Abstract
Objective
To compare the risk of subsequent oophorectomy among women who had hysterectomy for benign indications and those who did not.
Methods
Using Rochester Epidemiology Project resources, we compared the risk of oophorectomy through December 31, 2008 among 4,931 Olmsted County, Minnesota, women who had ovary-sparing hysterectomy for benign indications (cases) between 1965 and 2002 and 4,931 age-matched women who did not have hysterectomy (referents). The cumulative incidence of subsequent oophorectomy was estimated by the Kaplan-Meier method, and comparisons were evaluated by Cox proportional hazard models using age as the time scale to allow for complete age adjustment.
Results
The median follow-up for cases and referents was 19.6 and 19.4 years, respectively. At 10, 20, and 30 years after hysterectomy, the respective cumulative incidence of subsequent oophorectomy was 3.5%, 6.2%, and 9.2% among cases and 1.9%, 4.8%, and 7.3% among referents. The overall risk of subsequent oophorectomy among cases was significantly higher than among referents (hazard ratio [HR], 1.20; 95% CI, 1.02–1.42; P=.03). Furthermore, among cases, the risk of subsequent oophorectomy was significantly higher (HR, 2.15; 95% CI, 1.51–3.07; P<.001) in women who had both ovaries preserved compared with those who initially had one ovary preserved.
Conclusion
The incidence of oophorectomy after hysterectomy is only 9.2% at 30-year follow-up and is only 1.9 percentage points higher than the incidence of oophorectomy in referent women with intact reproductive organs.
Introduction
Ovarian cancer causes 14,000 deaths annually in the United States (1). Performing blood-based screening tests or sonographic surveillance has failed to improve early detection or survival. Prophylactic oophorectomy at the time of benign hysterectomy prevents subsequent ovarian malignancy. However, in those at low risk for ovarian malignancy, the absolute risk of ovarian cancer among women after ovarian preservation during benign hysterectomy is low (0.03%–1.96%) (2,3). A 24-year follow-up of the Nurses’ Health Study revealed that only 34 of 13,035 women (0.3%) who had ovarian preservation at the time of hysterectomy later succumbed to ovarian cancer (4). Furthermore, a growing body of evidence suggests that prophylactic oophorectomy is associated with increased overall mortality, coronary heart disease, dementia, osteoporosis, and other cancer mortality (4–7).
Women with ovarian preservation are at risk for future oophorectomy (8). This risk ranged from 2.9% to 7.7% in previous studies (5,9,10). However, this information was derived from studies with short follow-up that were not population based and lacked a comparison group (10–12). Moreover, in some series, subsequent oophorectomy was prompted by additional screening tests (eg, pelvic ultrasound) and thus may not reflect the true natural history of disease. An accurate appraisal of this question is necessary to refine our understanding of the risk-benefit ratio of prophylactic oophorectomy. We compared the risk of oophorectomy in a large community cohort of women who had hysterectomy for benign indications with age-matched referent women with intact uteri. In addition, the risk of pelvic reoperation was compared in women with one ovary compared with both ovaries preserved during hysterectomy.
Materials and Methods
This study used the data resources of the Rochester Epidemiology Project, which links and indexes the medical records of virtually all medical providers who serve the population of Olmsted County, Minnesota, including Mayo Clinic and its affiliated hospitals (Rochester Methodist and Saint Marys) and the Olmsted Medical Center and its affiliated community hospital (13). Since these institutions provide nearly all medical care for the community, this resource results in a comprehensive source of both inpatient and outpatient information for population-based epidemiologic research (14). The Rochester Epidemiology Project captures virtually all individuals who have lived in Olmsted Country from 1966 on. As of July 2009, the Rochester Epidemiology Project included information on 493,606 individuals and their respective medical records from 65 different health care providers (15). The vast majority of Olmsted County residents use the medical system, including 85% of women of all ages seen in the past 3 years and more than 90% of adults older than 70 years seen in the past year (16). According to statistics from 2000, the majority of Olmsted County residents are white (90.3%), which is similar to the remainder of Minnesota (89.4%) and higher than the United States overall (75.1%). In 2000, Olmsted County had a 2.7% black population and 2.4% Hispanic population (17).
After approval by the institutional review boards of both Mayo Clinic and Olmsted Medical Center, Rochester Epidemiology Project resources were used to identify all Olmsted County residents who underwent hysterectomy between January 1, 1965, and December 31, 2002 (18). The procedure type and indications were identified electronically, as were the route of hysterectomy, unilateral or bilateral ovarian preservation, the date of subsequent oophorectomy, and the date of last follow-up or death (18). As had been done previously, we randomly selected 100 women and manually compared the procedure type in the electronic medical record system to the procedure type listed in the surgical note; these two sources agreed in 83% of cases.
Of the 9,893 women with hysterectomy in this original cohort, 876 women (8.8%) who had not authorized the use of their medical records for research were excluded (19). We also excluded 914 women who had hysterectomy for malignant disease and 3,172 women who had no intact ovaries at the completion of their hysterectomy. The remaining 4,931 women (“cases”) were included for further analysis. For each hysterectomy case, one matched woman (“referent”) was randomly selected from all Olmsted County female residents born within 1 year of a case and seen at Mayo Clinic or Olmsted Medical Center in the same year that her matched case underwent hysterectomy (“index date”). As of the index date, the referent had not undergone hysterectomy and had at least one intact ovary. For each case and referent, the date of subsequent oophorectomy and date of last follow-up or death prior to December 31, 2008, were obtained electronically. For each referent, the date of subsequent hysterectomy prior to December 31, 2008, was also obtained.
Analysis was carried out using the Statistical Analysis System (SAS Institute, Inc, Cary, North Carolina). The Kaplan-Meier method was used to estimate the cumulative incidence of oophorectomy (1 minus the probability of survival free of oophorectomy) after the index date. The duration of follow-up was calculated from the index date to the date of the subsequent oophorectomy; otherwise the follow-up was censored at the date of last follow-up. The influence of hysterectomy (case compared with referent) on the risk of subsequent oophorectomy was evaluated based on fitting a Cox proportional hazards model using age as the time scale to allow for complete age adjustment. The association was summarized by calculating the hazard ratio (HR) and 95% confidence interval (CI) using the model estimated coefficient and its standard error estimated by the model. As a secondary analysis, the follow-up for a referent woman was censored at the date of a subsequent hysterectomy if the hysterectomy preceded an oophorectomy or their last follow-up date.
Among the cases, influence of age and calendar year on the risk of subsequent oophorectomy was assessed on the basis of fitting standard Cox models using time since hysterectomy as the time scale. The HR for the association between ovarian preservation (unilateral compared with bilateral) and risk of subsequent oophorectomy was estimated from a Cox model, again using age as the time scale to allow for complete age adjustment.
Our power calculations were completed based on reviewing past work with a similar cohort. We assumed that approximately 4,500 women would undergo hysterectomy with preservation of one or both ovaries. Because the reported frequency of reoperation after preservation of one or both ovaries during hysterectomy ranges from 3% to 7.6% (12), we anticipated that between 135 and 342 cases would have subsequent oophorectomy. Based on a sample size of 4,500 hysterectomy cases and 4,500 age-matched referents, the study was predetermined to have 80% power to detect an HR greater than 1.5 if a total of 200 women had subsequent oophorectomy, using sample size and power calculations for the 2-sided log-rank test (with a type I error level of 5%), as proposed by Schoenfeld (20).
Results
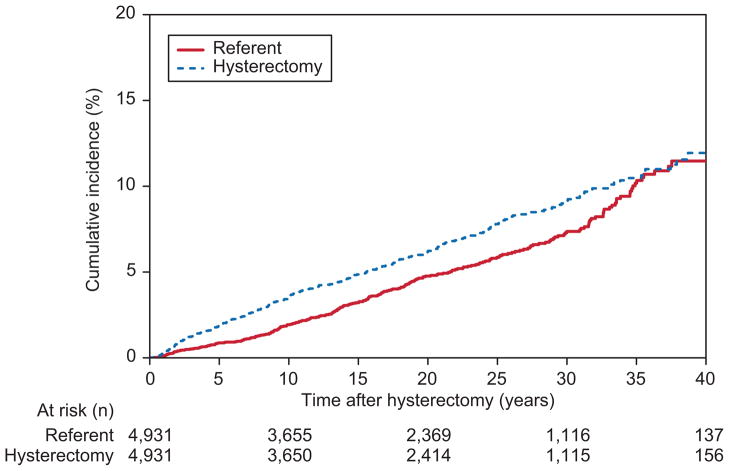
Within this cohort of 4,931 hysterectomy cases and 4,931 age-matched referents, both ovaries were intact at the index date in 79.5% of cases and 98.3% of referents. The mean age at the index date was 42.9 years (standard deviation [SD], 11.7 years) in cases and 43.6 years (SD, 12.1 years) in age-matched referents (Table 1). Approximately 74% of cases had vaginal hysterectomy; myomatous uterus was the most common indication for surgery (29.5%). Among the hysterectomy cases, the median duration of follow-up was 19.6 years (interquartile range [IQR], 9.6–29.0 years; range, less than 0.1–43.5 years), during which time 315 women had oophorectomy. The referents had a median follow-up of 19.4 years (IQR, 9.7–28.9 years); range, less than 0.1–43.5 years), during which time 247 women had oophorectomy (89 oophorectomy only and 158 oophorectomy concurrent with hysterectomy). The cumulative incidence of subsequent oophorectomy was 3.5%, 6.2%, and 9.2%, respectively, among the cases and 1.9%, 4.8%, and 7.3%, respectively, among the referents at 10, 20, and 30 years after the index date (Figure 1). The overall risk of subsequent oophorectomy among the cases was higher than that in the referents (HR, 1.20; 95% CI, 1.02–1.42; P=.03). In a secondary analysis, the follow-up for a referent woman was censored at the date of a subsequent hysterectomy. The result of the secondary analysis was similar to the primary analysis (HR, 1.22; 95% CI, 1.03–1.45; P=.02).
Table 1.
Summary of Demographic and Clinical Characteristics in Cases and Referents
| Characteristic | Hysterectomy Cases (n=4,931) | Age-Matched Referents (n=4,931) |
|---|---|---|
| Age at index date, year | ||
| Mean (SD) | 42.9 (11.7) | 43.6 (12.1) |
| Median (IQR) | 41 (36–47) | 41 (36–48) |
| Range | 11–93 | 11–93 |
| Ovary status at index date, n (%) | ||
| One intact | 86 (1.7) | 86 (1.7) |
| Both intact, but unilateral salpingo-oophorectomy with hysterectomy | 929 (18.8) | 0 |
| Both intact | 3,916 (79.5) | 4,845 (98.3) |
| Type of hysterectomy, n (%) | ||
| Vaginal | 3,632 (73.7) | |
| Abdominal | 1,263 (25.6) | |
| Other (laparoscopic) | 36 (0.7) | |
| Indication for hysterectomy, n (%) | ||
| Uterine leiomyomas | 1,454 (29.5) | |
| Precancerous condition | 1,162 (23.6) | |
| Uterine or vaginal prolapse | 903 (18.3) | |
| Menstrual disorders | 848 (17.2) | |
| Endometriosis | 338 (6.8) | |
| Inflammatory disease of pelvic organs | 138 (2.8) | |
| Other | 67 (1.4) | |
| Menopausal disorders | 21 (0.4) | |
SD, standard deviation; IQR, interquartile range.
Figure 1.
The cumulative incidence of subsequent oophorectomy among women who underwent hysterectomy (cases) and age-matched referent women (referents) from the same community.
Among the cases, age at the time of hysterectomy was associated with the risk of subsequent oophorectomy (HR, 1.62 per 10-year increase in age; 95% CI, 1.50–1.75; P<.001). The risk of subsequent oophorectomy was also related to the calendar period when hysterectomy was performed; that is, higher (HR, 1.35 [95% CI, 1.26–1.45] per 5-year increase in the calendar year; P<.001) for women who had hysterectomy more recently.
Either one ovary (1,015 women; mean age, 40.7 years [SD, 10.1 years]) or both ovaries (3,916 women; mean age, 43.5 years [SD, 12 years]) were preserved at hysterectomy. Among women with one preserved ovary, the cumulative incidence of subsequent oophorectomy was 1.2% at 2 years, 2.8% at 10 years, and 4.0% at both 20 and 30 years (Table 2). Among women with both ovaries intact, the cumulative incidence was 0.7% at 2 years, 3.7% at 10 years, 6.8% at 20 years, and 10.6% at 30 years. Hence, women in whom both ovaries were preserved at hysterectomy had an increased risk of reoperation (HR, 2.15; 95% CI, 1.51–3.07; P<.001) relative to women who did not have both preserved (ie, had only one ovary preserved).
Table 2.
Cumulative Incidence of a Subsequent Oophorectomy Among the Hysterectomy Cases, Separately by Level of Ovarian Preservation
| Ovarian Preservation at the Time of Hysterectomy | Number of Patients With Subsequent Oophorectomy | Cumulative Incidence of Subsequent Oophorectomy by Years After the Index Date, %*
|
||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| One ovary intact (n=1,015) | 34 | 1.2 | 1.7 | 2.8 | 3.2 | 4.0 | 4.0 | 4.0 |
| Both ovaries intact (n=3,916) | 281 | 0.7 | 1.9 | 3.7 | 5.3 | 6.8 | 8.8 | 10.6 |
The cumulative incidence of a subsequent oophorectomy is significantly different between the 2 groups (hazard ratio, 2.15; 95% confidence interval, 1.51–3.07; P<.001; based on a Cox model fit on an age scale).
Discussion
Although the cumulative incidence of a subsequent oophorectomy was higher among the cases (3.5%, 6.2%, and 9.2%) than in the referent group (1.9%, 4.8%, and 7.3%) at 10, 20, and 30 years, respectively, the absolute risk showed a minimal difference (less than 2 percentage points at 20-year- and 30-year follow-up). Moreover, the cumulative incidence of reoperation if both ovaries were preserved was only 10.6% at 30-year follow-up.. Our estimates of reoperation at 30 years are higher than the previously reported range of 2.9% to 7.7% (10–12). However, these studies had a shorter follow-up period (average duration of 4 years) and lacked a comparison group.
We theorize that disruption of ovarian blood flow as a result of hysterectomy may alter ovarian function, which could lead to adnexal pathology. In fact, menopause has been shown to occur earlier in premenopausal women having ovary-sparing hysterectomy (21,22). Alternatively, patients who undergo hysterectomy may have underlying conditions (eg, chronic pelvic pain) that predispose to subsequent surgery.
Approximately 75% of hysterectomies in this study were vaginal, which is higher than the proportion (30–35%) reported elsewhere in the United States (23,24). In general, it is easier to perform an oophorectomy during abdominal than vaginal hysterectomy. However, previous studies have shown that oophorectomy is feasible in 85% of attempted vaginal hysterectomies and that the likelihood of oophorectomy increases with surgical experience (25–27). Moreover, our concomitant oophorectomy rate of 39% (3,172/8,103) is comparable to that of other studies with predominantly abdominal hysterectomy (28,29).
Women in whom both ovaries were intact had a higher risk of subsequent oophorectomy. This difference was statistically significant even after adjusting for age. In contrast, an uncontrolled retrospective study using ultrasound surveillance reported a higher risk (7.6% compared with 3.5%) of subsequent oophorectomy in women with one intact ovary rather than both intact ovaries after hysterectomy (12). However, 78% of ovaries had normal histopathology, perhaps suggesting that the relationship between hysterectomy and reoperation was biased by surveillance procedures. Our study was not prone to this bias. The reason for this imbalance between unilateral and bilateral preservation may be related to the indication for hysterectomy during the index procedure. Unfortunately, a limitation of the current analysis is that we do not have full information regarding indications for the subsequent oophorectomy.
Although prophylactic oophorectomy during hysterectomy reduces the risk of ovarian cancer (30), women may have an increased risk of cardiovascular disease, dementia, and osteoporosis and higher all-cause and cancer-specific mortality (4–7). These risks are highest for women undergoing oophorectomy before the age of 45 years and decline thereafter until the early sixth decade (7). Furthermore, after a median follow-up of 15 years, there was a 54% increase in osteoporotic fractures among postmenopausal women who had oophorectomy at a median age of 62 years (31). Conversely, the incidence of ovarian cancer increases with age, peaking at the age of 70 years (1). While there is an ongoing debate about the pros and cons of oophorectomy, it is our professional opinion that it would be prudent to offer prophylactic oophorectomy only to women older than 60 years who are undergoing benign hysterectomy. Women with known risk factors for ovarian cancer are in a different category and should be counseled concerning the benefits of prophylactic oophorectomy (9,32).
The large sample size with a median follow-up of 19.5 years (IQR, 9.6–28.9 years; range, less than 0.1–43.5 years) allowed for accurate estimates of the incidence of subsequent oophorectomy, as well as sufficient power for comparisons. The use of a referent group allowed us to estimate the true effect of hysterectomy on the incidence of oophorectomy as opposed to other factors. In this study, the referent group (women who had not undergone hysterectomy) was chosen from the same community and matched by age to the hysterectomy patients. Strictly speaking, therefore, we cannot draw conclusions about the incidence of oophorectomy in the referent group as a whole, but only in comparison to the cases.
Between 2000 and 2004 in the United States, 3 million women underwent hysterectomy (23); therefore, the question of ovarian preservation is an important public health issue. Some gynecologists suggest that the ovaries be removed at the time of hysterectomy to avoid the risk of future surgery. This appears to be an unfounded concern, and women can be reassured that the odds are low that they will require a subsequent oophorectomy. Women of all age groups should be thoroughly counseled regarding the risks and benefits of ovarian preservation.
Acknowledgments
Supported in part by grant DK 78924 and made possible by the Rochester Epidemiology Project (AG 034676 from the National Institute on Aging), US Public Health Service.
The authors thank Shunaha Kim-Fine, MD for assistance in data collection. Editing, proofreading, and reference verification were provided by the Section of Scientific Publications, Mayo Clinic.
Footnotes
Presented in abstract form at the 37th Annual Society for Gynecologic Surgeons Meeting, San Antonio, Texas, April 12, 2011.
Financial Disclosure: The authors did not report any potential conflicts of interest.
The authors have no conflicts of interest to disclose.
References
- 1.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Clin Obstet Gynecol. 2007 Jun;50(2):354–61. doi: 10.1097/GRF.0b013e31804a838d. [DOI] [PubMed] [Google Scholar]
- 2.Christ JE, Lotze EC. The residual ovary syndrome. Obstet Gynecol. 1975 Nov;46(5):551–6. [PubMed] [Google Scholar]
- 3.Naylor AC. Hysterectomy: analysis of 2,901 personally performed procedures. S Afr Med J. 1984 Feb 18;65(7):242–5. [PubMed] [Google Scholar]
- 4.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol. 2009 May;113(5):1027–37. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006 Oct;7(10):821–8. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 6.Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008 Sep;14(3):111–6. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005 Aug;106(2):219–26. doi: 10.1097/01.AOG.0000167394.38215.56. [DOI] [PubMed] [Google Scholar]
- 8.Berek JS, Chalas E, Edelson M, Moore DH, Burke WM, Cliby WA, et al. Society of Gynecologic Oncologists Clinical Practice Committee. Prophylactic and risk-reducing bilateral salpingo-oophorectomy: recommendations based on risk of ovarian cancer. Obstet Gynecol. 2010 Sep;116(3):733–43. doi: 10.1097/AOG.0b013e3181ec5fc1. [DOI] [PubMed] [Google Scholar]
- 9.ACOG. ACOG Practice Bulletin No. 89. Elective and risk-reducing salpingo-oophorectomy. Obstet Gynecol. 2008 Jan;111(1):231–41. doi: 10.1097/01.AOG.0000291580.39618.cb. [DOI] [PubMed] [Google Scholar]
- 10.Dekel A, Efrat Z, Orvieto R, Levy T, Dicker D, Gal R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996 Sep;68(1–2):159–64. doi: 10.1016/0301-2115(96)00250-3. [DOI] [PubMed] [Google Scholar]
- 11.Zalel Y, Lurie S, Beyth Y, Goldberger S, Tepper R. Is it necessary to perform a prophylactic oophorectomy during hysterectomy? Eur J Obstet Gynecol Reprod Biol. 1997 May;73(1):67–70. doi: 10.1016/s0301-2115(97)02702-4. [DOI] [PubMed] [Google Scholar]
- 12.Plockinger B, Kolbl H. Development of ovarian pathology after hysterectomy without oophorectomy. J Am Coll Surg. 1994 Jun;178(6):581–5. [PubMed] [Google Scholar]
- 13.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012 Dec;87(12):1202–13. doi: 10.1016/j.mayocp.2012.08.012. Epub 2012 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996 Mar;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 15.Rochester Epidemiology Project: Olmsted County Population Overview [Internet] c2009–2011 [cited 2011 Oct 15]. Available from: http://www.rochesterproject.org/researchers/population-overview.php.
- 16.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, et al. Data Resource Profile: The Rochester Epidemiology Project (REP) medical records linkage system. Int J Epidemiol. 2012 Dec;41(6):1614–24. doi: 10.1093/ije/dys195. Epub 2012 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.StSauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012 Feb;87(2):151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babalola EO, Bharucha AE, Schleck CD, Gebhart JB, Zinsmeister AR, Melton LJ., 3rd Decreasing utilization of hysterectomy: a population-based study in Olmsted County, Minnesota, 1965–2002. Am J Obstet Gynecol. 2007 Mar;196(3):214e1–7. doi: 10.1016/j.ajog.2006.10.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melton LJ., 3rd The threat to medical-records research. N Engl J Med. 1997 Nov 13;337(20):1466–70. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 20.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983 Jun;39(2):499–503. [PubMed] [Google Scholar]
- 21.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005 Jul;112(7):956–62. doi: 10.1111/j.1471-0528.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 22.Moorman PG, Myers ER, Schildkraut JM, Iversen ES, Wang F, Warren N. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011 Dec;118(6):1271–9. doi: 10.1097/AOG.0b013e318236fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008 Jan;198(1):34e1–7. doi: 10.1016/j.ajog.2007.05.039. Epub 2007 Nov 5. [DOI] [PubMed] [Google Scholar]
- 24.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance: United States, 1994–1999. MMWR Surveill Summ. 2002 Jul 12;51(SS05):1–8. [PubMed] [Google Scholar]
- 25.Davies A, O’Connor H, Magos AL. A prospective study to evaluate oophorectomy at the time of vaginal hysterectomy. Br J Obstet Gynaecol. 1996 Sep;103(9):915–20. doi: 10.1111/j.1471-0528.1996.tb09912.x. [DOI] [PubMed] [Google Scholar]
- 26.Sheth SS. Adnexectomy for benign pathology at vaginal hysterectomy without laparoscopic assistance. BJOG. 2002 Dec;109(12):1401–5. doi: 10.1046/j.1471-0528.2002.00100.x. [DOI] [PubMed] [Google Scholar]
- 27.Gross CP, Nicholson W, Powe NR. Factors affecting prophylactic oophorectomy in postmenopausal women. Obstet Gynecol. 1999 Dec;94(6):962–8. doi: 10.1016/s0029-7844(99)00452-4. [DOI] [PubMed] [Google Scholar]
- 28.Asante A, Whiteman MK, Kulkarni A, Cox S, Marchbanks PA, Jamieson DJ. Elective oophorectomy in the United States: trends and in-hospital complications, 1998–2006. Obstet Gynecol. 2010 Nov;116(5):1088–95. doi: 10.1097/AOG.0b013e3181f5ec9d. [DOI] [PubMed] [Google Scholar]
- 29.Lowder JL, Oliphant SS, Ghetti C, Burrows LJ, Meyn LA, Balk J. Prophylactic bilateral oophorectomy or removal of remaining ovary at the time of hysterectomy in the United States, 1979–2004. Am J Obstet Gynecol. 2010 Jun;202(6):538e1–9. doi: 10.1016/j.ajog.2009.11.030. Epub 2010 Jan 13. [DOI] [PubMed] [Google Scholar]
- 30.Sightler SE, Boike GM, Estape RE, Averette HE. Ovarian cancer in women with prior hysterectomy: a 14-year experience at the University of Miami. Obstet Gynecol. 1991 Oct;78(4):681–4. [PubMed] [Google Scholar]
- 31.Melton LJ, 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003 May;18(5):900–5. doi: 10.1359/jbmr.2003.18.5.900. [DOI] [PubMed] [Google Scholar]
- 32.Finch A, Metcalfe KA, Chiang JK, Elit L, McLaughlin J, Springate C, et al. The impact of prophylactic salpingo-oophorectomy on menopausal symptoms and sexual function in women who carry a BRCA mutation. Gynecol Oncol. 2011 Apr;121(1):163–8. doi: 10.1016/j.ygyno.2010.12.326. Epub 2011 Jan 8. [DOI] [PubMed] [Google Scholar]