Abstract
Forty years ago, few cohort studies of cardiovascular disease (CVD) included women and fewer still included diabetes or glycemia as a risk factor. I describe here the Rancho Bernardo Study (RBS), a single-site, >40-year cohort study of sex differences in heart disease and how diabetes modifies women’s natural cardioprotection. More than 6000 participants were followed for morbidity and mortality, with nearly 3000 survivors (and death certificates for >85% of decedents).
In RBS more than half of diabetes was undiagnosed without an oral glucose tolerance test (OGTT); more women than men had isolated post-challenge hyperglycemia (IPH) as their only glucose evidence of diabetes; men had more diabetes than women, with higher fasting but lower post-challenge glucose levels than women; women with diabetes had more classical CVD risk factors than men; excess risk-factor clustering partially explained how diabetes eradicates female cardioprotection. Post-challenge glucose was a stronger CVD risk factor than fasting glucose. Endogenous insulin was not an independent CVD risk factor in women or men.
Men with higher testosterone levels developed less diabetes and had fewer metabolic syndrome components. In men higher total testosterone levels predicted a reduced risk of all-cause and CVD but not cancer mortality. In women both extremes of bioavailable testosterone predicted fatal coronary heart disease but not all-cause mortality.
Summary point estimates from large systematic reviews of individual data have replicated most RBS findings. Ongoing research can further clarify how diabetes modifies women’s cardioprotection from mid-life to old age.
Keywords: cardiovascular disease, cohort studies, coronary heart disease, diabetes, gender
Introduction
A 1985 review of hyperglycemia as a risk factor for coronary heart disease by Epstein(1) included 29 prospective studies of glycemia and heart disease risk, adjusted for cholesterol, smoking, and blood pressure, and found an independent association in 5 of 13 cohort studies using post-challenge hyperglycemia after different glucose loads and intervals. Glycemia was not associated with heart disease outcomes in studies using fasting plasma glucose (FPG) or casual glucose levels. Only four of these 29 studies included women! A 1999 review of glucose and incident cardiovascular events by Coutinho(2) included 20 published studies and nearly 100,000 individuals followed for an average of 12.5 years; the risk of incident cardiovascular disease increased with increasing glycemia beginning at glucose levels lower than the then presumed diagnostic threshold. Only two of these 20 studies included women! The Rancho Bernardo Study (RBS) began before the first WHO (1979) and ADA (1980) definitions of diabetes were created, designed in part to facilitate comparisons across populations.
Background of the Rancho Bernardo Study
The Rancho Bernardo Study (RBS) began as one of 12 Lipid Research Clinic (LRC) Prevalence Study sites sponsored by the National Heart Institute (now the National Heart Lung and Blood Institute). I was invited to be the epidemiologist for the San Diego LRC. We were the only North American site that measured height, weight, blood pressure, and fasting plasma glucose, and added 11 questions about lifestyle, medical history, classic cardiovascular disease (CVD) risk factors, and diabetes. We performed a house-to-house survey at baseline to find the target population (residents age 30 and older), which allowed us to calculate prevalence rates and study nonresponse bias.
LRC participants had moved from 38 states and Washington, D.C., to Rancho Bernardo, a new town in southern California. Like 95% of suburbs of the 1970s, a majority of residents were white, middle-class, and married; 90% had at least a high school education and health insurance. We viewed their education and social class homogeneity as an advantage, reducing differences in heart disease risk factors due to socioeconomic factors, and yielding reliable medical histories. My 1971 memo defending the choice of Rancho Bernardo as our LRC Prevalence Study location also noted the paucity of studies of community-dwelling “old people aged >50 years”.
We enrolled 82% of the target population age 30+ years in 1972-74. Within six weeks of their first brief clinical evaluation, 92% of a pre-selected subset (a 20% random sample plus those with hyperlipidemia) completed a second more extensive evaluation that included lifestyle, classic Framingham risk factors, most of the subsequently defined components of the metabolic syndrome (MetS), and a 24-hour diet recall using food models to quantify serving size and calories.
RBS, among the first cohort studies to focus on sex differences in diabetes and CVD, was inspired by a 1960s text book of medicine showing two unmodifiable CVD risk factors (age and sex) and four potentially modifiable risk factors (blood pressure, cholesterol, smoking, and diabetes). Sex and diabetes were the least well studied.
In 1984-87, 82% of surviving local community-dwelling RBS participants (who were then aged 45 to 100 years) attended a clinic visit funded by the NIDDK, when we first performed an oral glucose tolerance test (OGTT). Thereafter RBS research clinic visits were conducted every 2-5 years for six additional visits (repeated NIH R01 funding and four MERIT awards). About 95% of participants have been followed by mail or telephone for vital status to the present, and death certificates coded for >85% of decedents. Our expanded focus now includes sex differences in successful aging; quality of life; cognitive, physical, and emotional function; potentially modifiable behaviors; and co-morbidity.
The following is a personal history of highlights of our diabetes-CVD research; for brevity, only selected studies are noted. Nothing described here was done by a single person. Few epidemiologists work alone.
Early Studies
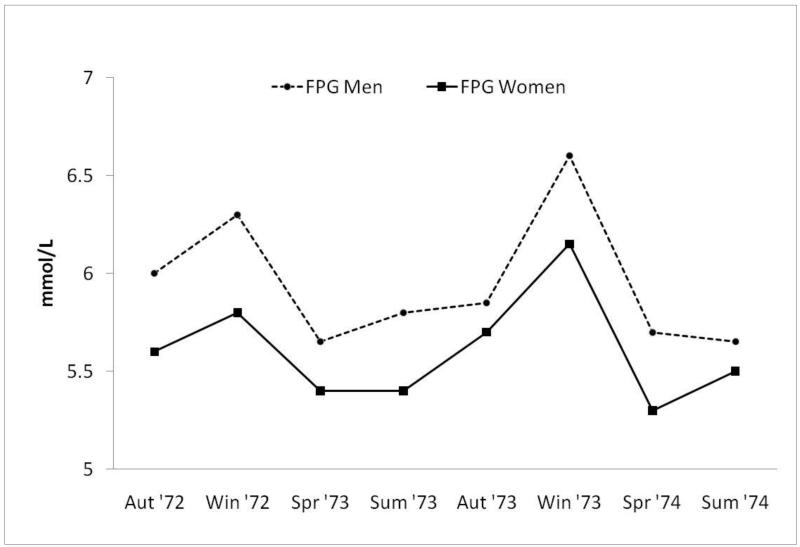
Early RBS publications showed that the prevalence of diabetes by history or FPG ≥140 mg/dl was nearly twice as high in men as in women(3). We also found that the prevalence of previously unknown diabetes, defined as FPG ≥140 mg/dl, was less than the prevalence of known diabetes, and that only 30% of known diabetics, approximately half of whom were using diabetes-specific medications, would have been correctly identified using the FPG cut-point criterion of ≥140 mg/dl[4]. We reported that FPG varied little by age in either sex, although men had higher FPG levels than women(3). There was striking seasonal variation in FPG based on local climatological data from the U.S. Department of Commerce(p = 0.03), not on individual exposure (Figure 1)(5)--possibly we missed early evidence for a vitamin D anti-diabetic effect.
Figure 1.
Seasonal variation in FPG levels occurring over a 2-year period (1972 – 1974); FPG (fasting plasma glucose).
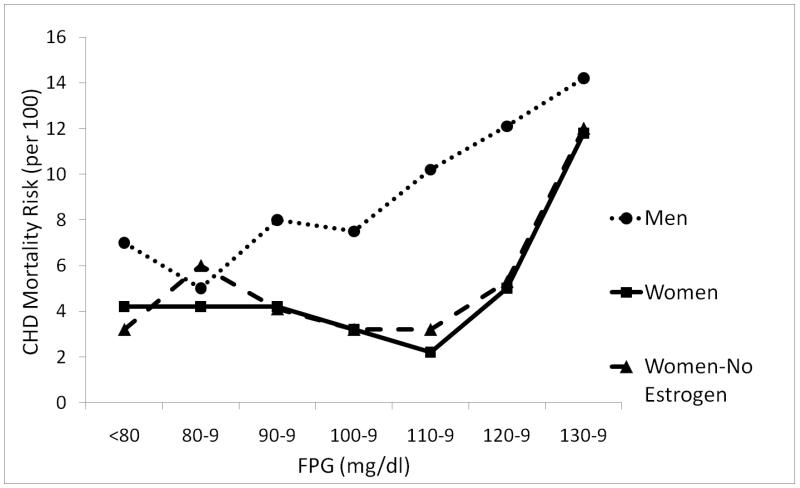
To separate glycemia from diabetes as a CVD risk factor, we studied participants without diabetes by history or high FPG (>140 mg/dl). Systolic blood pressure, body mass index, and triglycerides were higher at every incremental level of glucose in both sexes, and men had higher levels than women(6). In the same paper, the 14-year heart disease mortality risk in men showed a linear positive association throughout the entire range of baseline FPG levels; in contrast, in women there was a threshold association beginning at a glucose level around 100 mg/dl (Figure 2). This striking difference, where women appear to tolerate higher glucose levels than men, is concordant with their cardioprotection.
Figure 2.
Age-adjusted CHD mortality risk by FPG levels among nondiabetics aged 40 – 79 years, Rancho Bernardo, CA 1972-1987 (Scheidt-Nave C, Barrett-Connor E, Wingard DL, Cohn BA, Edelstein SL. Sex differences in fasting glycemia as a risk factor for ischemic heart disease death. Am J Epidemiol 1991; 133: 565-576, by permission of Oxford University Press) CHD (coronary heart disease); FPG (fasting plasma glucose).
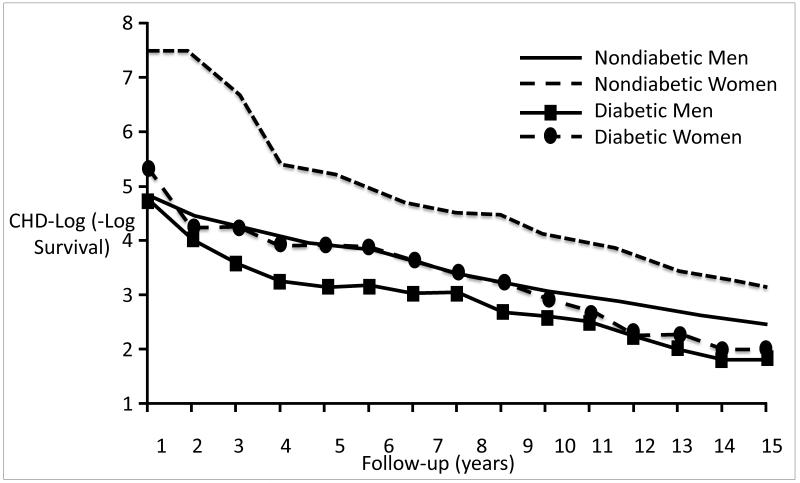
RBS men who had diabetes by history or FPG had a 2.4-fold excess risk of heart disease compared to men without diabetes, and women who had diabetes had a 3.5-fold excess risk compared to women without diabetes; these differences were independent of many covariates(7). The CHD sex-specific mortality differences by diabetes status based on 15-year log survival curves are shown in Figure 3(8). Women without diabetes at baseline had superior survival throughout compared to all other groups; women with diabetes had a survival risk similar to men without diabetes for the first 12 years of follow up, and thereafter had a risk similar to men with diabetes. These data dramatically illustrate how diabetes ultimately overrides female cardioprotection.
Figure 3.
Age-adjusted CHD –log(−log survival) by sex and diabetes; Rancho Bernardo,CA, 1972 through 1988. Curves were estimates by a Cox model blocked on both sex and diabetes status and adjusted for age (Barrett-Connor EL, Cohn BA, Wingard DL, et al. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA 1991;265:627-31 (Copyright ©1991 American Medical Association. All rights reserved); CHD (coronary heart disease).
Risk factor clusters
The proportion clustered in the 90th percentile of blood pressure, triglycerides, smoking, and obesity was almost twice as high among women compared to men (9, 10). When RBS data were pooled with sex-specific data from seven other cohort studies (11), this mini-meta-analysis showed large and statistically significant sex differences in coronary heart disease (CHD) before adjusting for clustering; these sex differences disappeared in cluster-adjusted analyses that showed similar adjusted relative risks of 2.3 in men and 2.9 in women. We concluded that most of the loss of female cardioprotection in the presence of diabetes could be explained by women’s higher prevalence of clustered classic CVD risk factors. Importantly, most of the observed sex difference in mortality was mediated by traditional and modifiable Framingham CVD risk factors.
Diagnosis of Diabetes and risk of CVD in the Glucose Tolerance Test Era
As expected, the RBS distribution of fasting plasma glucose (FPG) was much narrower than post-challenge glucose (PCG) (12), because most people spend more of their 24-hour day in the fed than in the fasting state. On the basis of limited range alone, it would be more difficult to show a glucose-CVD association with FPG than with PCG (even though PCG is known to have more day-to-day individual variation). There was a larger proportion of the population with categorically defined impaired glucose tolerance (IGT) than categorically defined impaired fasting glucose (IFG), even using a low FPG threshold of <100 mg/dl(13).
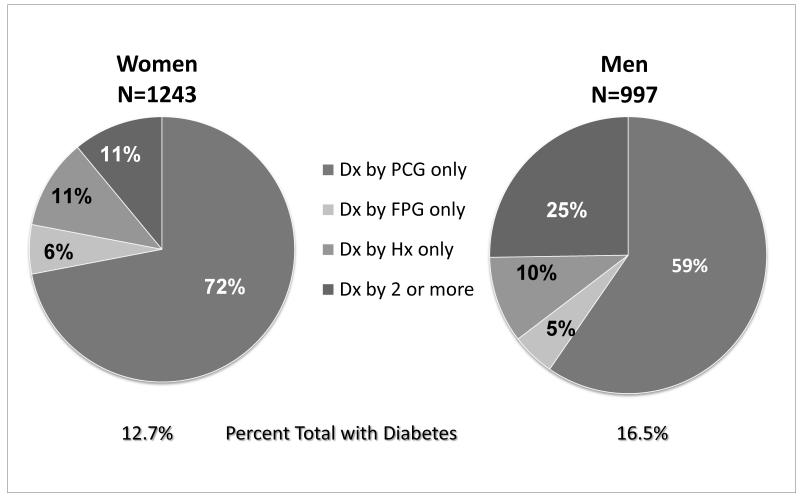
We were surprised how much diabetes in older adults would be missed without OGTT. As shown in pie graphs drawn from our 1990 paper (Figure 4) (12), the prevalence of diabetes (by any criteria) was 12.7% in women and 16.5% in men. Few of those with diabetes were diagnosed by history or FPG alone. Indeed, 72% of women and 59% men were diagnosed as diabetic only by isolated post-challenge hyperglycemia (IPH). In other words, without an OGTT we would miss more than half of older adults with undiagnosed diabetes(12). Similar results have now been reported in a NHANES representative U.S. sample (14).
Figure 4.
Frequency distribution of fasting and post – challenge plasma glucose among adults 50-89 years of age, Rancho Bernardo, CA 1984-1987. Drawn from data in D. Wingard et al., Diabetes Care, 1990;13(Suppl. 2):3-8. Dx (diagnosis); PCG (post-challenge glucose) ; FPG (fasting plasma glucose); HX (history).
We repeated this analysis after excluding participants who had known heart disease or diabetes; 70% of 125 women and 48% of 133 men had previously undiagnosed diabetes detected only by IPH (15). After a seven-year follow-up, women who had IPH had a multiply adjusted hazard ratio of 2.6 for CVD death and 2.2 for CHD death; no independent associations were seen in men(11). Similar results were reported from a pooled analysis of 13 prospective European cohort studies (six included women) with OGTT and a median 10-year follow-up; FPG alone did not identify individuals at increased risk of death(16).
Our finding that more than half of older people without known diabetes had diabetes based on IPH was recently confirmed in a 2010 meta-analysis of 102 prospective studies of older adults (including Ranch Bernardo); this meta-analysis also found that diabetes was a significantly stronger heart disease risk factor in women than men, and more strongly related to fatal than nonfatal myocardial infarction (17).
Because glycosylated hemoglobin (GHb) reflects usual glucose levels over the past 3-4 months, it is an excellent test of glucose control in patients with diabetes. Not surprisingly it was also a better CVD risk factor than glucose measured on a single day (18) (Table 1). Unfortunately, GHb was not good for diagnosing diabetes, such that one-third of participants with diabetes by FPG or PCG criteria would have been classified as nondiabetic using a GHb cutpoint of 6.5%(19). A similar low sensitivity was reported from NHANES in which the proportion with diabetes undiagnosed by glycosylated hemoglobin was 40% lower than the proportion diagnosed by single glucose cut-point criteria (14).
Table 1. GHb is a Better Predictor of 8-year Fatal CVD & CHD in Women but not Men w/o Diabetes.
| Men | Women | |
|---|---|---|
| HR (95% Cl) | HR (95% Cl) | |
| CVD mortality | ||
| GHb | 1.10 (0.61-1.97) | 2.61 (1.40-4.88) |
| FPG | 0.75 (0.39-1.46) | 1.30 (0.61-2.81) |
| PCG | 0.83 (0.47-1.45) | 1.01 (0.51-2.00) |
| CHD mortality | ||
| GHb | 1.25 (0.56-2.78) | 2.59 (1.14-5.85) |
| FPG | 0.98 (0.42-2.30) | 0.98 (0.34-2.80) |
| PCG | 0.90 (0.41-1.96) | 0.87 (0.34-2.21) |
Adjusted for age, systolic blood pressure, body mass index, LDL, HDL, triglycerides, smoking, anti-hypertension medications, and estrogen therapy use in women GHb (glycosylated hemoglobin); FPG (fasting plasma glucose) PCG (post-challenge glucose); CVD (cardiovascular disease); CHD (coronary heart disease); HR (hazard ratio); CI (confidence interval)
Adapted from S Park et al., Diabetes Care, 1996;19:450-456.
We suggest that earlier OGTT diagnosis will allow earlier intervention to prevent diabetes or reduce diabetes complications, and will improve our ability to determine whether diabetes or other CVD risk factors come first in the causal pathway to CVD by better exclusion of individuals with diabetes.
Insulin
In 1990 Stout (20) reviewed nearly 40 cross-sectional studies of patients with ischemia of the heart, lower limbs, or brain who showed increased insulin response to oral glucose load; he proposed that high circulating insulin levels were the cause of excess CVD in adults with Type 2 diabetes. In early cross-sectional analyses we showed than fasting insulin but not 2-hour insulin was significantly associated with low HDL and high triglyceride levels in both sexes, and this association with diabetic dyslipidemia was independent of age, BMI, waist/hip ratio, alcohol intake, smoking, and physical exercise(21).
Because most people with IGT or Type 2 diabetes also have hyperinsulinemia, it is difficult to distinguish the role of hyperinsulinemia from hyperglycemia (or other CVD risk factors) in the pathway to CVD. The case for endogenous insulin as a CVD risk factor is most convincing in individuals without diabetes, because fasting insulin is nearly identical to insulin resistance (calculated as HOMA-IR) only in persons without diabetes. When we reviewed studies that excluded individuals with diabetes, we found no prospective studies that showed an insulin-cardiovascular disease association in women, but very few studies reported women separately(22).
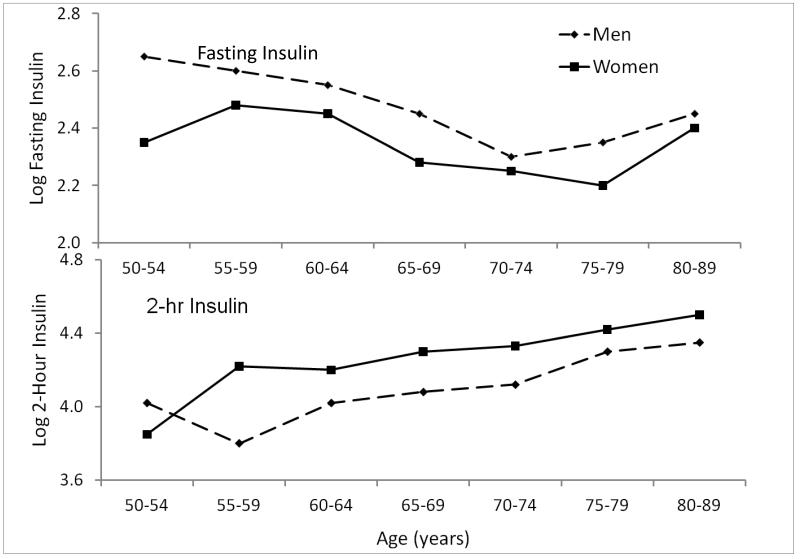
We did find intriguing sex differences in fasting and post-challenge insulin levels(23) (Figure 5). RBS men had higher fasting insulin levels than women, while women had higher 2-hour post-challenge insulin levels than men(24). The insulin sex differences did not explain the five-year risk of fatal cardiovascular disease in men or women. In fact, RBS men with higher post-challenge insulin levels were actually less likely to die, and the significant inverse association of 2-hour PCG with cardiovascular death persisted in multiply adjusted models (24). These results are concordant with results from a meta-analysis of 14 population-based studies, which found no association of fasting insulin with coronary heart disease, and only a weak association of post-challenge insulin (8 studies); these studies were mainly or exclusively in men(25). The meta-analysis authors concluded that any association between circulating insulin and CHD is much weaker than previously thought.
Figure 5.
Mean fasting & 2-hour insulin levels by age and sex, Rancho Bernardo Study, 1984-1987 (Ferrara A, Barrett-Connor E, Wingard DL, et al. Sex differences in insulin levels in older adults and the effect of body size, estrogen replacement therapy, and glucose tolerance status. The Rancho Bernardo Study, 1984-1987. Diabetes Care 1995;18:220-5, by permission of American Diabetes Association).
In the past, most insulin assays did not distinguish total insulin from proinsulin or c-peptide. Proinsulin, a precursor of intact insulin, is a marker for pancreatic beta cell exhaustion, released into the blood when beta cells can no longer produce enough intact insulin to control hyperglycemia. C peptide, derived from proinsulin, is theoretically preferable to insulin as an epidemiologic measure of insulin secretion, because it is less actively extracted by the liver. When we compared the cross-sectional association of intact insulin, proinsulin, and C peptide with prevalent heart disease in the 25% of men and 24% of women without diabetes (by history or OGTT criteria), only proinsulin was associated with prevalent heart disease in both sexes, as shown in Table 2(26). Subsequently, three prospective studies reported that only proinsuliin predicted a more than two-fold increased odds of heart disease; these studies could not address sex differences, because 100% of participants in two of the studies and 82% of participants in the third study were men(25).
Table 2. Odd Ratios (OR) and 95% CI for Insulin Variables and CHD From Multiple Logistic Regression Analyses: the Rancho Bernardo Study, 1992-1996.
| Variable* | OR | 95% Cl | P |
|---|---|---|---|
| Men | |||
| Fasting Insulin | 0.85 | 0.56-1.31 | 0.46 |
| Postchallenge Insulin | 1.17 | 0.83-1.66 | 0.36 |
| Proinsulin | 2.41 | 1.42-4.11 | 0.001 |
| C-peptide | 1.07 | 0.61-1.88 | 0.82 |
| Women | |||
| Fasting Insulin | 1.25 | 0.89-1.76 | 0.20 |
| Postchallenge Insulin | 1.44 | 1.06-1.96 | 0.02 |
| Proinsulin | 1.80 | 1.22-2.64 | 0.003 |
| C-peptide | 1.32 | 0.88-1.97 | 0.18 |
Covariates were age, BMI (body mass index), systolic blood pressure, HDL (high-density lipoprotein) cholesterol, and current estrogen use (women).
The log-odds for CHD increase for every 1-unit increase in the independent variable. confidence interval (CI); coronary heart disease (CHD)
footnote: Reprinted, with permission, from Oh et al., Circulation 2002;105:1311-1316.
Body size and shape
Obesity is the major risk factor for Type 2 diabetes and for vascular disease, as elegantly reviewed in West’s 1978 textbook(27). In RBS we examined this association in several ways, and mention only two here. To study maximum lifetime weight we used self-reported maximum weight (excluding pregnancy) and measured midlife height to calculate body mass index (BMI) correcting for height loss in old age. We found men and women had remarkably similar increased risk of diabetes associated with life-time maximum BMI(28).
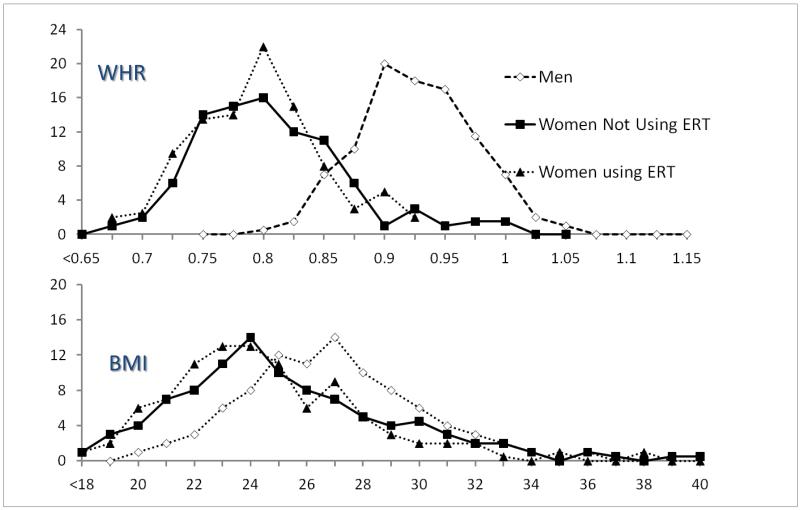
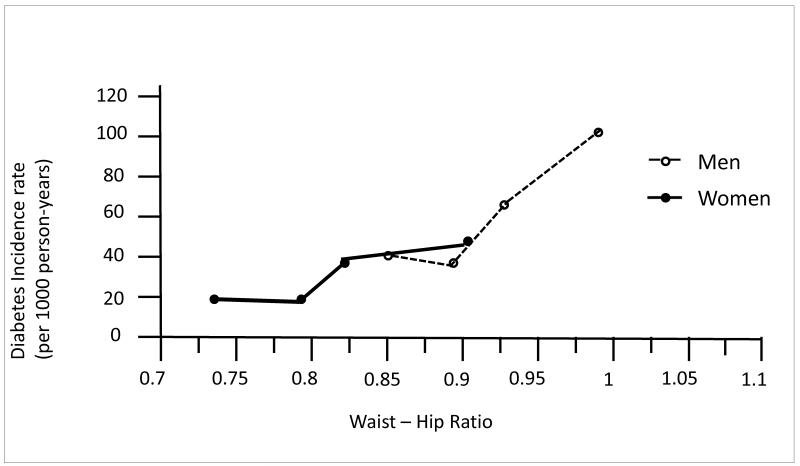
It is accepted that central/upper body obesity is a stronger risk factor for diabetes than BMI; the striking sex differences in waist girth in RBS are illustrated in Figure 6(23). Because there is so little overlap in waist girth by sex, it is statistically (and biologically) incorrect to adjust for sex-specific characteristics to study sex differences; this would be equivalent to controlling for presence of testes. A 1997 analysis (29) (used to calculate sample size for the Diabetes Prevention Trial [DPP]) showed a steady stepwise association between increasing central body fat distribution and the risk of diabetes in both sexes, an increase that parallels body size; the linear association shown in Figure 7 was redrawn from sex-specific data in our DPP paper and shows how similarities can be obscured by sex-specific analyses.
Figure 6.
Percent distribution of waist-hip ratio (WHR) and body mass index (BMI) by sex and ERT (estrogen replacement therapy), Rancho Bernardo, 1984-1987 (Ferrara A, Barrett-Connor E, Wingard DL, et al. Sex differences in insulin levels in older adults and the effect of body size, estrogen replacement therapy, and glucose tolerance status. The Rancho Bernardo Study, 1984-1987. Diabetes Care 1995;18:220-5, by permission of American Diabetes Association).
Figure 7.
Rancho Bernardo Study waist-hip ratio and 8-year diabetes Incidence (Edelstein S, Knowler W, Bain R,Andres R, Barrett-Connor EL, Dowse GK, Andres, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC, Collins VR, Hamman RF. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701-10, by permission of American Diabetes Association).
Because Type 2 diabetes may be unrecognized for years before diagnosis, it has been difficult to determine when weight gain (or loss) occurs in relation to diabetes onset. We evaluated weight measured in 725 men and women (without diabetes by OGTT in 1984-87) who had a second OGTT in1992-96(30). Insulin resistance, defined as the top quartile of fasting insulin or HOMA--which were highly correlated with each other in these participants without diabetes (r =0.98)(30)--was associated with a three-fold increased risk of losing 10 or more kg of body weight over the eight-year follow-up, independent of baseline weight (P < 0.01). These results suggest that hyperinsulinemia has a catabolic effect in older adults, compatible with Porte’s 20-year-old thesis that insulin acts as a catabolic hormone in the brain (31), different from its anabolic effects in peripheral tissues.
Metabolic syndrome
The classic metabolic syndrome (MetS) includes five CVD risk factors that are more common in individuals with diabetes than in those without. Five key factors were described in 1992 by Bierman(32), who recognized that almost all potentially modifiable CVD risk factors (except cigarette smoking and total cholesterol) were associated with diabetes. The choice of the initial components (high blood pressure, high triglycerides, low HDL cholesterol levels, waist girth or waist hip ratio, and high insulin levels or diabetes) was logical, but the cut-points defining normality were arbitrary.
In a RBS factor analysis we could not demonstrate that the MetS components represent a syndrome of clustered factors with a single underlying cause, such as insulin resistance or upper-body obesity; hypertension did not cluster with any of the other MetS components including insulin (33) (Table 3). MetS creates other problems in the context of studying sex differences because two of the universally accepted components, waist girth and HDL cholesterol, have been assigned cut-points that differ by sex.
Table 3. Factor Analysis of Components of the Insulin Resistance Syndrome Among 606 Men and 765 Women 50-89 years old; Rancho Bernardo, CA 1984-87.
| Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|
| Body Size | Glucose Tolerance | Lipids | Blood Pressure |
| Weight | Fasting Glucose | HDL | Systolic |
| Waist girth | 2-hour glucose | LDL | Diastolic |
| Fasting Insulin | 2-hour insulin | Triglycerides |
HDL (high-density lipoprotein), LDL (low density lipoprotein);
Adapted from D. Wingard, PhD, et al., University of California, San Diego, unpublished data presented at American Diabetes Association Scientific Sessions, June 8 – 11, 1996, San Francisco, CA.
In RBS, MetS was significantly associated with the 20-year risk of CHD mortality; in our first analysis the risk did not differ by age, sex, or diabetes status(34). MetS predicted the 4.5 year progression of coronary artery calcium (CAC), an estimate of plaque burden measured by electron beam computed tomography. Participants, whose mean age was 68 years, were excluded from our CAC follow-up study if they had known heart disease(35). MetS was present in 15.1% based on WHO-defined MetS, and in 11.8% by ATP III criteria. Hypertension was the only MetS component independently associated with CAC progression (OR 2.11) in the whole cohort. FPG independently predicted CAC progression only in women <65 years of age.
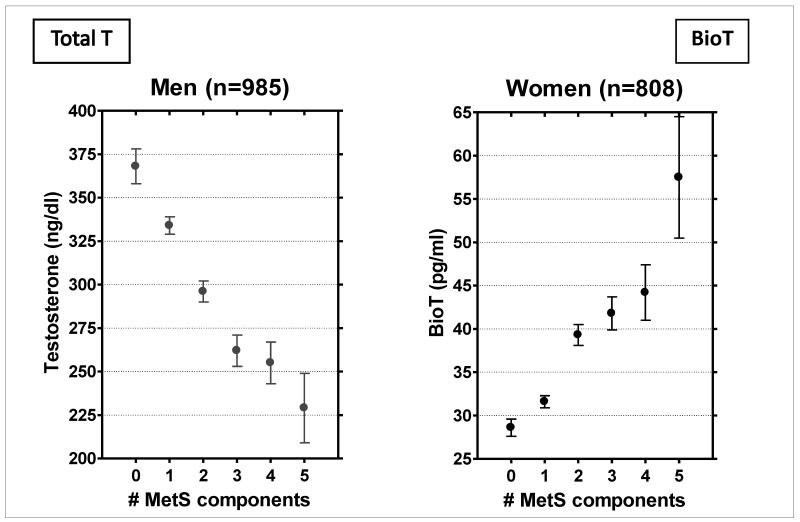
We examined the sex-specific number of the five classic components of the metabolic syndrome by sex hormone levels, shown in Figure 8(36) (unpublished) and found a steady stepwise association in opposite directions by sex: The more MetS components men had, the lower their total testosterone levels; the more MetS components women had, the higher their bioT levels. Because MetS is a precursor of diabetes, and male hormones appear to protect against diabetes and diabetes-related risk factors in men and not in women in RBS, diabetes (and its covariates) is a candidate explanation for the sex differences in cardiovascular disease. Because MetS is a widely accepted marker for diabetes and prediabetes, such that a majority of people with diabetes and about half of those with IGT have MetS, MetS and testosterone are clearly related to each other.
Figure 8.
Mean testosterone levels by the number of metabolic syndrome (MetS) components, Rancho Bernardo Study, 1984-1987 G.A. Laughlin, PhD, University or California, San Diego, unpublished data presented at 87th Annual Endocrine Society Meeting, June 24-27, 2006, Boston, MA. Bio T (bioavailable testosterone).
Another indication that MetS is not entirely satisfactory is the continuing attempt to improve it. In RBS, adding high sensitivity C-reactive protein (CRP) did not improve the ability of MetS to predict cardiovascular disease, but adding IL-6 did(34). Adding albuminuria improved the MetS 8-year prediction of CVD mortality, but only in women (37). Adding uric acid improved prediction of CVD risk but only in women with diabetes (38).
Despite its limitations, MetS played a central role in drawing attention of cardiologists and diabetologists to the link between diabetes and heart disease, and helped tear down the research and clinical silos that previously separated studies of causes and care for the increasing number of people at risk for both diabetes and CVD.
Subclinical heart disease
In 1990, we determined whether silent myocardial infarction (diagnosed mainly by major Q wave ECG) was more common in women than men with or without diabetes (diagnosed by OGTT and medical record review)(39). In this cross-sectional study, ECG evidence of asymptomatic heart disease was more common in those with diabetes than in those without diabetes. In both sexes the association was more pronounced for those with asymptomatic than symptomatic ECG abnormalities. An abnormal ECG in the absence of symptoms was more common in women than in men, differences that were not explained by age, weight, blood pressure, or medication use.
In 2010 we reported the first long-term prospective study of fatal CHD by glycemia status and angina symptoms defined by validated questionnaire. A 12 lead resting electrocardiogram using the Whitehall criteria (applied in the WHO Multinational Study of Diabetes and Vascular Disease criteria for classification of heart disease) was used to exclude women at baseline from the study of angina and diabetes as predictors of heart disease, and therefore ECG was not used as a predictor of future heart disease(40). Women with diabetes and angina had a three-to four-fold greater risk of dying from CHD than women who had diabetes without angina, independent of covariates. There were no independent associations in men. In both the cross-sectional and prospective studies, sex differences in subclinical heart disease extended to the two-thirds of individuals who did not know they had diabetes before their research clinic evaluation, evidence against diabetes detection bias as a cause of this association.
Sex hormones
A WHO analysis of CHD death rates for middle-aged men and women from 52 countries, using 1987 data (prior to adequate blood pressure or lipid treatment or widespread estrogen therapy)(41), clearly showed that men had more heart disease than women in every country despite large differences in country-specific rates. The ratio of male to female mortality in all countries was a constant with a mean ratio of 2.4 ± 0.8. These universal results make it unlikely that CHD sex differences are explained by lifestyle or occupation, and strongly suggest a common intrinsic factor. The most obvious candidate for the intrinsic factor has been endogenous sex hormones, either protective effects of estrogen or harmful effects of testosterone(42).
To understand divergent results it is important to know a little about sex hormone assays. Even old men usually have measurable testosterone levels, with 4-10 fold higher levels than postmenopausal women. Early studies often used commercially available sex hormone assays with low sensitivity for detecting the levels seen in postmenopausal women. Fortunately, Rancho Bernardo sex hormones were measured in a reproductive endocrinology laboratory (Dr. S.S.C .Yen) using organic solvent extraction and column chromatography prior to radioimmunoassay, which was more sensitive and specific than most available commercial assays of that (or this) era. We studied both total and bioavailable testosterone (bioT) and total and bioavailable estradiol (bioE). BioT and bioE are the non-sex hormone binding globulin (SHBG) fractions, thought to be transported more readily from blood into tissues.
We found that sex hormone associations differ by sex and by bioavailable versus total measurements. RBS men with IGT had lower total testosterone levels than men without IGT, and women with IGT (or diabetes) had higher bioT levels correlations than normoglycemic women(43). Table 4 shows that most correlations with risk factors were for total testosterone in men and for bioavailable testosterone in women (unpublished). The risk of diabetes (defined by two OGTTs 8 years apart) was increased in men if their baseline total T was in the lowest quartile, and in women if their baseline bioT was in the highest quartile(44).
Table 4.
CHD risk factors by total and bioavailable testosterone in men and women*
| Men (n= 834) | Women (n= 638) | |||
|---|---|---|---|---|
| Risk Factor | Total T** |
BioT** | Total T** |
BioT** |
| Age | -- | −.46 | .13 | -- |
| BMI | −.28 | -- | -- | .19 |
| Waist | −.29 | -- | -- | .18 |
| Total Chol | -- | -- | -- | .17 |
| LDL | -- | -- | -- | .14 |
| HDL* | .23 | -- | .20 | -- |
| Triglycerides* | −.34 | -- | −.15 | -- |
| SBP | −.10 | -- | -- | -- |
| DBP | −.13 | -- | .13 | .16 |
| HOMA IR* | −.24 | -- | -- | -- |
Only correlations of .13+ are shown.
Log-transformed for analysis, age-adjusted, all P≤ .01
CHD (coronary heart disease); HDL (high-density lipoprotein) ; LDL (low density lipoprotein); Total Chol (cholesterol); Total T (total testosterone); bio T (bioavailable testosterone); SBP (systolic blood pressure), DBP (diastolic blood pressure); HOMA IR (homeostasis model assessment for insulin resistance)
Adapted from G.A. Laughlin, PhD, University of California, San Diego, unpublished data, 2011.
Men Only Studies
RBS men had significant decrements in bioT with age independent of multiple covariates (45), and had a larger proportion of their total testosterone as bio T than women (presumably because women normally have higher levels of SHBG). Men aged ≥50 (46) showed no overall decrease in total T levels with age, in contrast to a striking decline in bioT (data not shown). In men a wide range of total and bioavailable testosterone was observed within each age group, such that some men in their 80s had higher testosterone levels than some men in their 50s (and vice-versa) (data not shown). A wide age distribution is an unexplained characteristic of many biologic variables in the elderly.
In an early publication based on sex hormones measured in 391 men aged 30-79 years, using 1973-5 blood samples(47), HDL levels were positively correlated and VLDL levels inversely correlated with testosterone levels independent of age, BMI, physical exercise, smoking, and alcohol intake. Mean HDL levels were 12% higher and VLDL levels were 40% lower in the highest versus the lowest quartile of testosterone. The observation that high levels of testosterone but not estrogen were associated with more favorable lipids and lipoproteins and estrogen levels was contrary to expectations.
In a small nested case-cohort study of 44 men with untreated diabetes and 88 age-matched men without diabetes (based on OGTT repeated 8 years apart), men with diabetes had significantly lower levels of total testosterone and bioT, compared to nondiabetic men(48).
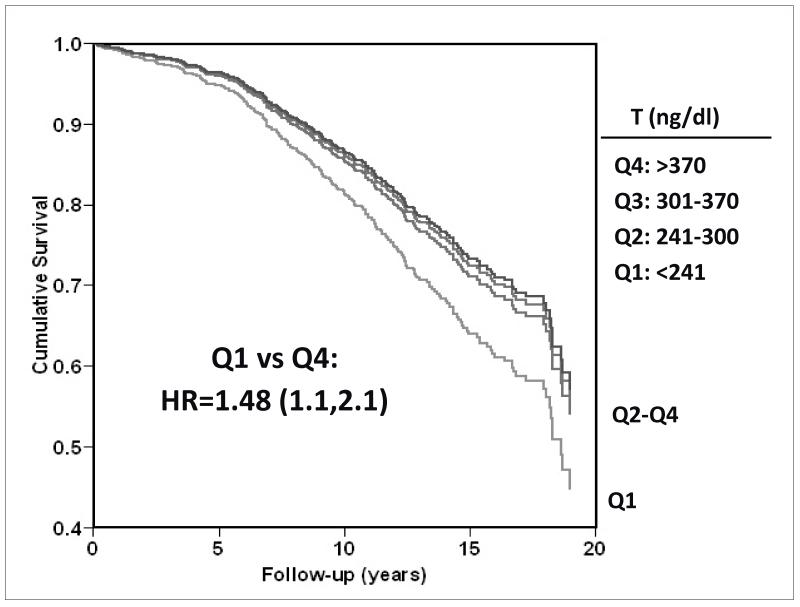
We more recently reported the association of low endogenous testosterone levels with CVD mortality in 794 RBS men aged 40-91 (median 73.6) years who had serum testosterone measurements (1984-1987) [Figure 9, drawn from data presented in (46)]; during an average 12-year follow up, 538 deaths occurred. Men whose total testosterone levels were in the lowest quartile (<241 ng/dl) were 40% (95% CI 1.14-1.71) more likely to die (all causes combined) than those with higher levels, independent of age, adiposity, and lifestyle. Additional adjustment for health status markers, lipids, lipoproteins, blood pressure, glycemia, adipocytokines, and estradiol levels had minimal effect on results, which were also independent of MetS, diabetes, and prevalent CVD. In stratified cause-specific analyses low testosterone predicted increased risk of death attributed to cardiovascular (HR 1.38; 95% CI 1.02-1.85) and respiratory disease (HR 2.29; 95% CI 1.25-4.20) but not cancer (HR 1.34; 95% CI 0.89-2.00). Results were similar for bioavailable testosterone.
Figure 9.
Twenty-year all-cause mortality by quartile (Q) of testosterone (T) among older Rancho Bernardo Study men (adjusted for age, BMI [body mass index], waist girth, alcohol, smoking, exercise)--drawn from data presented in (46).
Women only studies
Results in women were more complicated, in part because one-third of U.S. women in the age group of our cohort had a hysterectomy with or without oophorectomy, the latter often without their knowledge. This is important because after a natural menopause, ovarian follicles no longer make estrogen, but ovarian stromal cells continue to make testosterone. We validated oophorectomy status by medical records and found women who had had both their ovaries removed had 40% lower testosterone levels than intact women, and women who had had a hysterectomy without bilateral oophorectomy had 29% lower testosterone levels(49). The cause of the lower testosterone levels in women with ovarian conservation after hysterectomy is poorly understood.
One-third of women who had low total testosterone levels (which includes bioT) had had a hysterectomy and/or oophorectomy. In these women, low total testosterone was not associated with body mass index, hypertension, type 2 diabetes, or the metabolic syndrome; high bioT in intact women (i.e., women without oophorectomy or hysterectomy) was associated with more components of the metabolic syndrome while the reverse was true in men (Figure 8, unpublished). In early paper we found that women without diabetes had bioT levels that were positively associated with FPG but not with estrone or total estradiol (50).
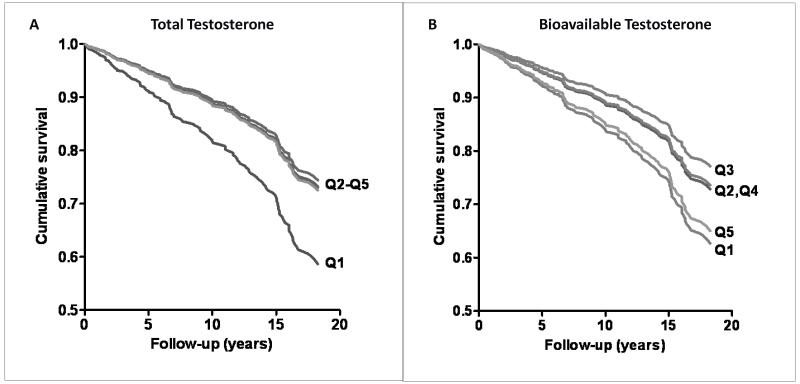
In a recent analysis in RBS women(51), low levels of total testosterone (which includes BioT) were significantly associated with incident CHD events, as shown in Figure 10A. A different pattern was seen for bioT, with an increased risk of cumulative 20-year CHD-events at both extremes (Figure 10B), such that the age-adjusted risk for the lowest and highest bioT quintiles relative to the middle third were 1.8 and 2.0, respectively. These data suggest that high bioT is harmful for women because of its association with obesity, diabetes, and the MetS components, while low total T may have a different mechanism.
Figure 10.
A. Total testosterone and 20-year incident CHD (coronary heart disease) in women (adjusted for age, BMI [body mass index], WHR [waist-hip ratio, smoking, exercise, alcohol). B. Bioavailable testosterone and 20-year incident CHD in women (adjusted for age, BMI, WHR, smoking, exercise, alcohol) (Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab 2010;95:740-7, Copyright 2010, The Endocrine Society). Q (quintile).
Further exploration of androgens and CHD in women will require sensitive and specific assays of both bioavailable and total testosterone, validated hysterectomy and oophorectomy status, and a population of women who were not almost universally treated with hormone therapy after hysterectomy (as were a majority of Rancho Bernardo women)(52).
Summary
The majority of deaths in adults with diabetes result from accelerated cardiovascular disease. The increasing incidence of obesity, diabetes, and CVD risk factors is expected to increase the burden of complications and costs to both individuals and society. Our 40 years of research have provided many new insights and highlighted major remaining gaps for understanding how diabetes eradicates women’s usual cardioprotection. We are particularly interested in looking at midlife biology, and biomarkers that may be reversible before, during, or after the menopause and prevent or delay CHD. Answers to these questions require well-characterized midlife cohorts with extended follow-up. Studies like RBS can continue to provide both training and discovery opportunities as long as the relevance of existing data remains an NIH funding priority.
Acknowledgments
Thank you to NHLBI, the NIDDK, and the NIA, who have at different times provided most of our funding, and the American Heart Association for critical grant support, including our first measurements of sex hormones. Thank you to all the faithful RBS participants. Thank you to the many graduate students, visiting scholars, colleagues who did most of the work on many of these papers. And a special thank you to Professor Joseph Stokes III who introduced me to Professor Dan Steinberg who invited me to be the epidemiologist for the Lipid Research Clinic Prevalence Study in Rancho Bernardo. And to Deborah Wingard, Michael Criqui, Kay-Tee Khaw, and Gail Laughlin, who were major contributors to the work reported here.
The Rancho Bernardo Study was funded by research grants AG07181 and AG028507 from the National Institute on Aging, and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Support:
The Rancho Bernardo Study was funded by research grants AG07181 and AG028507 from the National Institute on Aging, and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Abbreviations
- Bio T
(bioavailable testosterone)
- BMI
(Body mass index)
- CHD
(coronary heart disease)
- CVD
(cardiovascular disease)
- ERT
(estrogen replacement therapy)
- FPG
(fasting plasma glucose)
- GHb
(glycosylated hemoglobin)
- HOMA-IR
(homeostasis model for insulin resistance)
- IPH
(isolated postchallenge hyperglycemia)
- MetS
(metabolic syndrome)
- OGTT
(oral glucose tolerance test)
- PCG
(postchallenge glucose)
- SHBG
(sex hormone binding globulin)
- WHR
(waist-hip ratio)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Major Publications:
Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med 2002;162:1737-45.
Oh JY, Barrett-Connor E, Wedick NM. Sex differences in the association between proinsulin and intact insulin with coronary heart disease in nondiabetic older adults: the Rancho Bernardo Study. Circulation 2002;105:1311-6.
Jassal SK, Langenberg C, von Muhlen D, et al. Usefulness of microalbuminuria versus the metabolic syndrome as a predictor of cardiovascular disease in women and men>40 years of age (from the Rancho Bernardo Study). Am J Cardiol 2008;101:1275-80.
Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 2008;93:68-75.
Kramer CK, von Muhlen D, Gross JL, Laughlin GA, Barrett-Connor E. Blood pressure and fasting plasma glucose rather than metabolic syndrome predict coronary artery calcium progression: the Rancho Bernardo Study. Diabetes Care 2009;32:141-6.
Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab 2010;95:740-7.
No conflicts of interest to report.
References
- 1.Epstein FH. Hyperglycaemia as a risk factor for coronary heart disease. Monogr Atheroscler. 1985;13:92–7. [PubMed] [Google Scholar]
- 2.Coutinho M, Gerstein HC, Wang Y, et al. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–40. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-Connor E. Factors associated with the distribution of fasting plasma glucose in an adult community. Am J Epidemiol. 1980;112:518–23. doi: 10.1093/oxfordjournals.aje.a113021. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E. The prevalence of diabetes mellitus in an adult community as determined by history or fasting hyperglycemia. Am J Epidemiol. 1980;111:705–12. doi: 10.1093/oxfordjournals.aje.a112948. [DOI] [PubMed] [Google Scholar]
- 5.Suarez L, Barrett-Connor E. Seasonal variation in fasting plasma glucose levels in man. Diabetologia. 1982;22:250–3. doi: 10.1007/BF00281300. [DOI] [PubMed] [Google Scholar]
- 6.Scheidt-Nave C, Barrett-Connor E, Wingard DL, et al. Sex differences in fasting glycemia as a risk factor for ischemic heart disease death. Am J Epidemiol. 1991;133:565–76. doi: 10.1093/oxfordjournals.aje.a115928. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Wingard DL. Sex differential in ischemic heart disease mortality in diabetics: a prospective population-based study. Am J Epidemiol. 1983;118:489–96. doi: 10.1093/oxfordjournals.aje.a113654. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-Connor EL, Cohn BA, Wingard DL, et al. Why is diabetes mellitus a stronger risk factor for fatal ischemic heart disease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265:627–31. [PubMed] [Google Scholar]
- 9.Criqui MH, Barrett-Connor E, Holdbrook MJ, et al. Clustering of cardiovascular disease risk factors. Prev Med. 1980;9:525–33. doi: 10.1016/0091-7435(80)90047-x. [DOI] [PubMed] [Google Scholar]
- 10.Wingard DL, Suarez L, Barrett-Connor E. The sex differential in mortality from all causes and ischemic heart disease. Am J Epidemiol. 1983;117:165–72. doi: 10.1093/oxfordjournals.aje.a113527. [DOI] [PubMed] [Google Scholar]
- 11.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2002;162:1737–45. doi: 10.1001/archinte.162.15.1737. [DOI] [PubMed] [Google Scholar]
- 12.Wingard DLS P, Barrett-Connor E, McPhillips JB. Community - Based Study of Prevalence of NIDDM in Older Adults. Diabetes Care. 1990:13. doi: 10.2337/diacare.16.7.1022. [DOI] [PubMed] [Google Scholar]
- 13.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(Suppl 1):S62–7. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care. 2009;32:287–94. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett-Connor E, Ferrara A. Isolated postchallenge hyperglycemia and the risk of fatal cardiovascular disease in older women and men. The Rancho Bernardo Study. Diabetes Care. 1998;21:1236–9. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- 16.The DECODE study group. European Diabetes Epidemiology Group Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617–21. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. [PubMed] [Google Scholar]
- 17.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S, Barrett-Connor E, Wingard DL, et al. GHb is a better predictor of cardiovascular disease than fasting or postchallenge plasma glucose in women without diabetes. The Rancho Bernardo Study. Diabetes Care. 1996;19:450–6. doi: 10.2337/diacare.19.5.450. [DOI] [PubMed] [Google Scholar]
- 19.Kramer CK, Araneta MR, Barrett-Connor E. A1C and diabetes diagnosis: The Rancho Bernardo Study. Diabetes Care. 2010;33:101–3. doi: 10.2337/dc09-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stout RW. Insulin and atheroma. 20-yr perspective. Diabetes Care. 1990;13:631–54. doi: 10.2337/diacare.13.6.631. [DOI] [PubMed] [Google Scholar]
- 21.Laakso M, Barrett-Connor E. Asymptomatic hyperglycemia is associated with lipid and lipoprotein changes favoring atherosclerosis. Arteriosclerosis. 1989;9:665–72. doi: 10.1161/01.atv.9.5.665. [DOI] [PubMed] [Google Scholar]
- 22.Wingard DL, Barrett-Connor EL, Ferrara A. Is insulin really a heart disease risk factor. Diabetes Care. 1995;18:1299–304. doi: 10.2337/diacare.18.9.1299. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara A, Barrett-Connor E, Wingard DL, et al. Sex differences in insulin levels in older adults and the effect of body size, estrogen replacement therapy, and glucose tolerance status. The Rancho Bernardo Study, 1984-1987. Diabetes Care. 1995;18:220–5. doi: 10.2337/diacare.18.2.220. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara A, Barrett-Connor EL, Edelstein SL. Hyperinsulinemia does not increase the risk of fatal cardiovascular disease in elderly men or women without diabetes: the Rancho Bernardo Study, 1984-1991. Am J Epidemiol. 1994;140:857–69. doi: 10.1093/oxfordjournals.aje.a117174. [DOI] [PubMed] [Google Scholar]
- 25.Sarwar N, Sattar N, Gudnason V, et al. Circulating concentrations of insulin markers and coronary heart disease: a quantitative review of 19 Western prospective studies. Eur Heart J. 2007;28:2491–7. doi: 10.1093/eurheartj/ehm115. [DOI] [PubMed] [Google Scholar]
- 26.Oh JY, Barrett-Connor E, Wedick NM. Sex differences in the association between proinsulin and intact insulin with coronary heart disease in nondiabetic older adults: the Rancho Bernardo Study. Circulation. 2002;105:1311–6. doi: 10.1161/hc1102.105565. [DOI] [PubMed] [Google Scholar]
- 27.West K. Elsevier; New York: 1978. Epidemiology of Diabetes Mellitus and Its Vascular Compliations. [Google Scholar]
- 28.Holbrook TL, Barrett-Connor E, Wingard DL. The association of lifetime weight and weight control patterns with diabetes among men and women in an adult community. Int J Obes. 1989;13:723–9. [PubMed] [Google Scholar]
- 29.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997;46:701–10. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wedick NM, Mayer-Davis EJ, Wingard DL, et al. Insulin resistance precedes weight loss in adults without diabetes : the Rancho Bernardo Study. Am J Epidemiol. 2001;153:1199–205. doi: 10.1093/aje/153.12.1199. [DOI] [PubMed] [Google Scholar]
- 31.Porte D, Jr., Seeley RJ, Woods SC, et al. Obesity, diabetes and the central nervous system. Diabetologia. 1998;41:863–81. doi: 10.1007/s001250051002. [DOI] [PubMed] [Google Scholar]
- 32.Bierman EL. George Lyman Duff Memorial Lecture. Atherogenesis in diabetes. Arterioscler Thromb. 1992;12:647–56. doi: 10.1161/01.atv.12.6.647. [DOI] [PubMed] [Google Scholar]
- 33.Wingard D, Von Muhlen D, Barret-Connor E, et al. Factor Analysis of Proposed Components of the Insulin Resistance Syndrome [Abstract] ADA. 1996 [Google Scholar]
- 34.Langenberg C, Bergstrom J, Scheidt-Nave C, et al. Cardiovascular death and the metabolic syndrome: role of adiposity-signaling hormones and inflammatory markers. Diabetes Care. 2006;29:1363–9. doi: 10.2337/dc05-2385. [DOI] [PubMed] [Google Scholar]
- 35.Kramer CK, von Muhlen D, Gross JL, et al. Blood pressure and fasting plasma glucose rather than metabolic syndrome predict coronary artery calcium progression: the Rancho Bernardo Study. Diabetes Care. 2009;32:141–6. doi: 10.2337/dc08-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laughlin GA, Barrett-Connor E. Sex-Specific Association of the Metabolic Syndrome with the Serum Androgen to Estrogen Ratio in Older Adults: The Rancho Bernardo Study. Presented at 87th Annual Endocrine Society Meeting; Boston, MA. 2006. [Google Scholar]
- 37.Jassal SK, Langenberg C, von Muhlen D, et al. Usefulness of microalbuminuria versus the metabolic syndrome as a predictor of cardiovascular disease in women and men>40 years of age (from the Rancho Bernardo Study) Am J Cardiol. 2008;101:1275–80. doi: 10.1016/j.amjcard.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer CK, von Muhlen D, Jassal SK, et al. A prospective study of uric acid by glucose tolerance status and survival: the Rancho Bernardo Study. J Intern Med. 2010;267:561–6. doi: 10.1111/j.1365-2796.2010.02168.x. [DOI] [PubMed] [Google Scholar]
- 39.Scheidt-Nave C, Barrett-Connor E, Wingard DL. Resting electrocardiographic abnormalities suggestive of asymptomatic ischemic heart disease associated with non-insulin-dependent diabetes mellitus in a defined population. Circulation. 1990;81:899–906. doi: 10.1161/01.cir.81.3.899. [DOI] [PubMed] [Google Scholar]
- 40.Carpiuc KT, Wingard DL, Kritz-Silverstein D, et al. The association of angina pectoris with heart disease mortality among men and women by diabetes status: the Rancho Bernardo Study. J Womens Health (Larchmt) 2010;19:1433–9. doi: 10.1089/jwh.2009.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalin MF, Zumoff B. Sex hormones and coronary disease: a review of the clinical studies. Steroids. 1990;55:330–52. doi: 10.1016/0039-128x(90)90058-j. [DOI] [PubMed] [Google Scholar]
- 42.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–64. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 43.Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912–8. doi: 10.2337/diacare.23.7.912. [DOI] [PubMed] [Google Scholar]
- 44.Oh JY, Barrett-Connor E, Wedick NM, et al. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 45.Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–4. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 46.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khaw KT, Barrett-Connor E. Endogenous sex hormones, high density lipoprotein cholesterol, and other lipoprotein fractions in men. Arterioscler Thromb. 1991;11:489–94. doi: 10.1161/01.atv.11.3.489. [DOI] [PubMed] [Google Scholar]
- 48.Barrett-Connor E. Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med. 1992;117:807–11. doi: 10.7326/0003-4819-117-10-807. [DOI] [PubMed] [Google Scholar]
- 49.Laughlin GA, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:3561–8. doi: 10.1210/jcem.85.10.6861. [DOI] [PubMed] [Google Scholar]
- 50.Khaw KT, Barrett-Connor E. Fasting plasma glucose levels and endogenous androgens in non-diabetic postmenopausal women. Clin Sci (Lond) 1991;80:199–203. doi: 10.1042/cs0800199. [DOI] [PubMed] [Google Scholar]
- 51.Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab. 2010;95:740–7. doi: 10.1210/jc.2009-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kritz-Silverstein D, Von Muhlen DG, Ganiats TG, et al. Hysterectomy status, estrogen use and quality of life in older women: the Rancho Bernardo study. Qual Life Res. 2004;13:55–62. doi: 10.1023/B:QURE.0000015318.00707.53. [DOI] [PubMed] [Google Scholar]