Abstract
Allyl isothiocyanate (AITC) occurs in cruciferous vegetables that are commonly consumed by humans and has been shown to inhibit urinary bladder cancer growth and progression in previous preclinical studies. However, AITC does not significantly modulate cyclooxygenase-2 (Cox-2), whose oncogenic activity has been well documented in bladder cancer and other cancers. Celecoxib is a selective Cox-2 inhibitor and has been widely used for treatment of several diseases. Celecoxib has also been evaluated in bladder cancer patients, but its efficacy against bladder cancer as a single agent remains unclear. In a syngeneic rat model of orthotopic bladder cancer, treatment of the animals with the combination of AITC and celecoxib at low dose levels (AITC at 1mg/kg and celecoxib at 10mg/kg) led to increased or perhaps synergistic inhibition of bladder cancer growth and muscle invasion, compared with each agent used alone. The combination regime was also more effective than each single agent in inhibiting microvessel formation and stimulating microvessel maturation in the tumor tissues. The anticancer efficacy of the combination regime was associated with depletion of prostaglandin E2, a key downstream signaling molecule of Cox-2, caspase activation and downregulation of vascular endothelial growth factor in the tumor tissues. These data show that AITC and celecoxib complement each other for inhibition of bladder cancer and provide a novel combination approach for potential use for prevention or treatment of human bladder cancer.
Introduction
Allyl isothiocyanate (AITC) is a phytochemical known to possess antimicrobial and anticancer activities (1). It occurs in many cruciferous vegetables, which are commonly consumed by humans, such as Brussels sprouts, cabbage, cauliflower and kale, and is particularly abundant in mustard, horseradish and wasabi. In fact, AITC is mainly responsible for the pungent tastes of these vegetables. In plants, AITC is synthesized and stored as a glucosinolate (sinigrin) and is generated upon sinigrin hydrolysis by coexisting myrosinase, which, under normal circumstance, is physically segregated from its substrate (1). Myrosinase activity is also present in the intestinal microflora in animals and humans, and sinigrin that escapes the action of plant myrosinase may be converted to AITC in vivo (2), although our recent study indicates that <10% of orally dosed sinigrin is converted to AITC in F344 rats (3). In vivo, AITC is rapidly metabolized through the mercapturic acid pathway, and the metabolites, mainly the N-acetylcysteine conjugate, are disposed almost exclusively in urine (1). Many lines of evidence suggest that AITC is a highly promising agent for bladder cancer prevention and treatment as discussed below. First, the biological activities of AITC metabolites, including the N-acetylcysteine conjugate, which are believed to serve as carriers of AITC, resemble that of the parent compound (4,5). Second, not only is the bioavailability of AITC extremely high—more than 90% of an oral dose is absorbed—but also nearly 80% of orally dosed AITC is disposed in urine within 24 h (6–8), rendering the bladder perhaps the most exposed organ to AITC. Indeed, we have shown that concentrations of AITC equivalent are two to four orders of magnitude higher in the urine than in the blood in rats dosed orally with AITC (9,10). Thus, orally administered AITC is selectively delivered to bladder via urinary excretion. Third, we have shown that orally administered AITC, AITC-rich mustard seed powder or the N-acetylcysteine conjugate of AITC each inhibited bladder cancer growth and muscle invasion in an orthotopic rat bladder cancer model (3,9,10). Finally, given that bladder cancer originates predominately from the epithelial cells in the inner surface, intravesical drug delivery is a desirable approach in order to avoid or reduce systemic side effects of the drug. Indeed, all agents that are currently used to prevent recurrence of non-muscle invasive bladder cancer, e.g. Bacillus Calmette–Guerin (a live attenuated form of Mycobacterium bovis), are delivered intravesically via a urethral catheter. Importantly, intravesical delivery of AITC can be achieved by oral administration, thereby eliminating the need for urethral catheterization.
Our recent work has also revealed insight into the mechanism by which AITC targets cancer cells. We have shown that AITC promotes ubiquitination and degradation of α- and β-tubulin by binding to their cysteine residues and arrests cells in mitosis, which in turn leads to cell death by mitotic catastrophe through the mitochondrion-mediated apoptotic pathway via Bcl-2 phosphorylation (11). AITC is a new class of mitotic blocker, as its mechanism of action differs from that of taxol and vinblastine; the latter compounds work by modulating tubulin polymer stability. However, as shown in the present study, AITC does not modulate cyclooxygenase-2 (Cox-2). Cox-2 catalyzes the rate-limiting reactions in prostaglandin biosynthesis and is a well-known oncogene in a wide variety of cancers, including bladder cancer (12,13). Cox-2 expression was also found to be significantly associated with bladder cancer recurrence and progression (14,15). In animal models of bladder cancer, tumor growth could be significantly inhibited by Cox-2 inhibitors (16–20). Therefore, we hypothesized that combination of AITC with a Cox-2 inhibitor might lead to better outcome for inhibition of bladder cancer than AITC alone. Celecoxib, a well-known selective Cox-2 inhibitor, was chosen to test our hypothesis. Celecoxib has been evaluated in bladder cancer patients in clinical trials, but its efficacy as a single agent for bladder cancer prevention or treatment remains uncertain (21–23). Our present study shows that the combination of AITC with celecoxib at low dose levels causes strong inhibition of bladder cancer and is more effective than each agent used alone.
Materials and methods
Chemicals
AITC, thiazolyl blue tetrazolium bromide and soy oil were purchased from Sigma. AITC was redistilled before use. Celecoxib was purchased from LKT Laboratories.
Cell culture and cell proliferation assay
Rat bladder cancer cell line AY-27, which was originally established from a bladder cancer in F344 rat (24), was provided by Dr R.B.Moore at the University of Alberta in Canada. AY-27 cells were cultured as described previously (9). The effects of AITC and/or celecoxib on cell proliferation were measured using a previously published protocol (9). Briefly, AY-27 cells were grown in 96-well plates in the presence or absence of the test compound for 72h, followed by measurement of cell density by the thiazolyl blue tetrazolium bromide-based MTT assay.
Animals and a syngeneic orthotopic model of bladder cancer
The anticancer activities of celecoxib and/or AITC were evaluated in a rat orthotopic model of bladder cancer, as recently described (9). Briefly, female F344 rats (Harlan) at 9–10 weeks of age were anesthetized with ketamine and xylazine; next, their urinary bladders were treated via a catheter with 0.4ml of 0.1 N HCl for 15 s, neutralized immediately with 0.4ml of 0.1 N KOH for 15 s and washed afterwards with 0.9% saline. Each bladder was then instilled via a catheter with 1 × 106 AY-27 cells in 0.5ml serum-free medium, which was kept in the bladder for 1h. The rats were then randomly assigned to different experimental groups, and all treatments were initiated 1 day after cancer cell inoculation and continued for 21 days. AITC was freshly dissolved in soy oil, whereas celecoxib was first dissolved in a small amount of dimethyl sulfoxide and then diluted in soy oil (the final concentration of dimethyl sulfoxide was 7.5% by volume). Each agent was given orally in 0.5ml volume per 150g body weight. In rats that were treated with both AITC and celecoxib, the two agents were administered 5h apart. The corresponding control rats were also given the same amount of vehicle. One day after the last treatment, the animals were euthanized, and the bladders were removed and weighed. Half of each bladder was fixed in neutral buffered 10% formalin (EMD Chemical) or in IHC Zinc Fixative (BD Pharmingen), whereas the other half of each bladder was flash frozen and stored at −80°C. The animal protocol was approved by the Roswell Park Cancer Institute Animal Care and Use Committee.
Measurement of prostaglandin E2, caspase-3, -7, -10 and vascular endothelial growth factor
For measurement of prostaglandin E2 (PGE2), tumor tissues were homogenized in 0.1M phosphate buffer (pH 7.4), containing 1mM ethylenediaminetetraacetic acid and 10 µM indomethacin at 5mg tissue/ml. The homogenates were cleared by centrifugation, and the supernatants after further dilution were analyzed by the PGE2 Enzyme Immunoassay kit (Cayman Chemical) per manufacturer’s instruction. Culture media were diluted before analysis of PGE2 concentration.
For measurement of caspase activity and vascular endothelial growth factor (VEGF) level, tumor tissues were homogenized in CasPASE lysis buffer containing 1mM ethylenediaminetetraacetic acid as supplied in the CasPASE Apoptosis Assay kit (G-Biosciences) at 5mg tissue per 0.1ml buffer. The homogenates were cleared by centrifugation, and the supernatants were measured for caspase-3, -7 and -10 activity using Ac-DEVD-AFC as a substrate per manufacturer’s instruction. The supernatants were also used for determination of VEGF level using the VEGF Rat ELISA kit per manufacturer’s instruction (Abcam).
Western blot analysis
Cox-2 expression levels in cell lysates and tissue homogenates were measured by western blotting as described previously (10). Glyceraldehyde-3-phosphate dehydrogenase was used as a control. Antibodies against Cox-2 (sc-1745) and glyceraldehyde-3-phosphate dehydrogenase (MAB374) were purchased from Santa Cruz Biotechnology and Millipore, respectively. Densitometry analysis of protein bands on western blot was performed using the spot density analysis software provided with the Bio-Rad Molecular Imager Gel Doc XR system.
Histology, tumor microvessel density and vascular maturation index
For detection of tumor muscle invasion, formalin-fixed tissues were paraffin embedded, cut at ~4 µm and stained with hematoxylin and eosin. Three sections of ~10 µm apart from the midsection of each tumor were used to evaluate tumor muscle invasion. A Nikon 50i light microscope was used for the above analysis and for the immunohistochemical analyses described below.
Microvessel density was determined by staining CD31 in endothelial cells. Zinc-fixed specimens were paraffin embedded, cut at 4 µm, placed on charged slides, dried at 60°C for 1h before being deparaffinized in three changes of xylene and rehydrated using graded alcohols. Endogenous peroxidase was quenched with aqueous 3% hydrogen peroxide for 10min, followed by wash with phosphate-buffered saline with Tween 20 (PBS/T). The slides were then loaded on a DAKO autostainer where serum-free protein block (X0909; Dako) was applied for 5min, blown off, and the primary antibody (anti-CD31, #550300, 1:50 dilution; BD Pharmingen) was applied for 60min, followed by a biotinylated secondary goat-anti-mouse antibody (1:50 dilution; BD Pharmingen) for 30min. Streptavidin–horseradish peroxidase conjugate (43–4323; Invitrogen) was applied for 30min, followed by treatment with 3,3'-diaminobenzidine as a chromogen (Dako) for 5min. An isotype-matched IgG negative control was used on a duplicate slide at the same concentration of the primary antibody. Finally, the slides were counterstained with hematoxylin, dehydrated, cleared and cover slipped. Microvessel distribution counts were determined by counting all CD31-positive endothelial cell clusters in multiple high-power fields (×200) covering the non-necrotic areas of the whole tumor sections.
Immunohistochemical double staining was performed using CD31 and α-smooth muscle actin (SMA) for detection of endothelial cells and pericytes, respectively. Briefly, ~5 µm cryosections were fixed in cold zinc fixative (−4°C) for 20min, followed by endogenous peroxidase quenching with 3% hydrogen peroxide for 10min. After wash with PBS/T, serum-free protein block was applied for 5min, followed by 1h incubation with a rabbit polyclonal SMA antibody (abs5694, 1/200; Abcam) and a biotinylated goat anti-rabbit secondary antibody (1/250; Vector Labs) for 30min. Streptavidin–horseradish peroxidase conjugate (43–4323; Invitrogen) was applied for 30min, followed by treatment with 3,3'-diaminobenzidine as a chromogen (Dako) for 5min. After rinsing with PBS/T, the slides were treated with serum-free protein block for 5min and then incubated with the above-mentioned CD31 antibody for 60min, followed by incubation with the above-mentioned goat anti-mouse secondary antibody for 30min and then streptavidin–alkaline phosphatase (#434322; Invitrogen) for 20min. After rinsing with PBS/T, the slides were stained with the Fast Red chromogen (#TA-060-AL; Thermo Scientific) for 10min and then counterstained with hematoxylin. Endothelial cells (CD31) were stained pink, and pericytes (SMA) were stained brown. Isotype-matched negative controls were used on duplicate slides at the same concentration of the primary antibodies. For quantifying vascular maturation index (VMI), snapshots of the ×200 high-power fields were taken under identical setting using a Cannon EOS Digital Rebel XSi/EOS 450 D camera, followed by quantification of the CD31 pixels and the overlaying SMA pixels using the magic wand tool of Adobe Photoshop CS3 (Version 10). VMI was calculated based on the total pixels of CD31+ SMA+ areas and the pixels of CD31 in these areas in the double stained (CD31/SMA) tissue sections. CD31/SMA-based determination of VMI in tumor tissues has been widely used.
Statistical analysis
For continuous measurements, Student t-test and analysis of variance were used for two-group comparison and multigroup comparison, respectively. Multiple comparisons were adjusted using the Tukey–Kramer test. For binary measurement, Fisher exact test was used to compare two proportions. All tests were two sided and performed at a nominal significance level of 0.05, i.e. P value of 0.05 or lower was considered statistically significant.
Results
The effects of AITC and/or celecoxib on Cox-2, PGE2 and cell proliferation in cultured bladder cancer cells
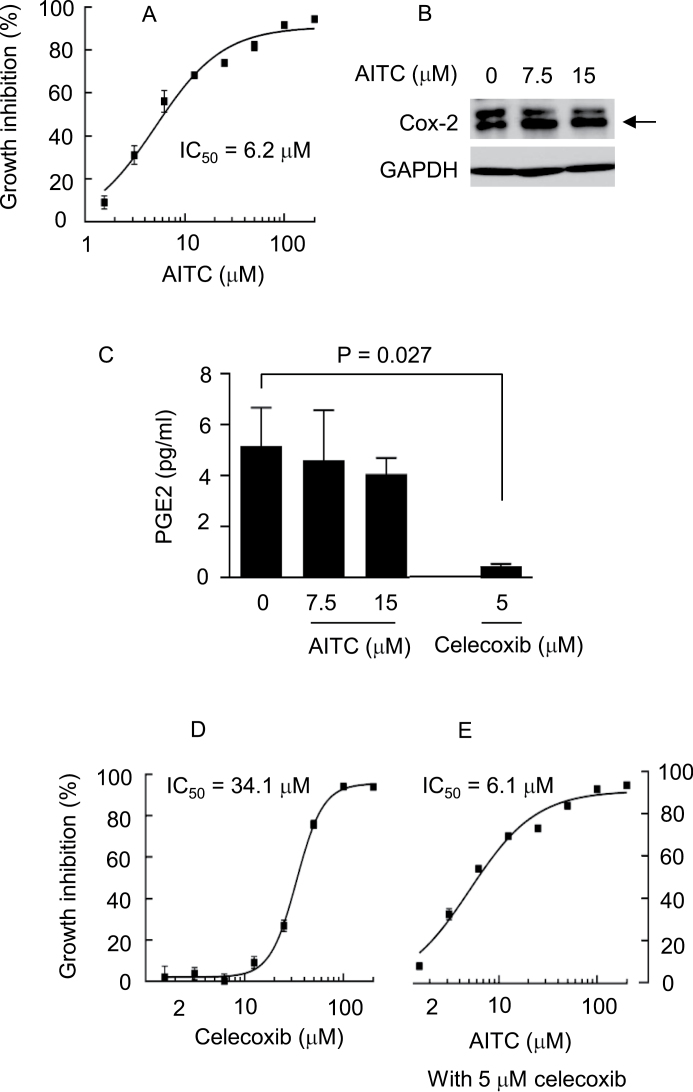
As mentioned before, in bladder cancer cells, we have shown previously that AITC modulates multiple cellular targets and causes mitotic arrest and mitotic catastrophe. However, at the concentrations where AITC was shown to exert the above-described effects and inhibited cancer cell growth (Figure 1A), it did not significantly mod-ulate Cox-2 expression (Figure 1B), nor did it significantly impact the production of PGE2 (Figure 1C), a key cancer-related prostaglandin generated in the Cox-1/Cox-2 pathway of arachidonic acid metabolism. In contrast, as expected, celecoxib, a Cox-2-selective inhibitor, was highly effective in depleting PGE2, as its level in the medium was reduced by 91% after treatment of AY-27 cells with celecoxib at 5 µM for 24h (Figure 1C). Interestingly, inhibition of Cox-2 by celecoxib and the ensuing decrease in PGE2 level did not lead to inhibition of cell proliferation, as no growth inhibition of AY-27 cells was detected after treatment with celecoxib at 5 µM for 72h, although, at much higher concentrations, celecoxib inhibited cell proliferation (IC50 of 34.1 µM; Figure 1D), which was apparently mediated by a Cox- 2-independent mechanism. A previous study also found that celecoxib at the concentrations of <25 µM did not inhibit the growth of multiple human bladder cancer cell lines in culture (20). Consistent with these results, growth inhibition of AY-27 cells by AITC was not affected by the addition of 5 µM celecoxib, as the IC50 of AITC in such a combination (6.1 µM; Figure 1E) was nearly identical to that of AITC as a single agent (6.2 µM; Figure 1A). Clearly, in cultured cells, Cox-2 inhibition by celecoxib does not enhance the growth inhibitory activity of AITC. Prostaglandins are excreted out of cells and function through G-protein-coupled membrane eicosanoid receptors. It is possible that excessive dilution of cell-excreted prostaglandins by the culture medium might have rendered it undetectable of any Cox-2-mediated effect of celecoxib on cell growth.
Fig. 1.
The effect of AITC and/or celecoxib on AY-27 cell proliferation, Cox-2 expression and PGE2 content. (A) Cells were grown in 96-well plates and treated with AITC for 72h before measurement of cell density by the MTT assay. Each value is mean ± SD (n = 8). IC50 was calculated from non-linear regression curve fit. (B and C) Cells were grown in six-well plates and treated with AITC or celecoxib for 24h, followed by measurement of Cox-2 level (indicated by the arrow) in cell lysates by western blotting, using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control (the results are representative of at least three samples), and medium level of PGE2 by an Enzyme Immunoassay kit (each value is mean + SD, n = 3). (D and E) Cells were grown in 96-well plates and treated with celecoxib or AITC plus celecoxib for 72h, followed by measurement of cell density by the MTT assay. IC50 was calculated from non-linear regression curve fit. Each value is mean ± SD (n = 8).
Combination of AITC and celecoxib versus a single agent for inhibition of bladder cancer in vivo
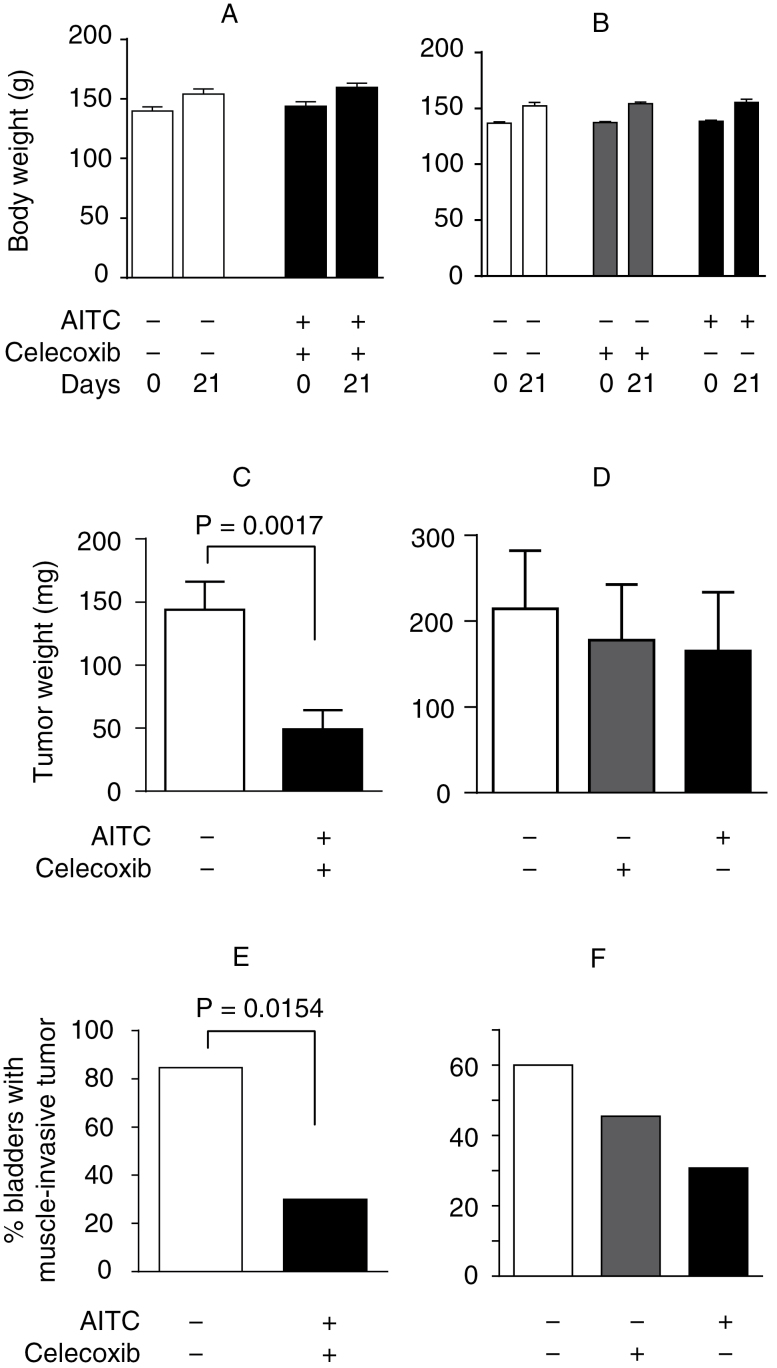
We have shown previously that daily oral administration of AITC at 1mg/kg body weight inhibited bladder cancer growth by 30% in rats inoculated orthotopically with AY-27 cells (9), but Cox-2 expression in the cancer tissues remained unchanged (data not shown). Despite the lack of a joint effect of the two agents in cultured bladder cancer cells as described above, we thought that combination of AITC and celecoxib might lead to a different outcome in vivo, as extracellular dilution of cell-excreted prostaglandins is probably limited. A previous study showed that bladder cancer incidence induced by a chemical carcinogen was significantly reduced in rats and mice fed with celecoxib in the diet at daily dose of ~30–40mg/kg body weight (18). A lower but clinically relevant dose of celecoxib (10mg/kg) was used in the present study. Thus, female F344 rats were inoculated with AY-27 cells intravesically via a urethral catheter (1 × 106 cells per rat); daily treatment with vehicle, AITC (1mg/kg) and/or celecoxib (10mg/kg) was initiated the next day and continued for 3 weeks. Both agents were administered to the animals via oral intubation, and in the combination group, the two agents were administered 5h apart in order to avoid potential direct interaction between them. The animals were killed 24h after the last dose, and the bladders were promptly collected. Two control groups were used: one group of rats were given the vehicle twice daily, to serve as the control for the two agents evaluated in combination, as the two agents were given to each animal 5h apart each day, whereas the second control group of rats were given the vehicle only once daily to serve as the control for each agent evaluated as single agents. Such a plan also allowed an adequate number of rats to be inoculated with a single preparation of AY-27 cell suspension in a relatively short period of time to ensure cell viability. The appearance and behavior of the animals were normal during the experiments, and their body weight gains over the experimental period were similar between the controls and the treatment groups (Figure 2A and B). Average bladder tumor weight was 63% lower in the rats treated with the combination of AITC and celecoxib than in the control rats (Figure 2C), and the number of rats bearing muscle-invasive tumor decreased 69% in the combination regimen group compared with the control group (Figure 2E). Bladder tumors in the second control group, with which AITC and celecoxib were evaluated as a single agent, appear to grow somewhat faster than that in the first control group (Figure 2D), but the difference was not statistically significant. Notably, twice as much soy oil was given to each rat in the first control group. Average tumor weight in the celecoxib-alone group and AITC-alone group was 17 and 23% lower, respectively, than in their corresponding control group, but the relatively large intragroup variation in tumor weight rendered each inhibition statistically insignificant (Figure 2D). Clearly, the combination regimen is far more efficacious in inhibiting tumor growth than each agent used alone. Both celecoxib and AITC also decreased the number of rats with muscle-invasive tumor by 24 and 49%, respectively (Figure 2F), but these decreases were also smaller than that in the combination group as described above.
Fig. 2.
Inhibitory effect of AITC and/or celecoxib on bladder cancer development and progression. Female F344 rats were inoculated intravesically with AY-27 cells to initiate orthotopic cancer growth. Oral administration of AITC (1mg/kg) and/or celecoxib (10mg/kg) once daily was started 1 day after cell inoculation and continued for 21 days. Two control groups were used: one group of rats were given the vehicle twice daily, to serve as the control for the two agents evaluated in combination, and the other control group of rats were given the vehicle once daily to serve as the control for the agents evaluated as single agents.(A and B) The initial and final body weights. Each value is mean + standard error of the mean (SEM) (11–16 rats per group). (C and D) Bladder tumor weight, which was calculated by subtracting the average normal bladder weight (48.4mg) from the tumor-bearing bladder weight. Each value is mean + SEM (11–16 rats per group). (E and F) Percentage of bladder with tumor invasion into the muscle tissue.
Molecular changes in bladder cancer tissues after treatment with AITC and/or celecoxib
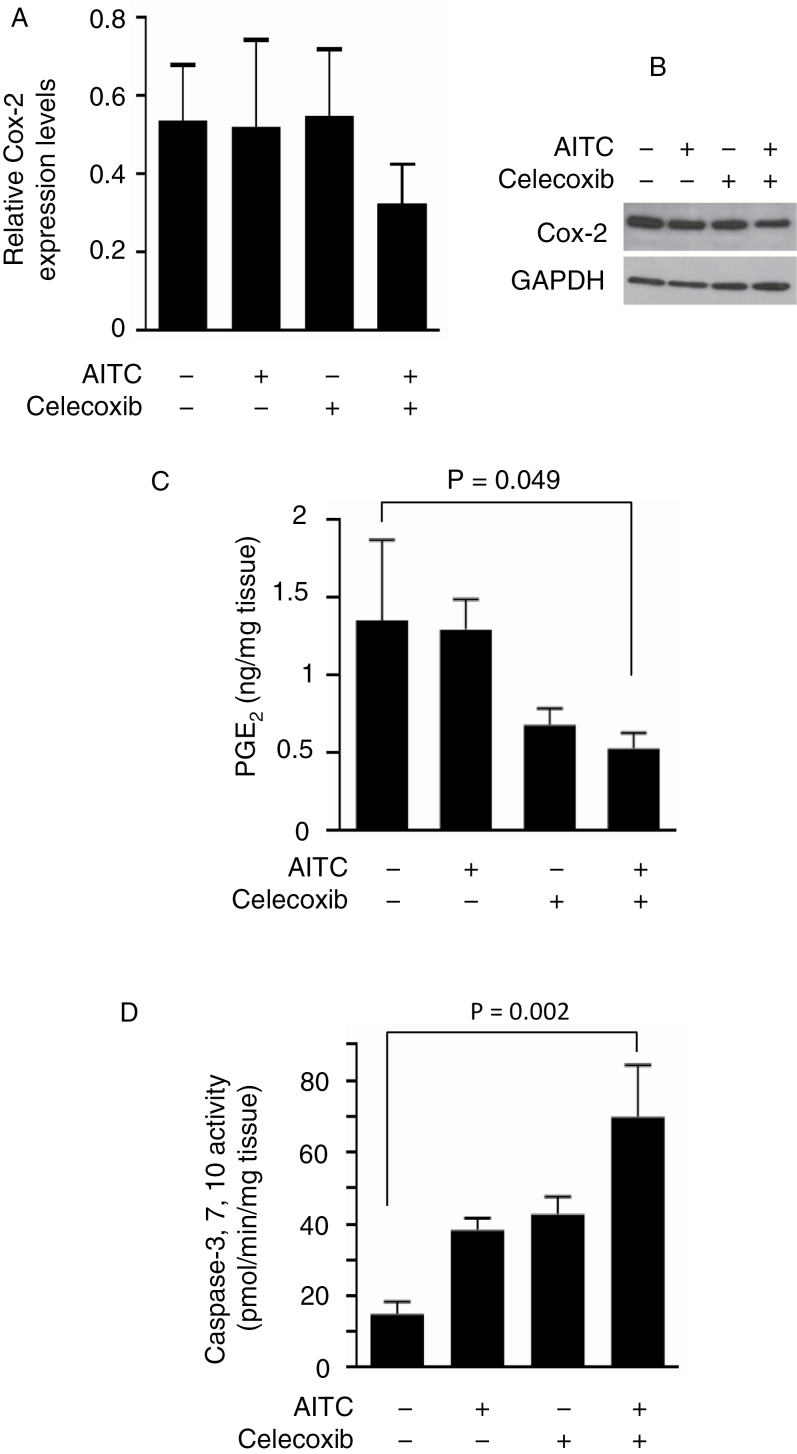
Preliminary experiments showed that there was not a significant difference between the two control groups mentioned above with regard to tumor tissue levels of the molecular parameters evaluated in the present study. Also, tumor growth rates between the two control groups were not statistically different. Thus, a single control group (rats treated with the vehicle twice daily) is presented for all molecular measurements, as well as for measurement of tissue microvessel density and VMI described later. As expected, in rats treated with AITC at 1mg/kg or celecoxib at 10mg/kg once daily for 3 weeks, Cox-2 expression in the bladder tumor tissues was not significantly affected (Figure 3A), as measured by western blotting. A representative western blot result is shown in Figure 3B. Cox-2 protein levels in the tumor tissues of rats treated with the combination of AITC and celecoxib were 39% lower than in the control rats (Figure 3A), albeit not statistically significant. PGE2 levels in the tumor tissues were measured by an ELISA. Although AITC appears to be totally ineffective on PGE2, treatments with celecoxib and celecoxib in combination with AITC were associated with 50 and 61% decreases in PGE2 level in the tumor tissues, respectively, the latter of which was statistically significant (Figure 3C). These results are consistent with the known ability of celecoxib to inhibit the catalytic activity of Cox-2 and also suggest that AITC may enhance the PGE2-lowering effect of celecoxib. Our results are consistent with a previous study, showing that a dose of 10–50mg/kg/day of celecoxib inhibited tissue PGE2 production (25).
Fig. 3.
Modulation of Cox-2, PGE2, caspase-3, -7 and -10 by AITC and/or celecoxib in tumor tissues in vivo. Bladder tumors were removed from rats, which were treated with AITC (1mg/kg) and/or celecoxib (10mg/kg) once daily for 21 days, from which tissue homogenates were prepared for analysis. (A and B) Western blot analysis of Cox-2 expression, using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control (four to five tumor specimens per group). Values shown in panel A were calculated based on densitometry scanning of the Cox-2 bands normalized by the GAPDH bands. A representative western blot result is shown in panel B. (C) PGE2 measurement by an Enzyme Immunoassay kit (4–11 tumor specimens per group). (D) Measurement of caspase-3, -7 and -10 activity by enzyme-linked immunosorbent assay (ELISA 9–12 tumor specimens per group). Each value is mean + standard error of the mean.
Caspase activity in the bladder tumor tissues was also measured, using the CasPASE Apoptosis Assay kit, which detects the combined activities of caspase-3, -7 and -10. AITC was shown previously to cause apoptosis of bladder cancer cells via mitochondria-mediated activation of caspase-3 (11) but is not known to modulate caspase-7 and -10. Thus, the changes detected by this assay kit may represent mainly, if not entirely, the effect of AITC on caspase-3. Three weeks of treatment with AITC (1mg/kg daily) or celecoxib (10mg/kg daily) increased caspase activity in the tumor tissue by 2.6- and 2.9-fold, respectively, although neither increase was statistically significant (Figure 3D). Combining AITC with celecoxib (same dose levels and treatment duration as in the single agent groups) increased the caspase activity 4.7-fold (Figure 3D), which is statistically significant. The results suggest that the combination regimen may be more effective in stimulating apoptosis in the tumor tissues than each agent alone.
Change in tumor vasculature after treatment with AITC and/or celecoxib
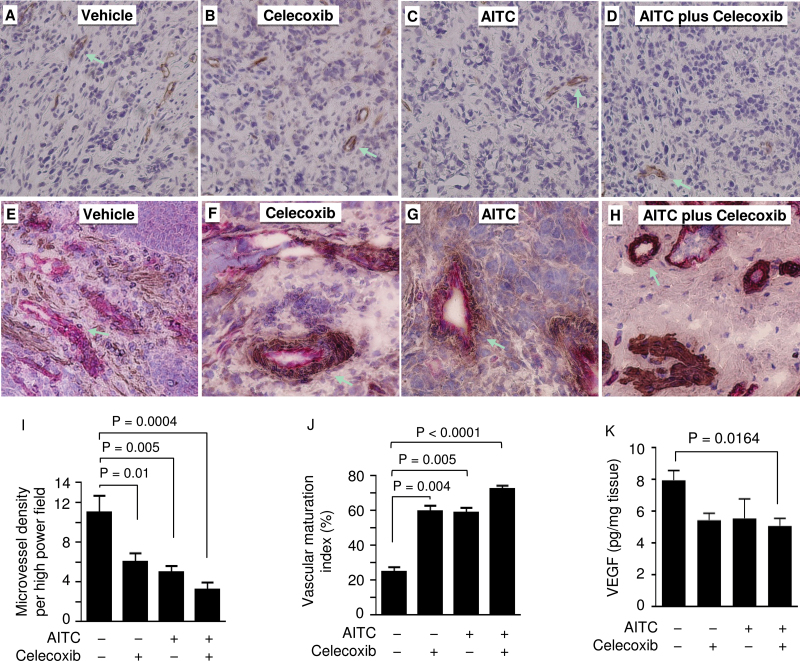
Our previous studies suggest that AITC may inhibit angiogenesis in bladder tumor tissues in vivo (3,10). On the other hand, Cox-2 was shown previously to stimulate angiogenesis (26). We examined the effect of AITC and/or celecoxib on microvessel density in the tumor tissues. Tumor microvessels were immunostained via endothelial cell marker CD31 (see Figure 4A–D for representative images). After 3 weeks of treatment with celecoxib at 10mg/kg or AITC at 1mg/kg, tumor tissue microvessel density decreased 46 or 55%, respectively, whereas the combination treatment of the two agents led to 71% decrease in tumor microvessel density (Figure 4I). Thus, AITC, in combination with celecoxib, was particularly effective in inhibiting tumor angiogenesis. Moreover, treatment with AITC and/or celecoxib also significantly increased tumor tissue vascular maturation (see Figure 4E–H for representative images of the double staining of endothelial cells and pericytes), and VMI increased 2.4-fold after treatment with each agent alone and 2.9-fold after the combination treatment (Figure 4J).
Fig. 4.
Effects of AITC and/or celecoxib on tumor tissue microvessel density, VMI and VEGF in vivo. Bladder tumors were removed from rats after treatment with AITC (1mg/kg) and/or celecoxib (10mg/kg) once daily for 21 days. (A–D) Representative images of CD31 staining (brown) in the tumor tissues (arrows point to selected microvessels; shown at ×100 magnification). (E–H) Representative images of double staining of CD31 (pink) and SMA (brown) in the tumor tissues (arrows point to selected microvessels; shown at ×200 magnification). (I) Tumor specimens (five to six specimens per group) were analyzed for microvessel density, examining up to 48 high-power fields (×200 magnification) per sample. Each value is mean + standard error of the mean (SEM). (J) Tumor specimens (three specimens per group) were analyzed for VMI, examining up to 16 high-power fields (×200 magnification). Each value is mean + SEM. (K) Tumor tissue homogenates were prepared and measured for VEGF by ELISA. Each value is mean + SEM (6–14 tumor specimens per group).
VEGF is well known to play an important role in tumor angiogenesis. Our previous studies suggest that AITC may downregulate VEGF in bladder tumor tissues (3,10). Celecoxib has also been shown to inhibit VEGF expression in bladder tumors (27) and other tumors (28). When VEGF level in the bladder tumor tissues was measured by ELISA in the present study, each agent alone or in combination was found to lower VEGF level by 30–36%, with the reduction in the combination group reaching statistical significance (Figure 4K).
Discussion
Both AITC and celecoxib as single agents showed antitumor activities in the syngeneic rat orthotopic model of bladder cancer. Similar AITC efficacy against bladder cancer was shown previously (9). In the present study, AITC and celecoxib were evaluated at 1 and 10mg/kg, respectively. Whether dose escalation of celecoxib would lead to increased antitumor efficacy in this model remains to be investigated, but dose escalation of AITC did not result in increased antitumor efficacy in our previous study (9). Combination of the two agents resulted in an increased, perhaps synergistic antitumor effect, inhibiting tumor growth by 63%, compared with 17% in celecoxib alone and 23% in AITC alone. The combination regimen also appears to be more effective than each agent alone with regard to inhibition of tumor muscle invasion, activation of apoptosis, inhibition of tumor microvessel formation and promotion of tumor vascular maturation. Moreover, although AITC itself showed no inhibitory activity against Cox-2 and PGE2 as expected, it appears to potentiate the PGE2-lowering activity of celecoxib. It is also worth noting that tumor vascular normalization has been shown to result in better intratumoral drug delivery (29,30); our results, therefore, suggest that AITC and/or celecoxib may be potentially useful for facilitating intratumoral delivery of agents currently used in bladder cancer treatment, such as mitomycin and platinum compounds.
Although the inhibitory mechanism of AITC against bladder cancer cells has been well studied as mentioned before, further study is needed to better understand the anticancer mechanism of celecoxib in bladder cancer. In cultured AY-27 cells in vitro, celecoxib at the concentration that caused marked decrease in PGE2 level did not inhibit cell growth and had no impact on cell growth inhibition by AITC, but at higher concentrations, celecoxib inhibited cell growth. In vivo, celecoxib both decreased PGE2 level in tumor tissue and exhibited antitumor activity, especially in combination with AITC. In view of the in vitro data, it remains a question as to what extent a Cox-2-dependent mechanism or Cox-2-independent mechanism may account for the antitumor activity of celecoxib in vivo. The mechanism by which AITC and celecoxib inhibit microvessel formation and induce microvessel normalization also requires further investigation. In the present study, tumor tissue levels of VEGF were reduced 30–36% after treatment with these agents, and the decrease was statistically significant in the combination group, suggesting that VEGF may play an important part in enabling the impact of AITC and celecoxib on tumor vasculature.
The present study shows that combination of AITC with celecoxib is more efficacious than each agent alone for inhibition of bladder cancer. Further evaluation of such a combination is warranted for potential clinical translation. Our findings also raised the question as to whether other Cox-2-targeting agents, e.g. aspirin, may also complement AITC for inhibition of bladder cancer. It has been reported that celecoxib and certain other Cox-2 inhibitors at high dose levels may cause cardiovascular toxicity (31–33). Combination with AITC may allow dose reduction for celecoxib or a similar compound, while at the same time achieving increased antitumor efficacy. In the present study, we observed no abnormalities of heart histology and no change in heart tissue PGE2 levels in the rats treated by the combination of AITC and celecoxib (data not shown). Moreover, since AITC is commonly consumed by humans via cruciferous vegetables and certain Cox-2 inhibitors, e.g. celecoxib and aspirin, are also widely taken by humans, our present finding suggests a need for epidemiological studies on the potential joint effect of the consumption of AITC-containing cruciferous vegetables and the use of Cox-2 inhibitors on bladder cancer and other cancers.
Funding
National Institutes of Health/National Cancer Institute (R01CA124627, R01CA164574, P30CA016056).
Acknowledgements
We acknowledge the Pathology Core Facility at Roswell Park Cancer Institute for their help with histology and immunohistochemical staining. We also thank Drs Yi Ding and Manivannan Ethirajan for technical assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- AITC
allyl isothiocyanate
- Cox-2
cyclooxygenase-2
- ELISA
enzyme-linked immunosorbent assay
- PBS/T
phosphate-buffered saline with Tween 20
- PGE2
prostaglandin E2
- SMA
α-smooth muscle actin
- VEGF
vascular endothelial growth factor
- VMI
vascular maturation index.
References
- 1. Zhang Y. (2010). Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res., 54, 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krul C., et al. (2002). Metabolism of sinigrin (2-propenyl glucosinolate) by the human colonic microflora in a dynamic in vitro large-intestinal model. Carcinogenesis, 23, 1009–1016 [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharya A., et al. (2010). Allyl isothiocyanate-rich mustard seed powder inhibits bladder cancer growth and muscle invasion. Carcinogenesis, 31, 2105–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang L., et al. (2004). Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J. Nutr., 134, 2004–2010 [DOI] [PubMed] [Google Scholar]
- 5. Tang L., et al. (2006). The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anticancer. Drugs, 17, 297–305 [DOI] [PubMed] [Google Scholar]
- 6. Ioannou Y.M., et al. (1984). Allyl isothiocyanate: comparative disposition in rats and mice. Toxicol. Appl. Pharmacol., 75, 173–181 [DOI] [PubMed] [Google Scholar]
- 7. Bollard M., et al. (1997). The disposition of allyl isothiocyanate in the rat and mouse. Food Chem. Toxicol., 35, 933–943 [DOI] [PubMed] [Google Scholar]
- 8. Munday R., et al. (2006). Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: critical nature of in vivo bioassay. Nutr. Cancer, 54, 223–231 [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharya A., et al. (2010). Inhibition of bladder cancer development by allyl isothiocyanate. Carcinogenesis, 31, 281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhattacharya A., et al. (2012). The principal urinary metabolite of allyl isothiocyanate, N-acetyl-S-(N-allylthiocarbamoyl)cysteine, inhibits the growth and muscle invasion of bladder cancer. Carcinogenesis, 33, 394–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geng F., et al. (2011). Allyl isothiocyanate arrests cancer cells in mitosis, and mitotic arrest in turn leads to apoptosis via Bcl-2 protein phosphorylation. J. Biol. Chem., 286, 32259–32267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohammed S.I., et al. (1999). Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res., 59, 5647–5650 [PubMed] [Google Scholar]
- 13. Klein R.D., et al. (2005). Transitional cell hyperplasia and carcinomas in urinary bladders of transgenic mice with keratin 5 promoter-driven cyclooxygenase-2 overexpression. Cancer Res., 65, 1808–1813 [DOI] [PubMed] [Google Scholar]
- 14. Mokos I., et al. (2006). Association of cyclooxygenase-2 immunoreactivity with tumor recurrence and disease progression in superficial urothelial bladder cancer. Tumori, 92, 124–129 [PubMed] [Google Scholar]
- 15. Kim S.I., et al. (2002). Association of cyclooxygenase-2 expression with prognosis of stage T1 grade 3 bladder cancer. Urology, 60, 816–821 [DOI] [PubMed] [Google Scholar]
- 16. Moon R.C., et al. (1993). Chemoprevention of OH-BBN-induced bladder cancer in mice by piroxicam. Carcinogenesis, 14, 1487–1489 [DOI] [PubMed] [Google Scholar]
- 17. Kitayama W., et al. (1999). Increased expression of cyclooxygenase-2 protein in rat urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Carcinogenesis, 20, 2305–2310 [DOI] [PubMed] [Google Scholar]
- 18. Grubbs C.J., et al. (2000). Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res., 60, 5599–5602 [PubMed] [Google Scholar]
- 19. Rao K.V., et al. (1996). Differential activity of aspirin, ketoprofen and sulindac as cancer chemopreventive agents in the mouse urinary bladder. Carcinogenesis, 17, 1435–1438 [DOI] [PubMed] [Google Scholar]
- 20. Mohammed S.I., et al. (2006). Cyclooxygenase inhibitors in urinary bladder cancer: in vitro and in vivo effects. Mol. Cancer Ther., 5, 329–336 [DOI] [PubMed] [Google Scholar]
- 21. Pagliarulo V., et al. (2008). Celecoxib for the treatment of non muscle invasive bladder cancer. Results from the first phase 11 clinical trial. Eur. Urol. Suppl., 7, –299 [Google Scholar]
- 22. Sabichi A.L., et al. (2011). A randomized controlled trial of celecoxib to prevent recurrence of nonmuscle-invasive bladder cancer. Cancer Prev. Res. (Phila)., 4, 1580–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelly J.D., et al. (2011). Boxing bladder cancer with COX-2-specific inhibition. Cancer Prev. Res. (Phila)., 4, 1534–1535 [DOI] [PubMed] [Google Scholar]
- 24. Cohen S.M., et al. (1981). Transplantation and cell culture of rat urinary bladder carcinoma. Invest. Urol., 19, 136–141 [PubMed] [Google Scholar]
- 25. Trifan O.C., et al. (2002). Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res., 62, 5778–5784 [PubMed] [Google Scholar]
- 26. Nie D., et al. (2002). Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell. Mol. Life Sci., 59, 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhawan D., et al. (2010). Effects of short-term celecoxib treatment in patients with invasive transitional cell carcinoma of the urinary bladder. Mol. Cancer Ther., 9, 1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei D., et al. (2004). Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res., 64, 2030–2038 [DOI] [PubMed] [Google Scholar]
- 29. Bhattacharya A., et al. (2008). Tumor vascular maturation and improved drug delivery induced by methylselenocysteine leads to therapeutic synergy with anticancer drugs. Clin. Cancer Res., 14, 3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bhattacharya A., et al. (2009). Inhibition of colon cancer growth by methylselenocysteine-induced angiogenic chemomodulation is influenced by histologic characteristics of the tumor. Clin. Colorectal Cancer, 8, 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hennekens C.H., et al. (2008). Cyclooxygenase-2 inhibitors and most traditional nonsteroidal anti-inflammatory drugs cause similar moderately increased risks of cardiovascular disease. J. Cardiovasc. Pharmacol. Ther., 13, 41–50 [DOI] [PubMed] [Google Scholar]
- 32. Solomon S.D., et al. ; APC and PreSAP Trial Investigators (2006). Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation, 114, 1028–1035 [DOI] [PubMed] [Google Scholar]
- 33. Bertagnolli M.M., et al. ; Adenoma Prevention with Celecoxib Study Investigators (2009). Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev. Res. (Phila)., 2, 310–321 [DOI] [PMC free article] [PubMed] [Google Scholar]