Abstract
Objective
To investigate if plasma DNA is elevated in patients with deep vein thrombosis (DVT) and to determine whether there is a correlation with other biomarkers of DVT.
Background
Leukocytes release DNA to form extracellular traps (ETs), which have recently been linked to experimental DVT. In baboons and mice, extracellular DNA co-localized with von Willebrand factor (VWF) in the thrombus and DNA appeared in circulation at the time of thrombus formation. ETs have not been associated with clinical DVT.
Setting
From December 2008 to August 2010, patients were screened through the University of Michigan Diagnostic Vascular Unit and were divided into three distinct groups: 1) the DVT positive group, consisting of patients who were symptomatic for DVT, which was confirmed by compression duplex ultrasound (n=47); 2) the DVT negative group, consisting of patients that present with swelling and leg pain but had a negative compression duplex ultrasound, (n=28); and 3) a control group of healthy non-pregnant volunteers without signs or symptoms of active or previous DVT (n=19). Patients were excluded if they were less than 18 years of age, unwillingness to consent, pregnant, on an anticoagulant therapy, or diagnosed with isolated calf vein thrombosis.
Methods
Blood was collected for circulating DNA, CRP, D-dimer, VWF activity, myeloperoxidase (MPO), ADAMTS13 and VWF. The Wells score for a patient’s risk of DVT was assessed. The Receiver Operating Characteristic (ROC) curve was generated to determine the strength of the relationship between circulating DNA levels and the presence of DVT. A Spearman correlation was performed to determine the relationship between the DNA levels and the biomarkers and the Wells score. Additionally the ratio of ADAMTS13/VWF was assessed.
Results
Our results showed that circulating DNA (a surrogate marker for NETs) was significantly elevated in DVT patients, compared to both DVT negative patients (57.7±6.3 vs. 17.9±3.5ng/mL, P<.01) and controls (57.7±6.3 vs. 23.9±2.1ng/mL, P<.01). There was a strong positive correlation with CRP (P<.01), D-dimer (P<.01), VWF (P<.01), Wells score (P<.01) and myeloperoxidase (MPO) (P<.01), along with a strong negative correlation with ADAMTS13 (P<.01) and the ADAMTS13/VWF ratio. The logistic regression model showed a strong association between plasma DNA and the presence of DVT (ROC curve was determined to be 0.814).
Conclusions
Plasma DNA is elevated in patients with deep vein thrombosis and correlates with biomarkers of DVT. A strong correlation between circulating DNA and MPO suggests that neutrophils may be a source of plasma DNA in patients with DVT.
INTRODUCTION
Extracellular DNA has recently been shown to contribute to thrombosis in animal models (1). Neutrophils and other leukocytes are known to release DNA fibers to form extracellular traps (ETs) (2–5). Neutrophils are one of the main inflammatory cell types that participate in acute deep vein thrombosis (DVT) (6). In experimental DVT in baboons (7) and mice (8, 9)e, ETs are a structural part of the thrombus and co-localize with von Willebrand factor (VWF). In vitro, ETs provide a scaffold and stimulus for thrombus formation (7, 10). ETs bind adhesion molecules such as (VWF) and fibrinogen, promote fibrin formation, platelet aggregation and act to recruit red blood cells. In vivo, it is possible that DNases in plasma degrade ET in venous thrombi liberating DNA fragments into circulation (11). Pertaining to experimental DVT, DNA appears in baboon (7) and murine plasma (8) at the time of thrombus formation. Our aim was to determine whether plasma DNA could be detected in patients with acute DVT and have the potential to serve as a diagnostic tool.
METHODS
Patient Stratification, Groups, and Inclusion / Exclusion Criteria
From December 2008 to August 2010, patients were screened through the University of Michigan Diagnostic Vascular Unit and were divided into three distinct groups: 1) the DVT positive group including patients who were symptomatic for DVT, which was confirmed by compression duplex ultrasound by standard criteria (see below, n=47), 2) the DVT negative group consisting of patients presenting with swelling and leg pain but having a negative compression duplex ultrasound, (n=28) and 3) a control group of healthy non-pregnant volunteers without signs or symptoms of active or previous DVT (n=19). Symptoms in the DVT negative group were determined in two third of the cases, caused by post-procedure pain, musculoskeleton disorders (osteoarthritis, fibromyalgia, etc.), cellulitis, and non-DVT associated edema. In one third of the cases the cause of the symptoms was less well defined. Controls were recruited randomly from the University of Michigan “Engage” website and underwent an ultrasound evaluation (upper and lower limbs) to exclude the presence of DVT at the time of their enrollment. Patients and controls demographics are listed in Table 1. Patient’s demographics were comparable except for patient location (out patients vs. in patients), active cancer and PE. The University of Michigan’s Institutional Review Board approved this protocol (HUM00024067) and all patients and controls signed written informed consent forms prior to enrollment.
Table I. Patient demographics.
Control: Control group; DVT (−): DVT negative group; DVT (+): DVT positive group. The p value for age was determined using one-way analysis of area. The p values for the rest of the variables were determined by Chi square test from a two-way frequency table. The first p value column represents the comparison of the 3 groups. The second p value column represents comparisons between DVT negative and DVT positive patients groups (control group excluded).
| Frequency or means | P values | ||||
|---|---|---|---|---|---|
| Group | Control (n=19) |
DVT (−) (n=28) |
DVT (+) (n=47) |
Comparisons between columns | |
| Column 1 | Column 2 | Column 3 | 1,2 and 3 | 2 and 3 | |
| AGE (mean in years old) | 35.4 | 56 | 57 | NS | NS |
| Gender (male) | 5 | 11 | 26 | NS | NS |
| Gender (female) | 14 | 17 | 21 | ||
| DVT Family history –no | 18 | 22 | 36 | NS | NS |
| DVT Family history –yes | 1 | 5 | 10 | ||
| DVT Family history –unknown | 0 | 1 | 1 | ||
| Race: Caucasian | 19 | 27 | 42 | NS | NS |
| Race: African American | 0 | 0 | 4 | ||
| Race: Asian | 0 | 1 | 1 | ||
| Previous DVT –no | 19 | 25 | 32 | NS | NS |
| Previous DVT –yes | 0 | 3 | 15 | ||
| Out patients | 19 | 26 | 31 | <.01 | <.01 |
| In patients | 0 | 2 | 16 | ||
| No surgery | 19 | 23 | 31 | <.05 | NS |
| Minor surgery | 0 | 2 | 2 | ||
| Major surgery | 0 | 3 | 14 | ||
| Active cancer –no | 19 | 23 | 28 | <.01 | <.05 |
| Active cancer –yes | 0 | 5 | 18 | ||
| Active cancer –unknown | 0 | 0 | 1 | ||
| Chemotherapy –no | 19 | 26 | 37 | <.05 | NS |
| Chemotherapy –yes | 0 | 2 | 10 | ||
| PE –no | 19 | 28 | 42 | <.05 | <.05 |
| PE –yes | 0 | 0 | 5 | ||
NS= Non-significant.
Color venous duplex ultrasound was performed at the time of patient recruitment. The criteria for a positive duplex ultrasound were the inability to compress the vein, evidence of venous dilatation, and an echo lucent pattern within the vein. Patients with proximal DVT (DVT at or above the knee) were recruited as DVT positive patients and those where ultrasound could not demonstrate a DVT were recruited as DVT negative patients. All ultrasound studies were performed at the Diagnostic Vascular Unit at the University of Michigan, an Intersocietal Commission for the Accreditation of Vascular Laboratories (ICAVL) approved laboratory. Patient demographics and clinical characteristics were recorded, including the Wells score along with all clinical risk factors associated with DVT.
Exclusion criteria were age younger than 18 years, unwillingness to consent, pregnancy, anticoagulant therapy, or a diagnosis of isolated calf vein thrombosis. This patient population was part of the “Michigan DVT Study” (12). Patient demographics and clinical characteristics were recorded, including the Wells score along with all clinical risk factors associated with DVT.
Sample Processing
At the time of enrollment/consent, venous blood was collected into 3.2 % sodium citrate tubes, centrifuged for 10 minutes at 2000 g at 4°C (except for plasma samples to determine ADAMTS13 and Von Willebrand factor (VWF) levels that underwent extra centrifugation [2 minutes at 15000 g at 25°C]), plasma was drawn off, snap frozen in liquid nitrogen and stored at −70°C. Patients that were positive for DVT had their blood samples drawn before anticoagulant therapy was initiated.
Quantification of plasma DNA
Plasma DNA was quantified as previously described (13). Plasma was diluted (1:10; v:v) in phosphate buffered saline (PBS, Invitrogen, Grand Island, NY). Diluted plasma was then mixed with 50-µl of PBS containing SytoxGreen (final concentration 2-µM, Invitrogen, Grand Island, NY) to label DNA. Fluorescence was recorded using fluorometer with a 485 excitation and 538 emission filter set (Fluoroskan, Thermo Fisher Scientific, Waltham, MA). Autofluorescence was considered as background and determined in samples mixed with PBS without SytoxGreen. DNA concentrations were calculated based on a standard curve of known concentrations of DNA (Invitrogen, Grand Island, NY).
Biomarker determinations
Soluble P-Selectin, D-dimer, and C-Reactive Protein Assays
Enzyme-linked immunosorbent assays were used to evaluate circulating sP-sel levels (Hyphen Biomed, Neuvillesur- Olse, France), D-dimer (Hyphen Biomed, Neuvillesur- Olse, France), and CRP levels (US Biological, Swampscott, Massachusetts). They were reported in ng/mL (sP-sel), mg/L (D-dimer), and µg/mL (CRP).. Samples were prepared according to the manufacturers’ instructions, run in duplicate, and read on an Elx808 plate reader (Biotek, Winooski, VT) at 450nm wavelength. Analyses were performed in a blinded fashion, as the samples were identified by a numbering system.
von Willebrand factor Assays
VWF: Activity was determined using INNOVANCE® VWF Ac Kit and the VWF: Antigen using vWF Ag® Kit (both Siemens Healthcare Diagnostics, Marburg, Germany) following manufacture instructions.
Quantification of myeloperoxidase (MPO)
MPO in plasma was quantified by ELISA (ZEN MPO ELISA, Invitrogen, Grand Island, NY) according to manufacturer’s instructions and measured in arbitrary units.
ADAMTS13 Activity
ADAMTS13 activity was determined by the slightly modified FRETS-VWF73 assay (14, 15). A normal human plasma pool (NHP; Swiss Red Cross Blood Services, Bern, Switzerland) was used for assay calibration. A dilution of NHP of 1:25 (v:v) in assay buffer (5mmol/L Bis-Tris, 25mmol/L CaC12, 0.005% Tween-20, pH 6.0 supplemented with lmmol/L Pefabloc SC, Boehringer, Mannheim, Germany) was arbitrarily set to correspond to 100%. Further assay calibration samples were obtained by serial pre-dilutions of NHP of 3:4 (75%), 1:2 (50%), 1:4 (25%), 1:10 (10%), and 1:20 (5%) in heat-inactivated NHP (30min at 56°C followed by 15min of centrifugation at 15,000g). Subsequently, all of these latter standard samples, as well as heat-inactivated NHP (0% ADAMTS13 activity) and all samples to be tested, were diluted 1:25 in assay buffer. 100-[j,L of diluted standard and patients’ samples were incubated in a white 96-well plate (NUNC, Roskilde, Denmark) for l0min at 37°C. Then l00-µL FRETS-VWF73 peptide substrate (4µmol/L), dissolved in assay buffer, was added to each well and the evolution of fluorescence was recorded at 37°C in a TEC AN GENios microplate reader (Tecan, Zurich, Switzerland) equipped with a 340nm excitation and a 450-nm emission filter. Fluorescence was recorded every 5min. The reaction rate was calculated by linear regression of fluorescence over time from 5 to 60min.
Wells score
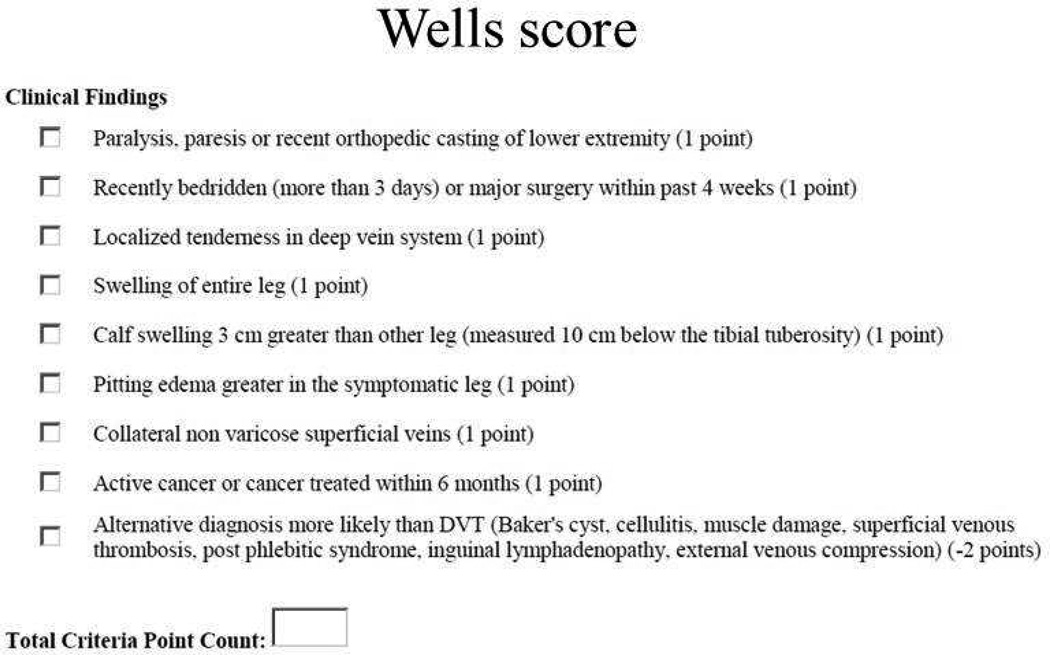
The Wells score for a patient’s risk of DVT was assessed as shown in Figure 1 (16).
Figure 1.
DVT Wells score.
Statistical Analysis
Data were analyzed using a one-way ANOVA with Tukey’s multiple comparison test, Wilcoxon rank sum test, multiple logistic regression, and chi-square tests, where appropriate. A value of P≤.05 was considered significant. Specifically, the data were analyzed as follows: First, student t-tests and one-way analysis of variance were performed comparing DNA values in the three groups. Then, a logistic regression model was utilized with DNA as the only independent variable and the Receiver Operating Characteristic (ROC) curve assessed the strength of the relationship between DNA and the presence of DVT. A Spearman correlation was performed to determine the relationship of the DNA value to the biomarkers, the Wells score and the ratio of ADAMTS13/VWF. Statistical analyses were performed using GraphPad Prism Software (GraphPad Prism version 5.01 for Windows, GraphPad Software, San Diego, CA) and SAS/IML software (SAS Institute, Inc.,Cary, NC).
RESULTS
Plasma DNA
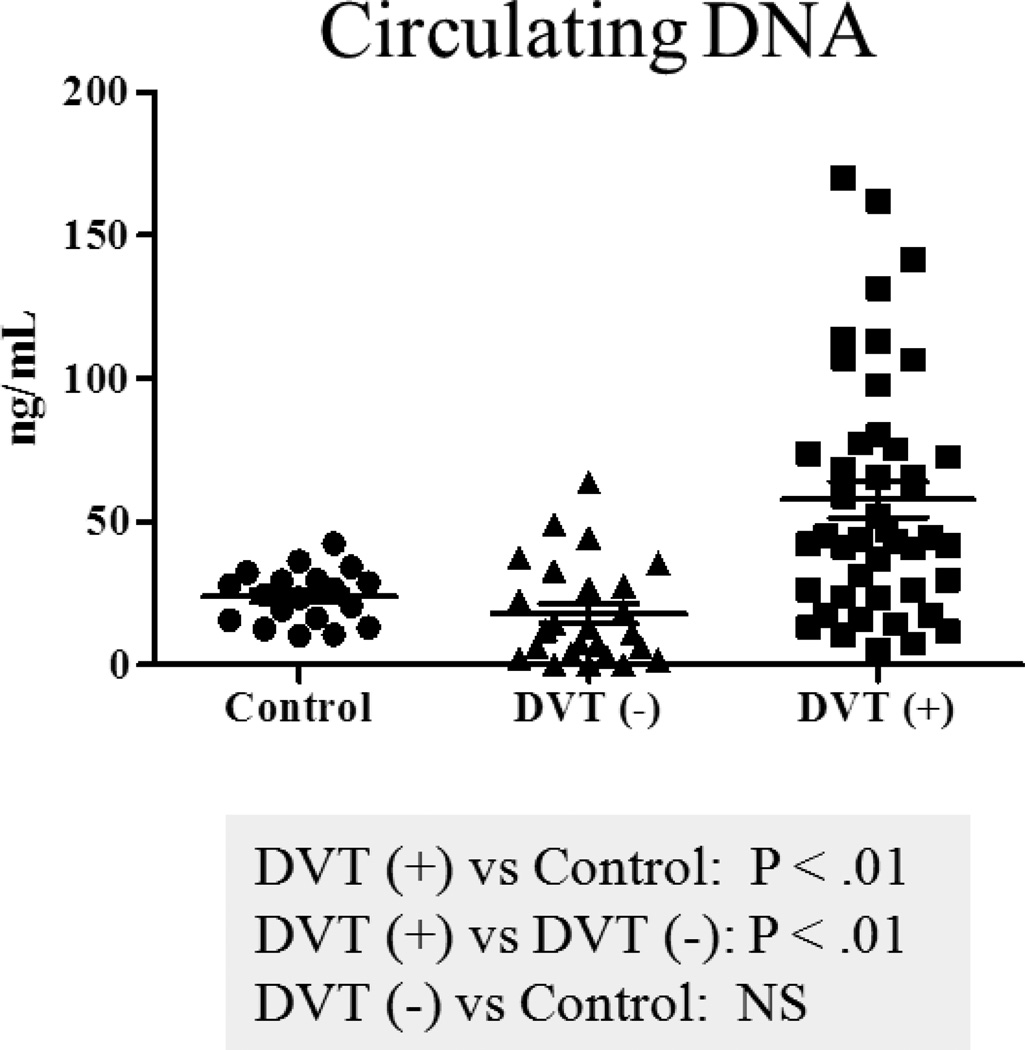
Plasma DNA was significantly increased in DVT positive patients, compared to controls (57.7±6.3 vs. 23.9±2.1ng/mL, P<.01), and also to DVT negative patients (57.7±6.3 vs. 17.9±3.5ng/mL, P<.01). In addition, plasma DNA was not significantly elevated in DVT negative patients when compared to controls (17.9±3.5 vs. 23.9±2.1ng/mL) (Figure 2). There was no significant correlation between an increase in plasma DNA and gender, DVT family history, race, previous DVT, surgery, cancer, chemotherapy or pulmonary embolism (PE).
Figure 2.
Comparison of circulating DNA levels in patients. DVT (+): DVT positive group; DVT (−): DVT negative group; Control: Control group. Bars: represent ± standard error of the mean.
Logistic regression model between plasma DNA and the presence of DVT and correlation between plasma DNA and biomarkers
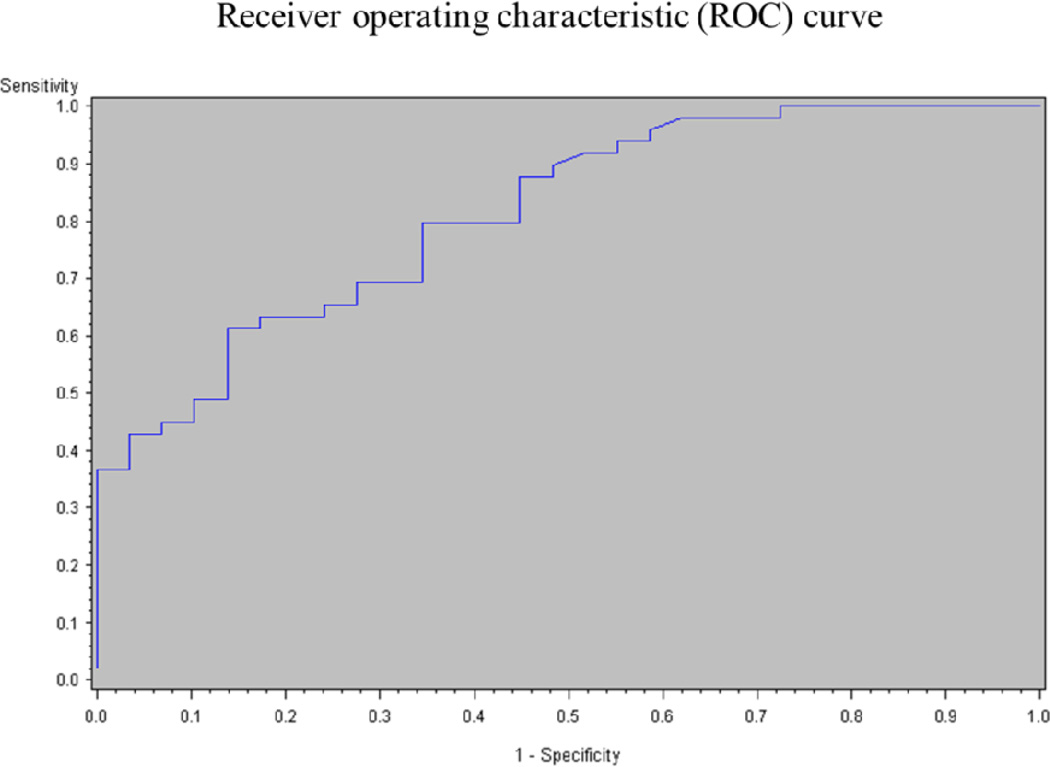
The logistic regression model showed a strong association between plasma DNA and the presence of DVT. From this analysis, the area under the ROC curve was determined to be 0.814, meaning that using DNA as a diagnostic tool for DVT has a specificity and sensitivity of around 81% (Figure 3). A positive correlation was found between circulating DNA levels and various biomarkers (see below in biomarkers section for individual results): CRP (P<.01), D-dimer (P<.01), VWF (P<.01), Wells score (P<.01) and MPO (P<.01) (Table 2). A significant negative correlation was observed between circulating DNA and ADAMTS13 (P<.01). No correlation was found between plasma DNA and sP-sel or plasma DNA and BMI (Table 2). In addition, using multivariable regression, with DNA as the dependent variable and D-dimer, VWF, CRP, sP-sel and ADAMTS13 as the independent variables, we identified that CRP, D-dimer and VWF were independently correlated with DNA (P<.05).
Figure 3.
Logistic regression model of DNA values
Table II. Spearman correlations in DVT positive patients.
Correlations between DNA and Wells score, BMI and biomarkers of DVT.
| Wells | BMI | CRP | D-dimer | sP-sel | VWF | ADAMTS13 | MPO | |
|---|---|---|---|---|---|---|---|---|
| DNA | 0.31699 | 0.11110 | 0.40075 | 0.46578 | 0.10710 | 0.30928 | −0.30204 | 0.50210 |
| P values | <.01 | NS | <.01 | <.01 | NS | <.01 | <.01 | <.01 |
NS= Nonsignificant.
Biomarkers
Soluble P-Selectin
sP-sel was significantly increased two-fold in DVT positive patients, compared to controls (71.8±4.5 vs. 41.5±5.3 ng/mL, P<.01), and was also elevated, but not significantly in the DVT positive group, compared to DVT negative patients (71.8±4.5 vs. 59.5±4.6ng/mL). Also DVT negative patients, showed significantly elevated sP-sel levels compared to controls (59.5±4.6 vs. 41.5±5.3ng/mL, P<.05).
D-dimer
D-dimer was significantly increased 22-fold in DVT positive patients versus controls (5.35±0.50 vs. 0.24±0.06mg/L, P<.01) and was also significantly elevated in the DVT positive group when compared to DVT negative patients (5.35±0.50 vs. 0.83±0.02mg/L, P<.05). In addition, D-dimer was significantly elevated in DVT negative patients, compared to controls (0.83±0.02 vs. 0.24±0.06mg/L, P<.01).
CRP
CRP levels were significantly increased 24-fold in DVT positive patients, compared to controls (4.94±0.80 vs. 0.19±0.07ng/mL, P<.01), and were also significantly elevated in the DVT positive group compared to DVT negative patients (4.94±0.80 vs. 0.57±0.23ng/mL, P<.01). In addition, CRP levels were not significantly elevated in DVT negative patients, compared to controls (0.57±0.23 vs. 0.19±0.070ng/mL).
VWF activity and antigen
Plasma VWF activity was significantly increased in DVT positive patients, compared to controls (233.7 ±17.3 vs.110.8±10.4%, P<.01), and was also elevated in the DVT positive group, compared to DVT negative patients (233.7 ±17.3 vs. 150.1±17.1%) while, VWF activity was not significantly different in DVT negative patients and controls (150.1±17.1vs. 110.8±10.4%).
Plasma VWF antigen was significantly increased in DVT positive patients, compared to controls (307.7 ±26.8 vs. 107.7±10.6%, P<.01), and was also elevated in the DVT positive group, compared to DVT negative patients (307.7 ±26.8 vs. 156.2±20.4%). In addition, VWF antigen was significantly elevated in DVT negative patients, compared to controls (156.2±20.4 vs. 107.7±10.6% activity, P<.05).
MPO
MPO plasma levels were significantly increased in DVT positive patients, compared to controls (31.7±3.6 vs. 5.7±0.9 Arbitrary Units (A.U.), P<.01), and were also significantly elevated in the DVT positive group compared to DVT negative patients (31.7±3.6 vs. 15.5±4.7A.U., P<.01). In addition, MPO levels were not significantly elevated in DVT negative patients, compared to controls (15.5±4.7 vs. 5.7±0.9A.U.).
ADAMTS13 activity
The ADAMTS13 levels were significantly decreased in DVT positive patients, compared to controls (87±3.6 vs. 104±4.4%, P<.01), and were also significantly decreased in the DVT positive group compared to DVT negative patients (87±3.6 vs. 102±4.2%, P<.05). There was no significant difference in the ADAMTS13 levels from DVT negative patients, compared to controls (102±4.2 vs. 104±4.4%).
ADAMTS13/VWF:activity ratios
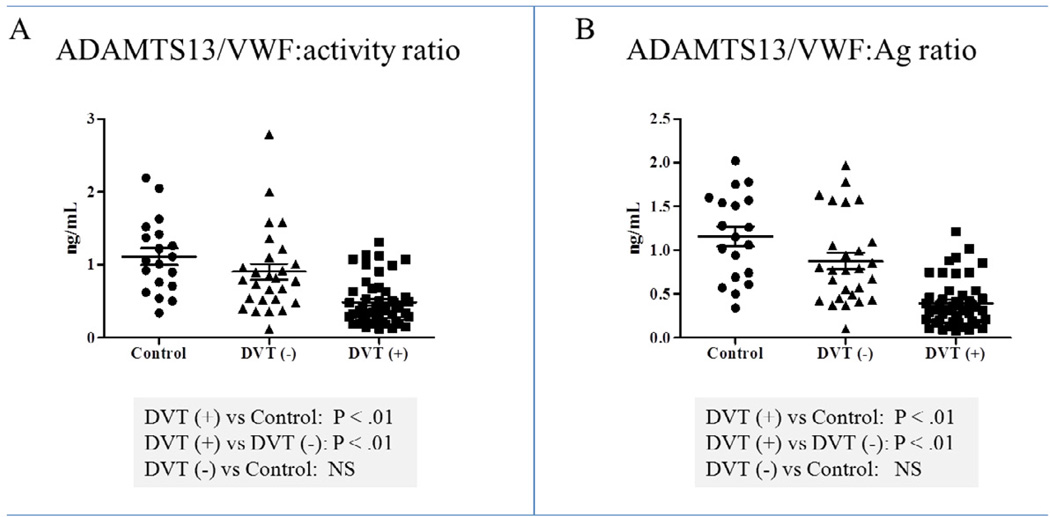
The ADAMTS13/VWF:activity ratios were calculated per individual patient sample and the means were: 1.11 (range 0.34 – 2.19) for controls ; 0.90 (range 0.12 – 2.79) for DVT negative patients and 0.48 (range 0.12 – 1.31) for DVT positive patients. The ADAMTS13/VWF:activity ratios were significantly decreased in DVT positive patients, compared to controls (0.48±0.04 vs.1.11±0.11, P<.01), and in the DVT positive group when compared to DVT negative patients (0.48±0.04 vs. 0.90±0.11, P<.01). There were no significant differences in the ADAMTS13/VWF activity ratios from DVT negative patients, compared to controls (0.90±0.11 vs. 1.11±0.11) (Figure 4A).
Figure 4.
ADAMTS13/VWF:activity (A) and ADAMTS13/VWF:Ag ratios (B) results. Control: Control group; DVT (−): DVT negative group; DVT (+): DVT positive group. Bars: represent ± standard error of the mean.
The ADAMTS13/VWF:Ag ratios were calculated per individual patient sample and the means were: 1.15 (range 0.34 – 2.02) for controls ; 0.88 (range 0.10 – 1.97) for DVT negative patients and 0.40 (range 0.08 – 1.21) for DVT positive patients. The ADAMTS13/VWF:Ag ratios were significantly decreased in DVT positive patients, compared to controls (0.40±0.04 vs.1.15±0.11, P<.01), in the DVT positive group when compared to DVT negative patients (0.40±0.04 vs. 0.88±0.09, P<.01). There were no significant differences in the ADAMTS13/VWF:Ag ratios from DVT negative patients, compared to controls (0.88±0.09 vs. 1.15±0.11) (Figure 4B).
Spearman correlations between plasma DNA and ADAMTS13/VWF activity ratio showed a significant negative correlation in the control group (Spearman r −0.530; P<.05), and not significant negative correlation in the DVT negative group (Spearman r −0.309) and the DVT positive group (Spearman r −0.245), respectively.
Spearman correlations between plasma DNA and ADAMTS13/VWF Ag ratios showed a significant negative correlation in the control group (Spearman r −0.493; P<.05) and in the DVT negative group (Spearman r −0.395; P<.05). There was a negative correlation in the DVT positive group (Spearman r −0.248).
DISCUSSION
Extracellular DNA has recently been reported to contribute to DVT through its interaction with endothelial cells, platelets, red blood cells and effects on the coagulation and thrombolysis processes (1). In addition, several recent reports indicate that extracellular DNA and histones contribute to thrombosis (2–4). DNA has been shown to promote fibrin formation by enhancing the activity of coagulation proteases of both the extrinsic and intrinsic pathway (8, 17, 18). Histones enhance thrombin generation in a dose-dependent fashion in the presence of fully activated platelets, an effect not dependent on tissue factor (19) which causes platelet aggregation in connection with adhesion molecules such as fibrinogen or VWF (20). Thus, histones induce platelet aggregation as well as platelet P-selectin, phosphatidylserine and factor V/Va expression (19). In addition, in the presence of histones, thrombin generation occurs independent of factor XII (19), and polyphosphate (which is released from activated platelets) is able to induce thrombin generation in plasma (21).
DNA and histones can be released from any apoptotic or necrotic nucleated cell including neutrophils. In addition, leukocytes can release extracellular DNA traps by a process termed NETosis, which is distinct from apoptosis and necrosis. Circulating DNA is a surrogate marker for NETs. Several studies have shown that plasma DNA is associated or correlates with plasma markers of neutrophils (such as MPO) indicating that neutrophil-derived DNA may contribute to plasma DNA. Neutrophils, which are the predominant nucleated cells in acute DVT, are able to release DNA and histones also in the form of ETs through coordinated cell death program, which is distinct from apoptosis and necrosis (22). ETs have been shown to participate in experimental DVT (7–9) as a structural part of in vivo thrombi in mice (8, 9) and baboons (7) and in in vitro thrombi (10) generated in a flow chamber that involve human platelets and neutrophils (7)(2). In addition, circulating DNA has been investigated as potential biomarker in the context of pulmonary embolism (23, 24). However, there are no current data supporting that this phenomenon occurs in human DVT. There is a clear limitation in obtaining tissue samples from the DVT site in humans, so measuring circulating components of the biological processes, such as circulating DNA, may help to understand human pathophysiology. Thus, we explored whether DNA would be increased in DVT-positive patients, in comparison to control subjects. DNA levels were observed to be significantly increased only in the DVT positive group, compared to both the control (more than 2-fold) and DVT negative group (more than 3fold) (Figure 2).
It remains to be seen whether the data in this manuscript will help in the prevention of DVT in high risk patients. However, the purpose of this study was to identify the presence of circulating DNA (and by inference NETs) in patients with DVT for the very first time the area under the ROC curve (0.814), performed using a logistic regression model, strongly suggests that elevated plasma DNA could predict the presence of DVT (Figure 3). To further investigate the correlation between increased levels of circulating DNA, and established DVT biomarkers (soluble P-selectin, D-dimer, CRP) and other markers of interest (MPO, VWF and ADAMTS13) in DVT positive patients, Spearman correlations demonstrated a significant positive correlation between circulating DNA and CRP, D-dimer, VWF, and the Wells score. In addition, CRP, D-dimer and VWF were independently associated with plasma DNA. Since ETs are the product of an inflammatory process, the correlation with CRP was expected. The correlation of D-dimer and DNA indicates that fibrinolysis and ETs degradation may occur in parallel, perhaps one facilitating the other. The correlation between plasma DNA and CRP levels and D-dimer, and the Wells score, support a relationship between circulating DNA, inflammation and DVT. Because NETs have been implicated in the pathogenesis of immune diseases, it is important to note that only one patient with positive DVT had a possible autoimmune disorder underlining DVT - the presence of Crohn’s disease.
It has been recently demonstrated that DNA co-localizes with VWF in thrombi from baboons (7) and mice (8). VWF was found to be crucial for DVT initiation and platelet recruitment in mice (25). In different clinical situations, such as sepsis, sickle cell disease, myocardial infarction and ischemic stroke (26–29) an inverse correlation between VWF levels and ADAMTS13 activity was observed. This is confirmed here as well, where we observed lower ADAMTS13/VWF ratio in DVT negative patients compared to controls, and the ratio were decreased even further in DVT positive patients. We documented a significant positive correlation between circulating DNA and VWF and a significant negative correlation between circulating DNA and ADAMTS13 (Table 2). This further supports a relationship between circulating DNA, platelets, and DVT.
We have recently reported an association between circulating DNA and systemic thrombosis(20). In patients with thrombotic microangiopathies (TMA), who suffer from life-threating systemic thrombotic events, we detected elevated levels of plasma DNA and an inverse correlation between plasma DNA and platelet counts. Plasma DNA was strongly elevated (up to several microgram per ml) (20). In DVT patients, the amount of plasma DNA was lower, compared to TMA patients. Importantly, in both studies, plasma DNA strongly correlated with plasma MPO, an enzyme stored in neutrophil granules, indicating that neutrophils may be the source of the circulating DNA.
A limitation of this study is the small number of patients and a much larger study will be necessary to determine clinical relevance of circulating DNA as a diagnostic biomarker. A second limitation is that the majority of the patients recruited in this study were outpatients and as such, the applicability of this study to inpatients cannot be determined. Finally, D-dimer and VWF Ag levels were elevated in the DVT negative patients. However, both D-dimer and VWF Ag levels may have been elevated due to the presence of comorbid disease including: active malignancy (6 patients), recent major operation (3 patients), active infection (1 patient), Crohn’s disease (1 patient), active malignancy and infection (1 patient), and end stage liver disease (1 patient).
In summary, we found that plasma DNA was significantly elevated in patients with acute DVT. This is the first report substantiating plasma DNA in clinical DVT. Further studies are warranted in order to understand the exact role of circulating DNA in the pathogenesis of DVT and the importance of degradation of DNA by DNases and VWF by ADAMTS13 in successful thrombolysis.
ACKNOWLEDGEMENTS
Drs Lämmle and Kremer Hovinga supported by the Swiss National Science Foundation (grant number 32003B-124892 to JA Kremer Hovinga and B Lämmle). Drs Thomas Wakefield and Denisa Wagner supported by the NIH (grant number HL095091).
APPENDIX
The Michigan Research Venous Group includes the authors and Nicole K. Baker, BS; Kenneth E. Guire, MA; Angela E. Hawley, BS; Elise P. DeRoo, BA; Cathy C. Stabler, RN, CCRP; Shirley K. Wrobleski, BS, LVT all from the Section of Vascular Surgery at the University of Michigan, Ann Arbor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012 Aug;32(8):1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004 Mar 5;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 3.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, et al. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe. 2010 Nov 18;8(5):445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008 Mar 15;111(6):3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 5.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008 Sep;14(9):949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 6.Stewart GJ, Ritchie WG, Lynch PR. Venous endothelial damage produced by massive sticking and emigration of leukocytes. Am J Pathol. 1974 Mar;74(3):507–532. [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010 Sep 7;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012 Jan;10(1):136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012 Apr 9;209(4):819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010 Aug;16(8):887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 11.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010 May 25;107(21):9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandy FC, Stabler C, Eliassen AM, Hawley AE, Guire KE, Myers DD, et al. Soluble P-Selectin for the Diagnosis of Lower Extremity Deep Venous Thrombosis. Journal of Vascular Surgery. 2012 doi: 10.1016/j.jvsv.2012.09.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lammle B. Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood. 2012 Aug 9;120(6):1157–1164. doi: 10.1182/blood-2012-02-412197. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremer Hovinga JA, Mottini M, Lammle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost. 2006 May;4(5):1146–1148. doi: 10.1111/j.1538-7836.2006.01904.x. [DOI] [PubMed] [Google Scholar]
- 15.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005 Apr;129(1):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [Evaluation Studies Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 16.Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997 Dec 20–27;350(9094):1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 17.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010 Dec 15;185(12):7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 18.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, et al. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011 Aug 18;118(7):1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011 Sep 29;118(13):3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011 Dec 22;118(26):6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007 Jan 15;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldhaber SZ, Hennekens CH, Markisz JA, McNeil BJ, Glynn MA, Bettmann MA, et al. Low sensitivity of plasma DNA in screening for pulmonary embolism. Am Rev Respir Dis. 1982 Aug;126(2):360–361. doi: 10.1164/arrd.1982.126.2.360. [DOI] [PubMed] [Google Scholar]
- 24.Vargo JS, Becker DM, Philbrick JT, Schoonover FW, Davis JS. Plasma DNA. A simple, rapid test for aiding the diagnosis of pulmonary embolism. Chest. 1990 Jan;97(1):63–68. doi: 10.1378/chest.97.1.63. [DOI] [PubMed] [Google Scholar]
- 25.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011 Jan 27;117(4):1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerolle N, Dunois-Larde C, Badirou I, Motto DG, Hill G, Bruneval P, et al. von Willebrand factor is a major determinant of ADAMTS-13 decrease during mouse sepsis induced by cecum ligation and puncture. J Thromb Haemost. 2009 May;7(5):843–850. doi: 10.1111/j.1538-7836.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- 27.Schnog JJ, Hovinga JA, Krieg S, Akin S, Lammle B, Brandjes DP, et al. ADAMTS13 activity in sickle cell disease. Am J Hematol. 2006 Jul;81(7):492–498. doi: 10.1002/ajh.20653. [DOI] [PubMed] [Google Scholar]
- 28.Andersson HM, Siegerink B, Luken BM, Crawley JT, Algra A, Lane DA, et al. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012 Feb 9;119(6):1555–1560. doi: 10.1182/blood-2011-09-380618. [DOI] [PubMed] [Google Scholar]
- 29.Kremer Hovinga JA, Zeerleder S, Kessler P, Romani de Wit T, van Mourik JA, Hack CE, et al. ADAMTS-13, von Willebrand factor and related parameters in severe sepsis and septic shock. J Thromb Haemost. 2007 Nov;5(11):2284–2290. doi: 10.1111/j.1538-7836.2007.02743.x. [DOI] [PubMed] [Google Scholar]