Abstract
The effectiveness of cancer therapeutics targeting signal transduction pathwaysis compromised by a diversity of mechanisms that drive de novo or acquired resistance. Two recent studies identify mTOR activation as a point of convergence of mechanisms that cause resistance to inhibitors of the Raf-MEK-ERK and PI3K signaling.
A critical turning point in the fight against advanced and metastatic melanomas occurred just over a decade ago with the discovery and characterization of the BRAFactivating mutationV600E inabout 60% of melanomas(Davies et al., 2002).This mutation causes constitutive activation of the B-Rafserine/threonine kinase, resulting inaberrant and persistent activation of the Raf-MEK-ERKmitogen-activated protein kinase cascade.Importantly, BRAFV600E correlated with poor prognosis in patients with metastatic melanoma.This prompted the development and clinical evaluation of Raf and MEK inhibitors for the treatment of BRAF mutant metastatic melanoma(Salama and Flaherty, 2013). The dramatic anti-tumor activities of these inhibitors led to FDA approval of two Raf (vemurafenib and dabrafenib) and one MEK (trametinib) inhibitor for the treatment of BRAF mutant melanoma(Chapman et al., 2011; Flaherty et al., 2012; Hauschild et al., 2012).Despite the clinical success of these inhibitors, resistance has limited their long-term clinical impact. Although patient selection based on BRAF mutation status defines the patient population that would benefit from Rafor MEK inhibition,20-50% of patients showed no initial response, suggesting de novoresistance in a significant subset of melanoma patients(Chapman et al., 2011; Hauschild et al., 2012). Furthermore, even for patients who do respond initially, within three months essentially all suffer fromrelapsed tumors that have acquired drug resistance.This has ledto numerous studies that haveidentifiedmultiple mechanisms of de novo and/or acquired resistance to Raf inhibition., with mechanisms that cause ERK reactivation downstream of the inhibitor block as well as ERK-independent mechanismshave been identified (Sullivan and Flaherty, 2013).
Corcoran et al. have recently identified a mechanism that may provide a more unifying model for the diverse mechanisms already identified(Corcoran et al., 2013). While decreased phosphorylation of ERK(pERK) has thus far been the standard used to gauge tumor sensitivity in both clinicaland preclinical studies, Corcoran et al.found thatrobust inhibition of pERK was still observed in melanoma cell lines resistant to Raf or MEK inhibitors, assayed by measuring growth inhibition and apoptosis induction. Instead, Corcoran et al.made an intriguing discovery that levels of ribosomal protein S6 (pS6)phosphorylation, a key componentdownstream of mTORC1,can be used as a marker of ERK-independent resistance to Rafand MEK inhibitor treatment. Analysis of melanoma cell lines with different sensitivities to vemurafenibindicated that while the common biomarkers pERK and pAKT responded similarly, pS6 decreased in sensitive lines but was sustained in insensitive lines even upon increasing doses of vemurafenib.To determine if MEK inhibition also required downregulation of pS6 for sensitivity, cells were treated with the MEK1/2 inhibitor selumetinib in the presence of activated mTOR, achieved by knockdown of Tsc2, a major negative regulator of mTORC1. This resulted in fewer apoptotic cells, signifying that mTOR activity protected cells against apoptosis induced by MEK inhibition. Combination of an mTORcatalytic inhibitor with vemurafenib increased cell death, further suggesting a combinatorial approach of Raf and mTOR inhibition may prove efficacious in vemurafenib-resistant melanomas. Preclinical modeling using mouse xenografts mirrored the cell line findings, with pERKdownregulation seen in both sensitive and insensitive tumors while pS6 downregulation was only observed in sensitive tumors.
The authors then addressed a critical issue of whether these cell culture and mouse model results could be translated to cancer patients. Most intriguingly, fine-needle aspiration (FNA) biopsies from the mouse xenograft tumors demonstrated real-time decreases in pS6 upon treatment, thisapproach was then advanced to successfully applied tomelanoma patients. In a time-sensitive setting where treatment choices and changes must be made quickly for the health of the patient, using FNAs to assess biomarker status is ideal, as it is minimally invasive and can be performed multiple times. FNAs were then used to probe pS6 and pERK response to vemurafenib in metastatic melanoma patients. This led to the promising result of an almost five-fold increase in progression-free survival seen in patients with decreased pS6 in their tumors compared to patients whose tumors did not. While these combined mTOR and Raf inhibition studies have shown efficacy in tumor cells and xenograft models, this approach still must be assessed in human patients. There is a trial currently recruiting for advanced cancers that will assess the combination of vemurafenib with the mTOR inhibitor everolimus. Hopefully the results from this clinical trial will support the data reported by Corcaran et al. showing improved patient outcome once both Raf and mTORC1 are blocked.
Notably, another study in the same issue of Science Translational Medicine by Elkabets et al. reveals mTOR-mediated resistance to p110α inhibition in PIK3CAmutant breast cancers(Elkabets et al., 2013). Preclinical and clinical evaluation indicated that PIK3CA mutation status provided an incomplete genetic marker for response to PI3K inhibition (Bendell et al., 2012; Maira et al., 2012). In these breast cancer cells, inhibition of mTOR by everolimus sensitized tumor cells to the p110α-specific inhibitor BYL719. Similar to the results reported by Corcoran et al., mTORC1 activity and pS6 were identified as important biomarkers to p110α inhibitor response. Interestingly, breast cancer cell lines with acquired resistance to BYL719 were established and these also displayed enhanced mTORC1 activity compared to their matching control cells indicating kinome reprogramming to p110α inhibitor treatment. Depletion of mTOR via shRNA from the acquired p110α inhibitor resistant cells was sufficient to prevent proliferation and a combination of BYL719 and mTORC1 inhibitor therapy prevented the tumorigenic growth of BYL719 resistant cells in mouse xenografts. Elkabets et al. also examined breast cancer patient biopsies from an ongoing phase I clinical trial of BYL719 treatment forPIK3CAmutant solid tumors. Strikingly, those patients who responded to BYL719 treatment showed a loss of pS6 staining intensity in their tumors as compared to biopsies before treatment began whereas those patients whose tumors did not respond to BYL719 treatment maintained high levels of pS6 during treatment. Interestingly, biopsies from two patients that initially responded to BYL719 therapy but later showed tumor progression displayed a return of pS6 to levels similar to that seen prior to any BYL719 treatment further implicating mTORC1 activation in the acquired resistance to BYL719/p110α therapy.
In summary, the findings from these two studies support mTOR activation as a key driver of resistance to PI3K inhibition in PIK3CA mutant breast cancer and resistance to Raf or MEK inhibition in BRAF mutant melanoma.It will be important to explore whether mTOR activation will act as a resistance mechanism to inhibitors of other signaling components in other cancer types. Additional patient analyses and combination inhibitor clinical trials will be needed to validate the importance of mTORC1 activation as a biomarker to predict patient response and mTOR inhibitor combination treatment to overcome resistance. mTOR is regulated downstream of both Raf and PI3K signaling and consequently may define a key point of convergence of the divergent resistance mechanisms that have been identified. Finally, the signaling mechanisms that cause mTOR activation to drive resistance as well as the downstream consequences of mTOR signaling that promote resistance are issues that remain to be fully elucidated.
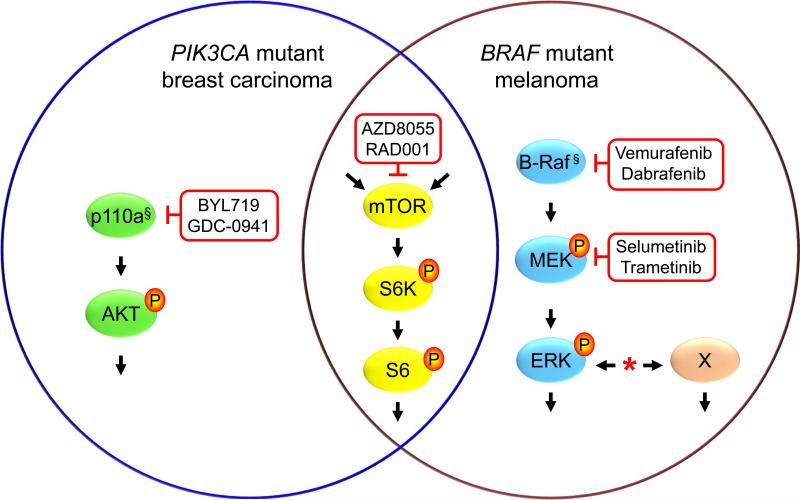
A subset of BRAF mutant melanomas possesses de novo resistances to Raf or MEK inhibitor therapy and essentially all cancers that are responsive initially develop acquired resistance. A diversity of mechanisms of resistance has been described (*), that most commonly causing ERK reactivation downstream of the inhibitor block or by activation of ERK-independent (X) mechanisms. Similarly, only a subset of PIK3CA(encodes p110α) mutant cancers isresponsive to p110α isoform selective (BYL719) or pan-class I (GDC-0941) PI3K inhibitors. In BRAF mutant (§) melanomas orPIK3CA(§) mutant breast carcinomas, activation of mTOR correlates with inhibitor resistance, and concurrent treatment with an allosteric (RAD001/Everolimus) or catalytic (AZD8055)mTOR inhibitor overcomes resistance. The phosphorylated state of S6, a substrate of mTORC1-activated S6 kinase, provides a marker for resistance and response. mTORC1 activation can be activated downstream of both PI3K and ERK as well as by other mechanisms, possibly providing a point of convergence for multiple mechanisms of resistance.
Figure 1.
mTOR-driven mechanisms of cancer cell resistance to Raf, MEK and PI3K inhibitors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bendell JC, Rodon J, Burris HA, de Jonge M, Verweij J, Birle D, Demanse D, De Buck SS, Ru QC, Peters M, et al. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran RB, Rothenberg SM, Hata AN, Faber AC, Piris A, Nazarian RM, Brown RD, Godfrey JT, Winokur D, Walsh J, et al. TORC1 Suppression Predicts Responsiveness to RAF and MEK Inhibition in BRAF-Mutant Melanoma. Science translational medicine. 2013;5:196ra198. doi: 10.1126/scitranslmed.3005753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, et al. mTORC1 Inhibition Is Required for Sensitivity to PI3K p110alpha Inhibitors in PIK3CA-Mutant Breast Cancer. Science translational medicine. 2013;5:196ra199. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Robert C, Hersey P, Nathan P, Garbe C, Milhem M, Demidov LV, Hassel JC, Rutkowski P, Mohr P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. The New England journal of medicine. 2012;367:107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr., Kaempgen E, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Molecular cancer therapeutics. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- Salama AK, Flaherty KT. BRAF in Melanoma: Current Strategies and Future Directions. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:4326–4334. doi: 10.1158/1078-0432.CCR-13-0779. [DOI] [PubMed] [Google Scholar]
- Sullivan RJ, Flaherty KT. Resistance to BRAF-targeted therapy in melanoma. European journal of cancer. 2013;49:1297–1304. doi: 10.1016/j.ejca.2012.11.019. [DOI] [PubMed] [Google Scholar]