Abstract
During February 2011–June 2012, invasive infection with Neisseria meningitidis serogroup W was identified in 11 persons in southeastern China. All isolates tested had matching or near-matching pulsed-field gel electrophoresis patterns and belonged to multilocus sequence type 11. The epidemiologic investigation suggested recent transmission of this clonal complex in southeastern China.
Keywords: Neisseria meningitidis, serogroup W135, meningococcal infections, multilocus sequence typing, China, bacteria
Neisseria meningitidis is a major public health threat in many parts of the world, including China. Since 2003, most meningococcal diseases in China have been caused by N. meningitidis serogroups A and C; only 3 cases of serogroup W meningococcal disease were reported before 2011 (1,2). However, during February 2011–June 2012, an increase in invasive disease caused by serogroup W N. meningitidis (11 cases total) was seen in southeastern China. To determine if this serogroup is emerging in China, we analyzed strains from 6 of the 11 infected patients reported during 2011–2012, from 16 of their close contacts, and from 3 serogroup W patients reported during 2006–2008.
The Study
Meningococcal disease is reportable in China. N. meningitidis isolates, cerebrospinal fluid (CSF), and blood samples from persons with invasive disease are forwarded to the Chinese Centers for Disease Control and Prevention (CDC) for serogroup determination by slide agglutination and/or PCR. Strains are further characterized by use of pulsed-field gel electrophoresis (PFGE) after NheI restriction enzyme digestion (3).
During February 2011–June 2012, we observed an increase in invasive meningococcal disease caused by N. meningitidis serogroup W in southeastern China. Of 11 cases total, 9 were diagnosed by strain isolation and 2 by PCR and real-time PCR of CSF samples (Table). Strains isolated from patients 1, 4, and 5 became nonviable during storage in the hospital laboratory. The 6 remaining strains (from patients 2, 3, 7, and 9–11) were submitted to the Chinese CDC along with 16 serotype W strains from close contacts of patients 4, 6, and 8–10. Thus, during 2011–2012, a total of 22 strains were submitted to the Chinese CDC, where they were confirmed as N. meningitidis serogroup W by slide agglutination with specific antiserum (Remel, Lenexa, KS, USA). In addition, CSF samples from patients 1, 4–6, and 8 were submitted and confirmed positive for N. meningitidis serogroup W by PCR and real-time PCR.
Table. Clinical and epidemiologic characteristics of patients with ST11 serogroup W meningococcal disease, China, 2011–2012*.
| Patient ID | Age, y/sex | Date of symptom onset | Province of onset | Patient occupation | Outcome | Method of diagnosis | ST11 W strains from close contacts (no.) |
|---|---|---|---|---|---|---|---|
| 1† | 16/M | 2011 Feb 12 | Guangxi | Student | Survived | Strain isolation/ PCR | No |
| 2 | 19/M | 2011 Apr 1 | Jiangsu | Factory worker | Died | Strain isolation | No |
| 3 | 19/F | 2011 Apr 20 | Zhejiang | Factory worker | Died | Strain isolation | No |
| 4† | 18/M | 2011 Apr 27 | Guangxi | Student | Survived | Strain isolation/ PCR | Yes (8) |
| 5† | 46/M | 2011 May 4 | Guangxi | Farmer | Survived | Strain isolation/ PCR | No |
| 6† | 22/F | 2011 Oct 13 | Guangdong | Factory worker | Survived | PCR | Yes (1) |
| 7 | 35/M | 2012 Feb 1 | Guangdong | Factory worker | Survived | Strain isolation | No |
| 8† | 23/M | 2012 Feb 2 | Guangxi | Factory worker | Survived | PCR | Yes (1) |
| 9 | 14/M | 2012 Feb 14 | Anhui | Student | Died | Strain isolation | Yes (4) |
| 10 | 3/M | 2012 Mar 27 | Henan | Student | Survived | Strain isolation | Yes (2) |
| 11 | 9/M | 2012 Jun 18 | Hunan | Student | Survived | Strain isolation | No |
*ID, identification; ST, sequence type. †Multilocus sequence typing results were obtained from cerebrospinal fluid but not from strains.
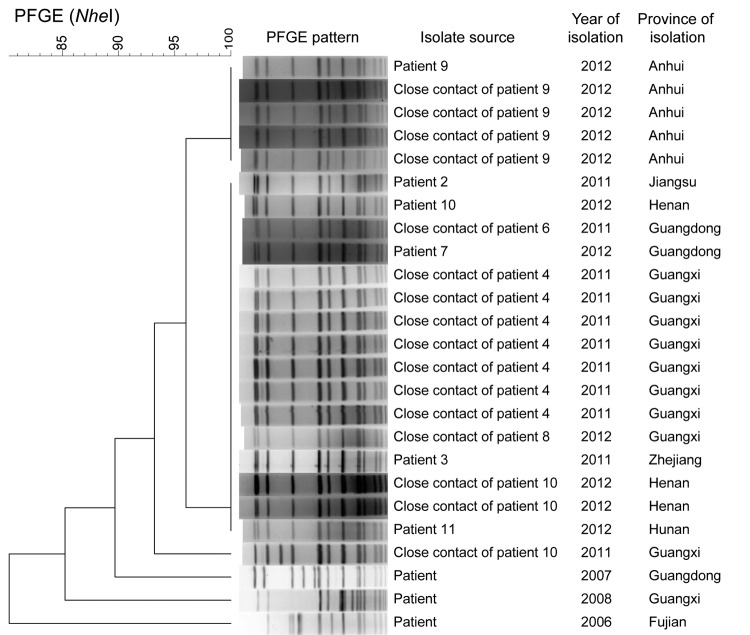
The 22 serogroup W strains from 2011–2012 were analyzed by PFGE; for comparison, 3 strains isolated from patients during 2006–2008 were also analyzed. PFGE patterns were distinguishable for 16 of the 22 strains from 2011–2012. Five strains associated with patient 9 and 1 strain isolated from a close contact of patient 4 had PFGE patterns that differed by 1 and 2 bands, respectively, indicating >94% similarity with the dominant pattern (Figure 1). PFGE patterns for the 3 isolates from 2006–2008 exhibited <90% similarity with those for isolates from 2011–2012, differing by 4–7 bands. All 22 isolates and 5 CSF samples from 2011–2012 were identified as sequence type (ST) 11 and PorA type P1.5,2, identical to the genotype of serotype W isolates associated with outbreaks reported in Saudi Arabia in 2000 and 2001 (4,5) and Burkina Faso in 2002 (6), sporadic cases in other countries (7–9), and the 3 cases identified in China during 2006–2008 (2).
Figure 1.
PFGE patterns for 22 Neisseria meningitidis serogroup W strains (6 from reported patients, 16 from close contacts) isolated during 2011–2012 and 3 isolated during 2006–2008, China. All isolates were sequence type 11 (determined by multilocus sequence typing) and PorA type P1.5,2. PFGE, pulsed-field gel electrophoresis. Scale bars indicates percent similarity.
Of the 11 patients with cases reported during 2011–2012, 4 resided in Guangxi Province, 2 in Guangdong Province, and 1 each in Jiangsu, Zhejiang, Anhui, Henan, and Hunan Provinces (Technical Appendix). The median age of patients was 20 years (range 3–46), 9 (81.8%) were male, and all denied recent foreign travel. Three of the 11 patients died of bacteremia. The epidemiologic investigation did not identify any common exposures, social settings, or other connections among the patients. Close contacts of all 11 patients were investigated, and no additional N. meningitidis infections were detected. Of the 11 reported cases, 5 occurred in or were associated with Laibin City, Guangxi Province: patients 1, 4, and 5 sought care in Laibin City; patient 3 sought care in Zhejiang Province on April 20, 2012, after having traveled to Zhejiang Province from Laibin City on March 26; and patient 8 sought care in Fangchenggang City, Guangxi Province, 10 days after a close contact (partner) had traveled to Laibin City (Technical Appendix). The partner of patient 8 was subsequently tested and identified as a carrier of ST11 serogroup W N. meningitidis.
A survey of N. meningitidis carriage was conducted among the healthy population of Laibin City in September 2011. A total of 1,311 persons 1–45 years of age were investigated, of whom 8.54% (112/1,311 persons) were positive for N. meningitidis carriage. Age groups and percentages of infected persons in each age group were 1–6 years (1.4%), 7–12 years (6.0%), 13–15 years (6.7%), 16–20 years (18.5%), and 21–45 years (1.0%). The serogroup for each strain was determined by use of slide agglutination and polyclonal antisera and PCR methods. Of the 112 N. meningitidis–positive samples, 20 (17.9%) were ST11 serogroup W, and of those 20 samples, 2, 4, and 14 were from persons 7–12, 13–15, and 16–20 years of age, respectively. All 20 strains exhibited indistinguishable PFGE patterns that matched the dominant pattern of the disease-associated strains. The carriage rate of ST11 serogroup W N. meningitidis reached 5.5% (11/200) among 200 students (16–20 years of age) in 1 school.
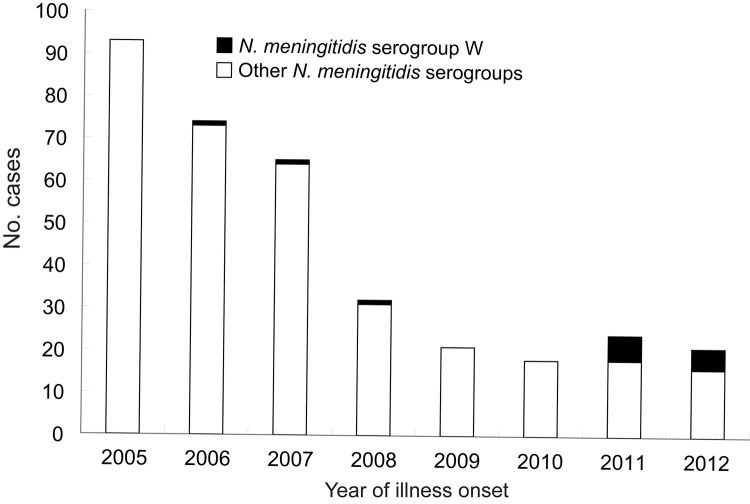
Since 2003, the annual incidence of meningococcal disease in China has stayed below 0.2 cases/100,000 population. Surveillance data in China suggest a historical trend for seasonal peaks of meningococcal disease during February–April. This peak season corresponds with the spring dry season in China, a time when tourists are most likely to visit the country, especially southern China. Among the 45 cases of meningococcal disease confirmed during 2011–2012 by meningococcal etiology and PCR methods, 11 (24.4%) were caused by serogroup W N. meningitidis (Figure 2); 8 of these 11 cases occurred during February–April. The 3 cases reported during 2006–2008 occurred during May, June, and October, respectively.
Figure 2.
Laboratory-confirmed cases of meningococcal disease, by Neisseria meningitidis serogroup and year of symptom onset, China, 2005–2012.
Conclusions
The incidence of serogroup W infections reported during February 2011–June 2012 represents a marked increase over that reported during 2005–2010. The emergence and spread of a new N. meningitidis serogroup in a region presents a challenge for the prevention and control of meningococcal disease, especially if vaccines used in the region do not cover all serogroups. ST7 serogroup A and ST4821 serogroup C N. meningitidis strains were identified as the 2 dominant lineages circulating in China during 2003–2008, causing >90% of meningococcal disease cases (1). Meningococcal polysaccharide vaccines A and C have been used in China for routine immunization since the outbreak of N. meningitidis serogroup C during 2003–2004. In some African countries, repeated vaccination against N. meningitidis serogroups A and C is thought to have led to a selective increase in the incidence of meningococci of other serogroups, thereby resulting in a changed profile of meningococcal disease (10–12). Therefore, meningococcal disease caused by N. meningitidis strains that belong to serogroups other than A and C, especially those that belong to hyperinvasive lineages, appears to be an emerging problem in China.
The 11 cases of meningococcal disease caused by ST11 serogroup W N. meningitidis strains described here had successively emerged in southeastern China; furthermore, ST11 serogroup W meningococci were isolated from close contacts of the patients and from healthy carriers. These observations suggest the possible establishment and spread of a clonal complex of serogroup W meningococci in southeastern China. Carriage and transmission of this strain have led to the emergence of ST11 serogroup W organisms as a cause of endemic meningococcal disease. Further epidemiologic and microbiological surveillance is needed for monitoring of meningococcal diseases caused by serogroup W in southeastern China and preventing the spread of this clone to other regions.
Figure showing distribution of 11 serogroup W meningococcal disease cases identified in China during February 2011–June 2012.
Acknowledgments
This study was supported by the National Natural Scientific Foundation (no. 81201332) from the Ministry of Science and Technology of the People’s Republic of China, grants from the Major State Basic Research Development Program of China (973 Program) (2011CB504900), and the Priority Project on Infectious Disease Control and Prevention (no. 2013ZX10004221 and 2012ZX10004215) from the Ministry of Health and the Ministry of Science and Technology of the People’s Republic of China.
Biography
Mr Zhou is a microbiologist at National Institute for Communicable Disease Control and Prevention, Chinese CDC, Beijing. His research interests include molecular subtyping of pathogenic bacteria and molecular epidemiology of meningococcal meningitis.
Footnotes
Suggested citation for this article: Zhou H, Liu W, Xu L, Deng L, Deng Q, Zhuo J, et al. Spread of Neisseria meningitidis serogroup W clone, China. Emerg Infect Dis. 2013 Sep [date cited]. http://dx.doi.org/10.3201/eid1909.130160
These authors contributed equally to this article.
References
- 1.Zhou H, Gao Y, Xu L, Li M, Li Q, Li Y, et al. Distribution of serogroups and sequence types in disease-associated and carrier strains of Neisseria meningitidis isolated in China between 2003 and 2008. Epidemiol Infect. 2012;140:1296–303 . 10.1017/S0950268811001865 [DOI] [PubMed] [Google Scholar]
- 2.Shao Z, Zhou H, Gao Y, Ren H, Xu L, Kan B, et al. Neisseria meningitidis serogroup W135, China. Emerg Infect Dis. 2010;16:348–9 . 10.3201/eid1602.090901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shao Z, Li W, Ren J, Liang XF, Xu L, Diao BW, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui Province, China. Lancet. 2006;367:419–23. 10.1016/S0140-6736(06)68141-5 [DOI] [PubMed] [Google Scholar]
- 4.Lingappa JR, Al-Rabeah AM, Hajjeh R, Mustafa T, Fatani A, Al-Bassam T, et al. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg Infect Dis. 2003;9:665–71. 10.3201/eid0906.020565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer LW, Reeves MW, Al-Hamdan N, Sacchi CT, Taha MK, Ajello GW, et al. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electrophoretic type-37 complex. J Infect Dis. 2002;185:1596–605. 10.1086/340414 [DOI] [PubMed] [Google Scholar]
- 6.Nathan N, Rose AM, Legros D, Tiendrebeogo SR, Bachy C, Bjørløw E, et al. Meningitis serogroup W135 outbreak, Burkina Faso, 2002. Emerg Infect Dis. 2007;13:920–3. 10.3201/eid1306.060940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemos AP, Harrison LH, Lenser M, Sacchi CT. Phenotypic and molecular characterization of invasive serogroup W135 Neisseria meningitidis strains from 1990 to 2005 in Brazil. J Infect. 2010;60:209–17. 10.1016/j.jinf.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle TJ, Mejia-Echeverry A, Fiorella P, Leguen F, Livengood J, Kay R, et al. Cluster of serogroup W135 meningococci, southeastern Florida, 2008–2009. Emerg Infect Dis. 2010;16:113–5. 10.3201/eid1601.091026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilic A, Urwin R, Li H, Saracli MA, Stratton CW, Tang YW. Clonal spread of serogroup W135 meningococcal disease in Turkey. J Clin Microbiol. 2006;44:222–4. 10.1128/JCM.44.1.222-224.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonkoua MC, Taha MK, Nicolas P, Cunin P, Alonso JM, Bercion R, et al. Recent increase in meningitis caused by Neisseria meningitidis serogroups A and W135, Yaoundé, Cameroon. Emerg Infect Dis. 2002;8:327–9. 10.3201/eid0803.010308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagneux SP, Hodgson A, Smith TA, Wirth T, Ehrhard I, Morelli G, et al. Prospective study of serogroup X Neisseria meningitidis outbreak in northern Ghana. J Infect Dis. 2002;185:618–26 . 10.1086/339010 [DOI] [PubMed] [Google Scholar]
- 12.Massenet D, Inrombe J, Mevoula DE, Nicolas P. Serogroup W135 meningococcal meningitis, northern Cameroon, 2007–2008. Emerg Infect Dis. 2009;15:340–2. 10.3201/eid1502.080988 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure showing distribution of 11 serogroup W meningococcal disease cases identified in China during February 2011–June 2012.