Abstract
Ranolazine, an anti-anginal compound, has been shown to significantly improve glycaemic control in large-scale clinical trials, and short-term ranolazine treatment is associated with an improvement in myocardial blood flow. As microvascular perfusion plays critical roles in insulin delivery and action, we aimed to determine if ranolazine could improve muscle microvascular blood flow, thereby increasing muscle insulin delivery and glucose use. Overnight-fasted, anaesthetized Sprague-Dawley rats were used to determine the effects of ranolazine on microvascular recruitment using contrast-enhanced ultrasound, insulin action with euglycaemic hyperinsulinaemic clamp, and muscle insulin uptake using 125I-insulin. Ranolazine's effects on endothelial nitric oxide synthase (eNOS) phosphorylation, cAMP generation and endothelial insulin uptake were determined in cultured endothelial cells. Ranolazine-induced myographical changes in tension were determined in isolated distal saphenous artery. Ranolazine at therapeutically effective dose significantly recruited muscle microvasculature by increasing muscle microvascular blood volume (∼2-fold, P < 0.05) and increased insulin-mediated whole body glucose disposal (∼30%, P= 0.02). These were associated with an increased insulin delivery into the muscle (P < 0.04). In cultured endothelial cells, ranolazine increased eNOS phosphorylation and cAMP production without affecting endothelial insulin uptake. In ex vivo studies, ranolazine exerted a potent vasodilatatory effect on phenylephrine pre-constricted arterial rings, which was partially abolished by endothelium denudement. In conclusion, ranolazine treatment vasodilatates pre-capillary arterioles and increases microvascular perfusion, which are partially mediated by endothelium, leading to expanded microvascular endothelial surface area available for nutrient and hormone exchanges and resulting in increased muscle delivery and action of insulin. Whether these actions contribute to improved glycaemic control in patients with insulin resistance warrants further investigation.
Key points
Ranolazine, an anti-anginal compound, improves glycaemic control in clinical trials and increases myocardial perfusion via a direct vasodilatatory effect on coronary arteries.
Skeletal muscle microvasculature controls the delivery of nutrient and hormones into muscle and their exchanges between plasma and muscle interstitium by providing microvascular exchange surface area.
In this study we examined whether ranolazine improves glycaemic control via exerting vasodilatatory action on the pre-capillary arterioles to recruit muscle microvasculature.
We demonstrate that ranolazine potently recruits muscle microvasculature, which expands microvascular endothelial surface area in muscle and results in increased muscle delivery and action of insulin.
The results help us better understand the physiological mechanism by which ranolazine improves glycaemic control and its potential as an insulin-sensitizing agent.
Introduction
Type 2 diabetes mellitus (T2DM) is associated with endothelial dysfunction and insulin resistance, two key factors that contribute to the accelerated cardiovascular complications seen in this patient population. Ranolazine ([(+)N-(2,6-dimethylphenyl)-4(2-hydroxy-3-(2-metho-xyphenoxy)-propyl)-1-piperazine acetamide dihydro-chloride]) is an active piperazine derivative that has an anti-ischaemic/anti-anginal property in patients with chronic angina (Clarke et al. 1993; Bagger et al. 1997; Chaitman, 2002, 2004). It reduces angina frequency and improves exercise performance (Chaitman et al. 2004b; Stone et al. 2006) and significantly improves exercise duration in patients with chronic angina (Chaitman et al. 2004a). The underlying mechanism was initially thought to be primarily through partial inhibition of fatty acid oxidation (McCormack et al. 1998; Zacharowski et al. 2001; Chaitman et al. 2004b). More recent evidence suggests that it reduces calcium overload in the ischaemic myocyte by inhibiting late sodium current (Song et al. 2004; Makielski & Valdivia, 2006). It is also likely that ranolazine exerts an anti-anginal effect via its well-known, direct vasodilatatory effect. In vivo studies have shown that short-term ranolazine treatment improves myocardial perfusion (Venkataraman et al. 2009) and injection of ranolazine into coronary or femoral arteries causes regional vasodilatation (Nieminen et al. 2011). Ex vivo study using isolated intrarenal arteries has demonstrated a direct vasodilatatory action as well (Deng et al. 2012).
Recently, two placebo-controlled, randomized clinical trials showed that ranolazine treatment was associated with a significant decrease in glycated haemoglobin (HbA1c) levels in both diabetic and non-diabetic subjects (Timmis et al. 2006; Morrow et al. 2009; Chisholm et al. 2010). After receiving 4 months of ranolazine treatment (1000 mg orally twice daily), subjects with HbA1c of 6–8% had a 0.28% reduction in HbA1c and 0.59% reduction for those subjects with HbA1c of 8–10%. The underlying mechanisms remain unknown. Although data from streptozotocin-induced diabetic mice indicated that ranolazine improves glucose homeostasis by promoting β-cell survival and increasing glucose-induced insulin secretion (Ning et al. 2011), it is unlikely that the β-cell protective effect played a major role in the observed decrease in HbA1c in the above-mentioned clinical trials as patients with T2DM were already hyperinsulinaemic and the effect of ranolazine on HbA1c was also apparent in non-diabetic subjects who had normal β-cell function (Morrow et al. 2009).
The microvasculature controls the delivery of nutrient and hormones into skeletal muscle and their exchanges between plasma and muscle interstitium. We and others have recently demonstrated that microvascular recruitment induced by exercise (Inyard et al. 2007, 2009), insulin (Coggins et al. 2001), mixed meal (Keske et al. 2009), angiotensin II receptor blocker (Chai et al. 2010) and glucagon-like peptide 1 (GLP-1) (Chai et al. 2012), by way of increasing microvascular exchange surface area, leads to increased insulin delivery to and its action in skeletal muscle (Clark, 2008; Barrett et al. 2009). Whether ranolazine improves glycaemic control via exerting vasodilatatory action on the pre-capillary arterioles to recruit microvasculature is unknown.
In the current study, we examined the effect of ranolazine on muscle microvascular recruitment, and insulin delivery to and its action in muscle. Our results indicate that ranolazine potently vasodilatates muscle microvasculature, which contributes to its glycaemic action.
Methods
Ethical approval
The study conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication No. 85-23, revised 1996). The study protocols were approved by the Animal Care and Use Committee of the University of Virginia, which conforms to the principles of UK regulations, as described in Drummond (2009).
Animal preparations and experimental protocols
Overnight-fasted adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 250–380 g were used in the study. Prior to the study rats were fed standard lab chow and water ad libitum and housed at 22 ± 2°C, on a 12 h light/dark cycle. Rats were anaesthetized with pentobarbital sodium (50 mg kg−1i.p.; Abbott Laboratories, North Chicago, IL, USA), placed in a supine position on a heating pad to ensure euthermia and intubated to maintain a patent airway. The carotid artery and the jugular vein were cannulated with polyethylene tubing (PE-50; Fisher Scientific, Newark, DE, USA) for arterial blood pressure monitoring, arterial blood sampling and various infusions. After a 30 min baseline period to ensure haemodynamic stability and a stable level of anaesthesia, rats were studied under one of the following four protocols (Fig. 1). At the end of each study, rats were killed via a pentobarbital sodium overdose.
Figure 1.
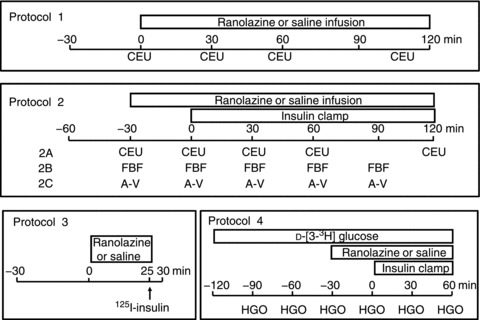
Experimental protocols
Protocol 1
Two groups of rats were studied under this protocol (five in each group). Baseline microvascular parameters, including microvascular blood volume (MBV), microvascular flow velocity (MFV) and microvascular blood flow (MBF), on the proximal adductor muscle group (adductor magnus and semimembranosus) of the right hindlimb were obtained using contrast-enhanced ultrasound (CEU) as previously described (Inyard et al. 2007, 2009). Rats then received a primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) i.v. infusion of ranolazine (Zacharowski et al. 2001; Dhalla et al. 2009) or the same volume of saline for 120 min. Microvascular parameters were again determined at 30, 60 and 120 min. Plasma samples were collected at −30, 0, 30, 60, 90 and 120 min and stored at −80°C for determination of plasma insulin concentrations using a rat insulin enzyme-linked immunosorbent assay kit (Mercodia AB, Uppsala, Sweden). At the end of study, rats were killed and gastrocnemius muscle and heart were freeze-clamped in liquid nitrogen for later measurement of Akt and ERK1/2 phosphorylation using Western blotting.
Protocol 2
Six groups of rats were studied under this protocol (five in each group). Each rat received a primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) infusion of ranolazine (Zacharowski et al. 2001; Dhalla et al. 2009) or the same volume of saline for 150 min (−30 to 120 min) with a systemic infusion of insulin (3 mU kg−1 min−1) superimposed from 0 to 120 min. Arterial blood glucose was determined every 10 min using an Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN, USA), and 30% dextrose (30% w/v) was infused at a variable rate to maintain blood glucose within 10% of basal. In protocol 2A, two groups of rats were included. Microvascular parameters including MBV, MFV and MBF were determined at −30, 0, 30, 60 and 120 min using CEU as in protocol 1. In protocol 2B, two groups of rats were included. Femoral artery blood flow (FBF) was measured at −30, −29, −25, −20, −10, 0, 30, 60 and 90 min using a flow probe (VB series 0.5 mm, Transonic Systems, Ithaca, NY, USA), as described previously (Vincent et al. 2004; Inyard et al. 2007, 2009). In protocol 2C, two groups of rats were included. Arterial–venous (A-V) glucose difference was determined at −30, 0, 30, 60 and 90 min as described previously (Rattigan et al. 1997).
Protocol 3
Two groups of rats were studied under this protocol (five in each group). Each rat received a primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) infusion of ranolazine (Zacharowski et al. 2001; Dhalla et al. 2009) or the same volume of saline for 25 min. Rats then received a bolus i.v. injection of 1.5 μCi 125I-insulin at time 25 min. Rats were killed 5 min afterwards and blood and gastrocnemius were obtained for determination of muscle insulin uptake.
Protocol 4
Two groups of rats were studied under this protocol (three in each group). Each rat received a primed (8 μCi) continuous infusion of d-[3–3H] glucose (0.11 μCi min−1; Amersham, Arlington Heights, IL, USA) from −120 to 60 min. A primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) infusion of ranolazine (Zacharowski et al. 2001; Dhalla et al. 2009) or the same volume of saline was initiated at −30 min. At time 0 min, each rat began to receive a systemic infusion of insulin (3 mU kg−1 min−1) which lasted for 60 min. Plasma glucose-specific activity was measured every 30 min. Blood glucose was monitored every 10 min during insulin infusion. Blood samples were collected every 30 min for determination of hepatic glucose output (HGO).
Throughout the study, mean arterial blood pressure (MAP) was monitored via a sensor connected to the carotid arterial catheter (Harvard Apparatus, Holliston, MA, USA and ADInstruments, Inc., Colorado Springs, CO, USA). Pentobarbital sodium was infused at a variable rate to maintain steady levels of anaesthesia and blood pressure throughout the study.
Muscle 125I-insulin clearance/uptake
125I-insulin was used as a tracer to track muscle clearance and uptake of native insulin, as descried previously (Inyard et al. 2007; Chai et al. 2011, 2012). In brief, protein-bound 125I in blood and muscle samples collected from protocol 3 was precipitated with 30% trichloroacetic acid, and radioactivity was measured using a gamma-counter. Muscle clearance rates of 125I-insulin were expressed as muscle 125I-insulin (d.p.m. g dry weight−1)/blood 125I-insulin (d.p.m. ml−1)/5 min. Muscle 125I-insulin uptake was expressed as 125I-insulin (d.p.m.)/muscle dry weight (g)/5 min.
Determination of hindleg glucose uptake
Carotid arterial and femoral venous blood glucose concentrations were determined using an Accu-Chek Advantage blood glucometer (Roche Diagnostics). Hindleg glucose uptake was calculated as the A-V glucose difference multiplied by average FBF.
Determination of HGO
At each time point, 50 μl plasma was collected and deproteinized with 30 μl 10% trichloroacetic acid solution. Forty microlitres of supernatant was dried at room temperature to eliminate tritiated water and then reconstituted with 100 μl distilled water. Radioactivity was counted in a liquid scintillation system. The glucose production (Ra) and HGO were calculated using the following formula: Ra= infusion rate of d-[3–3H]glucose (d.p.m. min−1)/plasma glucose-specific activity (d.p.m. mg glucose−1)/kg body weight; and HGO =Ra–Ri, where Ri represents the exogenous glucose infusion rate (Liu et al. 1993).
Culture of endothelial cells and determination of endothelial insulin uptake
Endothelial cell insulin uptake was assessed using 125I-insulin as previously reported (Dernovsek & Bar, 1985; Hachiya et al. 1987, 1988; Bertelsen et al. 2001). Briefly, bovine aortic endothelial cells (BAECs) in primary culture were purchased from Lonza (Walkersville, MD, USA). Cells between passages 3 and 8 were cultured in six-well plates until 80% confluence, serum starved for 18–22 h and then incubated with pre-warmed Hepes binding buffer (HBB) (0.1 m Hepes, 0.12 m NaCl, 5 mm KCl, 1.2 mm MgSO4, 8 mm glucose and 1% bovine serum albumin; pH 7.8) containing 200 pm125I-insulin in the presence or absence of unlabelled regular insulin (2 μm) or 3 μm of ranolazine at 37°C for 15 min. The reaction was stopped by transferring the culture plates onto ice. Cells were washed once with ice-cold HBB, then twice with ice-cold acid solution (0.5 m NaCl, 0.2 m acetic acid, pH 3.0) to remove surface-bound 125I-insulin, and then lysed with 0.5 ml 1 m NaOH on ice for 1 h. Aliquots of cell lysate were used for protein content determination and radioactivity quantification using a gamma counter. Endothelial insulin uptake (c.p.m. μg protein−1) was calculated after subtracting the non-specific binding and expressed as percentage of control.
Determination of intracellular cAMP contents in cultured endothelial cells
After 18–22 h starvation, BAECs with 80% confluence were treated with 100 nm insulin or various concentrations of ranolazine (1, 3 and 10 μm) in serum-free endothelial basic media for 15 min. Cells were lysed with 0.1 m HCl and intracellular cAMP contents were determined using a cAMP EIA kit (Cayman Chemical, Ann Arbor, MI, USA), as previously described (Fu et al. 2010).
Determination of protein phosphorylation
Phosphorylation of endothelial nitric oxide synthase (eNOS) in BAECs, and Akt and ERK1/2 in harvested muscle and heart samples from the animal studies were determined by Western blotting as described previously (Li et al. 2005, 2007; Chai et al. 2011). Primary antibodies against phospho-eNOS (Ser635), phospho-eNOS (Ser1177), total eNOS, phospho-Akt (Ser473), total Akt1, phospho-ERK (Thr202/Tyr204) and total ERK1/2 were obtained from Cell Signaling Technology (Beverly, MA, USA). All blots were developed using enhanced chemiluminescence (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA). Autoradiographic films were scanned densitometrically and quantified using ImageQuant 3.3 software. Both the total and the phosphospecific densities were quantified and the ratios of phosphospecific to total density were calculated.
Determination of ranolazine's vasorelaxant effect on pre-constricted small artery
Rat distal saphenous artery (≤ 250 μm) was dissected from six rats killed via CO2 overdose and each artery was cleaned of adhering connective tissues and cut into segments of ∼2 mm in length. Each segment was mounted in a Multi Myograph System (Danish Myo Technology, Aarhus, Denmark). The organ chamber was filled with 6 ml physiological salt solution (PSS) buffer (NaCl 130 mm, KCl 4.7 mm, CaCl2 1.6 mm, MgSO4 1.17 mm, KH2PO4 1.18 mm, NaHCO3 14.9 mm; EDTA 0.026 mm, glucose 5.5 mm; pH, 7.4), which was constantly bubbled with 95% O2–5% CO2 and maintained at 37°C. Each ring was stretched initially to 5 mN, an optimal tension, and then allowed to stabilize at baseline tone. In some arteries, the endothelium was mechanically removed by rubbing the luminal surface of the ring with human hair. Functional removal of the endothelium was verified by the lack of relaxation response to acetylcholine. After pre-constriction of the arterial ring with either 60 mm potassium or 2 μm phenylephrine, 3 mm ranolazine in 6 μl PSS buffer was added to the chamber (final concentration 3 μm) and changes in arterial tone were recorded.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Statistical analyses were performed with SigmaStat 11.0 software (Systat Software, Inc., Chicago, IL, USA), using either Student's t test or analysis of variance (ANOVA) with post-hoc analysis as appropriate. A P-value of < 0.05 was considered statistically significant.
Results
Ranolazine increases skeletal muscle microvascular recruitment
We and others have previously reported that microvascular recruitment is associated with increased muscle delivery of insulin and glucose use (Inyard et al. 2007, 2009; Chai et al. 2011, 2012) due to increased microvascular surface area. As ranolazine has been shown clinically to improve glycaemic control, we thus first examined whether ranolazine could also vasodilatate the pre-capillary and recruit microvasculature in muscle. Using CEU, we measured three important indices of microcirculation (MBV, MFV and MBF) before and after a primed continuous ranolazine infusion. As shown in Fig. 2, ranolazine potently and acutely increased muscle microvascular recruitment by increasing MBV within the first 30 min and this effect lasted for the entire 120 min infusion period (ANOVA, P < 0.05; Fig. 2A). MFV did not change (Fig. 2B). This led to an ∼2-fold increase in muscle MBF (ANOVA, P < 0.05; Fig. 2C). By contrast, these parameters did not change in control animals. Ranolazine priming induced an acute decrease in MAP (ANOVA, P < 0.05; Fig. 2D), which promptly returned to baseline. Ranolazine infusion did not alter the plasma insulin concentration (Table 1).
Figure 2. Effects of ranolazine on skeletal muscle microvascular recruitment.
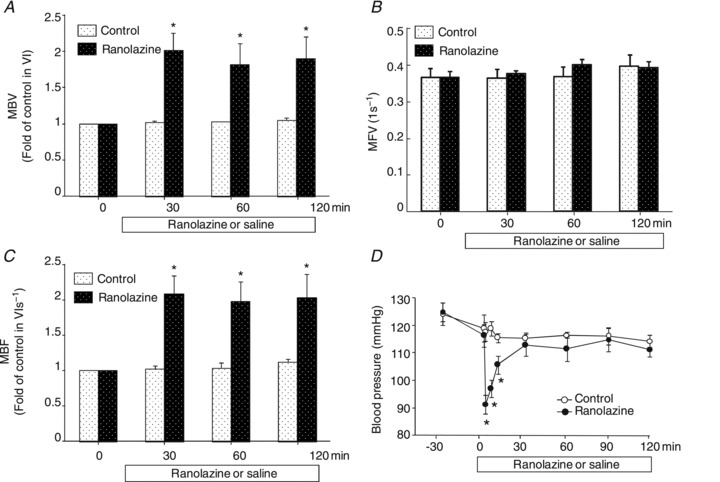
Each rat received a primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) i.v. infusion of ranolazine or the same volume of saline for 120 min. CEU measurements were made before and at 30, 60 and 120 min after the initiation of ranolazine infusion. A, MBV; B, MFV; C, MBF; D, MAP. n= 5 each. *P < 0.05 vs. control.
Table 1.
Plasma insulin concentrations
| Time (min) | Control (pm, mean ± SE) | Ranolazine (pm, mean ± SE) |
|---|---|---|
| −30 | 145.1 ± 6.7 | 150.6 ± 12.4 |
| 0 | 159.0 ± 6.1 | 135.1 ± 14.7 |
| 30 | 153.0 ± 8.9 | 136.7 ± 24.8 |
| 60 | 168.7 ± 10.0 | 156.7 ± 21.3 |
| 90 | 169.0 ± 8.5 | 154.0 ± 6.3 |
| 120 | 150.6 ± 15.7 | 165.8 ± 11.6 |
Ranolazine enhances insulin action in muscle
Knowing that ranolazine could indeed recruit muscle microvasculature, we next examined whether ranolazine could modulate insulin's action in muscle. Administration of ranolazine increased insulin-mediated whole body glucose disposal, as evidenced by a 30–60% increase in the glucose infusion rates (GIRs) during the first hour of insulin infusion (ANOVA, P < 0.005; Fig. 3A). Although the GIRs in the ranolazine group remained higher than the control group during the second hour of insulin infusion, the difference was not statistically significant. The overall GIR (area under the curve of GIR during insulin clamp) was ∼30% higher in the ranolazine group (Student's t test, P= 0.02; Fig. 3B). Blood glucose levels were comparable between the two groups during the insulin clamp (Fig. 3C). Similar to the protocol 1 CEU study, ranolazine priming again induced a prompt but transient decrease in arterial blood pressure (Fig. 3D).
Figure 3. Ranolazine augments insulin-stimulated whole body glucose disposal.
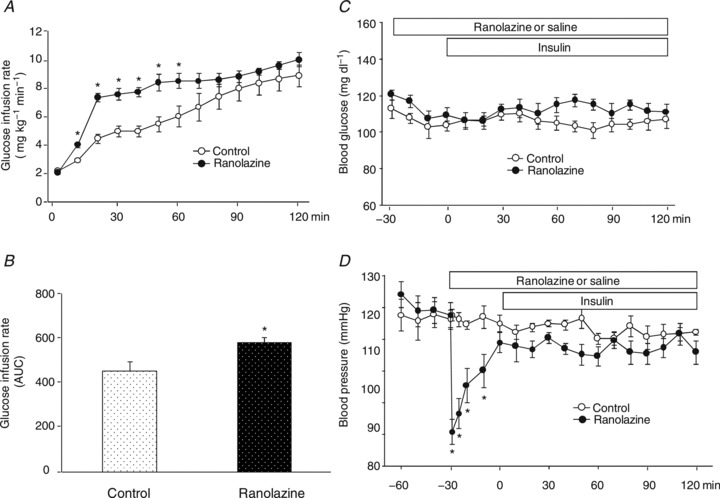
Each rat received a primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) i.v. infusion of ranolazine or the same volume of saline for 150 min. A euglycaemic insulin clamp (3 mU kg−1 min−1) was started 30 min after each rat began receiving ranolazine or saline (0 min) and continued for 120 min. A, time course of glucose infusion rate (GIR) during insulin clamp; B, area under the curve of GIR during insulin clamp; C, blood glucose concentrations; D, MAP. n= 5 each. *P < 0.05 vs. control.
The significant increase in GIR during the first hour prompted us to examine whether ranolazine and insulin have an additive effect on muscle microvascular recruitment. As shown in Fig. 4, insulin alone recruited muscle microvasculature and the effect lasted for 2 h. As ranolazine was given 30 min before insulin clamp, muscle MBV and MBF were significantly higher at 0 and 30 min in the ranolazine plus insulin group than the insulin alone group (ANOVA, P < 0.05). However, there was no difference at 60 and 120 min between the two groups. Although ranolazine increased FBF acutely, this effect was transient and FBF promptly returned back to baseline (within 15 min) and remained stable afterwards (Fig. 4D).
Figure 4. Effects of ranolazine on skeletal muscle microvascular recruitment and femoral blood flow during insulin clamp.
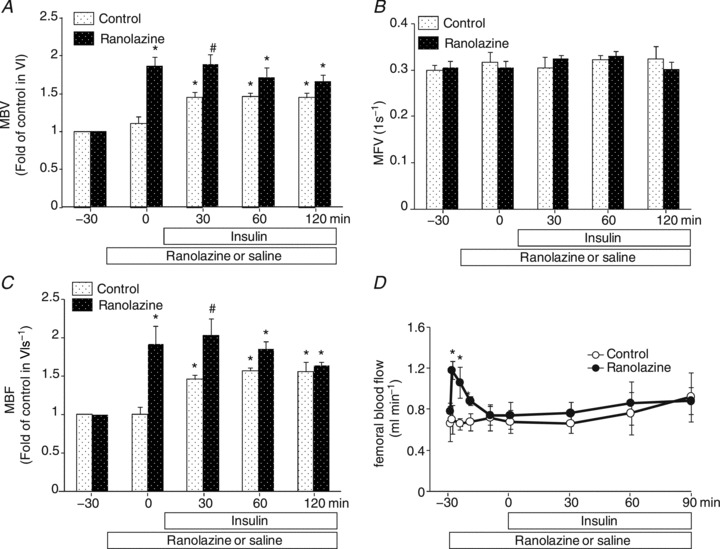
Each rat received a primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) i.v. infusion of ranolazine or the same volume of saline. A, MBV; B,MFV; C, MBF; D,FBF. n= 5 each. *P < 0.05 vs. baseline (−30 min). #P < 0.05 vs. insulin.
As skeletal muscle glucose use accounts for 70–80% of the whole body glucose disposal during insulin infusion (DeFronzo et al. 1981), we examined the effect of ranolazine on HGO and hindleg muscle glucose uptake to ensure the increase in GIR indeed represents increased muscle glucose uptake. As shown in Fig. 5A, insulin similarly suppressed HGO in both groups and the hindleg A-V glucose differences were significantly higher in the ranolazine vs. saline group at 30 and 60 min. We also examined the phosphorylation status of Akt and ERK1/2, two intermediate molecules in the insulin signalling pathways, in gastrocnemius and heart samples (Fig. 6). Insulin infusion significantly increased the phosphorylation of both Akt and ERK1/2 in skeletal and cardiac muscle in the control group (ANOVA, P < 0.05). Administration of ranolazine did not change either basal or insulin-induced muscle or heart Akt and ERK1/2 phosphorylation.
Figure 5. Effects of ranolazine on hepatic glucose output (HGO) and hindleg glucose uptake.
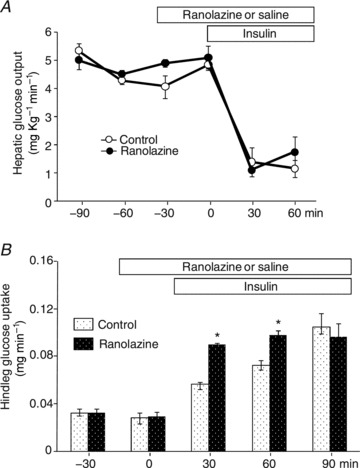
Each rat received a primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) i.v. infusion of ranolazine or the same volume of saline. HGO and hindleg glucose uptake were determined every 30 min. A, HGO (n= 3 each); B, hindleg glucose uptake (n= 5 each). *P < 0.05 vs. insulin.
Figure 6. Effect of ranolazine on insulin-mediated Akt and ERK1/2 phosphorylation.
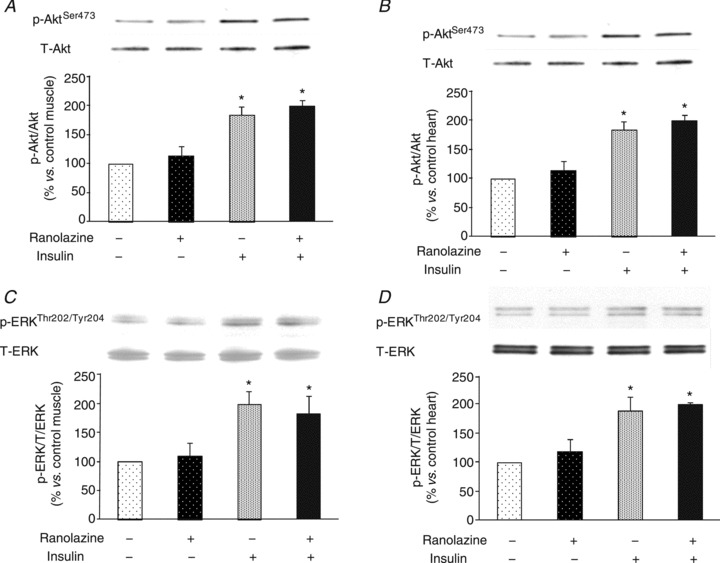
A, skeletal muscle Akt phosphorylation; B; heart muscle Akt phosphorylation; C, skeletal muscle ERK1/2 phosphorylation; D, heart muscle ERK1/2 phosphorylation. n= 4 each. *P < 0.05 vs. control.
Ranolazine increases muscle uptake of insulin
The increase in insulin-mediated GIRs in rats receiving ranolazine suggests an increased insulin action in the muscle. Previous studies have shown that an increase in muscle microvascular surface area increases muscle insulin delivery (Barrett et al. 2009, 2011) and interstitial insulin concentrations correlate better with insulin action (Chiu et al. 2008). To study whether ranolazine-induced microvascular recruitment is associated with increased muscle delivery of insulin, we used 125I-insulin to trace native insulin movement in vivo to determine muscle insulin uptake. As shown in Fig. 7, 25 min of ranolazine infusion significantly increased muscle clearance of insulin from blood (Fig. 7A, Student's t test, P= 0.005) and uptake of insulin (Fig. 7B, Student's t test, P= 0.03). These changes were not secondary to differential degradation of 125I-insulin as both the control and the ranolazine group showed similar degradation rates of 125I-insulin in blood and muscle (Fig. 7C).
Figure 7. Effect of ranolazine on skeletal muscle insulin uptake.
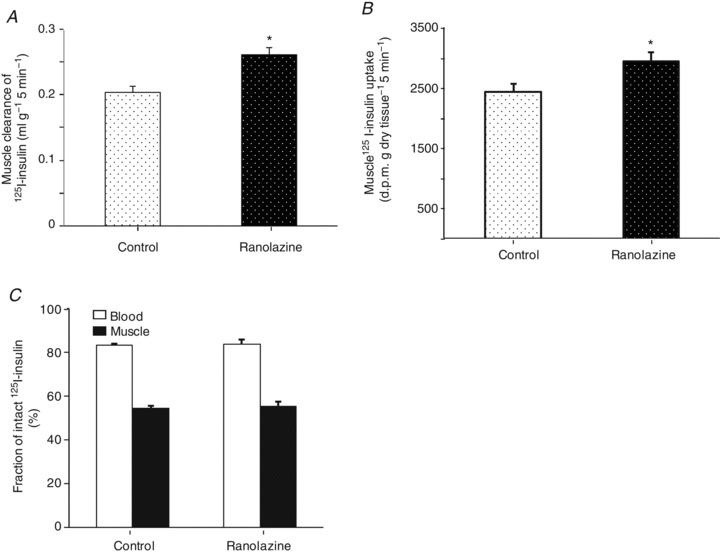
Each rat received a primed (10 mg kg−1) continuous (9.6 mg kg−1 h−1) infusion of ranolazine or the same amount of saline for 25 min, and a bolus i.v. injection of 125I-insulin (1.5 μCi) at 25 min. Blood and skeletal muscle were collected 5 min afterwards. Total and intact 125I-insulin was determined after TCA (30%) precipitation in both blood and muscle. A, muscle clearance of plasma insulin; B, muscle insulin uptake; C, fraction of intact 125I-insulin in blood and muscle. n= 5 each. *P < 0.05 vs. control.
Ranolazine induces relaxation on rat distal saphenous artery
Ranolazine has been shown to potently vasodilatate the intrarenal arteries (Deng et al. 2012). Given that it potently recruited muscle microvasculature, suggesting a vasodilatatory effect on the pre-capillary arterioles, we tested ranolazine's effect on isolated distal saphenous artery. We found that ranolazine at 3 μm, which is in the lower therapeutic dose range (2–8 μm), significantly dilatated phenylephrine pre-constricted arterial rings (by ∼40%, P < 0.01). Endothelium denudement partially abolished this vasodilatatory effect (ANOVA, P= 0.014; Fig. 8A and B). However, ranolazine at the same concentration had little effect on 60 mm potassium pre-constricted arterial rings (Fig. 8C and D).
Figure 8. Ranolazine causes arterial vasodilatation on arteries pre-constricted with phenylephrine but not high potassium.
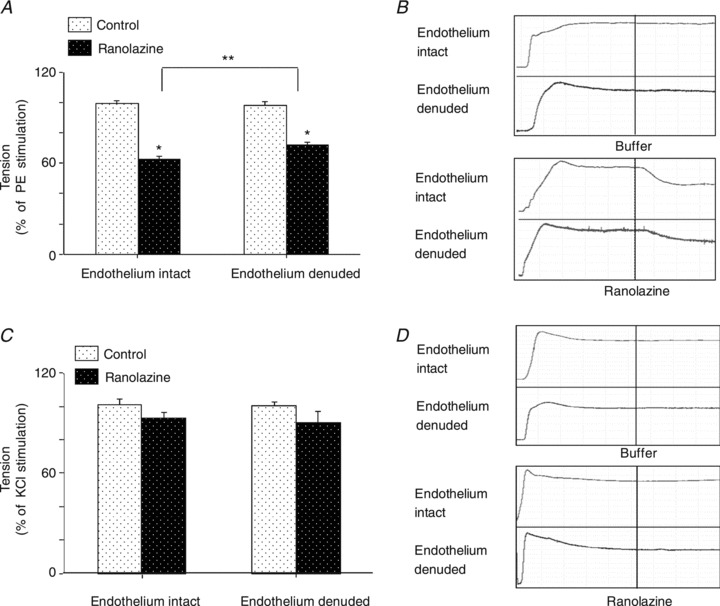
A and B, effects of ranolazine (3 μm) on isolated distal saphenous artery pre-constricted with phenylephrine (2 μm) and representative tomographic images. C and D, effects of ranolazine (3 μm) on isolated distal saphenous artery pre-constricted with potassium (60 mm) and representative tomographic images. n= 6 per group. *P < 0.01 vs. control, **P= 0.014 endothelium denuded vs. endothelium intact arteries.
Effects of ranolazine on eNOS phosphorylation, intracellular cAMP content and insulin uptake in cultured BAECs
As endothelium denudement partially abolished ranolazine's vasodilatatory effect and eNOS has been shown to play a pivotal role in the microvascular recruitment induced by several factors such as insulin, GLP-1 and losartan (Chai et al. 2011, 2012), we finally tested whether ranolazine could directly affect endothelial cells. Treatment of cultured BAECs with clinically relevant therapeutic concentrations of ranolazine (1 and 3 μm) significantly increased eNOS phosphorylation at both Ser635 (Fig. 9A) and Ser1177 (Fig. 9B; ANOVA, P < 0.05) and increased intracellular cAMP content (Fig. 9C; ANOVA, P < 0.05). However, ranolazine at 3 μm did not affect endothelial uptake of 125I-insulin (Fig. 9D).
Figure 9. Effect of ranolazine on eNOS phosphorylation, intracellular cAMP contents and 125I-insulin uptake in cultured BAECs.
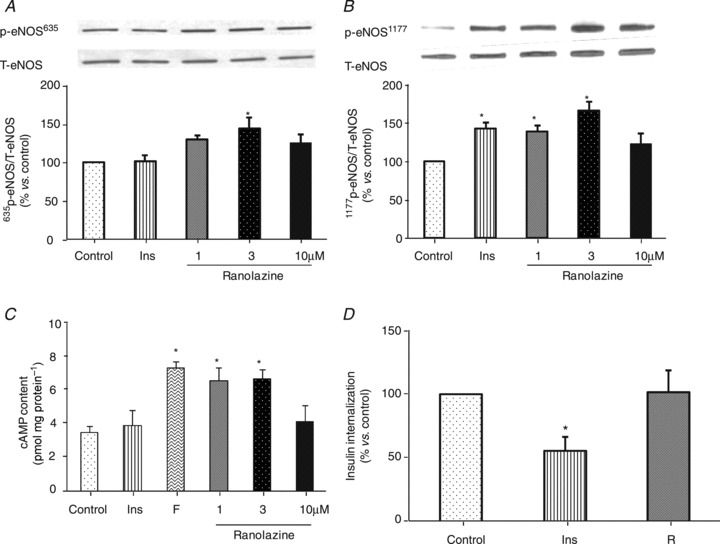
A, eNOS (Ser635) phosphorylation; B, eNOS (Ser1177) phosphorylation; C, intracellular cAMP contents. Insulin (Ins, 100 nm) and forskolin (F, 10 μm) were used as control. D, endothelial 125I-insulin uptake. Insulin (Ins, 2 μm) was used as positive control, R:3 μM ranolazine. n= 4–8. *P < 0.05 vs. control.
Discussion
Using a combination of in vivo animal study, ex vivo arterial myography and in vitro endothelial cell studies, we found in the current study that ranolazine at a therapeutically effective dose potently recruited muscle microvasculature and this was associated with increased muscle delivery and action of insulin. These findings are of clinical significance as ranolazine has been shown repeatedly in clinical trials to increase tissue (myocardium) blood flow and improve glycaemia (Timmis et al. 2006; Morrow et al. 2009; Venkataraman et al. 2009; Chisholm et al. 2010).
Insulin has been shown to potently relax the pre-capillary arterioles and increase muscle microvascular recruitment, leading to increased capillary exchange area for nutrient and hormones (Clark et al. 2003; Barrett et al. 2009, 2011). This action accounts for up to 40% of insulin-mediated whole body glucose disposal during insulin clamp (Vincent et al. 2003, 2004). Thus, increased muscle microvascular perfusion is a critical prerequisite for maximum insulin action in muscle. In humans with even moderate insulin resistance as seen in obesity or subjects receiving systemic lipid infusion, insulin fails to recruit muscle microvasculature (Clerk et al. 2006; Liu et al. 2009). In the current study, we found that ranolazine potently recruited muscle microvasculature, increased muscle insulin delivery and enhanced insulin-mediated glucose disposal during insulin clamp. These findings are entirely consistent with clinical trial findings that ranolazine improves glycaemia in patients with diabetes and provides new insight into the anti-diabetic effect of ranolazine.
Although high plasma concentrations (∼25 μm) of ranolazine decrease blood pressure in rats (Dhalla et al. 2009), therapeutically effective concentrations of ranolazine (2–8 μm) has no significant effect on blood pressure in both humans and rats (Chaitman et al. 2004a, b; Dhalla et al. 2009). Despite this, our results indicate that a low dose of ranolazine exerts a potent vasodilatatory action in both the in vivo (CEU study) and the ex vivo (isolated artery myograph) studies. Although we did not measure the plasma concentrations of ranolazine in the current study, previous animal studies have shown that a similar infusion rate leads to a steady-state plasma concentration of ∼8 μm (Dhalla et al. 2009). The decrease in MAP observed immediately after ranolazine priming, similar to previous reports (Dhalla et al. 2009), was probably due to the high plasma concentrations of ranolazine achieved after the bolus injection (∼25 μm; Dhalla et al. 2009). This initial decrease in MAP did not appear to have affected the overall action of ranolazine on muscle microvasculature. Our findings are also consistent with a prior report demonstrating in isolated intrarenal arteries that ranolazine as low as ∼1 μm has a vasodilatatory effect (Deng et al. 2012).
Previous results suggest that ranolazine exerts a vasodilatatory effect probably via a direct antagonism on the adrenergic receptors, not the L-type Ca2+ channel. Indeed, intracoronary or intrafemoral administration of ranolazine resulted in local vasodilatation and 70–90% increase in coronary or femoral blood flow and these effects were abolished with prazosin pretreatment (Nieminen et al. 2011). We have shown here that ranolazine significantly dilatated the isolated distal saphenous artery rings that were pre-constricted with phenylephrine but not with potassium. Raising extracellular potassium concentration induces vessel contraction by causing an elevation of the calcium level through the L-type Ca2+ channel in vascular smooth muscle cells (Karaki et al. 1997) but ranolazine only inhibits the L-type Ca2+ channel at very high concentrations (>30 μm; Deng et al. 2012). We did not test the effect of the classic α1 antagonists as these agents cause vasodilatation of the resistance arterioles, which lowers systemic blood pressure and may decrease capillary perfusion pressure. Treatment of obese hypertensive patients with prazosin for 12 weeks is associated with an increase in insulin-mediated glucose disposal during insulin clamp, suggesting an increased muscle insulin sensitivity (Pollare et al. 1988). Whether this is secondary to the increased microvascular recruitment remains to be examined.
As ranolazine is able to increase glucose-stimulated insulin secretion in rat and human islets in a glucose-dependent manner (Ning et al. 2011) and we and others have previously shown that insulin at physiological concentrations potently recruits muscle microvasculature (Clerk et al. 2002, 2004; Liu, 2007), it remains possible that the observed microvascular recruitment following ranolazine administration could be secondary to increased insulin secretion. However, this does not appear to be a likely mechanism as in the current study we found that plasma insulin levels remained unchanged before and after ranolazine infusion (Table 1). Together with our findings using the isolated distal saphenous artery, ranolazine probably exerts its vasodilatatory effect on the pre-capillary arterioles via a direct action on both the endothelium and the smooth muscle cells.
Although ranolazine induced a significant increase in muscle MBV and MBF over the entire 120 min of the study, the greatest increase in insulin-mediated glucose use occurred within 60 min after ranolazine infusion. This is not entirely surprising as we and others have previously shown that insulin per se facilitates its own delivery to muscle by increasing microvascular perfusion and trans-endothelial transport (Barrett et al. 2011) and it is likely that ranolazine and insulin combination increased muscle insulin delivery more efficiently than insulin alone in the first hour and the two groups had similar rates of insulin delivery in the second hour. Indeed, the ranolazine group had significantly higher MBV and MBF at 0 and 30 min than the insulin alone group while the two groups had similar degrees of microvascular blood flow afterwards (Fig. 4). The lack of changes in the phosphorylation of Akt and ERK1/2 in gastrocnemius and heart samples collected at the end of the insulin clamp is consistent with the finding of similar steady-state glucose infusion rates between the two groups.
In conclusion, ranolazin at therapeutic concentrations acutely recruits muscle microvasculature, thus leading to increased microvascular exchange surface area and muscle insulin delivery and action. This action may contribute to improved glycaemia in subjects treated with ranolazine. Whether ranolazine exerts a beneficial microvascular effect during chronic administration remains to be examined.
Translational perspective
Ranolazine, an anti-anginal drug, reduces angina frequency and improves exercise performance in patients with chronic angina. Patients on ranolazine also demonstrate better glycaemia. The current study shows that ranolazine potently recruits muscle microvasculature and increases muscle delivery and action of insulin in rats. These findings are of physiological importance as microvasculature controls the delivery of oxygen, nutrient and hormones into tissue and their exchanges between plasma and tissue interstitium by providing endothelial exchange surface area. Recent evidence confirms that microvasculature plays a crucial role in the regulation of oxygenation, insulin action and glucose metabolism in muscle. Increased muscle microvascular perfusion results in higher interstitial oxygen saturation. As for insulin, it has to be first delivered to muscle microcirculation and then transported through the endothelium to reach its receptors located on the muscle cell membrane. Muscle microvascular recruitment is a critical prerequisite for maximum insulin action in muscle and insulin-induced microvascular recruitment contributes up to 40% of insulin-mediated whole body glucose disposal during insulin clamp. This action is blunted or abolished in the presence of insulin resistance in both humans and laboratory animals. As ranolazine does not interact directly with insulin receptors and has the potential to increase tissue interstitial oxygenation and insulin delivery and action in the presence of vascular insulin resistance, its microvascular actions make it a particularly attractive agent for patients with diabetes and coronary artery disease. This also suggests that tissue microvasculature could be an important therapeutic target in this patient population and warrants further clinical studies.
Glossary
- A-V
arterial–venous
- BAECs
bovine aortic endothelial cells
- CEU
contrast-enhanced ultrasound
- eNOS
endothelial nitric oxide synthase
- FBF
femoral blood flow
- GIRs
glucose infusion rates
- GLP-1
glucagon-like peptide 1
- HbA1c
glycated haemoglobin
- HBB
Hepes-binding buffer
- HGO
hepatic glucose output
- MAP
mean arterial blood pressure
- MBF
microvascular blood flow
- MBV
microvascular blood volume
- MFV
microvascular flow velocity
- T2DM
type 2 diabetes mellitus
- PSS
physiological salt solution
Additional information
Competing interests
We declare no conflict of interest.
Author contributions
Z.F., L.Z., W. Chai. and Z.D. collected the data; W. Cao contributed to data analysis and discussion; Z.F. and Z.L. designed the study, analysed the data and wrote the manuscript.
Funding
This work was supported by American Diabetes Association grants 1-11-CR-30 and 9-09-NOVO-11 (to Z.L.), and National Institutes of Health Grant R01HL094722 (to Z.L.).
References
- Bagger JP, Botker HE, Thomassen A, Nielsen TT. Effects of ranolazine on ischemic threshold, coronary sinus blood flow, and myocardial metabolism in coronary artery disease. Cardiovasc Drugs Ther. 1997;11:479–484. doi: 10.1023/a:1007705707667. [DOI] [PubMed] [Google Scholar]
- Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52:752–764. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011;301:E252–263. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen M, Anggard EE, Carrier MJ. Oxidative stress impairs insulin internalization in endothelial cells in vitro. Diabetologia. 2001;44:605–613. doi: 10.1007/s001250051667. [DOI] [PubMed] [Google Scholar]
- Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, Liu Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–896. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Wang W, Dong Z, Cao W, Liu Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes. 2011;60:2939–2946. doi: 10.2337/db10-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, Liu Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension. 2010;55:523–530. doi: 10.1161/HYPERTENSIONAHA.109.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitman BR. Measuring antianginal drug efficacy using exercise testing for chronic angina: improved exercise performance with ranolazine, a pFOX inhibitor. Curr Probl Cardiol. 2002;27:527–555. doi: 10.1067/mcn.2002.129438. [DOI] [PubMed] [Google Scholar]
- Chaitman BR. Efficacy and safety of a metabolic modulator drug in chronic stable angina: review of evidence from clinical trials. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S47–64. doi: 10.1177/107424840400900105. [DOI] [PubMed] [Google Scholar]
- Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, Wang W, Skettino SL, Wolff AA. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004a;291:309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, Pepine CJ, Wang W, Nelson JJ, Hebert DA, Wolff AA. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004b;43:1375–1382. doi: 10.1016/j.jacc.2003.11.045. [DOI] [PubMed] [Google Scholar]
- Chisholm JW, Goldfine AB, Dhalla AK, Braunwald E, Morrow DA, Karwatowska-Prokopczuk E, Belardinelli L. Effect of ranolazine on A1C and glucose levels in hyperglycemic patients with non-ST elevation acute coronary syndrome. Diabetes Care. 2010;33:1163–1168. doi: 10.2337/dc09-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JD, Richey JM, Harrison LN, Zuniga E, Kolka CM, Kirkman E, Ellmerer M, Bergman RN. Direct administration of insulin into skeletal muscle reveals that the transport of insulin across the capillary endothelium limits the time course of insulin to activate glucose disposal. Diabetes. 2008;57:828–835. doi: 10.2337/db07-1444. [DOI] [PubMed] [Google Scholar]
- Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab. 2008;295:E732–750. doi: 10.1152/ajpendo.90477.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- Clarke B, Spedding M, Patmore L, McCormack JG. Protective effects of ranolazine in guinea-pig hearts during low-flow ischaemia and their association with increases in active pyruvate dehydrogenase. Br J Pharmacol. 1993;109:748–750. doi: 10.1111/j.1476-5381.1993.tb13637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerk LH, Rattigan S, Clark MG. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes. 2002;51:1138–1145. doi: 10.2337/diabetes.51.4.1138. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–1442. doi: 10.2337/db05-1373. [DOI] [PubMed] [Google Scholar]
- Clerk LH, Vincent MA, Lindner JR, Clark MG, Rattigan S, Barrett EJ. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev. 2004;20:3–12. doi: 10.1002/dmrr.414. [DOI] [PubMed] [Google Scholar]
- Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50:2682–2690. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Deng CY, Kuang SJ, Rao F, Yang H, Fang XH, Shan ZX, Li XH, Zhou ZL, Lin QX, Yang M, Wu SL, Yu XY, Lin SG. Effect of ranolazine on rat intrarenal arteries in vitro. Eur J Pharmacol. 2012;683:211–216. doi: 10.1016/j.ejphar.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Dernovsek KD, Bar RS. Processing of cell-bound insulin by capillary and macrovascular endothelial cells in culture. Am J Physiol Endocrinol Metab. 1985;248:E244–251. doi: 10.1152/ajpendo.1985.248.2.E244. [DOI] [PubMed] [Google Scholar]
- Dhalla AK, Wang WQ, Dow J, Shryock JC, Belardinelli L, Bhandari A, Kloner RA. Ranolazine, an antianginal agent, markedly reduces ventricular arrhythmias induced by ischemia and ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2009;297:H1923–1929. doi: 10.1152/ajpheart.00173.2009. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, Jia Z, Wang Y, Misra H, Liu D. Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151:3026–3037. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya HL, Halban PA, King GL. Intracellular pathways of insulin transport across vascular endothelial cells. Am J Physiol Cell Physiol. 1988;255:C459–464. doi: 10.1152/ajpcell.1988.255.4.C459. [DOI] [PubMed] [Google Scholar]
- Hachiya HL, Takayama S, White MF, King GL. Regulation of insulin receptor internalization in vascular endothelial cells by insulin and phorbol ester. J Biol Chem. 1987;262:6417–6424. [PubMed] [Google Scholar]
- Inyard AC, Chong DG, Klibanov AL, Barrett EJ. Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes. 2009;58:2457–2463. doi: 10.2337/db08-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes. 2007;56:2194–2200. doi: 10.2337/db07-0020. [DOI] [PubMed] [Google Scholar]
- Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- Keske MA, Clerk LH, Price WJ, Jahn LA, Barrett EJ. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care. 2009;32:1672–1677. doi: 10.2337/dc09-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Barrett EJ, Barrett MO, Cao W, Liu Z. Tumor necrosis factor-α induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology. 2007;148:3356–3363. doi: 10.1210/en.2006-1441. [DOI] [PubMed] [Google Scholar]
- Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology. 2005;146:4690–4696. doi: 10.1210/en.2005-0505. [DOI] [PubMed] [Google Scholar]
- Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab. 2007;293:E1250–1255. doi: 10.1152/ajpendo.00451.2007. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gardner LB, Barrett EJ. Insulin and glucose suppress hepatic glycogenolysis by distinct enzymatic mechanisms. Metabolism. 1993;42:1546–1551. doi: 10.1016/0026-0495(93)90149-i. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab. 2009;94:3543–3549. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makielski JC, Valdivia CR. Ranolazine and late cardiac sodium current–a therapeutic target for angina, arrhythmia and more. Br J Pharmacol. 2006;148:4–6. doi: 10.1038/sj.bjp.0706713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack JG, Stanley WC, Wolff AA. Ranolazine: a novel metabolic modulator for the treatment of angina. Gen Pharmacol. 1998;30:639–645. doi: 10.1016/s0306-3623(97)00301-7. [DOI] [PubMed] [Google Scholar]
- Morrow DA, Scirica BM, Chaitman BR, McGuire DK, Murphy SA, Karwatowska-Prokopczuk E, McCabe CH, Braunwald E. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119:2032–2039. doi: 10.1161/CIRCULATIONAHA.107.763912. [DOI] [PubMed] [Google Scholar]
- Nieminen T, Tavares CA, Pegler JR, Belardinelli L, Verrier RL. Ranolazine injection into coronary or femoral arteries exerts marked, transient regional vasodilation without systemic hypotension in an intact porcine model. Circ Cardiovasc Interv. 2011;4:481–487. doi: 10.1161/CIRCINTERVENTIONS.111.962852. [DOI] [PubMed] [Google Scholar]
- Ning Y, Zhen W, Fu Z, Jiang J, Liu D, Belardinelli L, Dhalla AK. Ranolazine increases β-cell survival and improves glucose homeostasis in low-dose streptozotocin-induced diabetes in mice. J Pharmacol Exp Ther. 2011;337:50–58. doi: 10.1124/jpet.110.176396. [DOI] [PubMed] [Google Scholar]
- Pollare T, Lithell H, Selinus I, Berne C. Application of prazosin is associated with an increase of insulin sensitivity in obese patients with hypertension. Diabetologia. 1988;31:415–420. doi: 10.1007/BF00271585. [DOI] [PubMed] [Google Scholar]
- Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997;46:1381–1388. doi: 10.2337/diab.46.9.1381. [DOI] [PubMed] [Google Scholar]
- Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48:566–575. doi: 10.1016/j.jacc.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2006;27:42–48. doi: 10.1093/eurheartj/ehi495. [DOI] [PubMed] [Google Scholar]
- Venkataraman R, Belardinelli L, Blackburn B, Heo J, Iskandrian AE. A study of the effects of ranolazine using automated quantitative analysis of serial myocardial perfusion images. JACC Cardiovasc Imaging. 2009;2:1301–1309. doi: 10.1016/j.jcmg.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–1423. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- Zacharowski K, Blackburn B, Thiemermann C. Ranolazine, a partial fatty acid oxidation inhibitor, reduces myocardial infarct size and cardiac troponin T release in the rat. Eur J Pharmacol. 2001;418:105–110. doi: 10.1016/s0014-2999(01)00920-7. [DOI] [PubMed] [Google Scholar]


