Abstract
Delirium is an acute change in awareness and attention and is common, morbid, and costly for patients and health care systems. While hyperactive delirium is easily identifiable, the hypoactive form is more common and carries a higher mortality. Hospital systems to address delirium should consist of 3 critical steps. First, hospitals must identify patients who develop or are at intermediate or high risk for delirium. Delirium risk may be assessed using known patient-based and illness-based risk factors, including preexisting cognitive impairment. Delirium diagnosis remains a clinical diagnosis that requires a clinical assessment that can be structured using diagnostic criteria. Hospital systems may be useful to efficiently allocate delirium resources to prevent and manage delirium. Second, it is crucial to develop a systematic approach to prevent delirium using multimodal nonpharmacologic delirium prevention methods and to monitor all high-risk patients for its occurrence. Tools such as the modified Richmond Agitation and Sedation Scale can aid in monitoring for changes in mental status that could indicate the development of delirium. Third, hospital systems can utilize established methods to assess and manage delirium in a standardized fashion. The key lies in addressing the underlying cause/causes of delirium, which often involve medical conditions or medications. With a sustained commitment, standardized efforts to identify and prevent delirium can mitigate the long-term morbidity associated with this acute change. In the face of changes in health care funding, delirium serves as an example of a syndrome where care coordination can improve short-term and long-term costs.
Keywords: delirium, quality improvement, hospital system, antipsychotic
Introduction
Delirium, an acute change in awareness and attention, is a common, morbid, and costly syndrome for patients and health systems. Delirium occurs in approximately 20% to 30% of general medical inpatients,1,2 10% to 48% of patients with stroke,3 70% to 83% of patients in the intensive care unit (ICU),4,5 and 80% of patients nearing end of life.6 The negative consequences of delirium are far-reaching, impacting patient and system-level hospital outcomes. Of interest to hospital systems is the risk of postoperative complications,7 elevated in-hospital mortality,8–10 and prolonged length of stay.8 Additionally, patients with delirium are at greater risk of readmission,11 institutionalization12 and long-term mortality.11–14 The high occurrence rates and wide-ranging negative outcomes of delirium in the inpatient setting highlight the need for hospital systems to take measures to identify, manage, and prevent the syndrome.
Delirium is characterized as an acute, fluctuating disturbance in awareness, attention, and sometimes perception; it is often causes by underlying medical conditions or over-/underdosing of medications. These disturbances develop acutely, usually over hours to days, and fluctuate over the course of a day, with periods of increased awareness and attention. Change in attention is the hallmark of cognitive change in delirium. The model of attention posed by Posner and Peterson includes “bottom-up” (awareness) and “top-down” (sustained attention) components.15 Bottom-up attention is a primal instinct where environmental stimuli trigger a cognitive response (ie, a nighttime flash of light results in increased concentration) and involves both cholinergic and adrenergic pathways.16 Alteration of the bottom-up attention, which relies on the reticular activating system of the brain stem, will cause environmental stimuli to be missed.17 The top-down attention involves more complex cognitive processes (ie, switching between 2 tasks).15 The top-down component of sustained attention is dependent on the integrity of the basal forebrain cholinergic system as well as the frontal and parietal regions of the right hemisphere.18 However, there are multiple contributing mechanisms to developing delirium; understanding the neuroanatomy can provide guidance to the psychomotor variants and guide strategies for assessment, prevention, and management.
The 2 primary variants of delirium include “hyperactive” or “agitated” delirium and “hypoactive” or “quiet” delirium. Although the hyperactive variant is less common, constituting only 25% of cases,19 it is often the most documented variant, because its disruptive nature is likely to interfere with the flow of care. The hypoactive variant may be underrecognized due to its quiescent, nondisruptive symptomatology. As such, the hypoactive subtype of delirium carries a higher mortality rate, likely due in part to its delayed recognition.20–22
Delirium is a clinical diagnosis requiring clinical assessment. However, diagnostic tools and algorithms may aid in its identification. Such algorithms employ the diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders (DSM). Among the algorithms, the most widely used is the Confusion Assessment Method (CAM).23 Based on the DSM III-R (Third Edition, Revised),24 the CAM is a reliable, sensitive, and specific algorithm for diagnosing delirium when compared to expert clinician examination.25 The CAM algorithm includes the following: acute onset and fluctuating course, inattention, and either disorganized thinking or alteration in consciousness.23 The CAM-ICU is a diagnostic tool for delirium that has been validated in both in the verbal and in the nonverbal ICU patients.26,27 The CAM-ICU adds objective assessments for attention, consciousness, and thought to the CAM.26 The advantages of the CAM-ICU are that it can be performed by trained nurses or physicians,28 can be repeated over time to detect fluctuation and changes, and has been associated with the ICU outcomes including mortality,29 length of stay,30 and cost.31
Developing Hospital-Wide Delirium Mitigation Systems
Diagnostic instruments aid in the clinical diagnosis of delirium, but hospital systems might employ alternative strategies to prevent, monitor for, and manage delirium to mitigate its negative consequences. A Commonwealth Group white paper identified 4 common elements to making sustained improvement: (a) focusing on a problem, (b) restructuring to facilitate improvement in the hospital, (c) making systematic measurements and improvements, and (d) developing policy and procedures to create sustained improvement in outcomes.32 For this multistep approach to be effective, strong commitment from hospital leadership is required and a grassroots investment in education and demonstration of the problem is needed. In this article, we will focus on 3 steps within systematic measurement and improvement that are particularly relevant to delirium. These are practical steps for hospitals to utilize, but effort devoted to implementation, education, and assessment is still required.
The use of hospital-wide systems to aid in the identification, prevention, and management of delirium may mitigate both patient morbidity and health system costs. This review describes a systematic approach to minimize, monitor for, and address delirium. Hospital-based technology systems have an important role in an overall delirium mitigation and management system. However, the medical literature has not seen the published results of a comprehensive electronic delirium management system. As such, health systems may consider looking toward automated methods for educating staff, assisting clinical decision making (initial diagnostic workup and medication ordering), and measuring delirium and its associated morbidity and surrogate markers (restraint use, acute antipsychotic use, etc).
Figure 1 describes a conceptual diagram of a hospital system approach to delirium. These steps are proposed as a sequential model for implementation, but due to overlap, there may need to be parallel implementation. Delirium prevention strategies work best on those patients at intermediate and at high risk for delirium Step 1 of a hospital-wide system is identifying these patients. Step 2 is developing a hands-on process for delirium prevention, followed by routine monitoring for delirium. Step 3 involves developing a standardized delirium management protocol.
Figure 1.
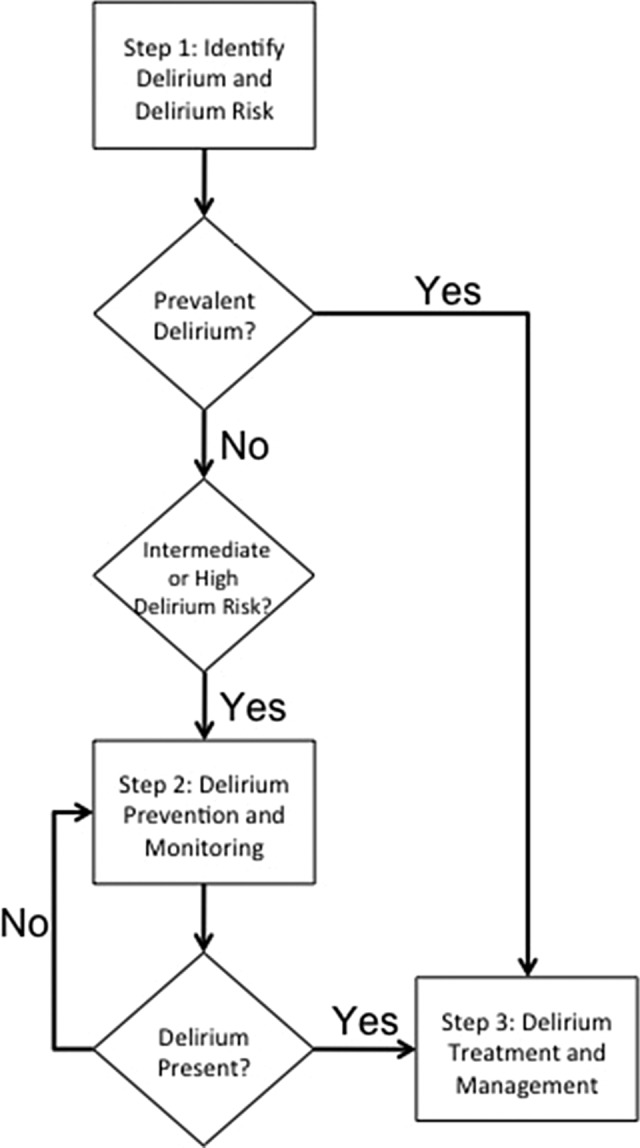
Developing a hospital system for delirium: key decision points and protocols.
Step 1: Identify Delirium and Delirium Risk
In older patients, delirium is highly prevalent (9%-15%) upon presentation to the emergency department33,34 and admission inpatient wards (14%-24%).2 Delirium that is present upon admission (prevalent delirium) requires prompt management (see section entitled step 3: Assessment of Delirium and Management of Associated Agitation). Patients without delirium should be identified for the risk of developing delirium during the hospitalization. Rules for the identification of delirium risk are validated for medical,35 surgical,9,36 and ICU patients.37 A recent meta-analysis by the National Center for Clinical Guidelines identified independent delirium risk factors as shown in Table 1.38 The key risk factors can be broken into patient-specific (age and vision impairment) and illness-based (fracture, infection, and severity of illness). Identification of delirium risk is important to target delirium prevention efforts toward those at intermediate and high risk. This targeting is designed to save both staff time and cost. Using an electronic medical record system, many of these delirium risk factors could be identified to develop an electronic delirium identification system.19
Table 1.
Independent Delirium Risk Factors Upon Admission: NICE Confidence.a
| Admission Characteristic | Odds Ratio (95% Confidence Interval) |
|---|---|
| Fracture | 6.6 (2.2-19.3) |
| Cognitive impairment | 6.3 (2.9-13.7) |
| Age >80 | 5.2 (2.6-10.4) |
| Severe illness | 3.5 (1.5-8.2) |
| Age >65 | 3.0 (1.2-7.7) |
| Infection | 3.0 (1.4-6.1) |
| Vision impairment | 1.7 (1.0-2.8) |
Abbreviations: NICE, National Institute for Health and Clinical Excellence. 38(p244)
aAdapted from Delirium: Diagnosis, Prevention and Management. Clinical Guideline 103.
Early identification of delirium is crucial to limit the morbidity and mortality associated with its occurrence. Inattention is at the core of delirium, and most tools used to screen for or diagnose delirium, including the CAM, are centered on this finding. As such, attention must be carefully assessed when considering a diagnosis of delirium. It is best assessed when formal testing, such as digit span, days of the week or months of the year backward, or serial 7s, is combined with interviewer observations instead of relying on either alone.39 Orientation questions alone generally are insensitive for diagnosing delirium and should not be used as the standard for detecting inattention.40
Preexisting cognitive impairment is one of the most common risk factors for developing delirium. A history of cognitive impairment is clear in patients who have dementia; however, many patients with dementia have never been diagnosed. Thus, not only is brief cognitive screening important upon admission to identify cognitively impaired patients who are at risk of developing delirium, but it is also important to perform in patients who become delirious while hospitalized after the delirium has fully resolved to look for underlying cognitive impairment. We recommend employing the Mini-Cog, a brief cognitive screen that is short and tests attention, memory, and executive function.41,42 Many other dementia screening tests exist; however, the Mini-Cog has the advantage of lack of education or language bias in addition to its brevity and high sensitivity, making it possible to incorporate into health care providers’ usual work flow. The Mini-Cog takes approximately 3 to 4 minutes to administer; and because of its simplicity, it can be performed even by nonphysicians.42 If Mini-Cog screening fails in a patient with nondelirium, further evaluation should be undertaken to determine whether the patient has underlying cognitive impairment, which may include more detailed cognitive testing, caregiver report regarding progression over time, imaging, and laboratory testing.
Step 2 Delirium Prevention and Monitoring Programs
NonPharmacological Prevention
Multimodal approaches to delirium prevention have been described in the literature.43–47 Marcantonio and colleagues evaluated the impact of proactive geriatric consultation on delirium occurrence in elderly individuals admitted for emergency surgical repair of hip fracture.48 The intervention focused on 10 components: central nervous system oxygen supply, fluid and electrolyte balance, pain management, reduction of unnecessary medications, adequate nutrition, regular bowel and bladder function, early mobilization and rehabilitation, prevention and management of postoperative complications, environmental stimuli, and appropriate delirium management. Not only was relative risk (RR) for delirium reduced (RR 0.6, 95% confidence interval [CI] 0.37-0.98), but RR of severe delirium was reduced as well (RR 0.40, 95% CI 0.18-0.89). The Hospital Elder Life Program (HELP) is an innovative program designed and tested by Inouye and colleagues to improve the care of hospitalized elders.49 The intervention consists of protocols to manage 6 delirium risk factors: cognitive impairment, sleep deprivation, immobility, visual impairment, hearing impairment, and dehydration. The protocol was found to reduce the odds of developing delirium (9.9% vs 15%; matched odds ratio 0.60, 95% CI 0.39-0.92), the total number of days with delirium (105 vs 161, P = .01), and the total number of delirious episodes (62 vs 90, P = .03).49 The HELP materials, including manuals, tools, and resources for institutions as well as caregivers, are available free of charge. Of importance to health systems and payers, the use of these materials was found to be cost effective in 73% of instances.50
Hospital-based systems or protocols should be developed for patients at risk for delirium. The systems should address specific delirium risk factors.49 For example, patients who are cognitively impaired are at risk for delirium and should be included in hospital systems that provide cognitive stimulation, including frequent orientation (clock, calendar, orientation board in room, and frequent orientation reminders from patient care staff), regular communication of the day’s events and plans, and avoiding medications that affect cognition. To avoid sleep deprivation in at risk patients, systems should be in place, including establishing a relaxing nighttime environment (warm drink, pleasant music, massage, and nighttime noise reduction such as silent pill crushers and vibrating beepers and alarms) and timing vital signs and procedures in a way that maximizes restfulness. Another important hospital-based procedure is to maximize sensory input, including providing glasses, magnifying devices, large-print books and telephone keypads, hearing aids, amplifiers, and provision of ear wax disimpaction. Patients should be systematically mobilized, aiding them in ambulation or active range-of-motion exercises at least 3 times daily, and restraints of all kinds (wrist restraints, bladder catheters, and intravenous catheters) should be minimized. Systems should be in place to ensure adequate pain control, bowel frequency, hydration, and nutrition.
Pharmacological Prevention
The pathophysiological causes of delirium are diverse, involving multiple pathways with acetylcholine, dopamine, glutamate, serotonin, and adrenergic pathways.51 The diversity of pathophysiological mechanisms results in significant confounding when performing studies with very tight control of entry criteria. Additionally, overlapping receptor pharmacology makes medication selection difficult. To date, the best evidence exists for high-risk procedures such as hip fractures, with both antipsychotics and acetylcholinesterase inhibitors undergoing testing.
The use of prophylactic antipsychotic medications in surgical and intensive care unit patients at high risk for delirium has not been extensively studied.46–48,52–54 Among surgical patients, data regarding the use of prophylactic antipsychotic medications and delirium occurrence is conflicting. In 1 study, utilization of prophylactic haloperidol has been shown to significantly reduce the duration (5.4 ± 4.91 vs 11.85 ± 7.56, mean difference 6.4 days, 95% CI = 4.0-8.0; P < .001), severity (mean difference at a maximum DRS-R-98 score of 4.0, 95% CI 2.0, 5.8, P < .001), and total hospital stay (17.7 ± 11.1 vs 22.6 ± 16.7, mean difference 5.5 days, 95% CI 1.4-2.3, P < .001) but not occurrence of delirium (RR 0.91, 95% CI 0.59, 1.42).55 In 2 additional studies of haloperidol, a reduced incidence of delirium ranging from 7.9% (P = .031) to 22% (significance not given) has been found.52,53 The use of prophylactic atypical antipsychotics has also been explored. Prakanrattana and colleagues evaluated the use of risperidone 1 mg in cardiac surgery patients administered immediately upon waking. The incidence of postoperative delirium was reduced in the risperidone group (11.1% vs 31.7% respectively, P = .009, RR = 0.35, 95% CI = 0.16-0.77).54 Larsen and colleagues conducted a randomized, double-blind, placebo-controlled trial of olanzapine 5 mg prior to and immediately following joint replacement surgery in elderly inpatients.56 Rates of delirium occurrence were lower for olanzapine-treated patients versus placebo (14.3% vs 40.2%, 95% CI 17.6-34.2, P < .0001). However, when delirium did occur in the study group, it lasted longer and was more severe.56 Another randomized double-blind placebo-controlled trial of haloperidol compared to ziprasidone and placebo for patients mechanically ventilated in the ICU showed that antipsychotic treatment did not improve the number of days alive without delirium or coma or increase adverse outcomes.57 Due to the paucity of evaluations, further studies are needed before delirium prophylaxis with antipsychotics becomes standard practice.
Procholinergic agents have also been investigated for prophylactic delirium risk reduction, but the results have been negative to equivocal. A multicenter randomized-controlled trial (RCT) of rivastigmine was stopped early because of increased risk of death (22% vs 8%, P = .07) and increased duration of delirium (5 vs 3 days, P = .06).58 In early trials of citicoline, the procholinergic agent failed to reduce delirium rates (11.76% vs 17.39%, P = .6). Dautzenberg and colleagues (2004) found a significant reduction in delirium (45.5%, n = 5 vs 88.9%, n = 8; P < .05) with chronic use of the cholinesterase inhibitor, rivastigmine, in elderly, hospital inpatients with dementia.59 However, an RCT of rivastigmine for delirium prevention in cardiac surgery found no effect of treatment on delirium incidence or cognitive performance.60 Donepezil has also been studied for prophylactic reduction of delirium occurrence in surgical inpatients but failed to reduce delirium occurrence (RR 1.2, 95% CI 0.48-3.00).61 In patients with hip fracture, donepezil had no impact on the rate of delirium, but all patients receiving donepezil developed adverse effects.62 In the absence of dementia requiring cholinergic therapy at baseline, the use of acetylcholinesterase inhibitors for delirium prophylaxis should be avoided.
As sleep dysregulation has been implicated as a delirium risk factor, the use of melatonin to regulate sleep cycles has been investigated for its impact on delirium prevention. In one study of hospitalized elders, the use of 0.5 mg of melatonin at night was associated with a reduction in delirium (12% vs 31%; P = .014; OR 0.19; 95% CI 0.06-0.62).63 The study failed to find an association of melatonin prophylaxis with symptom severity or length of stay (mean delirium severity score 11.4 ± 3.0, placebo 10.5 ± 5.3, P = .77; length of stay 18.5 ± 26.4 days vs 14.5 ± 21.6 days; P = .36). A second study of elderly patients undergoing hip replacement receiving prophylactic melatonin 5 mg, midazolam 7.5 mg, clonidine 0.1 mg, or placebo found a reduction in delirium occurrence in the 3 days following surgery with the use of melatonin (9.43% vs 33% for placebo P = .003; 44%, P = .245 for midazolam; and 37%, P = .629 for clonidine).64 A more definitive study of melatonin for delirium prophylaxis is nearing completion.65
Monitoring for Delirium
Defining mechanisms to identify the key events is critical for feedback and improvement within hospital systems. The development of delirium is a key event that could trigger a feedback cycle in such a system. For delirium, patient-care staff should be educated on delirium recognition so that measures to manage it can be instituted as quickly as possible after its onset. A standardized strategy for monitoring patients for acute changes in attention, awareness, or activities should be employed on a regular (at least daily) basis. The use of a mental status vital sign to identify changes rapidly has been recommended.66,67 The key to this recommendation is to have a rapid assessment of mental status in which changes can be easily detected, as they are with vital signs such as heart rate or blood pressure. Chester and colleagues found that longitudinal monitoring of consciousness using a modified version of the Richmond Agitation and Sedation Scale (m-RASS) can aid in the identification of delirium (see Table 2). Any score other than 0 on the m-RASS is abnormal. The m-RASS has a sensitivity of 64% and a specificity of 93% for delirium.1 However, its greatest value lies in its ability to monitor patients over time and identify changes; serial m-RASS measurements used to detect changes have a sensitivity of 85% and a specificity of 92% for incident delirium.
Table 2.
The Modified Richmond Agitation and Sedation Scale (mRASS).a
| Wake patient and gather attention | |
| State patient’s name, ask patient to open eyes and look at the speaker | |
| Ask open-ended question and observe response | |
| “Describe how you are feeling today” | |
| If the answer is short (<10 seconds), cue with another open-ended question | |
| If no response to verbal cue, provide physical stimulation by shaking shoulder | |
| Score mRASS scale | |
| −5 | Unarousable |
| −4 | Can’t stay awake |
| −3 | Difficult to wake |
| −2 | Wakes slowly |
| −1 | Wakes easily |
| 0 | Alert and calm |
| +1 | Restless |
| +2 | Slightly agitated |
| +3 | Very agitated |
| +4 | Combative |
a Adapted from Chester, Beth Harrington, and Rudolph .1
Step 3: Assessment of Delirium and Management of Associated Agitation
Assessment of Causes of Delirium
The presence of delirium should be considered a medical emergency, and all patients with delirium should be assessed promptly. The cornerstone of delirium management is to identify and treat the underlying causes for the delirium. As a result of its multifactorial nature, the use of a checklist should be included in the initial assessment and management of a patient with delirium to ensure completeness (see Figure 2). This checklist should include the following: interim history, physical examination, assessment for urine/stool retention or uncontrolled pain, targeted laboratory and other testing, and medication history, including medications recently administered or withdrawn. The clinician should begin with a broad differential diagnosis and systematically eliminate or treat potential causes. Table 3 offers an approach that utilizes the 6 Ms: medication use/underuse, metabolic, microorganisms, myocardial, mind, micturition. Delirium is often caused by multiple factors; thus, the search for contributors to delirium should not be terminated when a single cause has been identified, unless a differential checklist has been completed.
Figure 2.
Checklist for hospital acquired delirium assessment.
Table 3.
Differential Diagnosis of Delirium.
| Cause | Explanation |
|---|---|
| Medications | Use Anticholinergic medications Steroids Benzodiazepines Inhaled anesthetics Narcotics Underuse: Withdrawal from benzodiazepines, pain, and dementia medications Undertreated pain Alcohol withdrawal Lack of bowel regimen/constipation |
| Metabolic | Electrolyte abnormalities Glucose Sodium Calcium Uremia |
| Microorganisms | Infection Urinary tract infection Aspiration pneumonia Pressure ulcer Venous catheter infection |
| Myocardial | Myocardial infarction Pulmonary embolism Congestive heart failure Hypoxia/hypercarbia |
| Mind | Acute stroke Intracranial hemorrhage Brain mass/metastases Other psychiatric diagnosis |
| Micturition | Urinary retention Urinary catheters |
Management of Agitation Associated With Delirium
Medications administered for delirium do not treat the underlying cause of delirium. As noted in the review by Flaherty and colleagues, methodological limitations to studies evaluating antipsychotic efficacy in delirium have not supported the recommendation for their regular use.68 Medications may therefore only be useful in the management of hyperactive delirium symptoms. Tahir and colleagues’ study of quetiapine found that it had the potential to quickly reduce delirium symptom severity compared to placebo.69 Antipsychotics are considered to be the pharmacologic standard for the management of these symptoms.38 The affluence of evidence, high potency, low risk of sedation, lack of active metabolites, and variety of formulations make haloperidol the preferred agent.70 Antipsychotics, such as haloperidol, have been linked to possible motor and cardiovascular adverse effects such as extrapyramidal symptoms and QTc prolongation. As a result of the associated adverse effects and the increasing regulatory oversight of antipsychotic use,71 hospital systems should consider creation of pathways for appropriate pharmacological management of agitated delirium with appropriately dosed antipsychotics. For example, in older patients with hyperactive delirium, initial haloperidol dosing should begin at 0.25 to 0.5 mg and should increase slowly. However, younger patients may tolerate higher initial haloperidol doses and more rapid dosing escalation. Electronic systems such as prescribing alerts for potentially deliriogenic medications, algorithms to monitor delirium development, and best-practice tools for pharmacologic and nonpharmacologic management can be an important component of hospital delirium monitoring and management.
Although typical antipsychotics have been studied most extensively for the management of delirium, their risk for adverse effects has placed greater emphasis on the investigation and the clinical use of atypical antipsychotics.70 Although comparisons have revealed increased motor and cardiovascular effects with haloperidol use, methodological flaws in these studies make comparisons to atypical antipsychotics difficult to interpret.68 In general, small studies have found these atypical antipsychotics to be as effective as haloperidol in the management of delirium.72,73 Each of the atypical antipsychotics differs in their affinity for specific receptors, in their profile of action. As a result, these differing profiles may be used advantageously to guide symptom-directed therapy. However, though at times these medications are utilized to manage symptoms of hyperactive delirium, currently the data do not support the routine use of these drugs.
It should be noted that both classes of antipsychotics carry a black box warning label from the Food and Drug Administration (FDA) for their use in elderly patients with dementia and are not approved by FDA for the management of delirium. The April 2005 request from the FDA to apply the labeling to the atypical antipsychotics stems from a meta-analysis of 17 double-blind, placebo-controlled trials that found a 1.6- to 1.7-fold increase in mortality within this population. The analysis found that for every 100 elderly patients with dementia treated over 10 to 12 weeks, there may be 1 death due to the atypical medication use.74 As a result of several retrospective studies alluding to similar events with typical antipsychotics, the FDA expanded the warning in June 2008 to include the class.75,76 In these studies of both typical and atypical agents, dosage ranges and treatment durations were quite variable and often extended beyond that which is routinely recommended for management of delirious symptoms. The Centers for Medicare and Medicaid Services have increased regulatory oversight of antipsychotic medications in long-term care settings, a common destination of patients who develop delirium.77 As approximately 60% of patients with delirium may have an underlying dementia, limiting the long-term use and dosage of these agents should be recommended.
The role of benzodiazepines in the management of delirious symptoms may be in those patients requiring significant sedation, undergoing alcohol or benzodiazepine withdrawal, or with contraindication to antipsychotics. When used at doses lower than those used to produce significant sedation, paradoxical impairment in cognition and disinhibition may occur,78 thereby worsening delirious symptoms. While such reactions may more likely occur with high doses and long-acting agents, they may also occur despite previous stable doses of benzodiazepines.79,80 As such, the use of benzodiazepines should be considered a second-line modality, limited to those patients described above.
Conclusion
Delirium remains a clinical diagnosis. As delirium needs to be promptly evaluated and managed, hospital systems that promote the systematic review of potential contributors to delirium should be incorporated into the normal work flow. For instance, a high-risk alert should trigger nursing and physician interventions in every patient care instance, such as medication review, review of lines/tubes/restraints, or assessment of pain/constipation/urinary retention. Implementation of system-based screening measures that analyze patient risk factors and utilize frequent delirium assessment measures allow for early identification of those patients at high risk of delirium as well as those with early symptoms. These methods not only facilitate the targeting of high-risk patients for delirium prevention strategies but may also provide for the efficient use of intervention and monitoring parameters in the identification, severity reduction, and appropriate management of cases where delirium does occur.
Footnotes
Authors’ Note: The opinions represented here represent those of the authors and do not represent the opinions or policy of the Veterans Health Administration.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was unfunded. Drs Kostas and Rudolph are Department of Veterans Affairs (VA) employees. Dr Rudolph is funded by a VA Rehabilitation Research Career Development Award and a VA Patient Safety Center of Inquiry. Drs Kostas and Rudolph are funded by a VA Geriatrics and Extended Care T21 Alternative to Institutional Long Term Care grant. Dr Kostas is funded by a John A. Hartford Center of Excellence Award.
References
- 1. Chester JG, Beth Harrington M, Rudolph JL. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. J Hosp Med. 2012;7(5):450–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157–1165 [DOI] [PubMed] [Google Scholar]
- 3. Shi Q, Presutti R, Selchen D, Saposnik G. Delirium in acute stroke: a systematic review and meta-analysis. Stroke. 2012;43(3):645–649 [DOI] [PubMed] [Google Scholar]
- 4. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591–598 [DOI] [PubMed] [Google Scholar]
- 5. Pisani MA, Redlich CA, McNicoll L, Ely EW, Friedkin RJ, Inouye SK. Short-term outcomes in older intensive care unit patients with dementia. Crit Care Med. 2005;33(6):1371–1376 [DOI] [PubMed] [Google Scholar]
- 6. Zimmerman K, Rudolph J, Salow M, Skarf LM. Delirium in palliative care patients: focus on pharmacotherapy. Am J Hosp Palliat Care. 2011;28(7):501–510 [DOI] [PubMed] [Google Scholar]
- 7. Martin BJ, Buth KJ, Arora RC, Baskett RJ. Delirium as a predictor of sepsis in post-coronary artery bypass grafting patients: a retrospective cohort study. Crit Care. 2010;14(5): R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807–813 [DOI] [PubMed] [Google Scholar]
- 9. Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134–139 [PubMed] [Google Scholar]
- 10. Norkiene I, Ringaitiene D, Misiuriene I, et al. Incidence and precipitating factors of delirium after coronary artery bypass grafting. Scand Cardiovasc J. 2007;41(3):180–185 [DOI] [PubMed] [Google Scholar]
- 11. Koster S, Hensens AG, van der Palen J. The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg. 2009;87(5):1469–1474 [DOI] [PubMed] [Google Scholar]
- 12. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618–624 [DOI] [PubMed] [Google Scholar]
- 13. Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173–178 [DOI] [PubMed] [Google Scholar]
- 14. Gottesman RF, Grega MA, Bailey MM, et al. Delirium after coronary artery bypass graft surgery and late mortality. Ann Neurol. 2010;67(3):338–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42 [DOI] [PubMed] [Google Scholar]
- 16. Vincent SR. The ascending reticular activating system—from aminergic neurons to nitric oxide. J Chem Neuroanat. 2000;18(1-2):23–30 [DOI] [PubMed] [Google Scholar]
- 17. Johnson A, Proctor R. Attention: Theory and Practice. Thousand Oaks, CA: Sage Publications; 2004 [Google Scholar]
- 18. Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35(2):146–160 [DOI] [PubMed] [Google Scholar]
- 19. Rudolph JL, Harrington MB, Lucatorto MA, Chester JG, Francis J, Shay KJ. Validation of a medical record-based delirium risk assessment. J Am Geriatr Soc. 2011;59(suppl 2):S289–S294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levkoff SE, Evans DA, Liptzin B, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized-patients. Arch Intern Med. 1992;152(2):334–340 [DOI] [PubMed] [Google Scholar]
- 21. Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci. 2007;62(2):174–179 [DOI] [PubMed] [Google Scholar]
- 22. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467–2473 [DOI] [PubMed] [Google Scholar]
- 23. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948 [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders - III revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 25. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710 [DOI] [PubMed] [Google Scholar]
- 27. McNicoll L, Pisani MA, Ely EW, Gifford D, Inouye SK. Detection of delirium in the intensive care unit: comparison of confusion assessment method for the intensive care unit with confusion assessment method ratings. J Am Geriatr Soc. 2005;53(3):495–500 [DOI] [PubMed] [Google Scholar]
- 28. Truman B, Ely EW. Monitoring delirium in critically ill patients. Using the confusion assessment method for the intensive care unit. Crit Care Nurse. 2003;23(2):25–36 [PubMed] [Google Scholar]
- 29. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762 [DOI] [PubMed] [Google Scholar]
- 30. Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962 [DOI] [PubMed] [Google Scholar]
- 32. Hospital quality improvement: Strategies and lessons from U.S. hospitals The Commonwealth Fund, 2007. http://www.commonwealthfund.org/∼/media/Files/Publications/Fund%20Report/2007/Apr/Hospital%20Quality%20Improvement%20%20Strategies%20and%20Lessons%20From%20U%20S%20%20Hospitals/Silow%20Carroll_hosp_quality_improve_strategies_lessons_1009%20-pdf.pdf Accessed February 17, 2013
- 33. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248–253 [DOI] [PubMed] [Google Scholar]
- 35. Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. 1993;119(6):474–481 [DOI] [PubMed] [Google Scholar]
- 36. Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27(8):1297–304 [DOI] [PubMed] [Google Scholar]
- 38. National Institute for Health and Clinical Excellence Delirium: diagnosis, Prevention and Management, Clinical Guideline 103. Regents Park, London: National Clinical Guideline Centre at The Royal College of Physicians; 2010. [Google Scholar]
- 39. O'Keeffe ST, Gosney MA. Assessing attentiveness in older hospital patients: global assessment versus tests of attention. J Am Geriatr Soc. 1997;45(4):470–473 [DOI] [PubMed] [Google Scholar]
- 40. Stavros K, Rudolph JL, Jones RN, Marcantonio ER. Clinical assessment of attention in older adults: inattention to attention. J Am Geriatr Soc. 2008;56(suppl 2):S241–S24319016966 [Google Scholar]
- 41. Scanlan J, Borson S. The Mini-Cog: receiver operating characteristics with expert and naive raters. Int J Geriatr Psychiatry. 2001;16(2):216–222 [DOI] [PubMed] [Google Scholar]
- 42. Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive ‘vital signs' measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021–1027 [DOI] [PubMed] [Google Scholar]
- 43. Lundstrom M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622–628 [DOI] [PubMed] [Google Scholar]
- 44. Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52(1):79–90 [DOI] [PubMed] [Google Scholar]
- 45. Cole MG, McCusker J, Bellavance F, et al. Systematic detection and multidisciplinary care of delirium in older medical inpatients: a randomized trial. CMAJ. 2002;167(7):753–759 [PMC free article] [PubMed] [Google Scholar]
- 46. Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61(2):176–181 [DOI] [PubMed] [Google Scholar]
- 47. Naughton BJ, Saltzman S, Ramadan F, Chadha N, Priore R, Mylotte JM. A multifactorial intervention to reduce prevalence of delirium and shorten hospital length of stay. J Am Geriatr Soc. 2005;53(1):18–23 [DOI] [PubMed] [Google Scholar]
- 48. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516–522 [DOI] [PubMed] [Google Scholar]
- 49. Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676 [DOI] [PubMed] [Google Scholar]
- 50. Rizzo JA, Bogardus ST, Jr, Leo-Summers L, Williams CS, Acampora D, Inouye SK. Multicomponent targeted intervention to prevent delirium in hospitalized older patients: what is the economic value? Med Care. 2001;39(7):740–752 [DOI] [PubMed] [Google Scholar]
- 51. Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24(4):789–856 [DOI] [PubMed] [Google Scholar]
- 52. Kaneko T, Cai J, Ishikura T, Kobayashi M, Takuji N, Kaibara N. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica. 1999;42:179–184 [Google Scholar]
- 53. Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med. 2012;40(3):731–739 [DOI] [PubMed] [Google Scholar]
- 54. Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35(5):714–719 [DOI] [PubMed] [Google Scholar]
- 55. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658–1666 [DOI] [PubMed] [Google Scholar]
- 56. Larsen KA, Kelly SE, Stern TA, et al. Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: a randomized, controlled trial. Psychosomatics. 2010;51(5):409–418 [DOI] [PubMed] [Google Scholar]
- 57. Girard TD, Pandharipande PP, Carson SS, et al. Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38(2):428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Eijk MM, Roes KC, Honing ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376(9755):1829–1837 [DOI] [PubMed] [Google Scholar]
- 59. Dautzenberg PL, Mulder LJ, Olde Rikkert MG, Wouters CJ, Loonen AJ. Delirium in elderly hospitalised patients: protective effects of chronic rivastigmine usage. Int J Geriatr Psychiatry. 2004;19(7):641–644 [DOI] [PubMed] [Google Scholar]
- 60. Gamberini M, Bolliger D, Lurati Buse GA, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery—a randomized controlled trial. Crit Care Med. 2009;37(5):1762–1768 [DOI] [PubMed] [Google Scholar]
- 61. Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post-surgical delirium. Am J Geriatr Psychiatry. 2005;13(12):1100–1106 [DOI] [PubMed] [Google Scholar]
- 62. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59(suppl 2):S282–S288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Al-Aama T, Brymer C, Gutmanis I, Woolmore-Goodwin SM, Esbaugh J, Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trial. Int J Geriatr Psychiatry. 2011;26(7):687–694 [DOI] [PubMed] [Google Scholar]
- 64. Sultan SS. Assessment of role of perioperative melatonin in prevention and treatment of postoperative delirium after hip arthroplasty under spinal anesthesia in the elderly. Saudi J Anaesth. 2010;4(3):169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Jonghe A, van Munster BC, van Oosten HE, et al. The effects of melatonin versus placebo on delirium in hip fracture patients: study protocol of a randomised, placebo-controlled, double blind trial. BMC Geriatr. 2011;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Flaherty JH, Rudolph J, Shay K, et al. Delirium is a serious and under-recognized problem: why assessment of mental status should be the sixth vital sign. J Am Med Dir Assoc. 2007;8(5):273–275 [DOI] [PubMed] [Google Scholar]
- 67. Flaherty JH, Shay K, Weir C, et al. The development of a mental status vital sign for use across the spectrum of care. J Am Med Dir Assoc. 2009;10(6):379–380 [DOI] [PubMed] [Google Scholar]
- 68. Flaherty JH, Gonzales JP, Dong B. Antipsychotics in the treatment of delirium in older hospitalized adults: a systematic review. J Am Geriatr Soc. 2011;59(suppl 2):S269–S276 [DOI] [PubMed] [Google Scholar]
- 69. Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485–490 [DOI] [PubMed] [Google Scholar]
- 70. Lonergan E, Britton AM, Luxenberg J, Wyller T. Antipsychotics for delirium. Cochrane Database Syst Rev. 2007;(2):CD005594. [DOI] [PubMed] [Google Scholar]
- 71. Mitka M. CMS seeks to reduce antipsychotic use in nursing home residents with dementia. JAMA. 2012;308(2):119, 121 [DOI] [PubMed] [Google Scholar]
- 72. Han CS, Kim YK. A double-blind trial of risperidone and haloperidol for the treatment of delirium. Psychosomatics. 2004;45(4):297–301 [DOI] [PubMed] [Google Scholar]
- 73. Grover S, Kumar V, Chakrabarti S. Comparative efficacy study of haloperidol, olanzapine and risperidone in delirium. J Psychosom Res. 2011;71(4):277–281 [DOI] [PubMed] [Google Scholar]
- 74. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943 [DOI] [PubMed] [Google Scholar]
- 75. Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang PS. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775–786 [DOI] [PubMed] [Google Scholar]
- 77. McAvay GJ, Van Ness PH, Bogardus ST, Jr, et al. Older adults discharged from the hospital with delirium: 1-year outcomes. J Am Geriatr Soc. 2006;54(8):1245–1250 [DOI] [PubMed] [Google Scholar]
- 78. Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153(2):231–237 [DOI] [PubMed] [Google Scholar]
- 79. Khan LC, Lustik SJ. Treatment of a paradoxical reaction to midazolam with haloperidol. Anesth Analg. 1997;85(1):213–215 [DOI] [PubMed] [Google Scholar]
- 80. Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–1522 [PubMed] [Google Scholar]



