. Data were expressed as the fold change relative to control mice.
ACh and serotonin measurements
For the ACh measurement, segments of colon were placed in 1.5 ml lysing matrix D tubes (MPBio, Solon, OH, USA) and immediately frozen in liquid nitrogen. The amount of ACh in tissue was measured by spectrofluorometric assay using the ACh assay kit (Amplex Red; Molecular Probes, Eugene, OR, USA) according to the manufacturer's instructions. Protein concentration was assessed using the BCA protein assay kit (Pierce, Rockford, IL, USA). ACh concentration was expressed as micromoles of ACh per gram of protein. For the serotonin measurement, tissue samples were snap frozen in liquid nitrogen and stored at −80°C, homogenized manually and extracted with ice-cold methanol following a previously published protocol (Römisch-Margl et al. 2012). 5-HT was measured using the targeted metabolomic approach AbsoluteIDQ kit from BIOCRATES (BIOCRATES Life Sciences AG, Innsbruck, Austria), as described previously (Bogumil et al. 2008). We pipetted 10 μl of tissue extract onto the filter inserts of the 96-well kit plate (normalized by the applied milligrams of tissue fresh weight), and analysis was performed using an ABSciex QTrap 5500 mass spectrometer (Framingham, MA, USA) equipped with an Agilent 1200 Series HPLC (Santa Clara, CA, USA) and an HTC PAL autosampler controlled by the software Analyst 1.5. 5-HT was quantified by reference to an internal standard and expressed in micromoles.
Myenteric neurone recordings
We used voltage-sensitive dye imaging to record action potentials in myenteric neurones, as described previously (Reichardt et al. 2011). After loading the neurones with the voltage-sensitive dye Di-8-ANEPPS (Molecular Probes Mobitec, Göttingen, Germany), nicotine (nicotinic ACh receptor agonist; Sigma-Aldrich, Taufkirchen, Germany) and 2-methyl-5-HT (5-hydroxytryptamine type 3 receptor agonist; Tocris Bioscience, Ellisville, MI, USA) were applied by pressure pulse ejection from a micropipette (20 psi; pulse duration, 600 ms; ejection speed, 55 ± 27 nl s−1; distance to the ganglion, approximately 200 μm). Nicotine and 2-methyl-5-HT were applied at concentrations of 100 μm and 1 mm, respectively. The effects of the drugs were evaluated during a 3 s recording period and the drugs were applied 200 ms after the beginning of the recording. Recordings without drug application were used to identify neurones with spontaneous spike discharge. Recording periods were separated by 15–20 min. Relative changes in fluorescence (ΔF/F) are linearly related to changes in the membrane potential. The total number of neurones for each ganglion was determined by visual inspection of images from the Di-8-ANEPPS-stained ganglion. For analysis, the signals were superposed onto the image of the ganglion, thus allowing us to match signals to individual neurones.
We also used the neuroimaging technique to record responses of mechanosensitive neurones after mechanical stimulation by intraganglionic injections of small volumes of Krebs solution according to a previously published protocol (Mazzuoli & Schemann, 2012).
Statistics
The statistical analyses were performed with Sigma Plot 12.0 (Systat Software Inc., Erkrath, Germany). Normally distributed data were analysed using Student's t test and presented as the mean ± SD. Non-normally distributed data were analysed by the Mann–Whitney rank sum test and presented as the median, with the 25th and 75th percentiles given in parentheses. Correlations of non-normally and normally distributed data were analysed with the Spearman rank test and the Pearson test, respectively. The level of statistical significance was set at P < 0.05 (*P < 0.05; **P < 0.001). The numbers of data which are illustrated are given in the figure or figure legend rather than in the text.
Results
DIO mice showed increased body weight and faster colonic transit (12 weeks of feeding)
The two groups of mice had similar weights before the feeding regimens [ND: 16.8 g (14.0 g/18.9 g), n= 65; DIO: 17.6 g (14.1 g/19.0 g), n= 64]. After 12 weeks of feeding, DIO mice gained significantly more weight (38%) in comparison with ND mice (P < 0.001).
DIO mice exhibited a faster colonic transit in comparison with ND mice, as suggested by 33% faster bead expulsion (P= 0.014, Fig. 1A).
Figure 1. Diet-induced obesity accelerated colonic transit and increased mesenteric and epididymal adipose tissue mass, adipocyte size and expression of the adipokines leptin, interleukin-6 (IL-6) and monocyte chemoattractant protein 1 (MCP1) (12 weeks of feeding).
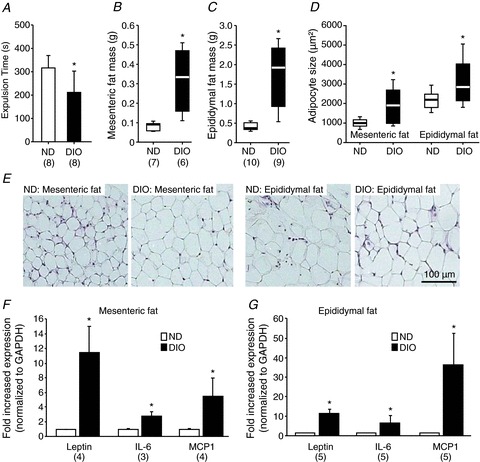
The shorter bead expulsion time indicated faster colonic transit in DIO mice (A). Mesenteric (B) and epididymal (C) fat mass was increased in DIO mice. Adipocyte sizes were also increased in both fat tissues (D). Images in (E) illustrate representative examples of adipocytes in mesenteric and epididymal fat revealed by haematoxylin and eosin staining; scale bar applies to all images. Expression of leptin, IL-6 and MCP1 was increased in DIO mice in both mesenteric (F) and epididymal (G) fat. Numbers in parentheses indicate number of animals. Asterisks mark significant changes, see text for P values. DIO, diet-induced obese mice; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ND, mice receiving normal diet.
DIO mice showed increased MAT and EAT mass, adipocyte size and increased expression of adipokines (12 weeks of feeding)
The mass of MAT was three times larger in DIO mice than in ND mice (P= 0.002, Fig. 1B). Similarly, the mass of EAT was increased almost five-fold in DIO mice (P < 0.001 compared with ND mice, Fig. 1C). Compared with ND mice both mesenteric and epididymal adipocyte sizes were increased in DIO mice (both P < 0.001, Fig. 1D and E). The mRNA expression of leptin, IL-6 and MCP1 was increased in MAT of DIO mice on average by 11.2, 4.0 and 4.8 times, respectively (P < 0.05, Fig. 1F), and in EAT by 10.8, 6.1 and 35.8 times, respectively (P < 0.05) (Fig. 1G).
DIO did not alter tissue integrity in the distal colon (12 weeks of feeding)
We evaluated inflammatory changes in the mucosa by scoring of the tissue pathology, measurement of the mucosal thickness and permeability, and investigation of the expression of markers for inflammation (TNF, IL-6, SAA3), chemotaxis (MCP1, IP-10) and apoptosis (Bcl2). The low histological scores in both groups did not suggest any signs of inflammation-driven pathology (Fig. 2A). Mucosal thickness was normal in DIO mice (Fig. 2B). The permeability was not altered in DIO relative to ND mice, as indicated by similar mucosal to serosal fluxes of sulfonic acid (Fig. 2C). Compared with ND mice, the mRNA expression of TNF-α, IL-6, SAA3, MCP1, IP-10 and Bcl2 was not changed in isolated epithelial cells of DIO mice (Fig. 2D). However, analysis of the full thickness colonic wall revealed a moderate but significantly increased expression of IL-6 and TNF, but not IL-1β, in DIO mice (Fig. 2E–G). Macrophage densities in mucosa/submucosa and muscle/myenteric plexus layers were similar in ND and DIO mice (Fig. 2H–J). The numbers of mast cells per crypt area tended to be smaller in the colons of DIO mice, but the difference did not reach significance (Fig. 2K).
Figure 2. Normal appearance, integrity and permeability of distal colon in DIO mice (12 weeks of feeding).
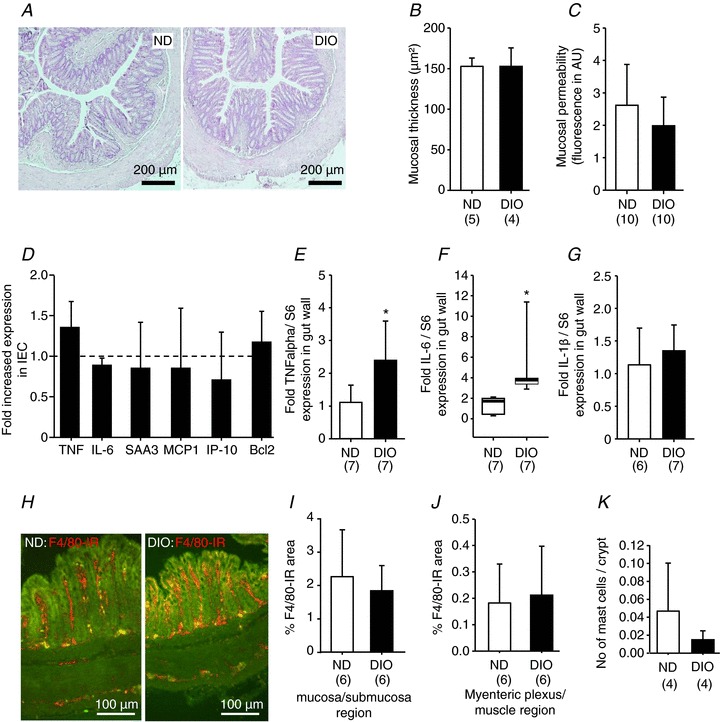
Haematoxylin and eosin staining revealed normal histology of the distal colon in DIO mice (A) and no increased mucosal thickness (B). Paracellular permeability was comparable between ND and DIO mice as mucosal to serosal translocation of fluorescein sulfonic acid was unchanged (C). The expression profile of markers for inflammation [tumour necrosis factor (TNF), interleukin-6 (IL-6), serum amyloid A 3 (SAA3)], chemotaxis [monocyte chemoattractant protein 1 (MCP1), interferon-inducible protein-10 (IP-10)] or apoptosis (B-cell lymphoma 2, Bcl2) was similar in ND (six animals) and DIO (four animals) mice (D). However, in extracts of the entire colonic wall (including fat tissue), the expression of TNF-α (E) and IL-6 (F), but not interleukin-1β (IL-1β) (G), was increased in DIO mice. Images in (H) are representative examples of F4/80 immunoreactive macrophages in cross-sections of the distal colon in ND and DIO mice. Analysis of macrophage densities in the mucosa/submucosa region (I) and myenteric plexus/muscle layer region (J) revealed no increased macrophage density in either region in DIO mice. Likewise, the number of CD117-positive mast cells was not altered significantly (K). Numbers in parentheses indicate number of animals. Asterisks mark significant changes, see text for P values. DIO, diet-induced obese mice; IEC, isolated epithelial cell; ND, mice receiving normal diet.
5-HT and ACh concentrations were increased in the distal colon of DIO mice (12 weeks of feeding)
Compared with ND mice, tissue concentrations of 5-HT and ACh were increased in DIO mice by 48% (P= 0.034) and 86% (P= 0.004), respectively (Fig. 3A and B). In the distal colon of DIO mice, the mRNA expression of TPH1 and TPH2 was increased threefold (P= 0.004) and twofold (P= 0.008), respectively (Fig. 3D and E). The expression levels of SERT and ChAT were comparable between the colons of ND and DIO mice (Fig. 3C and F). The number of EC cells per colonic crypt area was similar between DIO and ND mice (Fig. 3G and H). This coincided with the comparable PAX-4 mRNA expression in colon tissues of ND and DIO mice (Fig. 3I).
Figure 3. Tissue acetylcholine (ACh) and 5-hydroxytryptamine (5-HT) contents are increased in DIO mice, together with increased expression of tryptophan hydroxylase 1 (TPH1) and tryptophan hydroxylase 2 (TPH2), but not acetylcholine transferase (ChAT) or serotonin reuptake transporter (SERT) (12 weeks of feeding).
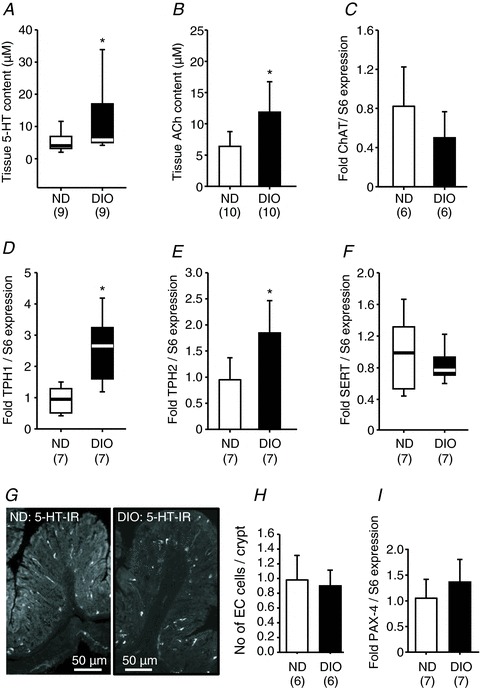
Colonic tissue content of 5-HT (A) and ACh (B) is significantly enhanced in DIO mice. These changes occurred without altered expression in ChAT (C, rate-limiting enzyme in the synthesis of ACh), but was associated with increased expression of TPH1 (D) and TPH2 (E), which are involved in the synthesis of 5-HT. SERT (F) expression was not changed in the colon of DIO mice. Images in (G) are representative examples of 5-HT immunoreactive enterochromaffin (EC) cells in the colonic mucosa of ND and DIO mice. Number of mucosal EC cells was similar in ND and DIO mice (H); note that 5-HT-positive mast cells in the lamina propria were not counted. Similar expression of paired box gene 4 (PAX4) in the colon of ND and DIO mice (I). Numbers in parentheses indicate number of animals. Asterisks mark significant changes, see text for P values. DIO, diet-induced obese mice; ND, mice receiving normal diet.
Applications of nicotine and 2-methyl-5-HT activated more myenteric neurones in the distal colon of DIO mice (12 weeks of feeding)
Spritz application of the nicotinic ACh receptor agonist nicotine and the 5-HT3 receptor agonist 2-methyl-5-HT evoked action potential discharge in myenteric neurones of both ND and DIO mice (Fig. 4A and D). Compared with ND mice, five times more neurones responded to nicotine (P= 0.018) and 2-methyl-5-HT (P= 0.033) in DIO mice (Fig. 4B and E). Although the proportion of nicotine- and 2-methyl-5-HT-responsive neurones was increased significantly in DIO mice, the evoked action potential frequencies were not different (Fig. 4C and F). Mechanical stimulation of myenteric neurones by intraganglionic volume injection activated RAMEN in both ND and DIO mice (Fig. 4G). We did not observe any difference in the proportion of RAMEN or in their spiking pattern (Fig. 4I). In the colon of ND and DIO mice, mechanical stimulation also activated very few slowly adapting mechanosensitive neurones; their proportion was 2.2% in ND mice (2 of 80) and 2.5% in DIO mice (2 of 89).
Figure 4. Nicotine and 2-methyl-5-hydroxytryptamine (2-methyl-5-HT) activated more colonic myenteric neurones in DIO mice (12 weeks of feeding).
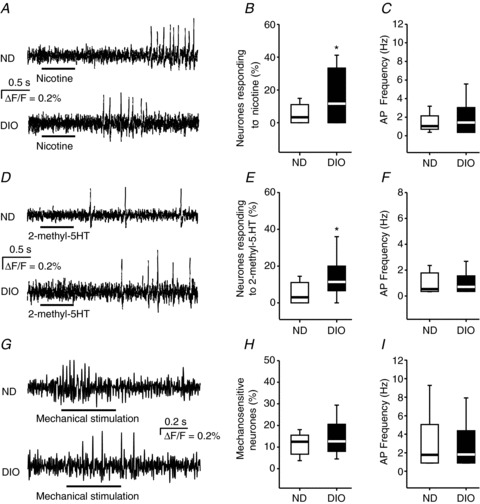
Myenteric neurones in ND and DIO mice fired action potentials (AP) in response to 500 ms pressure pulse application (indicated by the bars below the traces) of nicotine (A) and 2-methyl-5-HT (D). In DIO mice, more neurones responded to nicotine (B) and 2-methyl-5-HT (E), whereas the AP frequencies of the neurones responding to nicotine (C) or 2-methyl-5-HT (F) were similar (for nicotine: 41 of 235 neurones from 28 ganglia of six DIO mice and 18 of 277 neurones from 30 ganglia of six ND mice; for 2-methyl-5-HT: 33 of 223 neurones from 18 ganglia of six DIO mice and 14 of 209 neurones from 18 ganglia of six ND mice). Intraganglionic volume injection (indicated by the bar below the traces) caused a rapidly adapting spike discharge in colonic myenteric neurones of ND and DIO mice (G). The proportion of mechanosensitive neurones (H) as well as their spike discharge frequency (I) were similar in ND (six mice, 32 ganglia, 89 neurones) and DIO (six mice, 28 ganglia, 80 neurones) mice. Asterisks mark significant changes, see text for P values. DIO, diet-induced obese mice; ND, mice receiving normal diet.
Increased body weight correlated with neuronal sensitization, with leptin, MCP1 and IL-6 expression in mesenteric fat tissue and with 5-HT and ACh tissue levels (12 weeks of feeding)
There were positive correlations between body weight and several adipocyte markers and the degree of neuronal sensitization (Fig. 5). Correlations were analysed with data from ND and DIO mice. The expression of leptin, MCP1 and IL-6 was highly correlated with body weight. A modest but significant correlation was observed between body weight and 5-HT tissue content. A stronger correlation was observed between body weight and tissue ACh concentrations. The number of neurones which responded to 2-methyl-5-HT or to nicotine increased with body weight.
Figure 5. Increased body weight correlated with neuronal sensitization, with leptin, monocyte chemoattractant protein 1 (MCP1) and interleukin-6 (IL-6) expression in mesenteric fat tissue and with 5-hydroxytryptamine (5-HT) and acetylcholine (ACh) tissue levels (12 weeks of feeding).
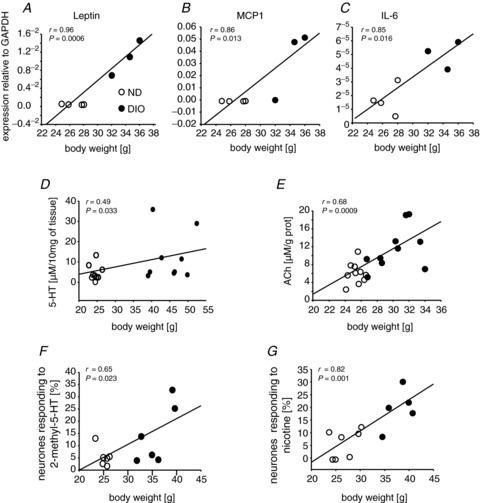
Leptin (A), MCP1 (B) and IL-6 (C) levels in fat tissue highly correlated with body weight. Body weight was also positively correlated with 5-HT (D) and ACh (E) tissue concentrations in the gut wall, as well as with the numbers of myenteric neurones responding to 2-methyl-5-hydroxytryptamine (2-methyl-5-HT) (F) and nicotine (G). Symbol key in (A) applies to all panels. DIO, diet-induced obese mice; ND, mice receiving normal diet.
DIO mice fed for 4 weeks showed no signs of inflammation or neuronal sensitization
To exclude the possibility that an early inflammation in the gut wall is resolved during the 12 week feeding period, we studied DIO mice fed for 4 weeks (Fig. 6). These mice gained significantly more weight than ND mice (25.9 ± 1.7 g, n= 16 versus 23.2 ± 1.4 g, n= 16; P < 0.001). This was associated with increased mesenteric fat mass (0.17 ± 0.7 g versus 0.11 ± 0.02 g; n= 8 each; P= 0.034) and increased epididymal fat mass (0.58 ± 0.07 g versus 0.28 ± 0.03 g; n= 8 each; P < 0.001). There were no signs of inflammation in the colonic wall because histological scoring, mucosal thickness and expression levels of proinflammatory cytokines in isolated epithelial cells were similar in DIO and ND mice (Fig. 6A and B). Accordingly, there were no changes in the density of macrophages in the mucosa/submucosa region or myenteric plexus/muscle regions (Fig. 6C). Although leptin, IL-6 (except in mesenteric fat) and MCP1 mRNA levels were significantly upregulated (Fig. 6D and E) in fat tissue, the increase was much smaller than in DIO mice fed for 12 weeks. Interestingly, we did not observe neuronal sensitization, as neither the number of myenteric neurones which responded to nicotine or 2-methyl-5-HT (Fig. 6F) nor the action potential discharge evoked by nicotine or 2-methyl-5-HT increased (Fig. 6G).
Figure 6. Lack of inflammation and neuronal sensitization in the colon of DIO mice fed for 4 weeks.
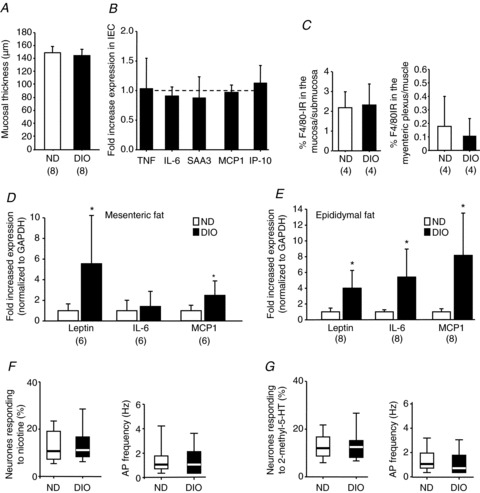
DIO mice fed for 4 weeks had normal mucosal thickness (A), normal expression of epithelial proinflammatory cytokines (B) and normal macrophage density (C). The expression levels of leptin, interleukin-6 (IL-6) and monocyte chemoattractant protein 1 (MCP1) in different fat tissues were increased (D, E); however, the fold increase was less than after 12 weeks of feeding (compare with Fig. 1F and G). The number of myenteric neurones responding to nicotine (F) or 2-methyl-5-hydroxytryptamine (2-methyl-5-HT) (G), as well as the spike discharge, did not differ between ND and DIO mice fed for 4 weeks (nicotine: eight DIO mice, 28 ganglia, 80 neurones and eight ND mice, 27 ganglia, 78 neurones; 2-methyl-5-HT: seven DIO mice, 29 ganglia, 63 neurones and seven ND mice, 19 ganglia, 53 neurones). Numbers in parentheses indicate number of animals. Asterisks mark significant changes, see text for P values. AP, action potential; DIO, diet-induced obese mice; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IEC, isolated epithelial cell; IP-10. interferon-inducible protein-10; ND, mice receiving normal diet; SAA3, serum amyloid A 3; TNF, tumour necrosis factor.
Discussion
Our study demonstrated functional alterations in the distal colon in a DIO mouse model. DIO mice showed faster colonic transit, enhanced sensitivity of myenteric neurones to nicotine and 2-methyl-5-HT, increased tissue content of ACh and 5-HT, and augmented expression of TPH1 and TPH2, without changes in SERT or ChAT expression. It has been suggested that alterations in the gut epithelium, together with impaired mucosal integrity and low grade inflammation, are associated or even causally linked to gastrointestinal symptoms in obesity (Ding & Lund, 2011). It is therefore important to note that the functional alterations in our mouse model occurred without significant signs of mucosal inflammation, impaired mucosal integrity or increased permeability. Our findings suggested neuronal plasticity in obese animals prior to the development of a pathological histology or abnormal mucosal functions. This questions the common assumption that mucosal inflammation and increased mucosal permeability initiate functional disorders in obesity.
As published previously (Baudry et al. 2012), our DIO mice did not exhibit increased plasma glucose concentrations after 12 weeks of high fat feeding, suggesting that these animals remained in a pre-diabetic state. The animals expressed greater adiposity, characterized by increased weight of mesenteric and epididymal fat. Adipocyte hypertrophy was associated with the increased expression of leptin, IL-6 and MCP1, which are considered to be typical proinflammatory adipokines (John et al. 2006). An enhanced production of these cytokines may lead to a systemic inflammation. However, we did not find evidence that cells in the gut wall contributed, as we did not detect inflammatory alterations in the epithelium or deeper layers (Piche et al. 2009). Indeed, histological tissue scores were normal and no different between DIO and ND mice. Moreover, expression levels of inflammatory and apoptotic markers, mucosal thickness and intestinal permeability were normal in the colonic epithelium of obese mice. We observed a moderate increase in IL-6 and TNF-α expression when we analysed the entire colonic wall. Our finding that these inflammatory markers were not upregulated in epithelial cells suggested that the epithelium did not contribute to the increased levels. Although other cells, such as muscle cells, may produce cytokines (Jarry et al. 2006), we believe that the increased IL-6 and TNF-α expression in the whole thickness colonic wall was caused by attached mesenteric fat. This interpretation is also supported by our finding in DIO mice that the macrophage density was normal in the lamina propria and muscle layers of the distal colon. An increase in macrophage density has been reported in the distal colon in another DIO mouse model (Erdelyi et al. 2009). There is no final explanation for the discrepant results, but one obvious difference is that our diet contained 36% fat volume (high in saturated fatty acids), whereas Erdelyi et al. (2009) used 20% fat (low in saturated fatty acids). Recently, it has been reported that large amounts of saturated fat in the diet induce less macrophage infiltration into fat tissue of mice than do low concentrations (Enos et al. 2013). Our findings in DIO mice fed for 4 weeks and 12 weeks demonstrated that, at least in our animal model, there were no inflammatory alterations in the colonic wall. This agrees with the lack of inflammation (upregulation of TNF-α) in the colon wall of DIO mice even after 16 weeks of feeding (Ding et al. 2010). However, the ileum of these mice was inflamed as early as at 6 weeks of high fat feeding. These and our data clearly indicate that the lack of inflammation after 12 weeks of feeding cannot be interpreted as an early onset inflammation that resolved over 12 weeks.
In view of the numerous, often discrepant, findings of altered gastrointestinal functions in obese animal models, we do not claim that the changes in our model are representative of DIO in general. Among others, the onset and duration of feeding of adipogenic diets and composition of the diets are known to affect functional alterations (Ding & Lund, 2011). For example, in our model, there was no change in mucosal TNF-α expression when feeding 5-week-old mice for 12 weeks with the adipogenic diet. However, feeding 8-week-old mice for 17 weeks increased TNF-α expression in the colonic mucosa (Liu et al. 2012). Interestingly, increased TNF-α expression has been observed in the proximal colonic wall of adult mice fed for 12 weeks, and this increase was associated with enhanced mucosal permeability (Lam et al. 2012). Longer feeding of an adipogenic diet probably results in more severe tissue alterations. For example, high fat feeding of 6-week-old mice for 15 weeks increased proximal colon permeability (Stenman et al. 2012).
We found increased levels of 5-HT, together with increased expression of TPH1 and TPH2, in the colonic tissues of DIO mice. TPH1 and TPH2 are rate-limiting enzymes in the biosynthesis of 5-HT in rodent EC and mast cells, and enteric neurones, respectively (Kuramoto et al. 2007; Li Z et al. 2011). The higher expression of TPH1 occurred without changes in the EC cell numbers; the expression of PAX-4, which is a regulator of EC cell development (Larsson et al. 1998), and SERT, which controls reuptake of 5-HT, was also not altered. These results suggested that the increased 5-HT content resulted from an enhanced synthesis of 5-HT. The stimulus causing enhanced 5-HT levels remains unknown; one possibility would be a direct stimulation of EC cells by fatty acids or bile acids, both of which are increased in the colonic lumen of obese mice (Kidd et al. 2008; Stenman et al. 2012). There are substantial region-specific effects of diet and/or metabolic imbalance on 5-HT metabolism. Rats fed for 16 weeks with a high fat diet exhibited decreased numbers of colonic EC cells, together with a reduced release of 5-HT, but normal TPH1 and SERT expression (Bertrand et al. 2012). The same group found increased EC cell numbers, together with a higher 5-HT availability and enhanced TPH1 and SERT expression, in the ileum of these rats (Bertrand et al. 2011). Although speculative, the increased expression of TPH2 in the colon of DIO mice suggested that enhanced neuronal 5-HT release might have contributed to the higher 5-HT tissue content. It is noteworthy that the tissue content of the most important excitatory neurotransmitter ACh was also increased.
In addition to increased tissue levels of 5-HT and ACh, we also found an augmented sensitivity of myenteric neurones to nicotine and 2-methyl-5-HT in DIO mice fed for 12 weeks. From a functional point of view, this sensitization of the ENS may lead to enhanced motor functions (Law et al. 2011), although it remains to be studied which particular neuronal population became sensitized. It remains open whether this was a consequence of the higher ACh and 5-HT levels in tissue or rather of the expression of nicotinic ACh or 5-HT3 receptors in a larger proportion of myenteric neurones. The increased responsiveness of myenteric neurones to 5-HT and ACh was not associated with enhanced sensitivity in sensory enteric circuits, because the activity of mechanosensitive myenteric neurones was not altered in DIO mice. Our results in the colon agreed with our findings in the same animal model that gastric myenteric neurones of DIO mice were sensitized based on their enhanced response to electrical field stimulation (Baudry et al. 2012). Feeding state-related alterations in enteric nerve activity have been reported recently (Roosen et al. 2012). The authors observed an increased sensitivity of myenteric neurones in guinea-pigs re-fed after an overnight fasting period. Interestingly, we found no neuronal sensitization in animals fed for 4 weeks. At this stage, we can only speculate that the relatively small increase in leptin and cytokine levels may not be sufficient to trigger neuronal sensitization. Both leptin and IL-6 activate myenteric neurones (Kelles et al. 2000; Reichardt et al. 2011). There was a strong correlation between body weight and number of neurones responding to nicotine or 2-methyl-5-HT and tissue levels of 5-HT and ACh. The modest weight gain in DIO mice fed for 4 weeks therefore agrees with the normal sensitivity of myenteric neurones to nicotine or 2-methyl-5-HT in these mice.
In addition to acting as a neurotransmitter, ACh dampens macrophage activation and thereby acts as an anti-inflammatory mediator (Wang et al. 2003). The increased tissue ACh level in DIO mice may therefore not only influence neurones, but may also activate the cholinergic anti-inflammatory pathway.
The increased tissue levels of ACh and 5-HT and the enhanced responsiveness of myenteric neurones to both excitatory mediators are in accordance with the faster colonic transit in DIO mice. This could represent the neuronal basis for colonic dysfunctions in obese humans (Delgado-Aros et al. 2008). Interestingly, we observed previously an increased gastric emptying in our DIO mouse model (Baudry et al. 2012), suggesting that enhanced intake and evacuation of food may contribute to the development of obesity.
An important aspect for future studies is to address the question of whether the altered functions observed in our pre-diabetic obese animals with no impaired mucosal integrity represent only the initial phase in the development of more severe inflammation, or whether these changes serve to counteract the threat posed by inflammatory signals from the adipose tissue.
Acknowledgments
We thank Birgit Kuch, Stephanie May, Alexander Haag and Klaus Michel for their precious help.
Glossary
- 2-methyl-5-HT
2-methyl-5-hydroxytryptamine
- 5-HT
5-hydroxytryptamine
- ACh
acetylcholine
- AP
action potential
- Bcl2
B-cell lymphoma 2
- ChAT
acetylcholine transferase
- DIO
diet-induced obesity/obese
- EAT
epididymal adipose tissue
- EC
enterochromaffin
- ENS
enteric nervous system
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IP-10
interferon-inducible protein-10
- MAT
mesenteric adipose tissue
- MCP1
monocyte chemoattractant protein 1
- ND
normal diet
- PAX-4
paired box gene 4
- RAMEN
rapidly adapting mechanosensitive enteric neurone
- SAA3
serum amyloid A 3
- SERT
serotonin reuptake transporter
- TNF-α
tumour necrosis factor-α
- TPH
tryptophan hydroxylase
Additional information
Competing interests
The authors have no competing interests.
Author contributions
All authors contributed to the final version of the manuscript. F.R., R.M., L.G., G.M., D.H., M.N. and M.S. designed the experiments, analysed and interpreted the data; F.R., C.B., S.K. and C.S. collected and analysed the data; M.N., R.M., D.H. and H.D. revised the article critically for important intellectual content. All authors approved the final version of the manuscript.
Funding
This study was supported by the Deutsche Forschungsgemeinschaft (DFG Sche 267/8–1, DFG HA 3148/3–1), the Agence Nationale pour la Recherche (ALIA 2009), the Fondation pour la Recherche Médicale and the French Ministère de l’Enseignement Supérieur.
References
- Baudry C, Reichardt F, Marchix J, Bado A, Schemann M, Bruley des Varranes S, Neunlist M, Moriez R. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and GDNF. J Physiol. 2012;590:533–544. doi: 10.1113/jphysiol.2011.219717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand R, Senadheera S, Markus I, Liu L, Howitt L, Chen H, Murphy TV, Sandow SL, Bertrand PP. A Western Diet increases serotonin availability in rat small intestine. Endocrinology. 2011;152:36–47. doi: 10.1210/en.2010-0377. [DOI] [PubMed] [Google Scholar]
- Bertrand RL, Senadheera S, Tanoto A, Tan KL, Howitt L, Chen H, Murphy TV, Sandow SL, Liu L, Bertrand PP. Serotonin availability in rat colon is reduced during a Western diet model of obesity. Am J Physiol Gastrointest Liver Physiol. 2012;303:G424–G434. doi: 10.1152/ajpgi.00048.2012. [DOI] [PubMed] [Google Scholar]
- Bogumil R, Koal T, Weinberger KM, Dammeier S. Massenspektrometrische Analyse von Blutplasma im Kitformat. Laborwelt. 2008;2:17–23. [Google Scholar]
- Delgado-Aros S, Camilleri M, Garcia MA, Burton D, Busciglio I. High body mass alters colonic sensory-motor function and transit in humans. Am J Physiol Gastrointest Liver Physiol. 2008;295:382–388. doi: 10.1152/ajpgi.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Lund PK. Role of intestinal inflammation as an early event in obesity and insulin resistance. Curr Opin Clin Nutr Metab Care. 2011;14:328–333. doi: 10.1097/MCO.0b013e3283478727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enos RT, Davis JM, Velázquez KT, McClellan JL, Day SD, Carnevale KA, Murphy EA. Influence of dietary saturated fat content on adiposity, macrophage behaviour, inflammation, and metabolism: composition matters. J Lipid Res. 2013;54:152–163. doi: 10.1194/jlr.M030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdelyi I, Levenkova N, Lin EY, Pinto JT, Lipkin M, Quimby FW, Holt PR. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr. 2009;139:2072–2078. doi: 10.3945/jn.108.104125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher TK, Geoghegan JG, Baird AW, Winter DC. Implications of altered gastrointestinal motility in obesity. Obes Surg. 2007;17:1399–1407. doi: 10.1007/s11695-007-9221-0. [DOI] [PubMed] [Google Scholar]
- Ho W, Spiegel BM. The relationship between obesity and functional gastrointestinal disorders: causation, association, or neither. Gastroenterol Hepatol (NY) 2008;4:572–578. [PMC free article] [PubMed] [Google Scholar]
- Hyland NP, Rybicka JM, Ho W, Pittman QJ, Macnaughton WK, Sharkey KA. Adaptation of intestinal secretomotor function and nutrient absorption in response to diet-induced obesity. Neurogastroenterol Motil. 2010;22:602–e171. doi: 10.1111/j.1365-2982.2010.01504.x. [DOI] [PubMed] [Google Scholar]
- Jarry A, Bach-Ngohou K, Masson D, Dejoie T, Lehur PA, Mosnier JF, Denis MG, Laboisse CL. Human colonic myocytes are involved in postischemic inflammation through ADAM17-dependent TNFalpha production. Br J Pharmacol. 2006;147:64–72. doi: 10.1038/sj.bjp.0706449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John BJ, Irukulla S, Abulafi AM, Kumar D, Mendall MA. Systematic review: adipose tissue, obesity and gastrointestinal diseases. Aliment Pharmacol Ther. 2006;23:1511–1523. doi: 10.1111/j.1365-2036.2006.02915.x. [DOI] [PubMed] [Google Scholar]
- Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. Erratum in: (2005) J Clin Invest 115, 1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelles A, Janssens J, Tack J. IL-1beta and IL-6 excite neurones and suppress cholinergic neurotransmission in the myenteric plexus of the guinea pig. Neurogastroenterol Motil. 2000;12:531–538. doi: 10.1046/j.1365-2982.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol. 2008;295:G260–G272. doi: 10.1152/ajpgi.00056.2008. [DOI] [PubMed] [Google Scholar]
- Kiely J, Kiely JM, Noh JH, Graewin SJ, Pitt HA, Swartz-Basile DA. Altered intestinal motility in leptin-deficient obese mice. J Surg Res. 2005;124:98–103. doi: 10.1016/j.jss.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Kuramoto H, Kadowaki M, Sakamoto H, Yuasa K, Todo A, Shirai R. Distinct morphology of serotonin-containing enterochromaffin (EC) cells in the rat distal colon. Arch Histol Cytol. 2007;70:235–241. doi: 10.1679/aohc.70.235. [DOI] [PubMed] [Google Scholar]
- Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. doi: 10.1371/journal.pone.0034233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson LI, St-Onge L, Houggard DM, Sosa-Pineda B, Gruss P. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev. 1998;79:153–159. doi: 10.1016/s0925-4773(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Law NM, Bharucha AE, Undale AS, Zinsmeister AR. Cholinergic stimulation enhances colonic motor activity, transit, and sensation in humans. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1228–G1237. doi: 10.1152/ajpgi.2001.281.5.G1228. [DOI] [PubMed] [Google Scholar]
- Li E, Zhou P, Petrin Z, Singer SM. Mast cell-dependent control of Giardia lamblia infections in mice. Infect Immun. 2004;72:6642–6649. doi: 10.1128/IAI.72.11.6642-6649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ma W, Wang S. Slower gastric emptying in high-fat diet induced obese rats is associated with attenuated plasma ghrelin and elevated plasma leptin and cholecystokinin concentrations. Regul Pept. 2011;171:53–57. doi: 10.1016/j.regpep.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Côté F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, Greenberg AS, Mason JB. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signalling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2012;23:1207–1213. doi: 10.1016/j.jnutbio.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA, Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol. 2012 doi: 10.1002/0471141755.ph0561s58. Chapter 5:Unit5.61. doi: 10.1002/0471141755.ph0561s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci. 2012;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuoli G, Schemann M. Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS One. 2012;7:e39887. doi: 10.1371/journal.pone.0039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58:196–201. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- Osinski MA, Bass P, Gaumnitz EA. Peripheral and central actions of orphanin FQ (nociceptin) on murine colon. Am J Physiol. 1999;276:G125–G131. doi: 10.1152/ajpgi.1999.276.1.G125. [DOI] [PubMed] [Google Scholar]
- Poylin V, Serrot FJ, Madoff RD, Ikrumuddin S, Lowry AC, Melton GB. Obesity and bariatric surgery: a systematic review of associations with defecatory dysfunction. Colorectal Dis. 2011;13:e92–e103. doi: 10.1111/j.1463-1318.2011.02584.x. [DOI] [PubMed] [Google Scholar]
- Raybould HE. Gut microbiota, epithelial function and derangements in obesity. J Physiol. 2012;590:441–446. doi: 10.1113/jphysiol.2011.222133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt F, Krueger D, Schemann M. Leptin excites enteric neurons of guinea-pig submucous and myenteric plexus. Neurogastroenterol Motil. 2011;23:e165–e170. doi: 10.1111/j.1365-2982.2010.01665.x. [DOI] [PubMed] [Google Scholar]
- Rodger CJ, Nicol L, Anderson JH, McKee RF, Finlay IG. Abnormal colonic motility: a possible association with urge fecal incontinence. Dis Colon Rectum. 2010;53:409–413. doi: 10.1007/DCR.0b013e3181cc55cc. [DOI] [PubMed] [Google Scholar]
- Römisch-Margl W, Prehn C, Bogumil R, Röhring C, Suhre K, Adamski J. A procedure for tissue sample preparation and metabolite extraction targeted for high-throughput metabolomics. Metabolomics. 2012;8:133–142. [Google Scholar]
- Roosen L, Boesmans W, Dondeyne M, Depoortere I, Tack J, Vanden Berghe P. Specific hunger- and satiety-induced tuning of guinea pig enteric nerve activity. J Physiol. 2012;590:4321–4333. doi: 10.1113/jphysiol.2012.231134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772–1782. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- Spångéus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes) Histol Histopathol. 2001;16:159–165. doi: 10.14670/HH-16.159. [DOI] [PubMed] [Google Scholar]
- Stenman LK, Holma R, Korpela R. High-fat-induced intestinal permeability dysfunction associates with altered fecal bile acids in mice. World J Gastroenterol. 2012;18:923–929. doi: 10.3748/wjg.v18.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasseli M, Chaumette T, Paillusson S, Monnet Y, Lafoux A, Huchet-Cadiou C, Aubert P, Hunot S, Derkinderen P, Neunlist M. Effects of oral administration of roterone on gastrointestinal functions in mice. Neurogastroenterol Motil. 2013;25:e183–e193. doi: 10.1111/nmo.12070. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]


