Abstract
Type 2 diabetes and obesity are very serious health problems in both developed and developing countries. Increased level of growth hormone (GH) is known to promote insulin resistance. Transgenic (Tg) mice over-expressing bovine GH are short-living and characterized, among others, by hyperinsulinemia and increased insulin resistance in comparison to normal (N) mice. Pioglitazone (PIO) is a member of the thiazolidinediones – group of insulin-sensitizing drugs which are selective agonists of peroxisome proliferator-activated receptor gamma (PPARγ). The aim of the study was to analyze the effects of PIO on the insulin signaling pathway in Tg and N mice. Plasma levels of insulin and glucose as well as hepatic levels of proteins involved in insulin signaling were analyzed by ELISA or western blot methods. Treatment with PIO decreased plasma level of glucose in N mice only. Similarly, PIO increased insulin sensitivity (expressed as the Relative Insulin Sensitivity Index; RISI) only in N mice. In the liver, PIO decreased the phosphorylation of IRS1 at a serine residue (Ser307-pS-IRS1), that inhibits insulin action, and had a tendency to increase tyrosine phosphorylation of IRS2 (Tyr-pY-IRS2) only in N mice but did not affect either of these parameters in Tg mice. Levels of total and phosphorylated mTOR were increased in Tg mice. Moreover, the AKT2 level was decreased by PIO in N mice only. In conclusion, the lack of improvement of insulin sensitivity in insulin-resistant Tg mice during PIO treatment suggests that chronically elevated GH level can inhibit the beneficial effects of PIO on insulin signaling.
Keywords: pioglitazone, insulin signaling, growth hormone, transgenic mice
INTRODUCTION
Diabetes, obesity and other non-communicable chronic diseases are very serious health problems in both developed and developing countries, leading to increased morbidity and premature mortality (Abegunde et al. 2007). Obesity per se constitutes one of the main reasons of insulin resistance and type 2 diabetes. Importantly, growth hormone (GH) which is a key regulator of growth and metabolism processes, may exert anti-insulinemic and diabetogenic actions. These effects are considered to be the key physiological effects of GH on carbohydrate and lipid metabolism (Davidson 1987). Increased GH level is known to promote insulin resistance in humans and laboratory animals (Hansen et al. 1986; Kopchick et al. 1999; Bartke 2003; Wang et al. 2007). For this reason, we decided to use in our study transgenic (Tg) mice over-expressing bovine GH (bGH) with the phosphoenolpyruvate carboxykinase (PEPCK) as a promoter (PEPCK-bGH mice). These giant mice are short-living and characterized, among others, by increased postnatal growth and adult body weight, organomegaly, reduced adiposity, various symptoms of accelerated aging, early onset of age-related changes in cognitive function, decreased plasma adiponectin, increased plasma resistin and cholesterol, elevated levels of TNF-α and IL-6 in adipocytes, hyperinsulinemia, increased insulin resistance (Bartke 2003; Wang et al. 2007), as well as depletion of very small embryonic-like stem cells (VSELs) from bone marrow (Kucia et al. 2013).
Pioglitazone (PIO) is an anti-diabetic drug which belongs to the thiazolidinedione (TZD) class – selective agonists of peroxisome proliferator-activated receptor gamma (PPARγ), which constitute a very important group of insulin-sensitizing drugs. It can exert beneficial antioxidant and anti-proliferative effects (Elte & Blickle 2007), as well as anti-tumor activity by inducing apoptosis, and may decrease the risk of cardiovascular events (Lincoff et al. 2007).
The aim of the study was to analyze the effects of PIO on the insulin signaling pathway [hepatic levels of insulin receptor (IR), insulin receptor substrate-1 (IRS1) – total and phosphorylated at a serine(307) residue (Ser307-pS-IRS1) (phosphorylation that inhibits insulin action), insulin receptor substrate-2 (IRS2) – phosphorylated at a tyrosine residue (Tyr-pY-IRS2)] in PEPCK-bGH Tg and normal mice. Moreover, plasma glucose and insulin levels were determined in these animals. Importantly, the influence of PIO on various components of insulin signaling pathway under chronically elevated GH level has not been, as far, analyzed.
Additionally, hepatic total mTOR (mammalian target of rapamycin; FKBP12-rapamycin-associated protein, FRAP1), phosphorylated mTOR (mTOR-pY) and AKT2 levels were assessed. It is known that hormones (insulin including), growth factors and other mitogens activate the PI3K/AKT/mTOR signaling cascade (Mamane et al. 2006). Furthermore, rapamycin – a natural macrolide, used in cancer therapy and as immunosuppressant drug as well, which inhibits mTOR, may lead to increase of lifespan in various species (Bjedov et al. 2010; Anisimov et al. 2011; Miller et al. 2011; Selman & Patridge 2012; Wilkinson et al. 2012). Therefore, it was also of interest to assess the effects of PIO on mTOR signaling.
MATERIALS AND METHODS
Animals and assessment of blood chemistry
Approximately 6 month old male mice over-expressing bovine growth hormone (PEPCK-bGH; Tg) and age matched normal (N) controls were randomly assigned to control or treatment groups. At the beginning of the study (“before treatment” groups), the mice were divided into four (4) experimental groups: normal (N) (10 animals), normal assigned to pioglitazone (PIO) treatment (N-PIO) (10 animals), transgenic (Tg) (10 animals), and transgenic assigned to PIO treatment (Tg-PIO) (10 animals). These group designations were used both before and after treatment and thus, “after treatment” N-PIO and Tg-PIO groups in which insulin, blood glucose, RISI and adiponectin were assessed, denote animals which had been receiving PIO treatment. Basal glucose, insulin and adiponectin levels as well as Relative Insulin Sensitivity Index (RISI) were measured at the start of the study. The RISI was calculated from the equation: 100/√ blood glucose level x insulin level. Then, the PIO treatment (20mg/kg of body weight per day for the period of 20 days) was started in groups: N-PIO and Tg-PIO. Pioglitazone (PIO) was incorporated in bacon flavored diet (pellets of modified LabDiet® Laboratory Rodent Diet 5001 with 0.2% Pioglitazone; TestDiet, Richmond, IN). Once daily PIO treated animals were provided with small piece of food containing drug (0.5 gram for Tg and 0.3 gram for N animals, corresponding to 20 mg PIO/kg of body weight on average per day). The mice in groups N and Tg (without PIO treatment) were fed unmodified Lab Diet 5001 chow (PMI Nutrition International, Richmond, IN).
The PEPCK-bGH (Tg) mice were originally produced by microinjecting the bGH structural gene fused with the promoter of the rat PEPCK gene into the pronuclei of fertilized mouse eggs (McGrane et al. 1988). The hemizygous Tg mice used in this study were produced by mating Tg males with normal C57BL/6 x C3H F1 hybrid females. The mice used in the study were housed under temperature- and light-controlled conditions (22 ± 2°C, 12 hr light/12 hr dark cycle). At the end of experiment, basal glucose, insulin and adiponectin levels as well as RISI were measured once more (see below). Half of animals from each experimental group was treated with insulin and the other half with saline before collecting tissues to assess stimulation of the phosphorylation of insulin receptor and downstream pathway of insulin action. All animal procedures were approved by the Laboratory Animal Care and Use Committee (LACUC) at the Southern Illinois University School of Medicine (Springfield, IL). After 20 days of PIO treatment, the animals were fasted overnight and fasting glucose levels were measured in blood collected from the tail vein using OneTouch Ultra glucometer (Life Scan, Milpitas, CA). Then, the animals were sacrificed and plasma obtained from blood collected by cardiac puncture was used for assessment of insulin using Rat/Mouse Insulin ELISA kit (Linco Research Inc., St. Charles, MO) following manufacturer’s protocols. Adiponectin levels were assayed using mouse adiponectin ELISA kit (Linco Research, St. Charles, MO). Total amount of insulin receptor (IR) in liver was assayed using IR (Total) Human ELISA kit (Invitrogen, Grand Island, NY). The assessment of above-mentioned four (4) parameters (glucose, insulin, adiponectin, total IR), as well as RISI was performed in all animals per group (i.e., in 10 mice). Phosphorylated IRS2 levels were assayed using PathScan® Phospho-IRS-2 (panTyr) Sandwich ELISA kit (Cell Signaling Technology, Inc., Danvers, MA). In this case, 5 animals per each subgroup (treated with saline or with insulin) were analyzed. Livers were rapidly collected, quickly frozen on dry ice and stored at −80°C until processed.
Protein extraction and Western blotting
Total proteins were obtained from tissue homogenates. Approximately 100 mg liver samples were homogenized in 1 ml ice-cold T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL), with Protease Inhibitor Cocktail Kit (Pierce Biotechnology, Rockford, IL), Phosphatase Inhibitor Cocktail 1 (Sigma-Aldrich, St. Louis, MO) and Phosphatase Inhibitor Cocktail 2 (Sigma-Aldrich, St. Louis, MO)]. After mixing, homogenates were centrifuged at 16000 rpm for 30 minutes. Protein concentrations were assessed using Pierce BCA (bicinchoninic acid) Protein Assay Kit (Pierce Biotechnology, Rockford, IL) in accordance with manufacturer’s protocol.
Western blot procedure was performed using the respective primary antibodies: total IRS1, phospho-IRS-1 (Ser307), total mTOR, phospho-mTOR (Ser2448), Akt2 (all from Cell Signaling Technology, Inc., Danvers, MA), and secondary goat antirabbit or antimouse antibodies (Calbiochem, La Jolla, CA). Monoclonal anti-β-actin antibody (Sigma-Aldrich Corp., St. Louis, MO) was used, after stripping the membrane, as a control for protein loading.
Western blotting analysis was performed according to the method described previously (e.g., Gesing et al. 2011), and six animals per group were analyzed. Photos of blots were taken with Image Reader LAS-4000 (FujiFilm, Tokyo, Japan) and quantified for statistical analysis using GeneTools software (SynGene, Cambridge, UK).
Statistical analysis
The data are expressed as mean ± Standard Error of the Mean (SEM). To evaluate the effects of the genotype and pharmacological intervention, two-way analysis of variance (ANOVA) was used. Additionally, we used the Bonferroni test – post hoc test for analyzing differences between group means. A value of p<0.05 was considered significant. All statistical calculations were conducted using SPSS version 17.0 (SPSS, Chicago, IL) with α=0.05. All graphs were made using Prism 4.02 (GraphPad Software, San Diego, CA).
RESULTS
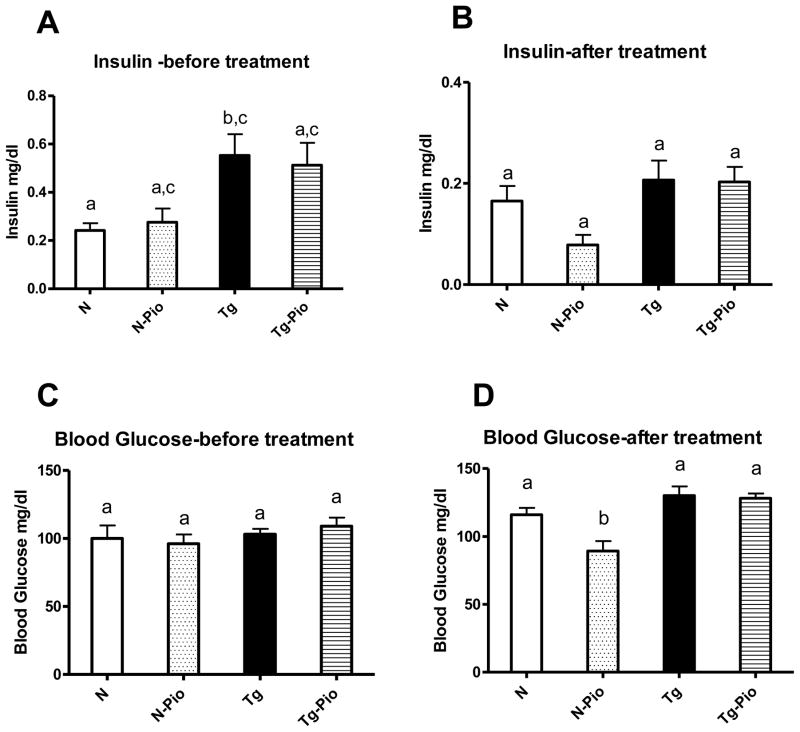
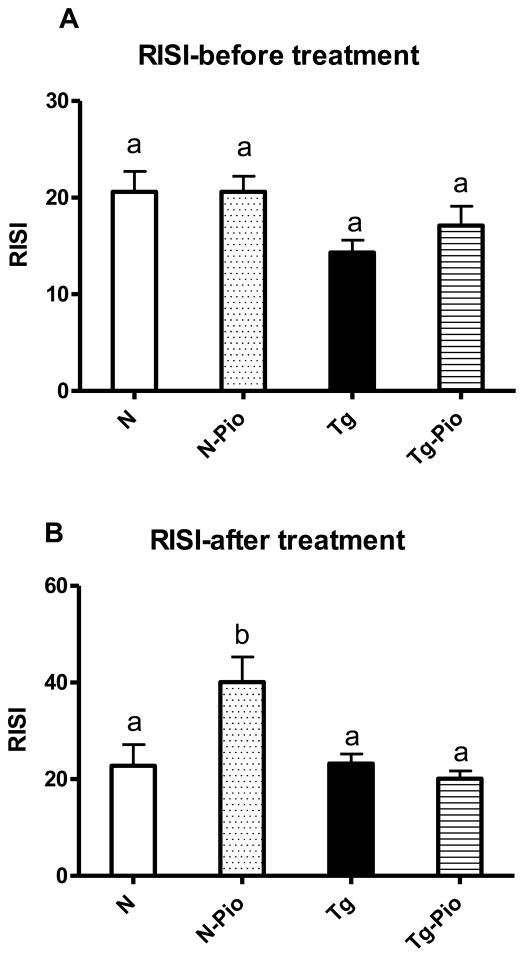
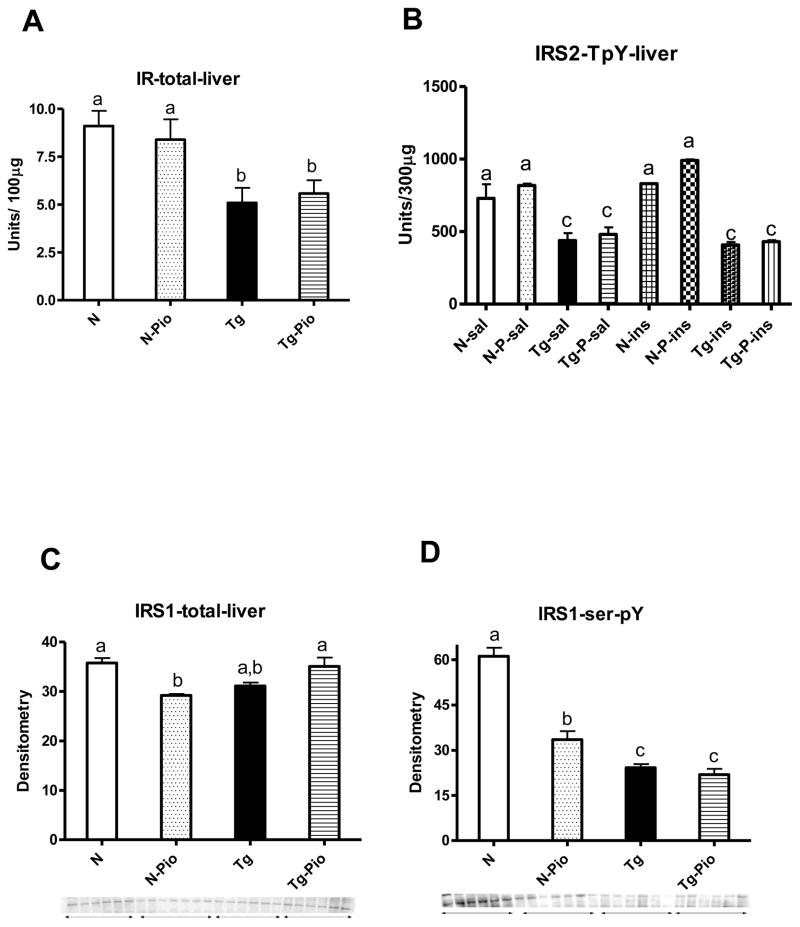
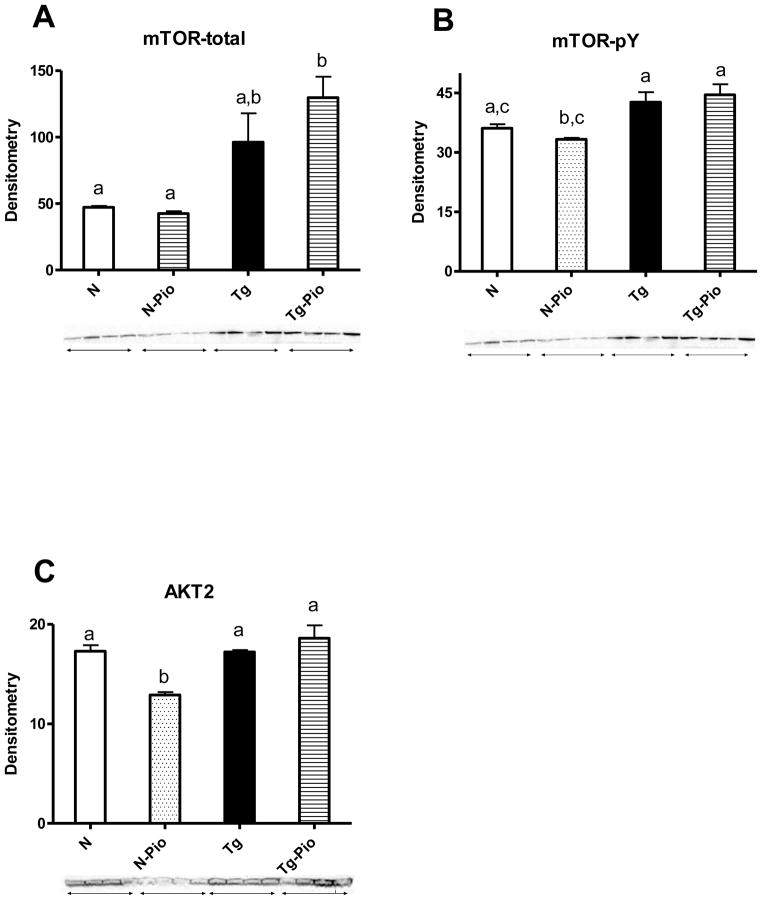
Before PIO treatment, plasma insulin level was increased in Tg mice, as compared to N animals (p=0.049) (Figure 1A). After treatment period, no changes between these two kinds of animals, not treated with PIO, were detected, although the overall effect of genotype (i.e., between pooled N and N-Pio mice and pooled Tg and Tg-Pio animals) was statistically significant with higher insulin levels in pooled Tg and Tg-Pio mice (p=0.022) (Figure 1B). The pioglitazone (PIO) treatment decreased the plasma level of glucose in normal (N) mice only (p=0.048), with no effects in transgenic (Tg) animals (Figure 1D). Before PIO treatment, no changes in blood glucose between N and Tg mice were observed (Figure 1C). Similarly, no differences between these two experimental groups, not treated with PIO, were seen after treatment period (Figure 1D). Expectedly, insulin sensitivity (as the Relative Insulin Sensitivity Index; RISI) was decreased in Tg mice (pooled Tg and Tg-Pio) as compared to N animals (pooled N and N-Pio) (p=0.012) (Figure 2A). The RISI indicated that PIO increased insulin sensitivity in N mice only (p=0.033) (Figure 2B). Before PIO treatment, plasma adiponectin level was decreased in Tg mice, as compared to N animals (p=0.0001) (Figure 3A). Similar observation was shown after treatment period (p=0.043) (Fig. 3B). The PIO treatment increased adiponectin level in plasma in N mice (p=0.003), as well as in Tg animals, although approaching only borderline significance (p=0.053) (Figure 3B). No effects of PIO treatment on total IR level in the liver in N or Tg mice were detected (Figure 4A). Importantly, the level of total IR was decreased in Tg mice in comparison with N animals (p=0.009) (Figure 4A). In the liver, PIO did not change the phosphorylation of Tyr-pY-IRS2 (although had a tendency to increase this parameter in N mice), but decreased the Ser307-pS-IRS1, the phosphorylation that inhibits insulin action, in N mice (p=0.0001) with no effects on either of these parameters in Tg mice (Figure 4B, 4D, respectively). No changes in Tyr-pY-IRS2 level in N mice (without PIO treatment) treated with saline as compared to N animals treated with insulin were detected (Fig. 4B). Similarly, in Tg mice (without and after PIO treatment), there were no differences between saline- and insulin-treated subgroups (Fig. 4B). Interestingly, PIO decreased total IRS1 level in liver of N mice (p=0.003) (Figure 4C). Furthermore, a tendency to the increase this parameter in PIO-treated Tg mice has been shown (p=0.14) (Figure 4C). No differences in total mTOR level between N and N-PIO, as well as between Tg and Tg-PIO mice were observed (Figure 5A). However, the level of mTOR was increased in Tg mice (pooled Tg and Tg-Pio) as compared with N animals (pooled N and N-Pio) (p=0.0001) (Fig. 5A). As similar to total mTOR, phosphorylated mTOR protein level was also increased in Tg mice (pooled Tg and Tg-Pio) in comparison to N animals (pooled N and N-Pio) (p=0.001) (Fig. 5B). Additionally, PIO decreased hepatic AKT2 level in N mice (p=0.011) with no effects observed in Tg mice (Figure 5C).
Fig. 1.
Plasma insulin level [mg/dl] before (A) and after (B) pioglitazone (PIO) treatment, blood glucose level [mg/dl] before (C) and after (D) PIO treatment in normal (N) and transgenic mice over-expressing bovine growth hormone (Tg). Values are means ± SEM. a, b – values that do not share the same letter in the superscript are significantly different (p<0.05).
Fig. 2.
The Relative Insulin Sensitivity Index (RISI) [100/√ blood glucose level x insulin level] before (A) and after (B) pioglitazone (PIO) treatment in normal (N) and transgenic mice over-expressing bovine growth hormone (Tg). Values are means ± SEM. a, b – values that do not share the same letter in the superscript are significantly different (p<0.05).
Fig. 3.
Plasma adiponectin level [mg/dl] before (A) and after (B) pioglitazone (PIO) treatment in normal (N) and transgenic mice over-expressing bovine growth hormone (Tg). Values are means ± SEM. a, b, c – values that do not share the same letter in the superscript are significantly different (p<0.05).
Fig. 4.
(A) Hepatic total insulin receptor (IR) protein level [units/100 μg protein] in normal (N) and transgenic mice over-expressing bovine growth hormone (Tg) without pioglitazone (PIO) treatment, and after PIO treatment in normal (N-Pio) and transgenic mice over-expressing bovine growth hormone (Tg-Pio); (B) hepatic insulin receptor substrate-2 (IRS2) phosphorylated at a tyrosine residue (IRS2-T pY) protein level [units/300 μg protein] in N mice treated with saline (N-sal) or insulin (N-ins) and Tg treated with saline (Tg-sal) or insulin (Tg-ins) without PIO treatment, and after PIO treatment in N mice treated with saline (N-P-sal) or insulin (N-P-ins) and Tg mice treated with saline (Tg-P-sal) or insulin (Tg-P-ins); (C) hepatic total insulin receptor substrate-1 (IRS1) protein level (using primary antibody from Cell Signaling Technology, Inc., Danvers, MA) in N and Tg mice without pioglitazone (PIO) treatment, and after PIO treatment in N (N-Pio) and Tg mice (Tg-Pio), (D) hepatic insulin receptor substrate-1 (IRS1) phosphorylated at a serine(307) residue (IRS1-ser-pY) protein level (using primary antibody from Cell Signaling Technology, Inc., Danvers, MA) in N and Tg mice without pioglitazone (PIO) treatment, and after PIO treatment in N (N-Pio) and Tg mice (Tg-Pio). Values are means ± SEM. a, b, c – values that do not share the same letter in the superscript are significantly different (p<0.05).
Fig. 5.
(A) Hepatic total mTOR protein level (using primary antibody from Cell Signaling Technology, Inc., Danvers, MA) in normal (N) and transgenic mice over-expressing bovine growth hormone (Tg) without pioglitazone (PIO) treatment, and after PIO treatment in normal (N-Pio) and transgenic mice over-expressing bovine growth hormone (Tg-Pio); (B) hepatic mTOR phosphorylated at a tyrosine residue (mTOR-pY) protein level (using primary antibody from Cell Signaling Technology, Inc., Danvers, MA) in N and Tg mice without pioglitazone (PIO) treatment, and after PIO treatment in N (N-Pio) and Tg mice (Tg-Pio); (C) hepatic AKT2 protein level (using primary antibody from Cell Signaling Technology, Inc., Danvers, MA) in N and Tg mice without pioglitazone (PIO) treatment, and after PIO treatment in N (N-Pio) and Tg mice (Tg-Pio). Values are means ± SEM. a, b, c – values that do not share the same letter in the superscript are significantly different (p<0.05).
DISCUSSION
Insulin resistance is a very serious health problem, which may lead to diabetes and obesity. One should emphasize that elevated GH as well as insulin-like growth factor-I (IGF- I) may lead to higher risk of mortality (Holdaway et al. 2004). Transgenic mice over- expressing GH are characterized, among others, by hyperinsulinemia and increased insulin resistance (Wang et al. 2007). Therefore, PEPCK-bGH mice constitute an excellent model system for the studies on the control of insulin sensitivity and insulin resistance during anti- diabetic treatment.
Krag et al. (2009) have shown that one of mechanisms which may be responsible for improvement of insulin sensitivity due to PIO treatment is the decrease of pro-inflammatory interleukin-6 (IL-6) level. Interleukin-6 is a cytokine, produced in adipose tissue (Fried et al. 1998), as well as in skeletal muscles, being one of important myokines (Pedersen & Febbraio 2008), involved in the regulation of insulin sensitivity. It has been previously reported that IL- 6 may inhibit the insulin signaling pathway by up-regulation of suppressors of cytokine signaling-3 (SOCS-3) – a marker of the activation of IL-6 signaling (reviewed in Coelho et al. 2013). In turn, SOCS-3, as well as SOCS-1, may lead - among others - to impaired insulin receptor substrate 1 (IRS1) and 2 (IRS2) tyrosine phosphorylation. Interestingly, Wan et al. (2012a) have shown that IL-6 may be involved in the regulation of mitochondrial function in adipose tissue, being an activator of adenosine monophosphate-activated protein kinase (AMPK) – one of key regulators of mitochondrial biogenesis. However, recent data has revealed that cytokine in question is not necessary for regulation of mitochondrial content in adipose tissue (Wan et al. 2012b). Nevertheless, there is a growing number of data showing a dual role of IL-6 in the regulation of insulin sensitivity (e.g., Jiang et al. 2013).
The mechanisms of action of PIO, relied on the decrease of plasma levels of glucose, and the increase of the Relative Insulin Sensitivity Index (RISI) in normal (N) mice only, as seen in our study, are consistent with well-known insulin-sensitizing properties of this drug. Puddu et al. (2012) have recently shown that PIO protects pancreatic islet cells (line HIT-T15) from the detrimental effects of Advanced Glycation End-Products (AGEs). Moreover, PIO is an effective drug in lowering glycated hemoglobin (HbA1C) (Russell-Jones et al. 2012).
One should recall that TZDs may decrease GH and IGF-I synthesis and levels, and as a consequence of this, attenuate anti-insulin activity (exerting by GH), what may lead to the improvement of insulin signaling pathway. Intriguingly, the doses of PIO, commonly used in the treatment of type 2 diabetes, did not improve GH and IGF-I levels in acromegalic patients (characterized by impaired insulin sensitivity) (Kim et al. 2012). These observations may be considered as consistent with our results showing lack of beneficial impact of PIO treatment in Tg mice. Nevertheless, it seems that the role of the interactions between GH and PIO requires further analyses.
The effects of PIO, leading to increased plasma adiponectin level in N mice agree with the results of the studies by Yu et al. (2002), showing increased adiponectin level after thiazolidinediones (TZDs) treatment. Pioglitazone also increased serum adiponectin level in 8-week high fructose diet-fed rats (Schaalan 2012) and in obese men (Powell et al. 2012). Adiponectin level was also increased in Wistar rats, fed high-fat insulin resistance-inducing diet, treated with PIO (Gong et al. 2012). Moreover, PIO treatment in these animals, fed a diet inducing derangements in insulin signaling pathway, led to increased level of adiponectin receptor type 2 and to decreased insulin resistance (Gong et al. 2012). The increase in adiponectin level in Tg mice, as a result of PIO treatment, seems, to some degree, to be consistent with the results of the study performed by Krag et al. (2008), showing increased adiponectin level in GH-deficient patients with GH replacement therapy, receiving PIO treatment. Concerning well-known anti-atherogenic properties of adiponectin, one should also recall the data of Saremi et al. (2013), who have recently reported that PIO may retard atherosclerosis progression in people with prediabetes. Interestingly, adiponectin-deficient mice are unresponsive to the anti-diabetic effects of TZDs (Nawrocki et al. 2006). Furthermore, the suppressive effects of PIO on angiotensin II-induced cardiac hypertrophy, as was seen in wild-type mice, were diminished in adiponectin-deficient mice (Li et al. 2010).
Decreased phosphorylation of IRS1 (Ser307-pS-IRS1) in the liver due to PIO treatment in N mice, constitutes beneficial effect of this drug because that kind of phosphorylation leads to inhibition of insulin action. Also, a tendency to increase phosphorylation of IRS2 (Tyr-pY- IRS2) in N animals seems to be beneficial for proper insulin signaling since it is known that opposite situation, i.e. inhibition of tyrosine phosphorylation of insulin receptor substrate proteins, caused by suppressors of cytokine signaling 1 (SOCS-1) and 3 (SOCS-3), may lead to insulin resistance (Ueki et al. 2004). Importantly, PIO may improve insulin sensitivity through the suppression of SOCS-3 (Kanatani et al. 2007). Interestingly, SOCS1 knockout mice are characterized by increased liver IRS2 expression and IRS2 tyrosine phosphorylation what may lead to enhanced hepatic insulin sensitivity (Jamieson et al. 2005).
Unexpected numerical (although not statistically significant) increase of total IRS1 level in the liver in Tg mice, potentially leading to the improvement of insulin signaling, and decrease of this substrate in the same organ in N mice, due to PIO treatment, seems to be quite difficult to interpret. However, Taniguchi et al. (2006) have shown that liver-specific deletion of the p85α regulatory subunit of PI3K, constituting the next downstream step (after IRS1) in insulin signaling pathway, may paradoxically improve the hepatic and peripheral insulin sensitivity. Importantly, the decreased level of total IR in Tg mice in comparison with N animals may be considered as consistent with well-known impaired insulin sensitivity in transgenic mice over-expressing GH.
One should emphasize that mTOR may integrate and coordinate various extracellular signals (Mamane et al. 2006). Importantly, reduced expression of genes associated with the mTOR signaling pathway has been shown in individuals from long-lived families in Leiden Longevity Study (Slagboom et al. 2011). Therefore, increased total mTOR level in short-lived Tg mice, as observed by us, may suggest an important role of TOR signaling in lifespan regulation. Also, increased phosphorylated mTOR protein level in Tg mice in comparison to N animals may confirm this relevant observation.
Presumably, the decrease of AKT2, enzyme involved in PI3K/AKT/mTOR signaling, can also be viewed as beneficial. This hypothesis could be confirmed by observation that AKT2 is the major isoform shown to be overexpressed in cancer in humans (reviewed by Hers et al. 2011). On the other hand, Garofalo et al. (2003) have unexpectedly shown that AKT2-deficient mice are insulin resistant with the tendency to development of diabetes in males. Therefore, the role of AKT2 in insulin sensitivity regulation requires further analysis.
Interestingly, the differences in insulin levels were no longer present between normal and transgenic mice without PIO treatment after treatment period (as compared to the beginning of the study). This could have been caused by the used method of blood collection. Initially, the blood was collected by orbital bleeding after brief isofluorane anaesthetic while the final collection was performed after ketamine/xylosine anesthesia with cardiac bleeding. Therefore, these two different methods of blood collection (differently stimulating the stress of the animals) could cause the difference in insulin values after treatment period when comparing N and Tg animals.
In summary, absence of effects of PIO treatment in transgenic mice over-expressing GH may suggest that chronically increased GH level may inhibit the beneficial effects of PIO on insulin signaling pathway. In contrast, PIO improved insulin signaling in animals with normal GH level. Therefore, one should hypothesize that PIO may not be useful in the management of impaired glucose tolerance or type 2 diabetes in patients with elevated GH levels. Presumably, higher doses of PIO would be required to exert beneficial effects on insulin signaling under conditions of GH overproduction. Further studies are needed to determine the therapeutic possibilities of PIO and explain how this anti-diabetic drug may exert beneficial effects.
Acknowledgments
FUNDING
The present study was supported by NIA, AG032290, AG 19899, AG031736 and U19 AG023122, Polish National Science Centre (DEC-2012/04/M/NZ4/00198) (grant No. 507/1- 107-05/507-10-050 of the Medical University of Lodz, Poland), and Polish Ministry of Science and Higher Education (N N401 042638).
Support provided by Takeda Pharmaceuticals U.S.A., Inc.
Footnotes
DECLARATION OF INTEREST
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–1938. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Bartke A. Can growth hormone (GH) accelerate aging ? Evidence from GH transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metabolism. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Archives of Medical Science. 2013;9:191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MB. Effect of growth hormone on carbohydrate and lipid metabolism. Endocrine Reviews. 1987;8:115–131. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- Elte JW, Blickle JF. Thiazolidinediones for the treatment of type 2 diabetes. European Journal of Internal Medicine. 2007;18:18–25. doi: 10.1016/j.ejim.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. Journal of Clinical Endocrinology and Metabolism. 1998;83:847–850. doi: 10.1210/jc.83.3.847. [DOI] [PubMed] [Google Scholar]
- Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. Journal of Clinical Investigation. 2003;112:197–208. doi: 10.1172/JCI200316885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesing A, Masternak MM, Wang F, Joseph AM, Leeuwenburgh C, Westbrook R, Lewinski A, Karbownik-Lewinska M, Bartke A. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:1062–1076. doi: 10.1093/gerona/glr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Li J, Li C, Mu Y, Xiao Y, Tian H, Pan C, Liu Y. The adipose tissue endocrine mechanism of the prophylactic protective effect of pioglitazone in high-fat diet-induced insulin resistance. The Journal of International Medical Research. 2012;40:1304–1316. doi: 10.1177/147323001204000409. [DOI] [PubMed] [Google Scholar]
- Hansen I, Tsalikian E, Beaufrere B, Gerich J, Haymond M, Rizza R. Insulin resistance in acromegaly: defects in both hepatic and extrahepatic insulin action. American Journal of Physiology. 1986;250:E269–E273. doi: 10.1152/ajpendo.1986.250.3.E269. [DOI] [PubMed] [Google Scholar]
- Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cellular Signalling. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Holdaway IM, Rajasoorya RC, Gamble GD. Factors influencing mortality in acromegaly. Journal of Clinical Endocrinology and Metabolism. 2004;89:667–674. doi: 10.1210/jc.2003-031199. [DOI] [PubMed] [Google Scholar]
- Jamieson E, Chong MM, Steinberg GR, Jovanovska V, Fam BC, Bullen DV, Chen Y, Kemp BE, Proietto J, Kay TW, et al. Socs1 deficiency enhances hepatic insulin signaling. Journal of Biological Chemistry. 2005;280:31516–31521. doi: 10.1074/jbc.M502163200. [DOI] [PubMed] [Google Scholar]
- Jiang LQ, Duque-Guimaraes DE, Machado UF, Zierath JR, Krook A. Altered response of skeletal muscle to IL-6 in type 2 diabetic patients. Diabetes. 2013;62:355–361. doi: 10.2337/db11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani Y, Usui I, Ishizuka K, Bukhari A, Fujisaka S, Urakaze M, Haruta T, Kishimoto T, Naka T, Kobayashi M. Effects of pioglitazone on suppressor of cytokine signaling 3 expression: potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes. 2007;56:795–803. doi: 10.2337/db06-1039. [DOI] [PubMed] [Google Scholar]
- Kim DD, Goh J, Panossian Z, Gamble G, Holdaway I, Grey A. Pioglitazone in acromegaly – an open-label, prospective study. Clinical Endocrinology (Oxf) 2012;77:575–578. doi: 10.1111/j.1365-2265.2012.04411.x. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Bellush LL, Coschigano KT. Transgenic models of growth hormone action. Annual Review of Nutrition. 1999;19:437–461. doi: 10.1146/annurev.nutr.19.1.437. [DOI] [PubMed] [Google Scholar]
- Krag MB, Nielsen S, Guo Z, Pedersen SB, Schmitz O, Christiansen JS, Jorgensen JO. Peroxisome proliferator-activated receptor gamma agonism modifies the effects of growth hormone on lipolysis and insulin sensitivity. Clinical Endocrinology (Oxf) 2008;69:452–461. doi: 10.1111/j.1365-2265.2008.03231.x. [DOI] [PubMed] [Google Scholar]
- Krag MB, Rasmussen LM, Hansen TK, Frystyk J, Flyvbjerg A, Moller N, Jorgensen JO. Peroxisome proliferator-activated receptor gamma (PPAR) agonism reduces the insulin-stimulated increase in circulating interleukin-6 in GH replaced GH-deficient adults. Clinical Endocrinology (Oxf) 2009;71:363–368. doi: 10.1111/j.1365-2265.2008.03483.x. [DOI] [PubMed] [Google Scholar]
- Kucia M, Masternak M, Liu R, Shin DM, Ratajczak J, Mierzejewska K, Spong A, Kopchick JJ, Bartke A, Ratajczak MZ. The negative effect of prolonged somatotrophic/insulin signaling on an adult bone marrow-residing population of pluripotent very small embryonic-like stem cells (VSELs) Age (Dordr) 2013;35:315–330. doi: 10.1007/s11357-011-9364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Shibata R, Unno K, Shimano M, Furukawa M, Ohashi T, Cheng X, Nagata K, Ouchi N, Murohara T. Evidence for the importance of adiponectin in the cardioprotective effects of pioglitazone. Hypertension. 2010;55:69–75. doi: 10.1161/HYPERTENSIONAHA.109.141655. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Journal of the American Medical Association. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- McGrane MM, de Vente J, Yun J, Bloom J, Park E, Wynshaw-Boris A, Wagner T, Rottman FM, Hanson RW. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. Journal of Biological Chemistry. 1988;263:11443–11451. [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. Journal of Biological Chemistry. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological Reviews. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. 10.1152/physrev. 90100.2007. [DOI] [PubMed] [Google Scholar]
- Powell LA, Crowe P, Kankara C, McPeake J, McCance DR, Young IS, Trimble ER, McGinty A. Restoration of adipose function in obese glucose-tolerant men following pioglitazone treatment is associated with CCAAT enhancer-binding protein β up-regulation. Clinical Science (Lond) 2012;123:135–146. doi: 10.1042/CS20110662. [DOI] [PubMed] [Google Scholar]
- Puddu A, Sanguineti R, Durante A, Viviani GL. Pioglitazone attenuates the detrimental effects of Advanced Glycation End-Products in the pancreatic beta cell line HIT-T15. Regulatory Peptides. 2012;177:79–84. doi: 10.1016/j.regpep.2012.05.089. [DOI] [PubMed] [Google Scholar]
- Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, Gonzalez JG, Chan M, Wolka AM, Boardman MK DURATION-4 Study Group. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naïve patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35:252–258. doi: 10.2337/dc11-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saremi A, Schwenke DC, Buchanan TA, Hodis HN, Mack WJ, Banerji M, Bray GA, Clement SC, Henry RR, Kitabchi AE, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arteriosclerosis, Thrombosis and Vascular Biology. 2013;33:393–399. doi: 10.1161/ATVBAHA.112.300346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaalan MF. Effects of pioglitazone and/or simvastatin on circulating TNFα and adiponectin levels in insulin resistance. J Immunotoxicology. 2012;9:201–209. doi: 10.3109/1547691X.2012.660998. [DOI] [PubMed] [Google Scholar]
- Selman C, Partridge L. A double whammy for aging ? Rapamycin extends lifespan and inhibits cancer in inbred female mice. Cell Cycle. 2012;11:17–18. doi: 10.4161/cc.11.1.18736. [DOI] [PubMed] [Google Scholar]
- Slagboom PE, Beekman M, Passtoors WM, Deelen J, Vaarhorst AA, Boer JM, van den Akker EB, van Heemst D, de Craen AJ, Maier AB, et al. Genomics of human longevity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:35–42. doi: 10.1098/rstb.2010.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Tran TT, Kondo T, Luo J, Ueki K, Cantley LC, Kahn CR. Phosphoinositide 3-kinase regulatory subunit p85α suppresses insulin action via positive regulation of PTEN. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12093–12097. doi: 10.1073/pnas.0604628103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Molecular and Cellular Biology. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- Wan Z, Ritchie I, Beaudoin MS, Castellani L, Chan CB, Wright DC. IL-6 indirectly modulates the induction of glyceroneogenic enzymes in adipose tissue during exercise. Public Library of Science One. 2012a;7:e41719. doi: 10.1371/journal.pone.0041719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z, Perry CG, Macdonald T, Chan CB, Holloway GP, Wright DC. IL-6 is not necessary for the regulation of adipose tissue mitochondrial content. Public Library of Science One. 2012b;7:e51233. doi: 10.1371/journal.pone.0051233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology. 2007;148:2845–2853. doi: 10.1210/en.2006-1313. [DOI] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]