Abstract
HIV-1 Nef is a multifunctional viral protein that contributes to higher plasma viremia and more rapid disease progression. Nef appears to accomplish this, in part, through modulation of T-cell activation; however, the results of these studies over the past 25 years have been inconsistent. Here, the history of contradictory observations related to HIV-1 Nef and its ability to modulate T-cell activation is reviewed, and recent reports that may help to explain Net’s apparent ability to both inhibit and activate T cells are highlighted.
Keywords: HIV-1, Nef, pathogenesis, replication, signal transduction, T-cell activation, T-cell receptor
HIV-1 is a major global health challenge. In 2011, approximately 34 million individuals were living with HIV-1, resulting in 2.5 million new infections and 1.7 million deaths [1]. HIV-1 replicates predominantly in CD4+ T cells, which serve a critical role as ‘helper’ cells for the immune system [2]. Loss of CD4+ cells as a result of infection impairs host immunity and increases morbidity and mortality associated with normally benign infections and cancers, resulting in AIDS [3,4]. The HIV-1 Nef protein contributes to high plasma viremia and disease progression [5–8], in part, through modulation of T-cell activation, including cytokine expression, transcription factor function and other signal transduction events [9]; however, the results of these studies have been inconsistent [10]. Here, the authors review the history of contradictory observations related to Nef and T-cell activation, and highlight new reports that may help to explain Nef’s apparent ability to both inhibit and activate T cells. A better understanding of the role of Nef in HIV-1 pathogenesis may enhance treatment strategies and assist in the development of novel approaches to curing HIV-1.
HIV-1 Nef: a ‘negative factor’?
Nef is expressed early following infection of the host cell [11]. This approximately 206-amino acid, 24–32-kDa protein can manipulate host cell processes, including receptor endocytosis, protein degradation and intracellular signaling, which enhances in vivo replication, progeny production and immune evasion [5,12,13]. Many Nef functions have been identified, of which downregulation of the viral entry receptor CD4 and host immune molecules HLA-I are most well characterized [5,12,13]. Nef is modified by myristoylation and it associates with intracellular membranes. Localization of Nef to the plasma membrane is required to downregulate CD4 and HLA-I. Cytoplasmic- and cytoskeleton-bound fractions of Nef may modulate other cellular activities through interactions with intracellular kinases, protein-sorting complexes and actin network components [14].
Early studies of HIV-1 ‘open reading frame b’ suggested that it inhibited virion production and viral replication [15,16], resulting in it being called ‘negative factor’ or Nef. Subsequent reports indicated that Nef silenced gene expression, supporting the notion that Nef dampens T-cell stimulation to prolong viral progeny production [17,18]. However, a series of later studies observed that Nef enhanced HIV-1 replication in primary cells. Spina et al. demonstrated that Nef-deleted viruses replicated poorly in quiescent CD4+ T cells [19] and Miller et al. highlighted the ability of Nef to enhance virion infectivity [20]. Therefore, contrary to its name, a consensus has emerged that Nef enhances infection. This model of Nef as a ‘positive factor’ is consistent with in vivo observations that Nef-deleted strains of HIV and SIV are less pathogenic [7,8,21].
Relevance of T-cell activation for HIV-1 pathogenesis
Antigen-stimulated (‘activated’) CD4+ T cells are highly permissive to HIV-1 infection [22,23], due, in part, to increased metabolic activity and reduced expression of intrinsic antiviral host restriction factors [24–26]. Maintenance of a ‘semi-activated’ state may be required for efficient production of viral progeny [27,28], since over-stimulation results in activation-induced cell death [29] that would shorten the lifespan of infected cells.
In addition to a productive replication cycle, HIV-1 establishes latent infection in resting CD4+ T cells, allowing persistence of proviral genomes, despite immune responses or initiation of drug therapy [30]. Recent studies have begun to elucidate the viral and cellular mechanisms involved in the establishment and maintenance of latency [30,31], and differences in T-cell activation state are likely to play a role. An improved understanding of the factors involved in regulating HIV-1 latency could provide new avenues for treatment or lead to strategies capable of eradicating the virus from an infected individual [30].
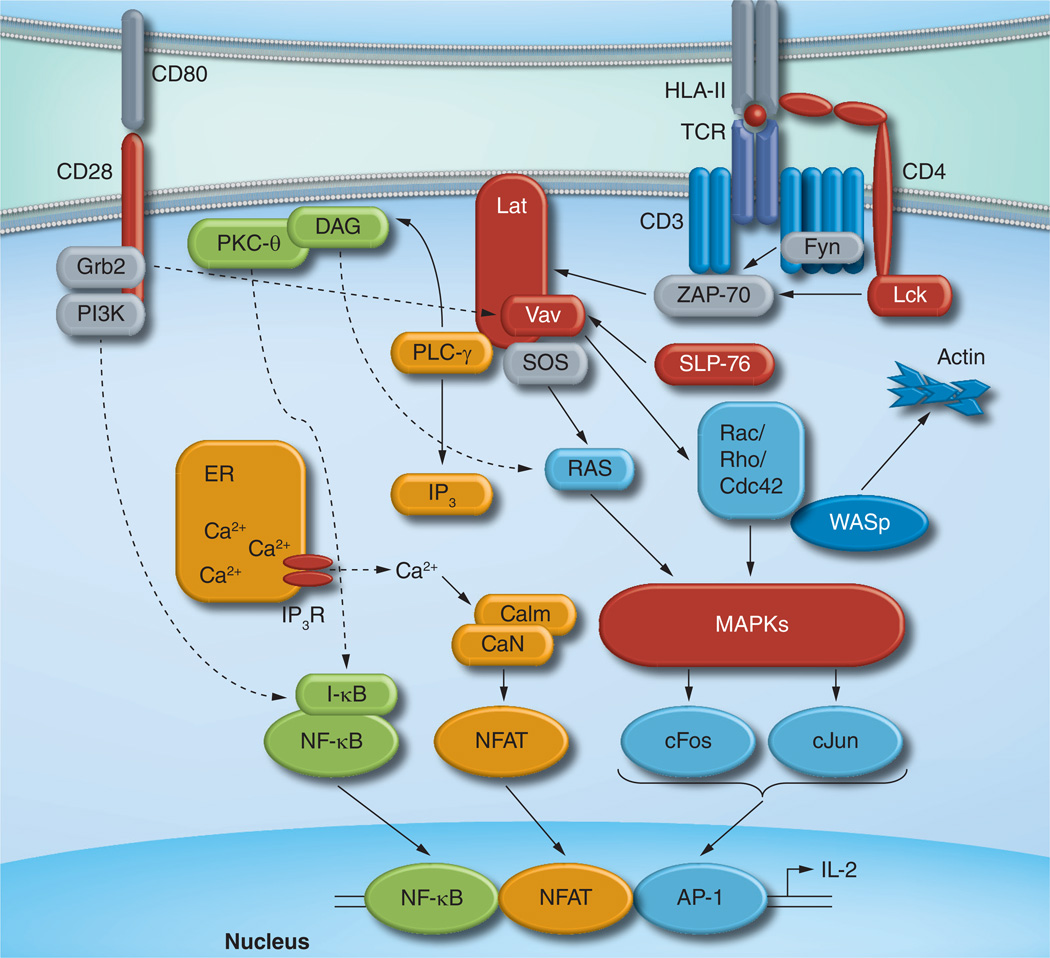
CD4+ T-cell activation occurs when its T-cell receptor (TCR)/CD3 complex engages a foreign peptide presented by HLA class II on an APC (Figure 1). TCR recognition initiates a series of events, including rearrangement of the actin cytoskeleton, stabilization of adhesion molecule binding and recruitment of new proteins to the plasma membrane [32]. This results in the formation of a TCR–APC contact zone, referred to as an ‘immune synapse’, which further enhances TCR signaling. A cascade of phosphorylation events ensues, involving p56-Lck and ZAP-70, adapter protein SLP-76, transmembrane protein LAT and others. These proximal events initiate distal signaling pathways, including MAPKs, PKC-θ and Ca2+/ Calcineurin, resulting in translocation of three critical transcription factors into the nucleus: nuclear factor of activated T cells (NFAT), AP-1 and NF-κB.
Figure 1. T-cell receptor signaling.
Recognition of peptide/HLA ligand by TCR leads to cellular activation and IL-2 production. The TCR/CD3 complex mediates proximal signaling events through recruitment of cellular kinases (Lck, Fyn and ZAP-70), adapter molecules (SLP-76) and the scaffold protein LAT. Distal events result in the activation of three key transcription factors (NFAT, NF-κB and AP-1) and their translocation into the nucleus. NFAT is triggered by CaN through a process that requires PLC-γ, IP3, release of Ca2+ from the ER and Calm (shown in orange). Activation of NF-κB requires degradation of I-κB, which is mediated by DAG and PKC-θ (shown in green) and enhanced by costimulatory signals provided by CD28. AP-1, consisting of cFos and cJun, is stimulated by MAPK cascades that are triggered by the Ras family GTPases, including Ras, Rac, Rho and Cdc42 (shown in blue). WASp initiates actin cytoskeletal rearrangement. Proteins whose activities are reported to be modulated by HIV-1 Nef are indicated in red.
Calm: Calmodulin; CaN: Calcineurin; DAG: Diacylglycerol; NFAT: Nuclear factor of activated T cells; TCR: T-cell receptor.
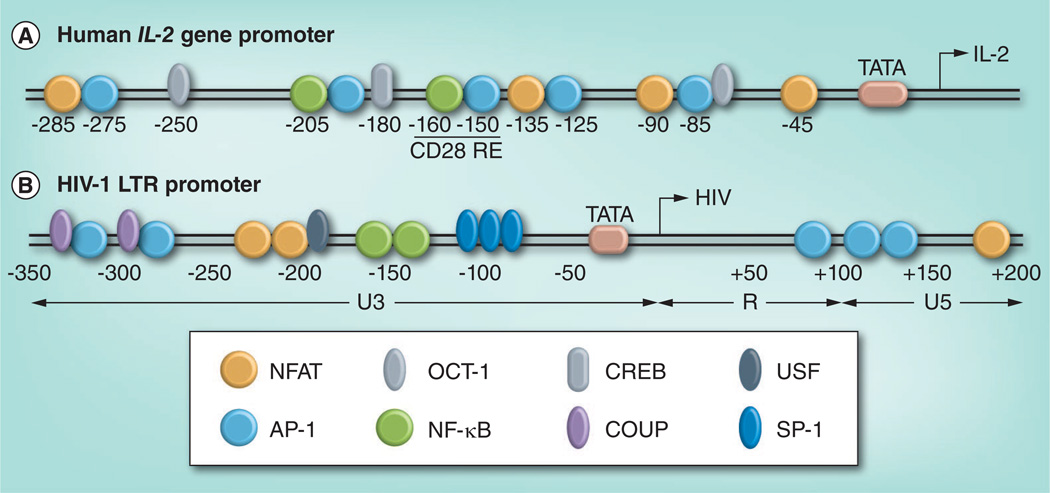
The affinity and kinetics of TCR binding determines the effectiveness of signaling [33]. Induction of IL-2 expression requires stimulation of NFAT, AP-1 and NF-κB transcription factors (Figure 2A), and this cytokine is therefore a hallmark of robust CD4+ T-cell activation. Secreted IL-2 enhances T-cell proliferation and is crucial for immunity against infection [34]. Notably, the HIV-1 long terminal repeat (LTR) promoter sequence also encodes binding sites for these transcription factors (Figure 2B) and TCR-mediated signaling events, among others, can reactivate latent proviral genomes [35].
Figure 2. Human IL-2 and HIV-1 long terminal repeat promoters.
The location of critical transcription factor binding sites in (A) the human IL-2 gene promoter and (B) the HIV-1 LTR sequence are indicated. In addition to NFAT, NF-κB and AP-1 (discussed in the text), constitutive transcription factors, OCT-1, COUP, USF-1 and SP-1, as well as CREB, contribute significantly to promoter activity.
COUP: Chicken ovalbumin upstream promoter; LTR: Long terminal repeat; NFAT: Nuclear factor of activated T cells; OCT-1: Octamer transcription factor 1; SP-1: Specificity protein 1; USF-1: Upstream stimulatory factor 1.
Effects of HIV-1 Nef on IL-2 gene expression
To investigate the impact of Nef on T-cell activation, its ability to modulate IL-2 has been assessed. Most reports indicate that Nef alters IL-2 expression; however, both positive and negative effects of Nef have been observed using similar experimental approaches.
Initial studies indicated that Nef reduced the ability of Jurkat T cells to induce IL-2 mRNA or to activate IL-2 promoter-driven gene expression following stimulation with phorbol 12-myristate 13-acetate (PMA)/phytohemagglutinin or CD3 antibody [36,37]. However, a series of reports observed the opposite effect, namely that Nef enhanced IL-2 expression. Rhee et al. found that Nef augmented CD3 -mediated activation of a murine T-cell hybridoma to produce IL-2 38]. Nef was subsequently shown to increase IL-2 secretion following CD3/CD28 stimulation using both Jurkat cells and peripheral blood mononuclear cell [39,40]. Other results using Jurkat cells indicated that Nef protein or HIV-1 infection enhanced IL-2 promoter-driven gene expression [41,42], and indicated that myristoylation, the proline-rich SH3 -binding motif, and the PAK2 binding motifs of Nef were required for this function [43].
Further complicating the story, Nef was shown to enhance IL-2 production in resting CD4+ cells following stimulation with PMA-ionomycin, but not CD3/CD28 [44]. Arhel et al. observed that HIV-1 Nef did not affect IL-2 production in virus-infected primary cells [45], and Thoulouze et al. found that although HIV-infected Jurkat cells displayed increased IL-2 production compared with uninfected cells, Nef-deleted viruses resulted in higher levels of IL-2 compared with wild-type HIV-1 [46].
Regulation of key transcription factors & signaling molecules by Nef
In the context of Nef and T-cell activation, the transcription factors NFAT, AP-1, and NF-κB, the tyrosine kinase p56-Lck, and the cytoskeleton modulator PAK2 have received greatest attention. We briefly review key observations for each here.
NFAT
The NFAT family of transcription factors is activated by TCR-mediated events through the Ca2+/calmodulin (Calm) pathway, and is critical for induction of IL-2 [47]. TCR binding initiates an inositol triphosphate (IP) signaling cascade, leading to an influx of calcium into the cytoplasm, which binds Calm and, in turn, activates the Calm-dependent serine/threonine phosphatase Calcineurin (CaN). Dephosphorylation of cytoplasmic NFAT family members NFATl and NFAT2 by CaN allows their translocation into the nucleus and their binding to promoter sequences 48]. Since NFAT binding sites are present in the HIV-1 LTR, over-expression of NFAT-induced viral replication in resting T cells [49,50].
Nef has most often been described to hyperactivate NFAT [51,52]. Wang et al. observed that Nef localized to membrane lipid rafts and that increased IL-2 secretion following CD3/CD28 stimulation was associated with enhanced NFAT signaling [40]. NFAT was also determined to contribute to increased Nef-mediated responsiveness of infected CD4+ T cells [53]. Other groups have observed similar results using NFAT promoter-driven luciferase constructs [42]. Nef may also activate NFAT in the absence of TCR signaling through synergistic interactions with the Ras/MAPK pathway [52] or through direct binding to IP3 receptors (IP3R), causing release of intracellular calcium stores [54]. TCR-independent activation of NFAT appears to be conserved among Nef alleles and is dependent on Nef motifs involved in membrane localization and SH3 binding [51]. Recent studies suggest that Nef’s ability to modulate NFAT following TCR activation may be dependent on the status of the cell, since Nef hyperinduces NFAT and IL-2 in quiescent T cells, but impairs NFAT and IL-2 in suboptimally activated T cells [55].
In contrast with these results, inhibition of NFAT-driven luciferase was observed when Jurkat T cells were transfected with a Nef-CD8 fusion protein and stimulated with peptide-pulsed APC [56]. Of interest, this function was associated with Nef-mediated modulation of Vav, possibly using PAK2 as an intermediary.
AP-1
The AP-1 transcription factor is a heterodimer of cFos and cJun proteins activated by MAPK. There are three major members of the MAPK family: ERK, JNK and p38. cFos is activated by ERK, while cJun is activated by JNK. MAPK cascades are essential for the regulation of cell differentiation, proliferation and death [57], and AP-1 binding contributes to the expression of numerous cellular genes, including IL-2 [58]. The HIV-1 genome encodes three adjacent AP-1 binding sites located within the R5 region of the LTR (Figure 2B) and three intragenic AP-1 sites within the pol gene, which contribute to viral replication [59]. Other studies have demonstrated that ERK activity is required for HIV-1 infectivity [60] and reverse transcription [61], as well as virion assembly and release [62].
Early studies reported that HIV-1 Nef inhibited AP-1 activity through an effect on TCR-dependent signaling [18,63]. Greenway et al. also demonstrated that Nef’s proline-rich domain bound to MAPK and inhibited its kinase activity [64]. These findings contrast with subsequent studies, which have described either little effect of Nef on AP-1 [65] or increased activation of MAPK by Nef. Fortin et al. reported higher AP-1 activity due to Tat and Nef in virus-infected T cells 53]. Schrager et al. indicated that Nef increased ERK activity following TCR stimulation [66]. These results are consistent with Witte et al, who reported that Nef enhanced Tat-mediated HIV transcription through an Lck-mediated process that required stimulation of ERK by PKC-8 [67]. Activation of cJun by JNK is also required for AP-1 function, and HIV-1 Nef has been reported to induce the JNK pathway through interaction with the guanine nucleotide exchange factor, Vav [68]. However, in other studies, Nef failed to enhance JNK activity in the presence or absence of TCR stimulation [66].
NF-κB
The NF-κB transcription factor plays a key role in cellular activation and proliferation. It is a heterodimer comprised of one ankyrin repeat-containing subunit (p50 or p52) and one trans-activation domain-containing subunit (p65/ RelA, RelB or c-Rel). Cytoplasmic NF-κB is sequestered by I-κB and activated by various stimuli, including TCR activation coupled with a costimulatory signal typically provided by CD28 [69]. Binding of CD28 to CD80 (or CD86) on the APC activates PI3K which triggers Akt and then I-κB kinase to phosphorylate I-κB, resulting in its ubiquitinylation and degradation, thereby releasing NF-κB for translocation to the nucleus [69]. NF-κB binding sites located in the HIV-1 LTR are critical for viral replication [70–73].
Consistent with early evidence that Nef inhibited IL-2, initial studies indicated that Nef suppressed NF-κB by interfering with a TCR-derived signal [63,74]. In contrast, other reports suggested that Nef enhanced NF-κB activity. Wang et al. observed that Nef recruitment to membrane lipid rafts promoted NF-κB [40]. Fortin et al. reported that Nef increased NF-κB nuclear translocation in Jurkat cells after stimulation with PMA-ionomycin or CD3/CD28 [53]. Yet another series of studies indicated that Nef had no effect on NF-κB: Collette et al. observed that CD28 signaling did not contribute to Nef-mediated modulation of IL-2 [75], and Yoon et al. reported that there was no influence of Nef on NF-κB or AP-1 [65]. Furthermore, Schrager et al. found that Nef did not alter I-kB phosphorylation in CD4+ T cells [66]. Echoing these results, a report by Witte et al. observed that induction of HIV-1 transcription by Nef involved Lck and PKC-θ recruitment to membrane lipid rafts, but resulted to activation of ERK rather than NF-κB 67]. In addition, Neri et al. demonstrated that Nef had no effect on early substrates in the NF-κB pathway (including I-κBα) or phosphorylation of NF-κB following stimulation with CD3/CD28 [55].
p56-Lck
NeF’s proline-rich motif binds to the SH3 domain of Lck, a member of the Src family of protein tyrosine kinases [76–78]. Lck is a ‘master regulator’ of TCR signaling that is recruited to the plasma membrane by CD4. Upon TCR engagement, Lck phosphorylates immunoreceptor tyrosine-based activation motifs on the TCR/CD3C chain. Phosphorylated TCR recruits ZAP-70 kinase, which is also phosphorylated by Lck. Activated ZAP-70 phosphorylates downstream signaling molecules, including transmembrane protein LAT and adapter protein SLP-76. Of note, Nef is reported to interact with other Src family kinases, including Hck and Fyn [79–81], which may alter intracellular signaling.
CD4-Lck binding & altered Lck localization
Bandres et al. observed that Nef-mediated downregulation of CD4 was more efficient in the presence of Lck [82]; however, other studies indicated that Nef and Lck interact with the same hydrophobic motif on CD4’s tail 83]. Furthermore, Nef’s proline-rich motif is dispensable for CD4 downregulation [78] and Nef’s ability to disrupt the CD4-Lck complex and to target CD4 to lysosomes for degradation are genetically separable [84,85]. Nef inhibits Lck clustering at the TCR, and Lck is instead retained in endosomal compartments [46,86,87]. Haller et al. further demonstrated that Nef uses distinct mechanisms to retain Lck within these compartments and to block Lck recruitment to the TCR [86], and Rudolph et al. have indicated that this function is conserved among HIV and SIV Nef proteins [88]. These results suggest that Nef displaces Lck from CD4 and redistributes it to an intracellular location.
Modulation of Lck phosphorylation
Several studies have indicated that Nef inhibits Lck phosphorylation and blocks its ability to activate downstream targets [64,76,77,89,90]. Reduced Lck phosphorylation was associated with Nef’s ability to disturb immune synapse formation [46,86], for which Nef’s proline-rich motif appeared to be essential [87]. However, Abraham et al. reported that Nef did not reduce Lck’s ability to phosphorylate its immediate downstream target, ZAP-70 [91]. Rather, they observed that Nef blocked subsequent steps required for TCR signaling: namely LAT recruitment to TCR microclusters, LAT phosphorylation, and the interaction between LAT and SLP-76 [91]. This is consistent with previous work by Haller et al, which also found that Nef inhibited the recruitment of phosphorylated LAT to the immune synapse [87]. In contrast, other studies have concluded that Nef activates Lck, which, in turn, stimulates PKC-8 and upregulates ERK signaling [67,92].
PAK2
P21-associated kinases (PAKs) are serine/threonine kinases that regulate the small GTPases Cdc42 and Rac. PAKs are involved in early signaling events that lead to MAPK [93], and they function in cytoskeleton remodelling and apoptosis [94]. PAK2 mutants that lack the kinase domain block TCR-induced CD69 upregulation, Ca2+ flux, NFAT activation and IL-2 production in T cells [95]. Sawai et al. first reported the presence of a Nef-associated kinase (NAK) [96], and Nef binding to NAK was found to depend on Cdc42 and Racl [97]. NAK was identified as a PAK family member [98–100], and Renkema et al. determined it to be PAK2 101]. Mutational analysis studies have identified amino acids within HIV-1 Nef that are critical in the interaction of Nef with PAK2. The Nef myristoylation residue G2, SH3-binding domain and the highly conserved arginine R105/R106 were shown to be required for Nef interaction with PAK2 [102,103]. Agopian et al. further demonstrated that Nef amino acids at positions 85, 89, 187, 188 and 191 (L, H, S, R and F in the clade B consensus, respectively) are critical for PAK2 association [104]. Using primary Nef isolates Foster et al. found that Nef mutants S189R and F193I were defective for Nef-FAK2 association [105]. Mutational analysis of PAK2 revealed that an intact Cdc42–Racl interactive binding motif was required for Nef–PAK2 interaction [106]. While the majority of Nef research has been conducted on PAK2, other studies have indicated that PAK1 contributes to NAK activity [107], and that PAK1 may be important for Nef-mediated enhancement of virion production [108].
Nef association with PAK2 is a conserved of property of HIV-1, HIV-2 and SIV. This interaction occurs in membrane lipid rafts and forms a multiprotein complex comprised of Nef, PAK2, PI3K, GTPases Rac and Cdc42, and Vavl [109,110], referred to as the Nef–PAK2 signalosome. Through interactions with PI3K, Nef–PAK2 may increase virion production [109] and prevent apoptosis [111]. More recently, it was observed that PAK2 binding is important for Nef-mediated enhancement of cellular activation and viral replication in primary T cells [43]. TCR-mediated remodeling of the actin cytoskeleton is necessary for cellular activation [112,113]. Nef associates with actin [114] and Nef-PAK2 can inhibit actin rearrangement [86,87]. Nef has been shown to modulate the key actin organizer WASp [87] and to deregulate the actinsevering factor cofilin [115,116], which may alter the immune synapse formation required for early events in TCR activation.
Effects on surface receptors that may contribute to T-cell activation CD3/TCR
HIV-2 and most SIV Nef alleles efficiently downregulate CD3/TCR [117,118]. This Nef activity, which has been shown to potently suppress T-cell activation in vitro, was lost in the lentiviral lineage that gave rise to HIV-1 [41]. The resulting lack of CD3 downregulation function by HIV-1 Nef has been suggested to contribute to the high pathogenicity observed with human infection compared with that of natural hosts [45]. A recent study demonstrated that HIV-2 Nef-mediated downregulation of CD3 and CD28 was associated with higher CD4 counts [119], but symptomatic HIV-2 patients also displayed Nef clones able to downregulate CD3 [120], and CD3 down-regulation alone was insufficient to explain SIV virulence [121].
CD4
Nef’s ability to downregulate CD4 is well characterized [122–124]. In addition to its role in viral entry, CD4 enhances TCR signaling by recruiting Lck to the immune synapse [125]. CD4 endocytosis is a normal response to TCR stimulation [10]; however, internalization of CD4 by Nef is independent of T-cell activation and has been suggested to modulate early events requiring Lck. Indeed several studies indicated that Nef-mediated downregulation of CD4 may alter TCR signaling [126,127], while other reports have determined that Nef-mediated inhibition of TCR signaling is independent of its CD4 downregulation function [46,51,128].
CD28
HIV and SIV Nef proteins downregulate cell-surface CD28 expression [119,129,130]. Costimulation provided by CD28 is necessary to elicit optimal antigen-specific T-cell activation, and absence of costimulation often results in T-cell anergy [131]. This Nef activity may contribute to pathogenesis, since in vivo reversion of H196Q, a codon important for CD28 downregulation [129], was associated with progression in SIV-infected rhesus macaques [132].
Nef-mediated activation of uninfected bystander T cells
HIV-1 Nef can also alter the activation of bystander uninfected T cells by modifying protein expression in APCs. In HIV-infected macrophages, a cell type targeted for HIV infection [133,134], HIV-1 Nef induces the production and release of two CC-chemokines, macrophage inflammatory proteins lα (CCL2) and 1β (CCL4), resulting in chemotaxis and activation of resting T lymphocytes, and thereby facilitating productive viral infection [135]. Furthermore HIV-1 Nef intersects the CD40 signaling pathway in macrophages, inducing the release of the soluble forms of intercellular adhesion molecule ICAM-1 and the coactivation molecule CD23, which, in turn, promotes interactions between B cells and T cells that render the T cell permissive to HIV-1 [136]. However, HIV-1-infected dendritic cells (DCs) cannot significantly increase resting CD4+ T-cell proliferation in DC–T-cell cocultures [137].
Soluble Nef can be detected in HIV-1-infected patient sera [138], and extracellular Nef may also contribute to T-lymphocyte activation. Indeed, recombinant Nef can enter monocytes and macrophages, and induce IL-15 synthesis resulting in activation of peripheral blood mononuclear cell [139]. Furthermore, it has been shown that recombinant Nef triggers a series of phenotypic and functional changes that promote DC differentiation, enhancing the ability of these cells to activate bystander CD4+ T cells [140].
Role of HIV-1 Nef in T-cell apoptosis
HIV-1 Nef is one of several viral proteins that have been described to modulate lymphocyte cell death. The role of Nef in apoptosis remains controversial, with both inhibitory and pro-apoptotic activities reported. The earliest study to assess the effects of HIV-1 Nef on apoptosis was by Fujii et al. [138]. This group observed that soluble Nef could bind to the surface of CD4+ T cells, and that the cross-linking of these proteins using anti-Nef antibodies was cytotoxic to uninfected cells [138]. These findings were substantiated by other studies reporting that soluble Nef bound not only to CD4+ T cells, but also CD8+ T cells, B cells, macrophages and neutrophils, and that cross-linking of membrane-bound Nef led to an apoptosis pathway that was independent of Fas (CD95) [141] and that could be blocked by serine/ threonine protein kinase inhibitors [142]. Lenassi et al. later described that this soluble Nef exited infected cells via exosomes to cause the apoptosis of bystander CD4+ T cells [143]. Various studies have reported enhancement of apoptosis by Nef through upregulation of cell surface Fas and Fas ligand [144,145], which could also serve as a mechanism to evade immune responses by initiating the death of antiviral CD8+ T cells [144]. Another mechanism employed by Nef to modulate apoptosis is by upregulation of the programmed death-1 surface protein, which appears to be dependent on the activation of p38/MAPK [146]. Nef also reduces the expression of antiapoptotic proteins Bcl-2 and Bcl-XL as a mechanism to increase apoptosis [147]. Finally, soluble Nef is reported to induce CXCR4-mediated apoptosis [148,149].
HIV-1 Nef has also been reported to inhibit apoptosis. In association with PAK and PI3Ks, Nef phosphorylates the cellular protein Bad to enhance its antiapoptotic properties [111]. In addition, Nef inhibits the function of ASK1 [150]. This prevents cell death that could result from cis-ligation of Nef-induced Fas and FasL on the surface of virus-infected cells, and simultaneously protecting the virus-infected cells from Fas and TNF-α death signaling pathways. It has also been reported that the survival of productively infected CD4+ T lymphocytes requires Nef expression by HIV-1, as well as activation by TNF-α expressed on the surface of macrophages [151]. Finally, Nef has been reported to bind the tumor suppressor protein p53 and to inhibit its ability to mediate apoptosis [152].
Making sense of discrepancies in the literature
Research on Nef and T-cell activation has yielded divergent results. On the one hand, substantial data suggests that Nef enhances activation –including increased nuclear translocation of transcription factors and IL-2 expression. On the other hand, Nef appears to disrupt TCR signaling – including reduced Lck activity, LAT phosphorylation and cytoskeletal reorganization. These contradictions are not new to the field and several factors have been proposed to contribute to these diverse observations.
Intracellular localization
Baur et al. investigated the importance of intracellular localization by fusing Nef to CD8 glycoprotein [89]. They observed that plasma membrane-targeted Nef activated TCR signaling and induced the NF-κB pathway, whereas cytoplasmic Nef inhibited both of these events 89]. The notion that Nef causes differential effects on T-cell activity depending upon its intracellular location remains an attractive hypothesis to resolve discrepancies in experimental results. While early work by Kaminchik et al. indicated that a naturally occurring N-terminally truncated Nef protein failed to localize to the plasma membrane [153], to our knowledge, potential differences in the localization of patient-derived Nef alleles has not been examined systematically. In addition, the concentration of Nef or the method used for protein expression (transient or stable) might also affect the relative localization of Nef within a cell, thereby shifting the balance of activating and inhibitory mechanisms.
Alternative signaling pathways
Over the past year, the Fackler group has reported a series of observations showing that, although Nef disrupts classical TCR signaling, this protein also organizes an alternative pathway that can enhance cellular activation. Abraham et al. demonstrated that Nef inhibited early events in TCR signaling downstream of ZAP-70, including LAT recruitment to TCR microclusters, SLP-76 activation and actin reorganization [91]. Notably, Nef also redirects Lck from the plasma membrane to the trans-golgi network and stimulates Lck-mediated activation of Ras/MAPK signaling [154]. A recent review by Abraham and Fackler provides additional discussion of this topic [155].
Cell type & intrinsic activation state
Numerous reports utilizing immortalized Jurkat T-cell lines have reported either Nef-mediated enhancement or inhibition of T-cell activation (Table 1), and it remains an important task to systematically evaluate and explain the contradictory results generated using presumably similar cells. Despite their utility as a well-established model for analysis of T-cell function, there are caveats to using Jurkat cells for Nef studies. Jurkat tumor cells display a constitutive partially active phenotype that manifests in an idiosyncratic expression profile of several signaling molecules, which has led to mischaracterization of PI3K–dependent signaling events [156] and of activation-dependent mechanisms of Nef-mediated HLA-I downregulation [157,158]. Therefore, Nef’s effect on activation in Jurkat cell lines may not be fully representative of its effect in primary cells. A positive role for Nef in HIV-1 replication was demonstrated in primary CD4+ T cells [19], and Nef-mediated enhancement of TCR signaling can contribute to this outcome [39,66]. Although these and other results using primary cells support an emerging consensus that Nef is a ‘positive factor’, Neri et al. recently reported that the intrinsic activation state of primary T cells is important for Nef function, since Nef was able to enhance IL-2 expression in quiescent CD4+ T cells, but not in preactivated cells [55]. Thus, it remains too simplistic to argue that Nef’s negative effects on T-cell signaling are merely artefacts of using Jurkat cell lines, or that Nef always promotes activation in primary cells. Subtle differences in the phenotype of primary T cells, how they are prepared or propagated, or inherent differences between cell lines may affect signaling pathways or protein interactions and tip the balance of Nef function in favor of T-cell activation or suppression.
Table 1.
Contradictory observations regarding HIV-1 Nef and T-cell activation†.
| Cell type | Expression system |
Nef effect | Stimulation (outcome) | Ref. |
|---|---|---|---|---|
| Jurkat cells |
Transient | Positive | PMA (NFAT); CD3/CD28 (ERK) | [51,52,56] |
| No effect | PMA/PHA (NF-κB, AP-1); CD3/CD28 (NFAT); APC (NF-κB) | [52,56,65] | ||
| Negative | PHA/PMA (NF-κB); CD3/CD28 (CD69, Actin, Lck); APC (NFAT) | [56,74,86,87,159] | ||
| Stable | Positive | CD3 (NF-κB); CD3/CD28 (NFAT, NF-κB, IL-2) | [39,40,42,89,159] | |
| Negative | PMA/PHA (NF-κB, IL-2, HIV LTR); PMA/CD2 (IL-2); CD3 (Ca2+, AP-1, NF-κB, TCR) | [36,63,74,89] | ||
| Virus infection | Positive | CD3/28 (NFAT, AP-1, NF-κB, IL-2) | [41,53] | |
| Negative | APC (IL-2, Lck) | [46] | ||
| Primary CD4+ T cells |
Transient | Positive | CD3/CD28 (IL-2) | [44] |
| Negative | PHA (Lck); IL-2 (proliferation) | [77] | ||
| Stable | Positive | CD3 (ERK); CD3/CD28 (IL-2) | [39,66] | |
| No effect | CD3/CD28 (NF-κB) | [66] | ||
| Virus infection | Positive | CD3/CD28 (IL-2, NFAT); APC (ERK) | [55,154] | |
| No effect | CD3/CD28 (Zap-70, NF-κB, Lck); APC (IL-2) | [45,55] | ||
| Negative | APC (Lck) | [46] | ||
Selected publications describing the effect of HIV-1 Nef on T-cell activation. Stimulation reagent(s) and outcome(s) measured are indicated.
APC: Antigen-presenting cell; CD3: Anti-CD3 antibody; CD28: Anti-CD28 antibody; Lck: Lymphocyte-specific protein tyrosine kinase; NFAT: Nuclear factor of activated T cells; PHA: Phytohemagglutinin; PMA: Phorbol 12-myristate 13-acetate; TCR: T-cell receptor.
Stimulation conditions
Most early experiments that observed Nef-mediated inhibition used PHA/PMA [36,74] or anti-CD3 antibody alone [38,63,89] to stimulate cells. One related study found that stimulation with anti-CD28 did not affect Nef’s inhibitory role [75]. By contrast, later studies have employed anti-CD3 and anti-CD28 that are either cross-linked [39,40,42,44,53], presented on beads [45,66,159] or bound to coverglass to mimic the immune synapse [86]; however, the stimulation dose in these assays may not reflect biological interactions with APC. This limitation may be overcome by activating T cells with peptide-pulsed APCs [45,46,56]. It would be of interest to systematically investigate whether reagents or dose can contribute to divergent findings.
Given that early and late signaling events require different kinetics, assay duration may be a complicating factor. Many studies noting a negative role for Nef in activation looked at transcription factor translocation or IL-2 transcription, requiring stimulation of 4 h [36,63,74] or less [89], while those assessing proximal TCR signaling events measured outcome after only minutes [45,46,55,86,89]. By contrast, many studies noting a positive role for Nef used IL-2 secretion or IL-2/NFAT-driven luciferase as activation markers, and often required longer assay times [39,40,55,159]. It would be useful to investigate whether longer stimulation conditions allow for compensatory processes or feedback loops.
Conclusion
Recent studies have attempted to reconcile a long history of divergent results by proposing more sophisticated models of HIV-1 Nef’s ability to modulate T-cell activation state. Rather than describing Nef as a ‘negative’ or a ‘positive’ factor, new results indicate that this protein may act in both capacities in virus-infected cells. By inhibiting proximal TCR-mediated signaling events, Nef can prevent activation-induced cell death and, thereby, extend the lifespan of virus-infected cells. By redirecting Lck and other kinases to intracellular compartments, Nef can stimulate transcription factor activity that is required to maintain viral gene expression and to enhance progeny virion production. Different in vitro experimental approaches may favor one of these mechanisms over the other, leading to seemingly contradictory observations; however, it is likely that both Nef functions contribute to the ability of this protein to promote in vivo HIV-1 replication and pathogenesis. Additional details of these mechanisms may provide novel targets for antiviral therapy that can counteract Nef’s impact on T-cell signaling.
Future perspective
Additional studies are necessary to characterize the two-pronged approach that Nef uses to modulate the activation state of virus-infected CD4+ T cells. Systematic analyses of methods used to assess Nef function should be performed to assess the impact of cell type, stimulation conditions, and Nef allele on outcome.
As mechanisms of Nef function are further defined, novel therapeutic strategies should be tested and the role of Nef in determining the balance between productive viral replication and establishment of latency should be revisited.
Nef & T-cell activation
Antigen-stimulated CD4+ T cells are highly permissive to HIV-1 infection and it is likely that Nef acts to maintain a ‘semi-activated’ state that is efficient for the production of viral progeny.
Early studies observed that Nef inhibited virion production and replication; however, later reports found that Nef enhanced viral pathogenesis and progression to AIDS.
Conflicting evidence of Nef’s role in modulating T-cell activation status has continued to accumulate – suggesting that Nef can function as both a ‘negative factor’ and as a ‘positive factor’.
Early T-cell receptor signaling events
Nef appears to inhibit early signaling events following T-cell receptor activation, including recruitment and activation of LAT, as well as actin reorganization.
Localization of Nef to the plasma membrane through myristoylation is likely to be required for this activity.
By blocking antigen-specific signaling, Nef may prevent activation-induced cell death and prolong the lifespan of virus-producing cells.
Alternative pathways for cellular activation
Nef also appears to increase stimulation of transcription factors and expression of host genes, such as IL-2.
Localization of Nef to intracellular compartments (endosomes) or interactions with cellular kinases (Lck and PAK2) are likely to be required for this activity.
Nef-mediated stimulation of gene expression may promote viral long terminal repeat function.
Footnotes
Financial & competing interests disclosure
The authors acknowledge research support from the Canadian Institutes for Health Research (CIHR), the NIH, the Global Health Research Initiative at the Canadian International Development Research Centre and Simon Fraser University. This work was undertaken, in part, thanks to funding from the Canada Research Chairs program (MA Brockman). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Joint United Nations Programme on HIV/AIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2012; Joint United Nations Programme on HIV/AIDS; Geneva, Switzerland. 2012. [Google Scholar]
- 2.Nakayamada S, Takahashi H, Kanno Y, O’Shea JJ. Helper T cell diversity and plasticity. Curr. Opin. Immunol. 2012;24(3):297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauci A, Bartlett JG, Goosby E. Early treatment of HIV-1 infection. Lancet. 1998;352(9144):1935. doi: 10.1016/s0140-6736(05)60429-1. [DOI] [PubMed] [Google Scholar]
- 4.Simon V, Ho DD, Abdool Karim Q. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet. 2006;368(9534):489–504. doi: 10.1016/S0140-6736(06)69157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landi A, Iannucci V, Nuffel AV, Meuwissen P, Verhasselt B. One protein to rule them all: modulation of cell surface receptors and molecules by HI V Nef. Curr. HIV Res. 2011;9(7):496–504. doi: 10.2174/157016211798842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arien KK, Verhasselt B. HIV Nef: role in pathogenesis and viral fitness. Curr. HIV Res. 2008;6(3):200–208. doi: 10.2174/157016208784325001. [DOI] [PubMed] [Google Scholar]
- 7.Deacon NJ, Tsykin A, Solomon A, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270(5238):988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl. J. Med. 1995;332(4):228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 9.Foster JL, Denial SJ, Temple BR, Garcia JV. Mechanisms of HIV-1 Nef function and intracellular signaling. J Neuroimmune Pharmacol. 2011;6(2):230–246. doi: 10.1007/s11481-011-9262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M. HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication. JBiosci. 2003;28(3):323–335. doi: 10.1007/BF02970151. [DOI] [PubMed] [Google Scholar]
- 11.Robert-Guroff M, Popovic M, Gartner S, Markham P, Gallo RC, Reitz MS. Structure and expression of tat-, rev-, and nef-specific transcripts of human immunodeficiency virus type 1 in infected lymphocytes and macrophages. JVirol. 1990;64(7):3391–3398. doi: 10.1128/jvi.64.7.3391-3398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster JL, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wonderlich ER, Leonard JA, Collins KL. HIV immune evasion disruption of antigen presentation by the HIV Nef protein. Adv. Virus Res. 2011;80:103–127. doi: 10.1016/B978-0-12-385987-7.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fackler OT, Krausslich HG. Interactions of human retroviruses with the host cell cytoskeleton. Curr. Opin. Microbiol. 2006;9(4):409–415. doi: 10.1016/j.mib.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Luciw PA, Cheng-Mayer C, Levy JA. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc. Natl Acad. Sci. USA. 1987;84(5):1434–1438. doi: 10.1073/pnas.84.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terwilliger E, Sodroski JG, Rosen CA, Haseltine WA. Effects of mutations within the 3’ orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. JVirol. 1986;60(2):754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad N, Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988;241(4872):1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- 18.Niederman TM, Hastings WR, Luria S, Bandres JC, Ratner L. HIV-1 Nef protein inhibits the recruitment of AP-1 DNA-binding activity in human T-cells. Virology. 1993;194(1):338–344. doi: 10.1006/viro.1993.1264. [DOI] [PubMed] [Google Scholar]
- 19.Spina CA, Kwoh TJ, Chowers MY, Guatelli JC, Richman DD. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. JExp. Med. 1994;179(1):115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller MD, Warmerdam MT, Gaston I, Greene WC, Feinberg MB. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 1994;179(1):101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kestler HW, 3rd, Ringler DJ, Mori K, et al. Importance of the nef gene for maintenance o high virus loads and for development of AIDS. Cell. 1991;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 22.Korin YD, Zack JA. Progression to the Gib phase of the cell cycle is required for completion of human immunodeficiency viru type 1 reverse transcription in T cells. JVirol. 1998;72(4):3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controllec at the level of T cell activation and proviral integration. EMBOJ. 1990;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baldauf HM, Pan X, Erikson E, et al. SAMHD1 restricts HIV-1 infection in resting CD4+ T cells. Nat. Med. 2012;18(11):1682–1689. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganesh L, Burstein E, Guha-Niyogi A, et al. The gene product Murrl restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426(6968):853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- 26.Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435(7038):108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 27.Song SK, Li H, Cloyd MW. Rates of shutdown of HIV-1 into latency: roles of the LTR and tat/rev/vpu gene region. Virology. 1996;225(2):377–386. doi: 10.1006/viro.1996.0612. [DOI] [PubMed] [Google Scholar]
- 28.Li XD, Moore B, Cloyd MW. Gradual shutdown of virus production resulting in latency is the norm during the chronic phase of human immunodeficiency virus replication and differential rates and mechanisms of shutdown are determined by viral sequences. Virology. 1996;225(1):196–212. doi: 10.1006/viro.1996.0588. [DOI] [PubMed] [Google Scholar]
- 29.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat. Rev. Immunol. 2007;7(7):532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- 30.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37(3):377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karn J. The molecular biology of HIV latency: breaking and restoring the Tat-dependent transcriptional circuit. Curr. Opin. HIV AIDS. 2011;6(1):4–11. doi: 10.1097/COH.0b013e328340ffbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat. Immunol. 2012;13(2):121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 33.Sykulev Y. T-cell receptor signaling kinetics takes the stage. Sci. Signal. 2010;3(153):pe50. doi: 10.1126/scisignal.3153pe50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181(11):7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 35.Chan JK, Greene WC. Dynamic roles for NF-κB in HTLV-I and HIV-1 retroviral pathogenesis. Immunol. Rev. 2012;246(1):286–310. doi: 10.1111/j.1600-065X.2012.01094.x. [DOI] [PubMed] [Google Scholar]
- 36.Luria S, Chambers I, Berg P. Expression of the type 1 human immunodeficiency virus Nef protein in T cells prevents antigen receptor-mediated induction of interleukin 2 mRNA. Proc. Natl Acad. Sci. USA. 1991;88(12):5326–5330. doi: 10.1073/pnas.88.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collette Y, Chang HL, Cerdan C, et al. Specific Thl cytokine down-regulation associated with primary clinically derived human immunodeficiency virus type 1 Nef gene-induced expression. J Immunol. 1996;156(1):360–370. [PubMed] [Google Scholar]
- 38.Rhee SS, Marsh JW. HIV-1 Nef activity in murine T cells. CD4 modulation and positive enhancement. J Immunol. 1994;152(10):5128–5134. [PubMed] [Google Scholar]
- 39.Schrager JA, Marsh JW. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl Acad. Sci. USA. 1999;96(14):8167–8172. doi: 10.1073/pnas.96.14.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang JK, Kiyokawa E, Verdin E, Trono D. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl Acad. Sci. USA. 2000;97(1):394–399. doi: 10.1073/pnas.97.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler M, Munch J, Kutsch O, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125(6):1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Fenard D, Yonemoto W, De Noronha C, Cavrois M, Williams SA, Greene WC. Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement. J Immunol. 2005;175(9):6050–6057. doi: 10.4049/jimmunol.175.9.6050. [DOI] [PubMed] [Google Scholar]
- 43.Olivieri KC, Mukerji J, Gabuzda D. Nef-mediated enhancement of cellular activation and human immunodeficiency virus type 1 replication in primary T cells is dependent on association with p21-activated kinase 2. Retrovirology. 2011;8:64. doi: 10.1186/1742-4690-8-64.The authors observed a critical role for PAK2 in Nef-mediated activation of primary CD4+ T cells
- 44.Keppler OT, Tibroni N, Venzke S, Rauch S, Fackler OT. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J Leukoc. Biol. 2006;79(3):616–627. doi: 10.1189/jlb.0805461. [DOI] [PubMed] [Google Scholar]
- 45.Arhel N, Lehmann M, Clauss K, Nienhaus GU, Piguet V, Kirchhoff F. The inability to disrupt the immunological synapse between infected human T cells and APCs distinguishes HIV-1 from most other primate lentiviruses. J . Invest. 2009;119(10):2965–2975. doi: 10.1172/JCI38994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity. 2006;24(5):547–561. doi: 10.1016/j.immuni.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Chow CW, Rincon M, Davis RJ. Requirement for transcription factor NFAT in interleukin-2 expression. Mol. Cell Biol. 1999;19(3):2300–2307. doi: 10.1128/mcb.19.3.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 49.Kinoshita S, Chen BK. Kaneshima H, Nolan GP. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell. 1998;95(5):595–604. doi: 10.1016/s0092-8674(00)81630-x. [DOI] [PubMed] [Google Scholar]
- 50.Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6(3):235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 51.Manninen A, Huotari P, Hiipakka M, Renkema GH, Saksela K. Activation of NFAT-dependent gene expression by Nef: conservation among divergent Nef alleles, dependence on SH3 binding and membrane association, and cooperation with protein kinase C-theta. J Virol. 2001;75(6):3034–3037. doi: 10.1128/JVI.75.6.3034-3037.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manninen A, Renkema GH, Saksela K. Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J Biol. Chem. 2000;275(22):16513–16517. doi: 10.1074/jbc.M910032199. [DOI] [PubMed] [Google Scholar]
- 53.Fortin JF, Barat C, Beausejour Y, Barbeau B, Tremblay MJ. Hyper-responsiveness to stimulation of human immunodeficiency virus-infected CD4+ T cells requires Nef and Tat virus gene products and results from higher NFAT, NF-κB, and AP-1 induction. J Biol. Chem. 2004;279(38):39520–39531. doi: 10.1074/jbc.M407477200. [DOI] [PubMed] [Google Scholar]
- 54.Manninen A, Saksela K. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. JExp. Med. 2002;195(8):1023–1032. doi: 10.1084/jem.20012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neri F, Giolo G, Potesta M, Petrini S, Doria M. The HIV-1 Nef protein has a dual role in T cell receptor signaling in infected CD4+ T lymphocytes. Virology. 2011;410(2):316–326. doi: 10.1016/j.virol.2010.11.018.Reports differential effects of Nef on T-cell signaling that depend upon the intrinsic cellular activation state
- 56.Tuosto L, Marinari B, Andreotti M, Federico M, Piccolella E. Vav exchange factor counteracts the HIV-1 Nef-mediated decrease of plasma membrane GM1 and NF-AT activity in T cells. Eur. J. Immunol. 2003;33(8):2186–2196. doi: 10.1002/eji.200323682. [DOI] [PubMed] [Google Scholar]
- 57.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways regulation and physiological functions. Endocr. Rev. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 58.Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117(25):5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Mak G, Franza BR., Jr In vitro study of functional involvement of Spl, NF-κB/Rel, and AP1 in phorbol 12-myristate 13-acetate-mediated HIV-1 long terminal repeat activation. J Biol. Chem. 1994;(48):30616–30619. [PubMed] [Google Scholar]
- 60.Yang X, Gabuzda D. Regulation of human immunodeficiency virus type 1 infectivity by the ERK mitogen-activated protein kinase signaling pathway. J. Virol. 1999;73(4):3460–3466. doi: 10.1128/jvi.73.4.3460-3466.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mettling C, Desmetz C, Fiser AL, Reant B, Corbeau P, Lin YL. Galphai protein-dependant extracellular signal-regulated kinase-1/2 activation is required for HIV-1 reverse transcription. AIDS. 2008;22(13):1569–1576. doi: 10.1097/QAD.0b013e32830abdaf. [DOI] [PubMed] [Google Scholar]
- 62.Hemonnot B, Carder C, Gay B, et al. The host cell MAP kinase ERK-2 regulates viral assembly and release by phosphorylating the p6gag protein of HIV-1. J. Biol. Chem. 2004;279(31):32426–32434. doi: 10.1074/jbc.M313137200. [DOI] [PubMed] [Google Scholar]
- 63.Bandres JC, Ratner L. Human immunodeficiency virus type 1 Nef protein down-regulates transcription factors NF-κB and AP-1 in human T cells in vitro after T-cell receptor stimulation. J. Virol. 1994;68(5):3243–3249. doi: 10.1128/jvi.68.5.3243-3249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greenway A, Azad A, Mills J, McPhee D. Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J. Virol. 1996;70(10):6701–6708. doi: 10.1128/jvi.70.10.6701-6708.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoon K, Kim S. Lack of negative influence on the cellular transcription factors NF-κB and AP-1 by the nef protein of human immunodeficiency virus type 1. J. Gen. Virol. 1999;80(Pt 11):2951–2956. doi: 10.1099/0022-1317-80-11-2951. [DOI] [PubMed] [Google Scholar]
- 66.Schrager JA, Der Minassian V, Marsh JW. HIV Nef increases T cell ERK MAP kinase activity. J. Biol. Chem. 2002;277(8):6137–6142. doi: 10.1074/jbc.M107322200. [DOI] [PubMed] [Google Scholar]
- 67.Witte V, Laffert B, Gintschel P, et al. Induction of HIV transcription by Nef involves Lck activation and protein kinase C theta raft recruitment leading to activation of ERK1/2 but not NF-κB. J. Immunol. 2008;181(12):8425–8432. doi: 10.4049/jimmunol.181.12.8425. [DOI] [PubMed] [Google Scholar]
- 68.Fackler OT, Luo W, Geyer M, Alberts AS, Peterlin BM. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell. 1999;3(6):729–739. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Verma IM. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 70.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 71.Levine BL, Mosca JD, Riley JL, et al. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science. 1996;272(5270):1939–1943. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- 72.Riley JL, Levine BL, Craighead N, et al. Naive and memory CD4 T cells differ in their susceptibilities to human immunodeficiency virus type 1 infection following CD28 costimulation: implicatip6s for transmission and pathogenesis. J. Virol. 1998;72(10):8273–8280. doi: 10.1128/jvi.72.10.8273-8280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cook JA, Albacker L, August A, Henderson AJ. CD28-dependent HIV-1 transcription is associated with Vav, Rac, and NF-κB activation. J. Biol. Chem. 2003;278(37):35812–35818. doi: 10.1074/jbc.M302878200. [DOI] [PubMed] [Google Scholar]
- 74.Niederman TM, Garcia JV, Hastings WR, Luria S, Ratner L. Human immunodeficiency virus type 1 Nef protein inhibits NF-κB induction in human T cells. J. Virol. 1992;66(10):6213–6219. doi: 10.1128/jvi.66.10.6213-6219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collette Y, Mawas C, Olive D. Evidence for intact CD28 signaling in T cell hyporesponsiveness induced by the HIV-1 nef gene. Eur. J. Immunol. 1996;26(8):1788–1793. doi: 10.1002/eji.1830260819. [DOI] [PubMed] [Google Scholar]
- 76.Collette Y, Dutartre H, Benziane A, et al. Physical and functional interaction of Nef with Lck. HIV-1 Nef-induced T-cell signaling defects. J. Biol. Chem. 1996;271(11):6333–6341. doi: 10.1074/jbc.271.11.6333. [DOI] [PubMed] [Google Scholar]
- 77.Greenway A, Azad A, McPhee D. Human immunodeficiency virus type 1 Nef protein inhibits activation pathways in peripheral blood mononuclear cells and T-cell lines. J. Virol. 1995;69(3):1842–1850. doi: 10.1128/jvi.69.3.1842-1850.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef viruses but not for down-regulation of CD4. EMBOJ. 1995;14(3):484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee CH, Leung B, Lemmon MA, et al. A single amino acid in the SH3 domain of Hck determines its high affinity and specificity in binding to HIV-1 Nef protein. EMBOJ. 1995;14(20):5006–5015. doi: 10.1002/j.1460-2075.1995.tb00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee CH, Saksela K, Mirza UA, Chait BT, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85(6):931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 81.Arold S, Franken P, Strub MP, et al. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure. 1997;5(0):1361–1372. doi: 10.1016/s0969-2126(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 82.Bandres JC, Shaw AS, Ratner L. HIV-1 Nef protein downregulation of CD4 surface expression: relevance of the lck binding domain of CD4. Virology. 1995;207(1):338–341. doi: 10.1006/viro.1995.1089. [DOI] [PubMed] [Google Scholar]
- 83.Salghetti S, Mariani R, Skowronski J. Human immunodeficiency virus type 1 Nef and p561ck protein-tyro sine kinase interact with a common element in CD4 cytoplasmic tail. Proc. Natl Acad. Sci. USA. 1995;92(2):349–353. doi: 10.1073/pnas.92.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim YH, Chang SH, Kwon JH, Rhee SS. HIV-1 Nef plays an essential role in two independent processes in CD4 down-regulation: dissociation of the CD4-p56(lck) complex and targeting of CD4 to lysosomes. Virology. 1999;257(1):208–219. doi: 10.1006/viro.1999.9642. [DOI] [PubMed] [Google Scholar]
- 85.Laguette N, Bregnard C, Bouchet J, Benmerah A, Benichou S, Basmaciogullari S. Nef-induced CD4 endocytosis in human immunodeficiency virus type 1 host cells: role of p561ck kinase. J. Virol. 2009;83(14):7117–7128. doi: 10.1128/JVI.01648-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haller C, Rauch S, Fackler OT. HIV-1 Nef employs two distinct mechanisms to modulate Lck subcellular localization and TCR induced actin remodeling. PLoS ONE. 2007;2(11):e1212. doi: 10.1371/journal.pone.0001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haller C, Rauch S, Michel N, et al. The HIV-1 pathogenicity factor Nef interferes with maturation of stimulatory T-lymphocyte contacts by modulation of N-Wasp activity. J. Biol. Chem. 2006;281(28):19618–19630. doi: 10.1074/jbc.M513802200. [DOI] [PubMed] [Google Scholar]
- 88.Rudolph JM, Eickel N, Haller C, Schindler M, Fackler OT. Inhibition of T-cell receptor-induced actin remodeling and relocalization of Lck are evolutionarily conserved activities of lentiviral Nef proteins. J. Virol. 2009;83(22):11528–11539. doi: 10.1128/JVI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baur AS, Sawai ET, Dazin P, Fantl WJ, Cheng-Mayer C, Peterlin BM. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1(5):373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 90.Collette Y, Dutartre H, Benziane A, Olive D. The role of HIV1 Nef in T-cell activation: Nef impairs induction of Thl cytokines and interacts with the Src family tyrosine kinase Lck. Res. Virol. 1997;148(1):52–58. doi: 10.1016/s0923-2516(97)81914-0. [DOI] [PubMed] [Google Scholar]
- 91.Abraham L, Bankhead P, Pan X, Engel U, Fackler OT. HIV-1 Nef limits communication between linker of activated T cells and SLP-76 to reduce formation of SLP-76-signaling microclusters following TCR stimulation. J. Immunol. 2012;189(4):1898–1910. doi: 10.4049/jimmunol.1200652.Examined the impact of Nef on peptide-induced T-cell receptor signaling. The authors observed a significant disruption of proximal T-cell receptor signals in the presence of Nef that was associated with reduced SLP-76 microclusters and actin remodeling
- 92.Wolf D, Giese SI, Witte V, et al. Novel (n) PKC kinases phosphorylate Nef for increased HIV transcription, replication and perinuclear targeting. Virology. 2008;370(1):45–54. doi: 10.1016/j.virol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 93.Trono D, Wang JK. Nef and PAK: virulence factor and cellular accomplice. Chem. Biol. 1997;4(1):13–15. doi: 10.1016/s1074-5521(97)90232-5. [DOI] [PubMed] [Google Scholar]
- 94.Chan PM, Manser E. PAKs in human disease. Prog. Mol. Biol. Transl Sci. 2012;106:171–187. doi: 10.1016/B978-0-12-396456-4.00011-0. [DOI] [PubMed] [Google Scholar]
- 95.Chu PC, Wu J, Liao XC, et al. A novel role for p21-activated protein kinase 2 in T cell activation. J. Immunol. 2004;172(12):7324–7334. doi: 10.4049/jimmunol.172.12.7324. [DOI] [PubMed] [Google Scholar]
- 96.Sawai ET, Baur A, Struble H, Peterlin BM, Levy JA, Cheng-Mayer C. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc. Natl Acad. Sci. USA. 1994;91(4):1539–1543. doi: 10.1073/pnas.91.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu X, Wu X, Plemenitas A, et al. CDC42 and Racl are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr. Biol. 1996;6(12):1677–1684. doi: 10.1016/s0960-9822(02)70792-6. [DOI] [PubMed] [Google Scholar]
- 98.Nunn MF, Marsh JW. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 1996;70(9):6157–6161. doi: 10.1128/jvi.70.9.6157-6161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sawai ET, Cheng-Mayer C, Luciw PA. Nef and the Nef-associated kinase. Res. Virol. 1997;148(1):47–52. doi: 10.1016/s0923-2516(97)81913-9. [DOI] [PubMed] [Google Scholar]
- 100.Sawai ET, Khan IH, Montbriand PM, Peterlin BM, Cheng-Mayer C, Luciw PA. Activation of PAK by HIV and SIV Nef: importance for AIDS in rhesus macaques. Curr. Biol. 1996;6(11):1519–1527. doi: 10.1016/s0960-9822(96)00757-9. [DOI] [PubMed] [Google Scholar]
- 101.Renkema GH, Manninen A, Mann DA, Harris M, Saksela K. Identification of the Nef-associated kinase as p21-activated kinase 2. Curr. Biol. 1999;9(23):1407–1410. doi: 10.1016/s0960-9822(00)80086-x. [DOI] [PubMed] [Google Scholar]
- 102.Wiskerchen M, Cheng-Mayer C. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology. 1996;224(1):292–301. doi: 10.1006/viro.1996.0531. [DOI] [PubMed] [Google Scholar]
- 103.Sawai ET, Baur AS, Peterlin BM, Levy JA, Cheng-Mayer C. A conserved domain and membrane targeting of Nef from HIV and SIV are required for association with a cellular serine kinase activity. J. Biol. Chem. 1995;270(25):15307–15314. doi: 10.1074/jbc.270.25.15307. [DOI] [PubMed] [Google Scholar]
- 104.Agopian K, Wei BL, Garcia JV, Gabuzda D. A hydrophobic binding surface on the human immunodeficiency virus type 1 Nef core is critical for association with p21-activated kinase 2. J. Virol. 2006;80(6):3050–3061. doi: 10.1128/JVI.80.6.3050-3061.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foster JL, Molina RP, Luo T, et al. Genetic and functional diversity of human immunodeficiency virus type 1 subtype B Nef primary isolates. J. Virol. 2001;75(4):1672–1680. doi: 10.1128/JVI.75.4.1672-1680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Renkema GH, Manninen A, Saksela K. Human immunodeficiency virus type 1 Nef selectively associates with a catalytically active subpopulation of p21-activated kinase 2 (PAK2) independently of PAK2 binding to Nek or beta-PIX. J. Virol. 2001;75(5):2154–2160. doi: 10.1128/JVI.75.5.2154-2160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fackler OT, Lu X, Frost JA, et al. p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol. Cell Biol. 2000;20(7):2619–2627. doi: 10.1128/mcb.20.7.2619-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen DG, Wolff KC, Yin H, Caldwell JS, Kuhen KL. “UnPAKing” human immunodeficiency virus (HIV) replication: using small interfering RNA screening to identify novel cofactors and elucidate the role of group I PAKs in HIV infection. J. Virol. 2006;80(1):130–137. doi: 10.1128/JVI.80.1.130-137.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Linnemann T, Zheng YH, Mandic R, Peterlin BM. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology. 2002;294(2):246–255. doi: 10.1006/viro.2002.1365. [DOI] [PubMed] [Google Scholar]
- 110.Rauch S, Pulkkinen K, Saksela K, Fackler OT. Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vavl via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity. J. Virol. 2008;82(6):2918–2929. doi: 10.1128/JVI.02185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wolf D, Witte V, Laffert B, et al. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 2001;7(11):1217–1224. doi: 10.1038/nm1101-1217. [DOI] [PubMed] [Google Scholar]
- 112.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 2007;7(2):131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 113.Huang Y, Burkhardt JK. T-cell-receptor-dependent actin regulatory mechanisms. J. Cell. Sci. 2007;120(Pt 5):723–730. doi: 10.1242/jcs.000786. [DOI] [PubMed] [Google Scholar]
- 114.Fackler OT, Kienzle N, Kremmer E, et al. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur. J. Biochem. 1997;247(3):843–851. doi: 10.1111/j.1432-1033.1997.00843.x. [DOI] [PubMed] [Google Scholar]
- 115.Stolp B, Abraham L, Rudolph JM, Fackler OT. Lentiviral Nef proteins utilize PAK2-mediated deregulation of cofilin as a general strategy to interfere with actin remodeling. J. Virol. 2010;84(8):3935–3948. doi: 10.1128/JVI.02467-09.Indicates that Nef’s interaction with PAK2 results in deregulation of the actin-severing factor cofilin
- 116.Stolp B, Reichman-Fried M, Abraham L, et al. HIV-1 Nef interferes with host cell motility by deregulation of Cofilin. Cell Host Microbe. 2009;6(2):174–186. doi: 10.1016/j.chom.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 117.Bell I, Ashman C, Maughan J, Hooker E, Cook F, Reinhart TA. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 1998;79(Pt 11):2717–2727. doi: 10.1099/0022-1317-79-11-2717. [DOI] [PubMed] [Google Scholar]
- 118.Howe AY, Jung JU, Desrosiers RC. ζ-chain of the T-cell receptor interacts with nef of simian immunodeficiency virus and human immunodeficiency virus type 2. J. Virol. 1998;72(12):9827–9834. doi: 10.1128/jvi.72.12.9827-9834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khalid M, Yu H, Sauter D, et al. Efficient Nef-mediated downmodulation of TCR-CD3 and CD28 is associated with high CD4+ T cell counts in viremic HIV-2 infection. J. Virol. 2012;86(9):4906–4920. doi: 10.1128/JVI.06856-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Munch J, Schindler M, Wildum S, et al. Primary sooty mangabey simian immunodeficiency virus and human immunodeficiency virus type 2 nef allAes modulate cell surface expression of various human receptors and enhance viral infectivity and replication. J. Virol. 2005;79(16):10547–10560. doi: 10.1128/JVI.79.16.10547-10560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schindler M, Munch J, Brenner M, Stahl-Hennig C, Skowronski J, Kirchhoff F. Comprehensive analysis of nef functions selected in simian immunodeficiency virus-infected macaques. J. Virol. 2004;78(19):10588–10597. doi: 10.1128/JVI.78.19.10588-10597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anderson S, Shugars DC, Swanstrom R, Garcia JV. Nef from primary isolates of human immunodeficiency virus type 1 suppresses surface CD4 expression in human and mouse T cells. J. Virol. 1993;67(8):4923–4931. doi: 10.1128/jvi.67.8.4923-4931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia JV, Alfano J, Miller AD. The negative effect of human immunodeficiency virus type 1 Nef on cell surface CD4 expression is not species specific and requires the cytoplasmic domain of CD4. J. Virol. 1993;67(3):1511–1516. doi: 10.1128/jvi.67.3.1511-1516.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350(6318):508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 125.Holdorf AD, Lee KH, Burack WR, Allen PM, Shaw AS. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat. Immunol. 2002;3(3):259–264. doi: 10.1038/ni761. [DOI] [PubMed] [Google Scholar]
- 126.Mariani R, Skowronski J. CD4 down-regulation by nef alleles isolated from human immunodeficiency virus type 1-infected individuals. Proc. Natl Acad. Sci. USA. 1993;90(12):5549–5553. doi: 10.1073/pnas.90.12.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Skowronski J, Parks D, Mariani R. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 1993;12(2):703–713. doi: 10.1002/j.1460-2075.1993.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iafrate AJ, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16(4):673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bell I, Schaefer TM, Trible RP, Amedee A, Reinhart TA. Down-modulation of the costimulatory molecule, CD28, is a conserved activity of multiple SIV Nefs and is dependent on histidine 196 of Nef. Virology. 2001;283(1):148–158. doi: 10.1006/viro.2001.0872. [DOI] [PubMed] [Google Scholar]
- 130.Swigut T, Shohdy N, Skowronski J. Mechanism for down-regulation of CD28 by Nef. EMBO J. 2001;20(7):1593–1604. doi: 10.1093/emboj/20.7.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009;229(1):12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Whatmore AM, Cook N, Hall GA, Sharpe S, Rud EW, Cranage MP. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J. Virol. 1995;69(8):5117–5123. doi: 10.1128/jvi.69.8.5117-5123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 134.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276(5320):1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 135.Swingler S, Mann A, Jacque J, et al. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat. Med. 1999;5(9):997–103. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Swingler S, Brichacek B, Jacque JM, Ulich C, Zhou J, Stevenson M. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature. 2003;424(6945):213–219. doi: 10.1038/nature01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.St Gelais C, Coleman CM, Wang JH, Wu L. HIV-1 Nef enhances dendritic cell-mediated viral transmission to CD4+ T cells and promotes T-cell activation. PloS ONE. 2012;7(3):34521. doi: 10.1371/journal.pone.0034521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fujii Y, Otake K, Tashiro M, Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 1996;393(1):93–96. doi: 10.1016/0014-5793(96)00859-9. [DOI] [PubMed] [Google Scholar]
- 139.Quaranta MG, Camponeschi B, Straface E, Malorni W, Viora M. Induction of interleukin-15 production by HIV-1 nef protein: a role in the proliferation of uninfected cells. Exper. Cell Res. 1999;250(1):112–121. doi: 10.1006/excr.1999.4494. [DOI] [PubMed] [Google Scholar]
- 140.Quaranta MG, Tritarelli E, Giordani L, Viora M. HIV-1 Nef induces dendritic cell differentiation: a possible mechanism of uninfected CD4+ T cell activation. Exper. Cell Res. 2002;275(2):243–254. doi: 10.1006/excr.2002.5497. [DOI] [PubMed] [Google Scholar]
- 141.Okada H, Takei R, Tashiro M. HIV-1 Nef protein-induced apoptotic cytolysis of a broad spectrum of uninfected human blood cells independently of CD95(Fas) FEBS Lett. 1997;414(3):603–606. doi: 10.1016/s0014-5793(97)01080-6. [DOI] [PubMed] [Google Scholar]
- 142.Okada H, Takei R, Tashiro M. Inhibition of HIV-1 Nef-induced apoptosis of uninfected human blood cells by serine/threonine protein kinase inhibitors, fasudil hydrochloride and M3. FEBS Lett. 1998;422(3):363–367. doi: 10.1016/s0014-5793(98)00045-3. [DOI] [PubMed] [Google Scholar]
- 143.Lenassi M, Cagney G, Liao M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11(1):110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu XN, Screaton GR, Gotch FM, et al. Evasion of cytotoxic T lymphocyte (CTL) responses by nef-dependent induction of Fas ligand (CD95L) expression on simian immunodeficiency virus-infected cells. J. Exper. Med. 1997;186(1):7–16. doi: 10.1084/jem.186.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zauli G, Gibellini D, Secchiero P, et al. Human immunodeficiency virus type 1 Nef protein sensitizes CD4+ T lymphoid cells to apoptosis via functional upregulation of the CD95/CD95 ligand pathway. Blood. 1999;93(3):1000–1010. [PubMed] [Google Scholar]
- 146.Muthumani K, Choo AY, Shedlock DJ, et al. Human immunodeficiency virus type 1 Nef induces programmed death 1 expression through a p38 mitogen-activated protein kinase-dependent mechanism. J. Virol. 2008;82(23):11536–11544. doi: 10.1128/JVI.00485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rasola A, Gramaglia D, Boccaccio C, Comoglio PM. Apoptosis enhancement by the HIV-1 Nef protein. J. Immunol. 2001;166(1):81–88. doi: 10.4049/jimmunol.166.1.81. [DOI] [PubMed] [Google Scholar]
- 148.Huang MB, Jin LL, James CO, Khan M, Powell MD, Bond VC. Characterization of Nef-CXCR4 interactions important for apoptosis induction. J. Virol. 2004;78(20):11084–11096. doi: 10.1128/JVI.78.20.11084-11096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.James CO, Huang MB, Khan M, Garcia-Barrio M, Powell MD, Bond VC. Extracellular Nef protein targets CD4+ T cells for apoptosis by interacting with CXCR4 surface receptors. J. Virol. 2004;78(6):3099–3109. doi: 10.1128/JVI.78.6.3099-3109.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Geleziunas R, Xu W, Takeda K, Ichijo H, Greene WC. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature. 2001;410(6830):834–838. doi: 10.1038/35071111. [DOI] [PubMed] [Google Scholar]
- 151.Mahlknecht U, Deng C, Lu MC, et al. Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence. J. Immunol. 2000;165(11):6437–6446. doi: 10.4049/jimmunol.165.11.6437. [DOI] [PubMed] [Google Scholar]
- 152.Greenway AL, Mcphee DA, Allen K, et al. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J. Virol. 2002;76(6):2692–2702. doi: 10.1128/JVI.76.6.2692-2702.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kaminchik J, Bashan N, Itach A, Sarver N, Gorecki M, Panet A. Genetic characterization of human immunodeficiency virus type 1 nef gene products translated in vitro and expressed in mammalian cells. J. Virol. 1991;65(2):583–588. doi: 10.1128/jvi.65.2.583-588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pan X, Rudolph JM, Abraham L, et al. HIV-1 Nef compensates for disorganization of the immunological synapse by inducing trans-golgi network-associated Lck signaling. Blood. 2012;119(3):786–797. doi: 10.1182/blood-2011-08-373209.Describes the ability of Nef to redirect Lck to an intracellular location in order to trigger an alternative signaling pathway that could contribute to T-cell activation
- 155.Abraham L, Fackler OT. HIV-1 Nef: a multifaceted modulator of T cell receptor signaling. Cell. Commun. Signal. 2012;10(1):39. doi: 10.1186/1478-811X-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Astoul E, Edmunds C, Cantrell DA, Ward SG. PI 3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol. 2001;22(9):490–496. doi: 10.1016/s1471-4906(01)01973-1. [DOI] [PubMed] [Google Scholar]
- 157.Dikeakos JD, Atkins KM, Thomas L, et al. Small molecule inhibition of HIV-1-induced MHC-I down-regulation identifies a temporally regulated switch in Nef action. Mol. Biol. Cell. 2010;21(19):3279–3292. doi: 10.1091/mbc.E10-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yi L, Rosales T, Rose JJ, Chowdhury B, Knutson JR, Venkatesan S. HIV-1 Nef binds a subpopulation of MHC-I throughout its trafficking itinerary and down-regulates MHC-I by perturbing both anterograde and retrograde trafficking. J. Biol. Chem. 2010;285(40):30884–30905. doi: 10.1074/jbc.M110.135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu X, Schrager JA, Lange GD, Marsh JW. HIV Nef-mediated cellular phenotypes are differentially expressed as a function of intracellular Nef concentrations. J. Biol. Chem. 2001;276(35):32763–32770. doi: 10.1074/jbc.M101025200. [DOI] [PubMed] [Google Scholar]