Abstract
In skeletal muscle hormone-sensitive lipase (HSL) has long been accepted to be the principal enzyme responsible for lipolysis of intramyocellular triacylglycerol (IMTG) during contractions. However, this notion is based on in vitro lipase activity data, which may not reflect the in vivo lipolytic activity. We investigated lipolysis of IMTG in soleus muscles electrically stimulated to contract ex vivo during acute pharmacological inhibition of HSL in rat muscles and in muscles from HSL knockout (HSL-KO) mice. Measurements of IMTG are complicated by the presence of adipocytes located between the muscle fibres. To circumvent the problem with this contamination we analysed intramyocellular lipid droplet content histochemically. At maximal inhibition of HSL in rat muscles, contraction-induced breakdown of IMTG was identical to that seen in control muscles (P < 0.001). In response to contractions IMTG staining decreased significantly in both HSL-KO and WT muscles (P < 0.05). In vitro TG hydrolase activity data revealed that adipose triglyceride lipase (ATGL) and HSL collectively account for ∼98% of the TG hydrolase activity in mouse skeletal muscle, other TG lipases accordingly being of negligible importance for lipolysis of IMTG. The present study is the first to demonstrate that contraction-induced lipolysis of IMTG occurs in the absence of HSL activity in rat and mouse skeletal muscle. Furthermore, the results suggest that ATGL is activated and plays a major role in lipolysis of IMTG during muscle contractions.
Key points
In skeletal muscle hormone-sensitive lipase (HSL) is considered the only enzyme responsible for breakdown of intramyocellular triacylglycerol (IMTG) during contractions. This notion is based on indirect measures in which important cellular events are not taken into account.
Using two histochemical techniques to measure breakdown of IMTG during contractions in isolated skeletal muscles we found that IMTG was decreased (1) in rat muscles during acute pharmacological blockade of HSL, and (2) in muscles of HSL knockout mice.
We demonstrated that adipose triglyceride lipase (ATGL) and HSL collectively account for at least 98% of the total TG lipase activity in mouse muscle, and other TG lipases accordingly seem of negligible importance for breakdown of IMTG.
In conclusion, breakdown of IMTG occurs in the contracting muscle in the absence of HSL activity. Our data suggest that ATGL is activated during contractions and plays a major role in breakdown of IMTG.
Introduction
In most mammalian cell types triacylglycerol (TG) is stored in lipid droplets (LDs), which until recently were viewed as inert depots of fat. However, these LDs are now recognized as functional organelles consisting of a core of TG and cholesteryl esters surrounded by a phosholipid monolayer and with several regulatory proteins associated with the LD surface (reviewed by Walther & Farese, 2009). In skeletal muscle, fatty acids (FAs) for oxidation can be derived from uptake of plasma albumin-bound FAs, from FAs liberated from very low-density lipoprotein-TG or from lipolysis of intramyocellular TG (IMTG) (Kiens, 2006). Lipolysis of IMTG has been found in skeletal muscle in response to adrenaline, exercise and during contractions of isolated muscles (Bergstrom et al. 1973; Spriet et al. 1986; Peters et al. 1998; Roepstorff et al. 2005). Furthermore, in skeletal muscle TG lipase activity is increased both by adrenaline and by local factors in response to contractions (Langfort et al. 1999, 2000).
Hormone-sensitive lipase (HSL) was long considered the only and rate-limiting enzyme responsible for lipolysis of TG in adipose tissue and in most other tissues (Zechner et al. 2009). However, studies on HSL-deficient mice (HSL-knockout (KO)) revealed that these animals accumulated diacylglycerol (DAG) rather than TG in adipose, muscle and testis tissues in response to fasting (Osuga et al. 2000; Haemmerle et al. 2002). Thus, these studies suggested that TG lipases other than HSL exist. In line with this, three groups independently discovered a novel lipase called adipose triglyceride lipase (ATGL, also named desnutrin and calcium-independent phospholipase A2ζ (iPLA2ζ)) (Zimmermann et al. 2004; Villena et al. 2004; Jenkins et al. 2004). The specific activity of ATGL for TG is 10 times higher than that for DAG (Zimmermann et al. 2004), while HSL preferentially hydrolyses DAG with a 10-fold higher lipolytic rate compared to TG (Fredrikson et al. 1981). The current view of lipolysis in adipose tissue is that the lipases act sequentially to regulate TG hydrolysis: ATGL hydrolyses the first ester bond on TG, HSL then cleaves the next FA from DAG, and finally monoacylglycerol lipase (MGL) hydrolyses the last ester bond on monoacylglycerol to release FA and glycerol (Zechner et al. 2009).
In skeletal muscle HSL accounts for 20–60% of TG hydrolase activity during resting conditions (Langfort et al. 1999, 2000; Roepstorff et al. 2004; Watt et al. 2004b; Alsted et al. 2009) but HSL is considered the primary lipase activated by contractions and adrenaline stimulation (Langfort et al. 1999, 2000; Watt et al. 2004b; Roepstorff et al. 2004). This notion is based on in vitro activity measurements, where the contraction or adrenaline-induced increase in TG lipase activity was completely blocked when adding an HSL antibody to the assay media (Langfort et al. 1999, 2000; Watt et al. 2004b). However, the in vitro activity assay does not include changes in important regulatory events such as translocation of lipases to the lipid droplets and interaction with lipid droplet-associated proteins, and therefore may not entirely reflect the acute activation of muscle TG lipases in vivo. Indeed, HSL translocation to the lipid droplets has been demonstrated in rat skeletal muscle during contractions (Prats et al. 2006). In addition, in several human studies dissociations between in vitro HSL activity and net change in IMTG content during exercise have been observed, as increased HSL activity was not always accompanied by a decrease in IMTG (Roepstorff et al. 2004; Watt et al. 2004a, 2006). This may reflect the fact that lipases other than HSL are at play or alternatively that the in vitro activity measurement is not the correct approach to evaluate acute TG lipase activation in skeletal muscle. In addition, no direct evidence of HSL being the principal contraction-activated TG lipase exists, as lipolysis of IMTG during contractions has not been studied using muscles of HSL-KO mice.
Knowledge of ATGL in skeletal muscle is limited, but ATGL protein expression and activity have been demonstrated in both rodent and human skeletal muscle (Lass et al. 2006; Alsted et al. 2009; Jocken et al. 2008). The functional importance of ATGL for basal TG hydrolysis in skeletal muscle is highlighted by the finding of massive IMTG accumulation in ATGL-KO mice (Haemmerle et al. 2006) and is supported by findings of an increased TG hydrolase activity and decreased TG content in myotubes overexpressing ATGL (Badin et al. 2011). In addition, mutations of the human genes of both ATGL and comparative gene identification 58 (CGI-58, also named α/β-hydrolase fold domain-containing protein 5, ABHD5), an activating protein of ATGL, have been found in patients with neutral lipid storage disease with myopathy, which is characterized by TG accumulation in various tissues including skeletal muscle (Chanarin et al. 1975; Lefevre et al. 2001; Fischer et al. 2007; Kobayashi et al. 2008) suggesting a defect of ATGL function. These data strongly suggest that ATGL has an important role for skeletal muscle TG hydrolysis. In a recent study an increased co-immunoprecipitation of CGI-58 with ATGL was found in response to contractions in isolated rat skeletal muscle, indicating that ATGL was activated (MacPherson et al. 2013). However, the functional significance of this interaction was not demonstrated and direct evidence of ATGL being activated during skeletal muscle contractions is lacking.
The assessment of IMTG content in mice and rats, using a biochemical approach, involves technical difficulties due to the presence of adipocytes located between the fibres (Donsmark et al. 2005). This exogenous adipose tissue is not easily dissected from rodent muscles and may contaminate the sample, thus making changes in IMTG content hard to detect (Donsmark et al. 2005). To circumvent the problem we analysed IMTG content using two histochemical techniques to measure lipolysis during acute electrically stimulated contractions.
In the present study we investigated whether contraction-induced lipolysis of IMTG occurs in the absence of HSL activity in skeletal muscle. Our hypothesis was that lipases other than HSL (such as ATGL) contribute significantly to contraction-induced lipolysis of IMTG.
Methods
Ethical approval
All experiments were approved by the Danish Animal Experimental Inspectorate and complied with the ‘European Convention for the Protection of Vertebrate Animals used for Experiments and other Scientific Purposes’ (council of Europe no. 123, Strasbourg, France, 1985).
Animals
Male Wistar rats and C57Bl/6J/SV129 hybrid mice were used for the ex vivo experiments. HSL-KO and ATGL-KO mice were generated by targeted homologous recombination of the HSL and ATGL genes, respectively, as previously described (Grober et al. 2003; Haemmerle et al. 2006). ATGL-KO mice were backcrossed onto a C57Bl/6J background for more than 10 generations. Mice that were used in the ex vivo experiments were males, littermates with a mixed genetic background (C57Bl/6J and SV129) (Mulder et al. 2003). The animals were kept on a 12/12 h light–dark cycle and received a standard chow diet and water ad libitum. Two days prior to the experiments the rats received a fat-rich diet (60% energy of fat; D12492, Research Diets, Inc., New Brunswick, NJ, USA). The selection of 2 days of high fat diet feeding was chosen during the optimization of the experimental protocol to induce an increased fat metabolism during contractions. Animals were fed ad libitum prior to all experiments.
Ex vivo muscle incubations
For time course and HSL inhibitor experiments fed male Wistar rats (55–65 g) were anaesthetized by i.p. injection of sodium pentobarbital (6 mg/100 g body weight). Following induction of anaesthesia, the soleus (SOL) muscles with intact tendons tied with silk sutures were dissected free, the adipose tissue surrounding the tendons carefully removed and the isolated muscles were transferred to incubation chambers (Multi Myograph system 610 M; Danish Myo-Technology A/S, Aarhus, Denmark) where they were attached to hooks connected to a force transducer. In the incubation chambers the muscles were suspended at resting tension and preincubated for 60 min in warmed (30°C), gassed (95% O2 and 5% CO2) Krebs-Ringer-Henseleit buffer containing 5 mm glucose, 1 mm pyruvate and 0.1% FA-free bovine serum albumin (BSA). In the HSL inhibitor experiments the muscles were preincubated for 60 min in the presence of either a mono-specific, small molecule inhibitor of HSL (76-0079, Novo Nordisk, Bagsvaerg, Denmark) or corresponding concentrations of vehicle (DMSO, 0.4%). This inhibitor is highly specific towards HSL as previously shown in mouse white adipose tissue extracts, intact fat pads and liver extracts (Schweiger et al. 2006; Reid et al. 2008). In addition, in the present study the specificity of the inhibitor in muscle lysates was demonstrated (see below). Following the preincubation period the medium was changed, and one muscle of each animal was stimulated electrically (D330 MultiStim System, Digitimer Ltd, Welwyn Garden City, UK) every second with 200 ms trains of 0.2 ms impulses delivered at 100 Hz (30–40 V) for 20 min to induce maximal tetanic contractions. During the first few seconds of the electrical stimulation, the length of the muscle and the voltage of stimulation were adjusted to achieve maximal power output (recorded using Chart 5.0 software, AD Instruments, Sydney, NSW, Australia), which indicates that the entire fibre population was recruited to a maximal extent.
Initially the effect of the HSL inhibitor was tested by measuring the increase of DAG content in muscles contracted for 15 min with increasing concentrations (1–3 mm) of the inhibitor in the medium. The inhibition was considered maximal when adding a higher dose of the inhibitor to the incubation medium did not elevate DAG content further. In addition, control experiments with volumes of DMSO corresponding to the volume of the inhibitor or incubation with no additions to the media were conducted. In other experiments, the effect of contraction time on IMTG hydrolysis was determined by stimulating the muscles for 5 or 20 min. In all experiments, the SOL muscle of one leg of the animal was used for electrical stimulation while the muscle from the contralateral leg served as a basal control.
At the end of the stimulation period the muscles were either mounted in Tissue-Tek (Sakura Finetek, Zoeterwoude, the Netherlands) and frozen in isopentane for histochemical analyses or directly frozen in liquid nitrogen for Western blotting and glycogen measurements. All samples were stored at −80°C.
To further investigate skeletal muscle lipolysis of IMTG in the absence of HSL, SOL muscles from HSL-KO and WT littermate mice were excised, preincubated for 30 min and electrically stimulated every 2 s with 200 ms trains of 0.2 ms impulses delivered at 100 Hz (30–40 V) for 15 min. Using the same stimulation protocol for mouse muscles as for rats resulted in no or a very low force output (<20 mN) in the last 5–7 min. Therefore, the stimulation protocol was modified for the mouse muscles.
At the end of the stimulation period the mouse muscles were either pinned down with intact tendons and fixed in ice cold fixative as described below (Morphological quantification of IMTG in mouse skeletal muscles) or directly frozen in liquid nitrogen for Western blotting and glycogen measurements. The latter samples were stored at −80°C.
After muscle excision the animals were killed by cervical dislocation while anaesthetized.
All materials were from Sigma-Aldrich (St Louis, MO, USA) unless stated otherwise.
Analyses
Morphological quantification of IMTG in rat skeletal muscles
To avoid contamination by exogenous adipose tissue surrounding the muscle fibres, which is often found with biochemical analyses of IMTG, a morphological approach to determine IMTG content was used. In a previous study, this analysis was performed on single fibres of rat SOL muscles, showing that cytoplasmic LDs are homogeneously distributed between the regions just beneath the sarcolemma and between the myofilaments and respond similarly to contraction and epinephrine stimulations (Prats et al. 2006). In the present study, the analysis was applied to muscle cross sections, to be able to include more fibres in the analysis and be able to distinguish between type I and type II fibres in terms of IMTG turnover. The muscles mounted in Tissue-Tek were cut in 10 μm thick transverse sections using a microtome cryostat at −25°C (HM 500 OM, Microm Laborgeräte GmbH, Walldorf, Germany), collected on Superfrost Plus glass slides (Thermo Fisher Scientific, Waltham, MA, USA) and immediately transferred to ice cold fixative (4% formaldehyde and 0.15% picric acid) (Stefanini et al. 1967) in which they remained for 1 h. The muscle sections were washed three times for 10 min in PBS and incubated for 1 h at room temperature with 4,4-difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s- indacene (BODIPY 493/503; 20 μg ml−1 in PBS) (Invitrogen, Eugene, OR, USA) and a mouse monoclonal anti-myosin heavy chain I antibody (M 8421) labelled with a Zenon Mouse IgG labelling kit (Z25006, Invitrogen) followed by another three 10 min washes in PBS. After the final wash the muscle sections were mounted in Vectashield (H-1000, Vector Laboratories, Burlingame, CA, USA) and analysed by confocal microcopy.
Confocal microscopy imaging and quantification
Images were obtained on a Leica confocal microscope (TCS SP2, Leica Microsystems, Mannheim, Germany) using an HCX plan APO ×63/1.32 oil objective. Confocal images were taken with a separation of 0.35 μm in the z-plane. For quantification, an average projection of four planes was taken from each area, and for visualization a maximal projection of four planes was used. From each muscle section a minimum of 120 fibres were analysed by drawing a line around each fibre and measuring fluorescence within each fibre. Quantification of lipid droplet staining was performed on TIFF mages using the freeware image software ImageJ 1.45e. A threshold value for the intensity of staining was established for all images and lipid droplets were quantified as total positive staining per muscle fibre area (total lipid staining per area), average area of the LDs (average LD size) and the number of positive IMTG stainings per fibre area (number of LDs per 200 μm2).
Morphological quantification of IMTG in mouse skeletal muscles
The method that was used to fix and stain LDs in rat skeletal muscle gave only very little or no staining in samples from mouse skeletal muscle. Fixation and staining of intracellular LDs in various tissues for imaging by electron microscopy has previously been performed with glutaraldehyde and osmium tetroxide (OsO4) in combination with p-phenylenediamine (PPD). As an example, PPD enhanced the staining intensity of LDs fixed by OsO4 in rat uterine epithelium (Boshier et al. 1984). In the present study we adopted this technique for staining of LDs in cross sections of plastic embedded mouse skeletal muscle and imaging by light microscopy.
Mouse soleus muscles were pinned down with intact tendons and fixed in ice cold 2% glutaraldehyde in 0.15 m sodium phosphate buffer (pH 7.35). The muscles were transferred to fresh fixative and incubated overnight and washed for 2 days in 0.1 m sodium phosphate buffer. Post fixation was performed with 2% OsO4 (0972B, Polysciences Inc., Warrington, PA, USA) in 0.1 m sodium phosphate buffer for 2.5 h at room temperature followed by washing in 0.1 m sodium phosphate buffer. The samples were washed twice for 5 min in 50% acetone and en block mordanted with 2% uranyl acetate in 50% acetone for 20 min. Dehydration was performed using increasing concentrations of acetone (50, 70 and 90% and twice 100%) for 5 min each. The samples were infiltrated and embedded in epoxy resin (Poly/Bed 812, 08791, Polysciences Inc.) followed by polymerization at 55–60°C for 3 days. Transverse sections of 2 μm thickness were cut using an ultramicrotome (Leica Ultracut UCT) and mounted on Superfrost Plus glass slides (Thermo Fisher Scientific). The sections were post stained with 1% PPD (P-6001) in 2-propanol and methanol (1:1) for 10 min, followed by two washes in methanol for 15 and 10 s, respectively. The sections were air dried overnight and mounted in Eukitt (Electron Microscopy Sciences, Hatfield, PA, USA). Examination and imaging of the sections were performed with a Leica DMRXE microscope (Leica Microsystems, Ballerup, Denmark) using an HCX plan APO ×63/1.32 oil objective and Leica application suite software version 2.8.1.
From each section a minimum of 85 muscle fibres were analysed by drawing a line around each fibre and analysing LD staining inside the muscle fibre. A threshold value for the intensity of staining was established for all images and LDs were quantified as total positive staining per muscle fibre area (total lipid staining per area), average area of the LDs (average LD size) and as the number of positive IMTG stainings per fibre area (number of LDs per 200 μm2). Images were analysed using the freeware software ImageJ 1.45e (http://rsbweb.nih.gov/ij/index.html).
DAG content
Muscle DAG content was determined in freeze dried and dissected muscle tissue using the method of Folch et al. (1957) and as previously described (Hoeg et al. 2011). Briefly, lipids were extracted by homogenizing 1 mg of freeze dried and dissected muscle tissue in 1 ml methanol and 2 ml chloroform using a polytron homogenizer (Kinematica, PT 1200, Lucerne, Switzerland) followed by addition of 3 ml NaCl (0.9%, w/v) to the extraction mixture. After centrifugation for 10 min at 1922g, the lower phase was collected and evaporated under a steam of nitrogen and the resulting pellet was dissolved in chloroform. The lipids were then separated by thin layer chromatography (TLC) by loading the dissolved pellet along with an internal DAG standard on silica plates (Merck, Darmstadt, Germany) and consecutively immersing these in two separate solvents, chloroform/methanol/acetic acid/water (50:50:5:5) followed by petroleum ether/diethyl ether/acetic acid (120:25:1.5), each containing butylated hydroxytoluene (50 mg l−1). The DAG bands were visualized by immersing the plate in a 10% copper sulphate pentahydrate and 8% phosphoric acid solution. Lipid bands were developed at 120°C and quantified using a Kodak Image station (2000MM, Kodak, Glostrup, Denmark) according to internal standards.
Muscle lysate preparation
Freeze dried and dissected muscle tissue was homogenized in an ice-cold buffer (1:80 w/v) containing 50 mm Hepes (pH 7.5), 150 mm NaCl, 20 mm sodium pyrophosphate, 20 mmβ-glycerophosphate, 10 mm NaF, 2 mm sodium orthovanadate, 2 mm EDTA, 1 mm EGTA, 1% Nonidet P-40, 10% glycerol, 2 mm phenylmethanesulfonyl fluoride, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin and 3 mm benzamidine using a tissue lyser (TissueLyser II, Retsch GmbH, Haan, Germany). Muscle lysates were prepared as previously described (Alsted et al. 2009) and stored at −80°C until analysis. The lysate protein content was assessed using the bicinchoninic acid method (Pierce, Rockford, IL, USA).
Western blotting
Protein expression and phosphory- lation were determined using SDS-PAGE and Western blotting techniques. For these purposes the following antibodies were used: anti-ATGL (AF5365, R&D Systems, Minneapolis, MN, USA), anti-CGI-58 (NB110–41576, NOVUS Biologicals, Littleton, CO, USA), anti-HSL (kindly donated by Dr Cecilia Holm, Department of Cell and Molecular Biology, University of Lund, Sweden), anti-G(0)/G(1) switch gene 2 (G0S2) protein (HPA010016), anti-phospho-Thr172-AMP-activated pro- tein kinase (AMPK) (2531, Cell Signaling Technology, Beverly, MA, USA), anti-AMPKα2 (kindly donated by Dr. G. Hardie, Division of Molecular Physiology, University of Dundee, Dundee, UK), anti-phospho-Thr56 eukaryotic elongation factor 2 (eEF2) (2331, Cell Signaling Technology), anti-eEF2 (sc-13003, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-phospho-Ser221-acetyl-CoA-carboxylase-β (ACCβ) (07–303, Millipore, Billerica, MA, USA) and anti-Actin (Cat. No. A 2668). Total ACCβ was stained with horseradish peroxidase (HRP)-conjugated streptavidin and for other protein membranes were incubated with appropriate HRP-conjugated secondary antibodies. Antigen–antibody complexes were visualized using enhanced chemiluminescence (IMMOBILION™ Western, Millipore) and quantified using a Kodak Image station 2000MM.
TG hydrolase activity assay
TG hydrolase activities were performed as previously described with modifications (Alsted et al. 2009). Briefly, quadriceps muscles from mice (ATGL-KO, HSL-KO and their respective WT littermates) were homogenized on ice in a buffer containing 0.25 m sucrose, 1 mm EDTA, 1 mm dithiothreitol, 40 mmβ-glycerophosphate, 10 mm sodium pyrophosphate, 20 μg ml−1 leupeptin, 20 μg ml−1 antipain, 6.25 μg ml−1 pepstatin A and 0.31 μm okadaic acid (pH 7.0). The supernatant was recovered after centrifugation of the homogenate at 18,500g at 4°C for 45 s and used for TG hydrolase activity measurements. The substrate containing triolein and tri [9,10 (n)-3H]-olein (NET-431, PerkinElmer Life Sciences, Boston, MA, USA) was emulsified with phosphatidylcholine/phosphatidylinositol (3:1) in 3 ml of 0.1 m potassium phosphate buffer (pH 7.0) and 1 ml of 20% FA-free BSA in 0.1 m potassium phosphate buffer (pH 7.0) by sonication (Branson digital sonifier 250, Danbury, CT, USA). For TG hydrolase activities, 50 μg of a lysate pool of eight animals was preincubated for 30 min with different concentrations of the HSL inhibitor (76-0079, Novo Nordisk) or corresponding concentrations of DMSO as vehicle. The mixture was then incubated in a shaking water bath for 60 min at 37°C with 100 μl [3H]-triolein substrate (167 nmol, 1.25 × 104 c.p.m.), enzyme dilution buffer (20 mm potassium phosphate, pH 7.0, 1 mm EDTA, 1 mm dithioerythritol and 0.02% FA-free BSA) in a total volume of 200 μl. All samples were run in triplicate and the results are representative of three independent experiments. The reaction was terminated by adding 3.25 ml of methanol/chloroform/heptane (10:9:7) and 1 ml of 0.1 m potassium carbonate, 0.1 m boric acid, pH 10.5. After the mixture was centrifuged at 1100g for 20 min, 1 ml of the upper phase was removed and the radioactivity of the released 3H-labelled fatty acids was determined by liquid scintillation counting (Tri-Carb 2500 TR, PerkinElmer, Waltham, MA, USA).
Glycogen
Muscle glycogen content was measured on rat muscle homogenates (200 μg) and on whole mouse muscles and determined as glycosyl units after acid hydrolysis (Lowry & Passonneau, 1972).
Calculations and statistics
The total lipid droplet staining intensity per muscle area as well as the number and size of the LDs were obtained from 6–7 different areas of the fibre cross sections and were used to calculate the mean values of the whole muscle. Statistical evaluations were performed either by a one-way or two-way ANOVA with or without repeated measures where appropriate. When the test indicated significant differences, the Tukey's post hoc test was used for further analysis (Sigma Stat 11.0). Data are expressed as mean ± SEM. Differences between groups were considered statistically significant at P < 0.05.
Results
Effects of contraction time on IMTG content in rat skeletal muscle
A morphological approach using BODIPY 493/503 staining of LDs and confocal microscopy imaging was used to determine IMTG content. To evaluate the effect of contraction time on changes in IMTG content, rat SOL muscles were stimulated to contract for either 5 or 20 min and the intensity of the LD staining per area was used as a measure of IMTG content. In response to contraction a significantly decreased IMTG content was observed after 5 min (20%, P < 0.05) and a further decrease was observed after 20 min (77%, P < 0.05; Fig. 1A, B). The number of LDs per fibre area was unchanged from basal to 5 min of contractions while it was decreased to 55% of basal after 20 min (P < 0.05 vs. basal and 5 min; Fig. 1C). The average size of the LDs was decreased to a similar extent after 5 min and 20 min of stimulation (P < 0.05; Fig. 1D). Fibre type-specific analysis revealed a similar pattern of reductions in total IMTG content, number of LDs per area and average LD size in both type I and type II fibres (Table S1). Thus, the small decrease in IMTG after 5 min could be ascribed to a reduction in the LD size, while the further decrease in IMTG content following 20 min of contraction was due to a reduction in the number of lipid droplets.
Figure 1. Effects of contraction time on IMTG degradation in rat soleus muscles.
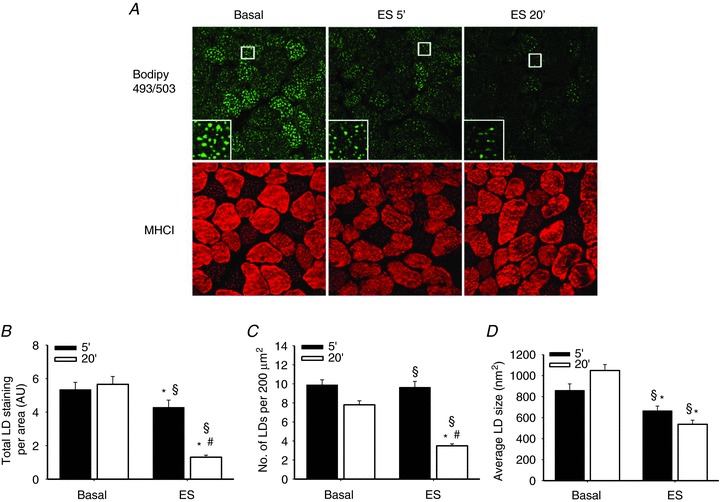
A, representative images of muscles in the basal state and electrically stimulated (ES) for 5 and 20 min, respectively. Results are expressed as total lipid doplet (LD) staining per area (B), number of LDs per 200 μm2 muscle fibre area (C) and average LD size (nm2) (D) (n= 8); data are mean ± SEM. *P < 0.05 compared with corresponding basal; #P < 0.05 compared with ES 5 min; §P < 0.05 interaction between time and ES.
The NNC 76-0079 is specific for HSL in skeletal muscle and inhibits HSL ex vivo
To test the specificity of the NNC 76-0079 inhibitor for HSL (76-0079) in skeletal muscle, in vitro TG hydrolase activity was assayed in lysates of quadriceps muscles from WT and HSL-KO mice in the presence of increasing concentrations of the inhibitor. In lysates from WT mice the inhibitor reduced TG hydrolase activity to levels seen in lysate from HSL-KO mice (40% of WT control, P < 0.001), whereas the inhibitor had no effect in lysate from HSL-KO mice (Fig. 2A). During contractions of rat soleus muscles ex vivo the inhibition of HSL upon addition of the NNC 76-0079 was demonstrated as an increase in muscle DAG content. In contracted muscles, which were preincubated with 76-0079, a modest but significant increase in DAG content compared to the contralateral basal muscle was observed (P < 0.05; Fig. 2B). The DAG content was increased by 7–12% when using inhibitor concentrations of 1–3 mm, whereas no increase in DAG content was found in muscles preincubated with either DMSO (vehicle, VEH) or without either inhibitor or vehicle. These data indicate that HSL was maximally inhibited at the 2 mm concentration of the inhibitor ex vivo; hence, this concentration was used in further experiments.
Figure 2. Effects of HSL inhibition in vitro and ex vivo.
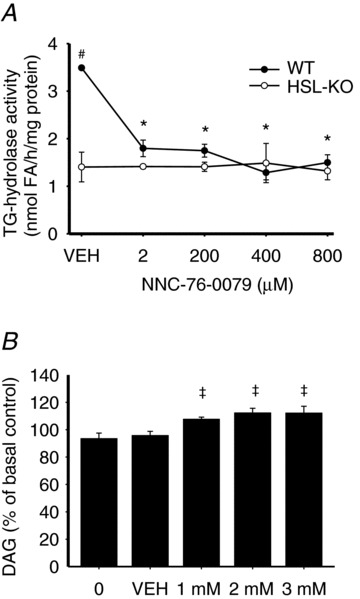
A, TG hydrolase activity in lysates of quadriceps muscles from HSL-KO and wild type (WT) mice incubated with vehicle (VEH) or increasing doses of a mono-specific, small molecule HSL inhibitor (76-0079). Data are expressed as mean ± SEM. B, effect of HSL inhibition on muscle DAG content in electrically stimulated rat soleus muscles (n= 6). Data are expressed as mean percentage of the basal control muscle ± SEM. *P < 0.001 compared with VEH within WT; #P < 0.05 compared with HSL-KO at the corresponding dose; ‡P < 0.05 compared with ES 0 and VEH.
Acute inhibition of HSL does not influence contraction-induced IMTG breakdown, glycogen breakdown or muscle contractile properties in rat skeletal muscle
In response to contractions the IMTG content in rat soleus muscles decreased to the same extent in the presence of the HSL inhibitor or VEH (72 and 75%, respectively, P < 0.001; Fig. 3A). In addition, the number of LDs per area and the average LD size were significantly decreased (P < 0.001), with no differences observed between the groups (Fig. 3B and C). Furthermore, in type I and type II fibres of the contracted muscles, IMTG degradation as well as reductions in the number of LDs per area and average LD size were identical regardless of whether the HSL inhibitor or VEH was present in the incubation medium (P < 0.05) (Table S2). Glycogen content was reduced in contracted muscles (P < 0.05) to the same extent in the presence of the HSL inhibitor or VEH (Fig. 3D). Force production by the muscles throughout the 20 min of stimulation was identical in the inhibitor and the VEH incubated muscles (Fig. 3E) and no differences in time to peak tension or total relaxation time were observed (Table S3).
Figure 3. Acute inhibition of HSL does not impair contraction-induced IMTG breakdown, glycogen breakdown or force production.
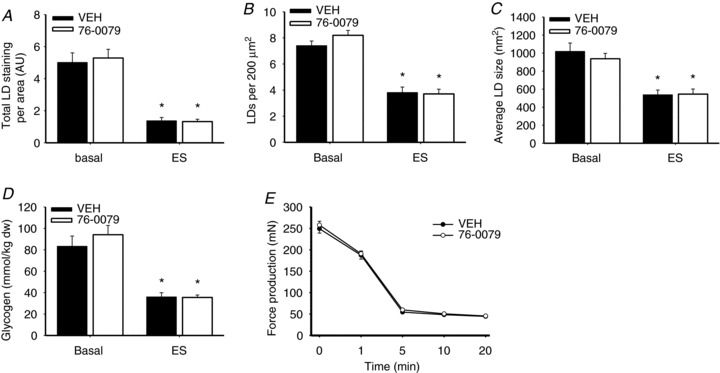
A–C, rat soleus muscles were stimulated electrically to contract (ES) for 20 min or remained in the basal state in the presence of the specific HSL inhibitor 76-0079 (2 mm) or vehicle (VEH). Results are expressed as total LD staining per area (A), number of LDs per 200 μm2 muscle fibre area (B) and average LD size (nm2) (C) (n= 9). D and E, glycogen content before and after ES (n= 9) (D) and force production during ES (n= 18) (E) in rat soleus muscles in the presence of the specific HSL-inhibitor 76-0079 (2 mm) or VEH. Data are expressed as mean ± SEM. *P < 0.001 compared with corresponding basal.
Contraction-induced IMTG breakdown is slightly impaired while glycogen breakdown and muscle contractile properties are not affected in HSL-KO mice
To further investigate lipolysis of IMTG in the absence of HSL, we measured IMTG staining before and after contractions ex vivo in soleus muscles of HSL-KO mice. As the method that was used to fix and stain LDs in rat skeletal muscle resulted in very little or no staining in samples from mouse skeletal muscle, we adopted and developed a technique based on a previously used electron microscopy protocol for LD staining in cross sections of mouse skeletal muscle, to image LDs with bright-field light microscopy. A representative image of LD staining in cross sections from basal and ex vivo stimulated soleus muscles of HSL-KO mice and WT littermates is shown in Fig. 4A. IMTG content decreased significantly with contractions in muscles of both HSL-KO and WT mice (P < 0.05), the decrease being slightly less in the former (53 and 63% decrease for HSL-KO and WT, respectively, P < 0.05; Fig. 4B). In addition, the average LD size and the number of LDs per fibre area were significantly reduced (P < 0.05), but no differences between genotypes were observed (Fig. 4C and D). Likewise, glycogen content in response to contractions was decreased (P < 0.05) to a similar extent in muscles of HSL-KO and WT littermates (Fig. 4E). Muscle force production during electrical stimulation did not differ between genotypes (Fig. 4F). In addition, no differences in time to peak tension or total relaxation time were found (Table S4).
Figure 4. Contraction-induced IMTG breakdown is slightly impaired while glycogen breakdown and muscle contractile properties are not altered in HSL-KO mice.
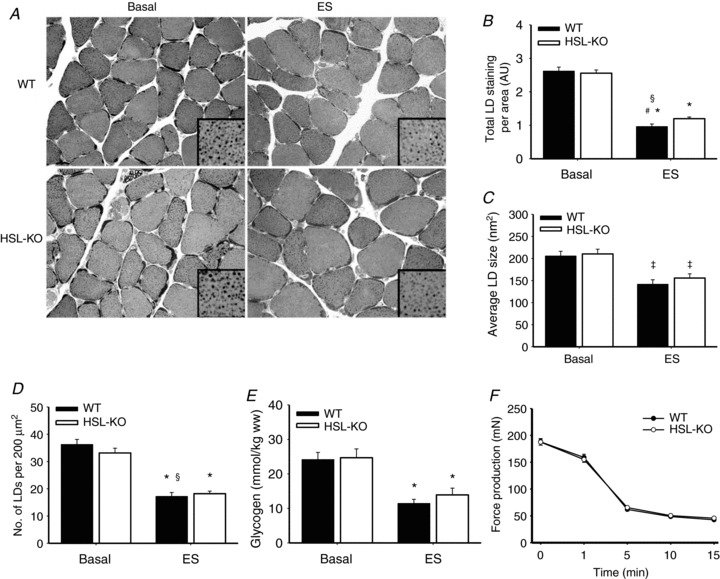
A, representative images of LDs stained in cross sections from basal and electrically stimulated (ES) muscles of HSL-KO mice and WT littermates. Results are expressed as total LD staining per area (B), average LD size (nm2) (C) and number of LDs per 200 μm2 muscle fibre area (D) (n= 8). E and F, glycogen content (n= 8) before and after ES (E) and force production (n= 16) during ES (F) in soleus muscles of HSL-KO and WT littermates. Data are expressed as mean ± SEM. *P < 0.05 compared with corresponding basal; #P < 0.05 compared with HSL-KO, same condition; §P < 0.001 interaction between genotype and ES; ‡P < 0.001 main effect of ES.
Activation of contraction responsive signalling pathways in rat and mouse skeletal muscle
To examine whether the HSL inhibitor exerted any unspecific effects and if any side effects due to the lack of HSL activity occurred, the phosphorylation status of three known contraction responsive proteins, AMPK, ACCβ (a downstream target of AMPK) and eEF2 (a downstream target of eEF2 kinase) was assessed. Muscle contraction-induced significant increases in phosphorylations of AMPK Thr172 (P < 0.001), ACCβ Ser218 (P < 0.001) and eEF2 Thr56 (P < 0.001) (Fig. 5A–D). These changes were similar in the inhibitor and VEH incubated rat soleus muscles. In mouse soleus muscles contraction increased phosphorylations of AMPK Thr172 (P < 0.001), ACCβ Ser212 (P < 0.01) and eEF2 Thr56 (P < 0.01) to a similar extent in HSL-KO and WT littermates (Fig. 5E–H). Thus, neither absence of HSL activity nor the HSL inhibitor per se seemed to influence contraction-induced changes in protein signalling in skeletal muscle.
Figure 5. Effects of contractions on muscle signalling proteins.
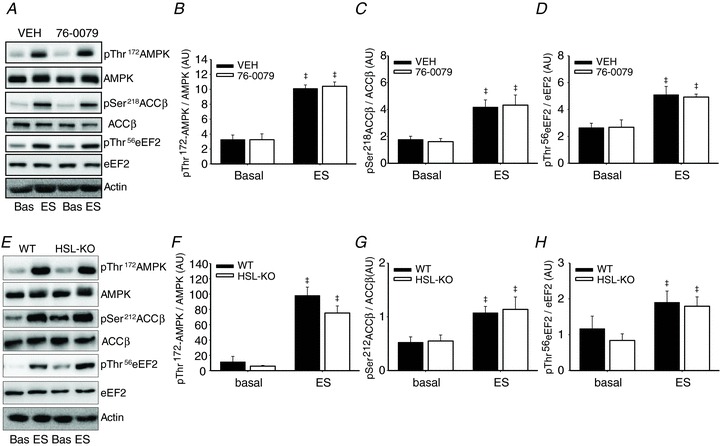
Immunoblotting of rat soleus muscles before and after 20 min of electrically stimulated (ES) contractions in the presence of the specific HSL inhibitor 76-0079 (2 mm) or VEH (A–D) and mouse soleus muscles of HSL-KO and WT (E–H) before and after ES. Values for protein phosphorylation are corrected for the corresponding total protein. Data are expressed as mean ± SEM, n= 8–9 per group. ‡P < 0.01 main effect of ES. AU, arbitrary units.
TG hydrolase activity and protein expressions in mouse skeletal muscle
To investigate whether lipases other than ATGL and HSL could contribute to lipolysis of IMTG, we analysed TG hydrolase activities in lysates of quadriceps muscles from ATGL-KO and WT in the presence of the HSL inhibitor or VEH (Fig. 6A). At maximal inhibition of HSL in ATGL-KO lysates, TG hydrolase activity was below 2% of WT control lysates (P < 0.001). Thus, collectively ATGL and HSL account for at least 98% of the TG hydrolase activity in mouse skeletal muscle, and other TG lipases seem of little or no importance for lipolysis of IMTG. The finding that lipolysis of IMTG was intact in HSL-KO mice might suggest compensatory changes in protein expressions of ATGL or co-regulatory factors. However, no differences in the protein expression of ATGL between HSL-KO and WT littermates were found (Fig. 6B and C). In addition, the protein expressions of CGI-58 and G0S2 protein, which are positive and negative regulators of ATGL activity, respectively, did not differ between genotypes (Fig. 6D and E).
Figure 6. TG hydrolase activities and protein expressions.
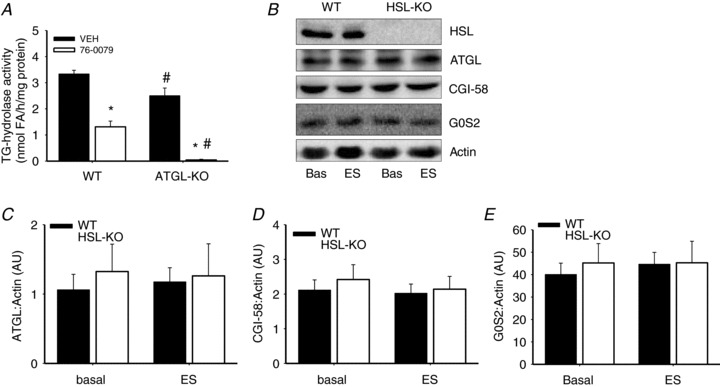
A, TG hydrolase activity in lysates of quadriceps muscles from ATGL-KO and wild-type (WT) littermates incubated with the specific HSL inhibitor 76-0079 or vehicle (VEH). B–E, protein expression of ATGL (C) and ATGL activity regulating proteins CGI-58 (D) and G0S2 (E) in mouse soleus muscles of HSL-KO and WT mice before and after electrically stimulated (ES) contractions. No HSL protein was detected in muscles of HSL-KO mice (B). For protein expression, values are normalized to actin to correct for potential unequal protein loading. Data are expressed as mean ± SEM, n= 8 per group. *P < 0.001 compared with VEH, same genotype; #P < 0.001 compared with WT at the corresponding dose. AU, arbitrary units.
Discussion
HSL has long been considered the only lipase activated during muscle contractions. However, the present study is the first to investigate the effect of acute HSL inhibition and deletion of the HSL gene, respectively, on contraction-induced lipolysis of IMTG in intact skeletal muscle. At maximal pharmacological inhibition of HSL in rat skeletal muscles ex vivo, contraction-induced lipolysis of IMTG was identical to that seen in control muscles. This was so in both type I and type II fibres. In addition, IMTG staining decreased significantly with contractions in muscles of both HSL-KO and WT, the decrease being slightly less in the former. In vitro TG lipase activity measurements revealed that ATGL and HSL collectively account for at least 98% of the TG hydrolase activity in mouse skeletal muscle, and other TG lipases accordingly seem of negligible importance for lipolysis of IMTG. The present study is the first to demonstrate that contraction-induced lipolysis of IMTG occurs and is almost intact in the absence of HSL activity in rat and mouse skeletal muscle. Together, these data strongly suggest that ATGL-mediated lipolysis of IMTG, although not directly measured, plays a physiologically significant role in contraction-induced lipolysis in skeletal muscle.
The finding of maintained contraction-induced lipolysis of IMTG when HSL was acutely inhibited or in muscles of HSL-KO mice suggests that HSL is not the only TG lipase activated by contractions in rat skeletal muscle. This result is in contrast to the general view of HSL being entirely responsible for the contraction-induced lipolytic activity (Langfort et al. 2000; Roepstorff et al. 2004; Watt et al. 2004b). This notion, however, is based on in vitro activity measurements, where the increase in TG lipase activity, measured in homogenates of contracted or adrenaline-stimulated muscles, was blocked when adding an anti-HSL antibody to the assay media (Langfort et al. 1999, 2000; Watt et al. 2004b). However, the in vitro TG lipase assay may not fully reflect the acute activation of muscle TG lipases and lipolytic activity in vivo, which is illustrated by comparing two previously published papers. Thus, Langfort et al. (2000) found that HSL activity, although increased after 5 min, was not increased above basal in homogenates of rat skeletal muscle, contracted for 10 and 60 min. By contrast, using the same experimental setup Prats et al. (2006) found that HSL translocated to one-third of the LDs and colocalized with Adipose differentiating related protein (ADRP) in parallel with a 60% reduction of IMTG content after 15 min of contractions. These findings suggest either that HSL was activated, but this could not be detected in the in vitro activity assay in the study by Langfort et al. (2000), or that other lipases than HSL are activated during contractions. In addition, while the protein kinase A-mediated phosphorylation of HSL increases the in vitro activity of the enzyme approximately 2-fold, this cannot account for the 50–100-fold release of FA and glycerol upon β-adrenergic stimulation of adipocytes (Nilsson et al. 1980; Fredrikson et al. 1981), suggesting that translocation and allosteric mechanisms are important for regulation of lipolytic activity.
The results of the present study imply that ATGL was activated during contractions in rat skeletal muscle. This interpretation is based on the findings that only 2% of total TG lipase activity remained when HSL was maximally inhibited in muscle lysates of ATGL-KO mice. Thus, ATGL and HSL collectively account for at least 98% of the TG lipase activity in mouse skeletal muscle, and other TG lipases accordingly are of negligible importance for lipolysis of IMTG. This finding is in line with results obtained in white adipose tissue, showing that ATGL and HSL together account for more than 95% of the TG lipase activity (Schweiger et al. 2006). In adipose tissue ATGL activity has been found to be regulated positively by CGI-58 (Lass et al. 2006) and negatively by G0S2 (Yang et al. 2010) and it could be hypothesized that the expression of these proteins was altered in the present study to compensate for the absence of HSL activity. However, neither ATGL, CGI-58 nor G0S2 protein contents differed between muscles of HSL-KO and WT mice. These analyses were performed on samples from muscle tissue dissected free from visible adipose tissue. Contamination from adipose tissue could, however, remain a possibility, as we may have missed a difference between genotypes in expression of these proteins. In adipose tissue, ATGL was previously thought to be important for lipolysis only in the basal state (Langin et al. 2005). However, ATGL is now also considered important for adrenaline-stimulated lipolysis in adipose tissue as demonstrated both in adipocytes in vitro (Haemmerle et al. 2006; Schweiger et al. 2006) and by an apparent defect in FA release from the adipose tissue to the circulation during treadmill running in ATGL-KO compared to WT mice (Huijsman et al. 2009; Schoiswohl et al. 2010). In addition, a study on human adipocytes showed that ATGL activity was rate limiting for TG hydrolysis in the basal condition and during adrenaline stimulation (Bezaire et al. 2009). Thus, the result of the present study indicating that ATGL is activated during contractions in skeletal muscle suggests that ATGL is not merely a basal lipase.
In the present study the specificity of the NNC 76-0079 inhibitor for HSL was tested using an in vitro TG hydrolase activity assay in skeletal muscle lysates of HSL-KO mice and WT littermates. In lysate from WT mice the inhibitor reduced TG hydrolase activity to levels seen in lysate from HSL-KO mice, whereas the inhibitor had no effect in lysate from the latter. These data are in line with previously published results, showing a potent effect of the inhibitor in white adipose tissue lysates, isolated fat pads and liver lysates from ATGL-KO mice, with no effects on HSL-KO fat tissue or extracts (Schweiger et al. 2006; Reid et al. 2008). In muscles incubated ex vivo, inhibition of HSL resulted in an increased DAG content during contractions. Importantly, the HSL inhibitor did not have non-specific effects on force production, rate of fatigue, calcium signalling as determined by eEF2 Thr56 phosphorylation or AMPK signalling, which are factors known to be influenced by skeletal muscle contractions (Winder & Hardie, 1996; Rose et al. 2005) Therefore, we conclude that the compound NNC 76-0079 inhibits HSL in skeletal muscle and does not exert unspecific effects in contracting muscles ex vivo.
Using a morphological approach, IMTG content was found to decrease with time during contractions. After 20 min of electrically stimulated contractions, IMTG content, the number of LDs per muscle area and the average LD size were all decreased significantly. This is in line with a previous study in rats, in which a decrease of IMTG content was found after 15 min of contractions, assessed by staining of LDs in single fibres (Prats et al. 2006). The extent to which IMTG was degraded ex vivo is above what is normally found during in vivo exercise in human skeletal muscle (Watt et al. 2004a; Roepstorff et al. 2005). This can be explained by the high intensity of the contractions performed by the muscles and by FA being absent from the incubation media, which may enhance lipolysis of IMTG, as previously found in human skeletal muscle (Watt et al. 2004a). In the present study, muscle glycogen was broken down to a similar extent in muscles treated with either HSL inhibitor or vehicle, which supports the findings that IMTG was degraded to a similar extent in these muscles. Because muscle force production was similar, a compensatory increase of glycogen utilization would be expected to occur if lipolysis of IMTG was attenuated. Corresponding with these findings, glycogen degradation and force production in muscles of HSL-KO mice were similar to findings in WT mice, while lipolysis of IMTG was almost intact. Enhanced utilization of glycogen was previously observed in muscles of HSL-KO mice during low-intensity treadmill running (Huijsman et al. 2009). In another study, no difference in muscle glycogen utilization between HSL-KO and WT mice was observed during high-intensity running until exhaustion (Fernandez et al. 2008). Thus, the present findings of a maintained force production and similar glycogen degradation during contractions support that IMTG breakdown was intact in the absence of HSL activity.
In response to contractions, an increased phospho- rylation of AMPK Thr172 and its downstream target ACCβ Ser218/212 was found in both rat and mouse skeletal muscles, indicating AMPK activation (Park et al. 2002). AMPK is thought to be a negative regulator of lipolysis by phosphorylating HSL on Ser565 (Garton et al. 1989), and this phosphorylation has been demonstrated in skeletal muscle (Donsmark et al. 2004; Roepstorff et al. 2004; Watt et al. 2006). However, increased AMPK activity in parallel with increased hydrolysis of IMTG has been demonstrated before (Watt et al. 2004b; Smith et al. 2005), and conflicting results regarding the role of HSL Ser565 phosphorylation for contraction-induced lipolysis of IMTG exist (Donsmark et al. 2004; Roepstorff et al. 2004; Watt et al. 2006). In addition, it was recently found that AMPK can phosphorylate ATGL in vitro at Ser406, and that this phosphorylation increases lipolysis in adipocytes (Ahmadian et al. 2011). However, in mixed human skeletal muscle, ATGL Ser404 phosphorylation was not affected by a submaximal exercise bout, and pharmacological activation of AMPK in C2C12 myotubes did not affect ATGL phosphorylation at Ser406, suggesting that AMPK is not an upstream kinase of ATGL in skeletal muscle (Mason et al. 2012). Nevertheless, the results of the present study suggest that AMPK does not exert a major negative effect on lipolysis in skeletal muscle during high-intensity contractions.
In summary, the present study shows that contraction induced lipolysis of IMTG occurs in the absence of HSL activity. The fact that this was seen during acute pharmacological blockade of HSL in rat skeletal muscle as well as in HSL gene deficient mouse muscle indicates, for the first time, that HSL is not the only lipase activated by contractions and that ATGL seems to be activated as well.
Acknowledgments
We acknowledge the skilled technical assistance of Peter Albers, Irene B. Nielsen and Gerda Hau.
Glossary
- ACCβ
acetyl-CoA-carboxylase-β
- AMPK
AMP-activated protein kinase
- ATGL
adipose triglyceride lipase
- BODIPY 493/503
4,4-difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s-indacene
- CGI-58
comparative gene identification 58
- DAG
diacylglycerol
- eEF2
eukaryotic elongation factor 2
- FA
fatty acid
- HSL
hormone-sensitive lipase
- IMTG
intramyocellular triacylglycerol
- KO
knockout
- LDs
lipid droplets
- MGL
monoacylglycerol lipase
- PPD
p-phenylenediamine
- TG
triacylglycerol
- TLC
thin-layer chromatography
- G0S2
G(0)/G(1) switch gene 2
Additional information
Competing interests
None.
Author contributions
Conception and design of experiments: T.J.A., H.G. and B.K. Collection, analysis and interpretation of data: T.J.A., T.P., C.P., A.K.S., L.H., P.S., H.G. and B.K. Drafting the article or revising it critically for important intellectual content: all authors. The experiments were performed at the Department of Exercise and Sport Sciences University of Copenhagen, Denmark, and at the Department of Biomedical Sciences, University of Copenhagen, Denmark. All authors approved the final version of the manuscript.
Funding
This study was supported by the Danish Agency of Science, Technology and Innovation and the Ministry of Food, Agriculture and Fisheries, and also by The Novo Nordisk Foundation, The Lundbeck Research Foundation, The Nordea Foundation and the Danish Medical Research Council. This work was carried out as a part of the research program of the UNIK: Food, Fitness & Pharma for Health and Disease (see http://www.foodfitnesspharma.ku.dk). The UNIK project is supported by the Danish Ministry of Science, Technology and Innovation.
Supplementary material
Table S1
Table S2
Table S3
Table S4
References
- Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsted TJ, Nybo L, Schweiger M, Fledelius C, Jacobsen P, Zimmermann R, Zechner R, Kiens B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am J Physiol Endocrinol Metab. 2009;296:E445–453. doi: 10.1152/ajpendo.90912.2008. [DOI] [PubMed] [Google Scholar]
- Badin PM, Louche K, Mairal A, Liebisch G, Schmitz G, Rustan AC, Smith SR, Langin D, Moro C. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes. 2011;60:1734–1742. doi: 10.2337/db10-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J, Hultman E, Saltin B. Muscle glycogen consumption during cross-country skiing (the Vasa ski race) Int Z Angew Physiol. 1973;31:71–75. doi: 10.1007/BF00693727. [DOI] [PubMed] [Google Scholar]
- Bezaire V, Mairal A, Ribet C, Lefort C, Girousse A, Jocken J, Laurencikiene J, Anesia R, Rodriguez AM, Ryden M, Stenson BM, Dani C, Ailhaud G, Arner P, Langin D. Contribution of adipose triglyceride lipase and hormone-sensitive lipase to lipolysis in hMADS adipocytes. J Biol Chem. 2009;284:18282–18291. doi: 10.1074/jbc.M109.008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshier DP, Holloway H, Kitchin LF. A comparison of standard lipid staining techniques used in electron microsco- pic studies of mammalian tissues. Stain Technol. 1984;59:83–89. doi: 10.3109/10520298409113837. [DOI] [PubMed] [Google Scholar]
- Chanarin I, Patel A, Slavin G, Wills EJ, Andrews TM, Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975;1:553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsmark M, Langfort J, Holm C, Ploug T, Galbo H. Contractions induce phosphorylation of the AMPK site Ser565 in hormone-sensitive lipase in muscle. Biochem Biophys Res Commun. 2004;316:867–871. doi: 10.1016/j.bbrc.2004.02.140. [DOI] [PubMed] [Google Scholar]
- Donsmark M, Langfort J, Holm C, Ploug T, Galbo H. Hormone-sensitive lipase as mediator of lipolysis in contracting skeletal muscle. Exerc Sport Sci Rev. 2005;33:127–133. doi: 10.1097/00003677-200507000-00005. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Hansson O, Nevsten P, Holm C, Klint C. Hormone-sensitive lipase is necessary for normal mobilization of lipids during submaximal exercise. Am J Physiol Endocrinol Metab. 2008;295:E179–186. doi: 10.1152/ajpendo.00282.2007. [DOI] [PubMed] [Google Scholar]
- Fischer J, Lefevre C, Morava E, Mussini JM, Laforet P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fredrikson G, Stralfors P, Nilsson NO, Belfrage P. Hormone-sensitive lipase of rat adipose tissue. Purification and some properties. J Biol Chem. 1981;256:6311–6320. [PubMed] [Google Scholar]
- Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- Grober J, Lucas S, Sorhede-Winzell M, Zaghini I, Mairal A, Contreras JA, Besnard P, Holm C, Langin D. Hormone-sensitive lipase is a cholesterol esterase of the intestinal mucosa. J Biol Chem. 2003;278:6510–6515. doi: 10.1074/jbc.M208513200. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- Hoeg LD, Sjoberg KA, Jeppesen J, Jensen TE, Frosig C, Birk JB, Bisiani B, Hiscock N, Pilegaard H, Wojtaszewski JF, Richter EA, Kiens B. Lipid-induced insulin resistance affects women less than men and is not accompanied by inflammation or impaired proximal insulin signalling. Diabetes. 2011;60:64–73. doi: 10.2337/db10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijsman E, van de Par C, Economou C, van der Poel C, Lynch GS, Schoiswohl G, Haemmerle G, Zechner R, Watt MJ. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am J Physiol Endocrinol Metab. 2009;297:E505–513. doi: 10.1152/ajpendo.00190.2009. [DOI] [PubMed] [Google Scholar]
- Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- Jocken JW, Smit E, Goossens GH, Essers YP, van Baak MA, Mensink M, Saris WH, Blaak EE. Adipose triglyceride lipase (ATGL) expression in human skeletal muscle is type I (oxidative) fibre specific. Histochem Cell Biol. 2008;129:535–538. doi: 10.1007/s00418-008-0386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev. 2006;86:205–243. doi: 10.1152/physrev.00023.2004. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Inoguchi T, Maeda Y, Nakashima N, Kuwano A, Eto E, Ueno N, Sasaki S, Sawada F, Fujii M, Matoba Y, Sumiyoshi S, Kawate H, Takayanagi R. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J Clin Endocrinol Metab. 2008;93:2877–2884. doi: 10.1210/jc.2007-2247. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem J. 2000;351:207–214. doi: 10.1042/0264-6021:3510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J. 1999;340:459–465. [PMC free article] [PubMed] [Google Scholar]
- Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Ryden M, Arner E, Sicard A, Jenkins CM, Viguerie N, van H, V, Gross RW, Holm C, Arner P. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005;54:3190–3197. doi: 10.2337/diabetes.54.11.3190. [DOI] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin–Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, Lakhdar H, Wollenberg A, Verret JL, Weissenbach J, Ozguc M, Lathrop M, Prud’Homme JF, Fischer J. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin–Dorfman syndrome. Am J Hum Genet. 2001;69:1002–1012. doi: 10.1086/324121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A flexible system of enzymatic analysis. London: Academic Press; 1972. [Google Scholar]
- MacPherson RE, Ramos SV, Vandenboom R, Roy BD, Peters SJ. Skeletal muscle PLIN proteins, ATGL and CGI-58, interactions at rest and following stimulated contraction. Am J Physiol Regul Integr Comp Physiol. 2013;304:R644–650. doi: 10.1152/ajpregu.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RR, Meex RC, Lee-Young R, Canny BJ, Watt MJ. Phosphorylation of adipose triglyceride lipase Ser404 is not related to 5′-AMPK activation during moderate-intensity exercise in humans. Am J Physiol Endocrinol Metab. 2012;303:E534–541. doi: 10.1152/ajpendo.00082.2012. [DOI] [PubMed] [Google Scholar]
- Mulder H, Sorhede-Winzell M, Contreras JA, Fex M, Strom K, Ploug T, Galbo H, Arner P, Lundberg C, Sundler F, Ahren B, Holm C. Hormone-sensitive lipase null mice exhibit signs of impaired insulin sensitivity whereas insulin secretion is intact. J Biol Chem. 2003;278:36380–36388. doi: 10.1074/jbc.M213032200. [DOI] [PubMed] [Google Scholar]
- Nilsson NO, Stralfors P, Fredrikson G, Belfrage P. Regulation of adipose tissue lipolysis: effects of noradrenaline and insulin on phosphorylation of hormone-sensitive lipase and on lipolysis in intact rat adipocytes. FEBS Lett. 1980;111:125–130. doi: 10.1016/0014-5793(80)80776-9. [DOI] [PubMed] [Google Scholar]
- Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci U S A. 2000;97:787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. Phosphorylation–activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol. 2002;92:2475–2482. doi: 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- Peters SJ, Dyck DJ, Bonen A, Spriet LL. Effects of epinephrine on lipid metabolism in resting skeletal muscle. Am J Physiol. 1998;275:E300–309. doi: 10.1152/ajpendo.1998.275.2.E300. [DOI] [PubMed] [Google Scholar]
- Prats C, Donsmark M, Qvortrup K, Londos C, Sztalryd C, Holm C, Galbo H, Ploug T. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J Lipid Res. 2006;47:2392–2399. doi: 10.1194/jlr.M600247-JLR200. [DOI] [PubMed] [Google Scholar]
- Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Jr, Huang LS. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff C, Vistisen B, Donsmark M, Nielsen JN, Galbo H, Green KA, Hardie DG, Wojtaszewski JF, Richter EA, Kiens B. Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. J Physiol. 2004;560:551–562. doi: 10.1113/jphysiol.2004.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepstorff C, Vistisen B, Kiens B. Intramuscular triacylglycerol in energy metabolism during exercise in humans. Exerc Sport Sci Rev. 2005;33:182–188. doi: 10.1097/00003677-200510000-00006. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Broholm C, Kiillerich K, Finn SG, Proud CG, Rider MH, Richter EA, Kiens B. Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J Physiol. 2005;569:223–228. doi: 10.1113/jphysiol.2005.097154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoiswohl G, Schweiger M, Schreiber R, Gorkiewicz G, Preiss-Landl K, Taschler U, Zierler KA, Radner FP, Eichmann TO, Kienesberger PC, Eder S, Lass A, Haemmerle G, Alsted TJ, Kiens B, Hoefler G, Zechner R, Zimmermann R. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J Lipid Res. 2010;51:490–499. doi: 10.1194/jlr.M001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- Smith AC, Bruce CR, Dyck DJ. AMP kinase activation with AICAR further increases fatty acid oxidation and blunts triacylglycerol hydrolysis in contracting rat soleus muscle. J Physiol. 2005;565:547–553. doi: 10.1113/jphysiol.2004.081687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriet LL, Heigenhauser GJ, Jones NL. Endogenous triacylglycerol utilization by rat skeletal muscle during tetanic stimulation. J Appl Physiol. 1986;60:410–415. doi: 10.1152/jappl.1986.60.2.410. [DOI] [PubMed] [Google Scholar]
- Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967;216:173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- van Loon LJ, Manders RJ, Koopman R, Kaastra B, Stegen JH, Gijsen AP, Saris WH, Keizer HA. Inhibition of adipose tissue lipolysis increases intramuscular lipid use in type 2 diabetic patients. Diabetologia. 2005;48:2097–2107. doi: 10.1007/s00125-005-1889-x. [DOI] [PubMed] [Google Scholar]
- Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- Walther TC, Farese RV., Jr The life of lipid droplets. Biochim Biophys Acta. 2009;1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004a;287:E120–127. doi: 10.1152/ajpendo.00542.2003. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Chan S, Garnham A, Kemp BE, Febbraio MA. β-Adrenergic stimulation of skeletal muscle HSL can be overridden by AMPK signalling. FASEB J. 2004b;18:1445–1446. doi: 10.1096/fj.03-1067fje. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Yang X, Lu X, Lombes M, Rha GB, Chi YI, Guerin TM, Smart EJ, Liu J. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 2010;11:194–205. doi: 10.1016/j.cmet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


