Abstract
Therapeutic stimulation of angiogenesis to re-establish blood flow in ischemic tissues offers great promise as a treatment for patients suffering from cardiovascular disease or trauma. Since angiogenesis is a complex, multi-step process, different signals may need to be delivered at appropriate times in order to promote a robust and mature vasculature. The effects of temporally regulated presentation of pro-angiogenic and pro-maturation factors were investigated in vitro and in vivo in this study. Pro-angiogenic factors vascular endothelial growth factor (VEGF) and angiopoietin 2 (Ang2) cooperatively promoted endothelial sprouting and pericyte detachment in a three-dimensional in vitro EC-pericyte co-culture model. Pro-maturation factors platelet-derived growth factor B (PDGF) and angiopoietin 1 (Ang1) inhibited the early stages of VEGF- and Ang2-mediated angiogenesis if present simultaneously with VEGF and Ang2, but promoted these behaviors if added subsequently to the pro-angiogenesis factors. VEGF and Ang2 were also found to additively enhance microvessel density in a subcutaneous model of blood vessel formation, while simultaneously administered PDGF/Ang1 inhibited microvessel formation. However, a temporally controlled scaffold that released PDGF and Ang1 at a delay relative to VEGF/Ang2 promoted both vessel maturation and vascular remodeling without inhibiting sprouting angiogenesis. Our results demonstrate the importance of temporal control over signaling in promoting vascular growth, vessel maturation and vascular remodeling. Delivering multiple growth factors in combination and sequence could aid in creating tissue engineered constructs and therapies aimed at promoting healing after acute wounds and in chronic conditions such as diabetic ulcers and peripheral artery disease.
Introduction
Therapeutic angiogenesis, the promotion of new blood vessel formation to re-establish adequate perfusion in ischemic tissues, offers great promise as a treatment for patients suffering from cardiovascular disease and acute injuries [1–3]. Many recent strategies have concentrated on delivering single factors involved in the initial stages of blood vessel formation, such as vascular endothelial growth factor (VEGF)[4, 5]. However, blood vessels that sprout during the initial stages of angiogenesis must be stabilized in order to prevent regression and promote maturation of the nascent microvascular network into therapeutically functional vasculature[6, 7]. Despite significant progress[8], promoting a robust angiogenic response and creating mature vasculature remain goals of vascular medicine and, more broadly, of regenerative medicine and tissue engineering.
Sprouting angiogenesis is a remodeling process in which blood vessels form via sprouting from pre-existing vessels. This normal, physiological event occurs in the embryo during development as well as in adults during wound healing, reproductive cycling, and inflammation. In response to physiologic stress due to injury, ischemic tissues secrete signaling factors, which (1) activate endothelial cells (EC) and pericytes to degrade the mural wall as well as cause pericyte detachment from the endothelium; (2) promote sprouting of endothelial cells toward ischemic areas guided by growth factor gradients; (3) lead to the anastomosis of immature endothelial sprouts to form immature vasculature and (4) guide the maturation of vessels through recruitment of mural cells and deposition of extracellular matrix around the now maturing blood vessels (Fig. 1). The complex, multi-step nature of this process suggests that the presentation of multiple signals at appropriate times is necessary to promote a robust, mature, and functional blood vessel network[7, 9, 10].
Figure 1.
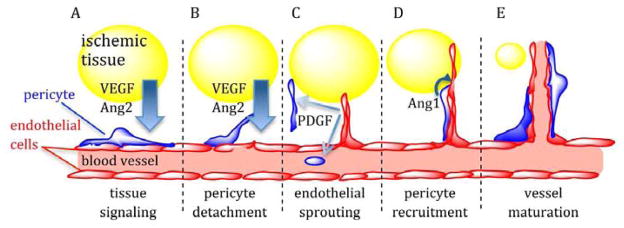
Model of select growth factor signaling during angiogenesis. (A) Ischemic tissues (yellow) release pro-angiogenic factors such as VEGF and Ang2, creating growth factor gradients that signal blood vessels to increase capacity. (B) Pro-angiogenic factor signaling destabilizes EC-pericyte interactions, promoting pericyte detachment from the endothelium and EC sprouting away from existing vessels. (C) As sprouts grow and penetrate hypoxic tissues, they release PDGF, which activates and recruits pericytes from surrounding tissue and progenitor cells from the blood stream to nascent vessels. (D) Pericyte-derived Ang1 antagonizes the Ang2 receptor (Tie2) and serves as a stabilizing factor that strengthens pericyte-EC interactions and promotes vessel maturation and lumen formation (E).
Previous studies have taken advantage of in vitro models of early angiogenesis to study the mechanisms of angiogenesis, and as a tool to predict the efficacy of therapeutic intervention[11]. These models generally involve culture of ECs under conditions that promote EC sprouting or tubule formation on 2D surfaces or inside of 3D matrices. These models have illuminated the roles of pro-angiogenic factors[4, 5, 12–16] and angiogenesis inhibitors[17, 18] as well as other angiogenesis-related signaling pathways[13, 19]. However, in vitro models rarely include mural cells and those that do[20, 21] generally do not take into account mural cell behavior and their response to therapeutic growth factors. In addition to inhibiting the angiogenic response of endothelial cells[22, 23], pericytes are suggested to control vessel contractility, tone and diameter[23–27] and to secrete factors necessary for endothelial survival and proliferation[28, 29]. Pericytes and endothelial cells act as a functional and physical unit through the establishment of cell-cell heterotypic contacts, and synthesis and secretion of growth factors that promote their mutual survival[29–31].
Multiple factors that promote the initial and maturation phases of angiogenesis have been identified, and the co-administration of both types of factors enhances blood vessel regeneration [32–37]. VEGF is a potent mitogen for endothelial cells and initiates their sprouting and proliferation to form new, immature vessel sprouts[38, 39]. While VEGF can initiate angiogenesis, additional factors are required to promote vessel maturation[34, 40, 41]. Platelet-derived growth factor B (PDGF) encourages maturation of the nascent vessels by activating and recruiting pericytes and smooth muscle cells that associate with endothelial sprouts, stabilizing them, and preventing regression[42–45]. The angiopoietins, -1 (Ang1) and -2 (Ang2), have opposing functions and compete for the same Tie-2 receptor[46]. Ang1 is a stabilizing factor that strengthens the interactions between pericytes/smooth muscle cells and ECs. In contrast, Ang2 weakens those same interactions, destabilizing blood vessels[46–48]. Importantly, factors that promote later stages of vascularization have been demonstrated to inhibit the early angiogenic stage. Specifically, PDGF inhibits VEGFmediated angiogenesis in matrigel plugs and in chorioallantoic membranes, and inhibits VEGFmediated pericyte migration in vitro[49]. The pro-maturation factor Ang1 serves to antagonize the interaction of the pro-angiogenesis factor Ang2 and its cognate receptor, Tie-2[48].
The need to sustain angiogenic factor signaling for extended periods of time has motivated the development of a number of polymer systems that provide a sustained and localized release of VEGF and other factors[50–56]. These systems have demonstrated significant enhancement in the extent of angiogenesis, as compared to those utilizing bolus factor delivery, and improved function of the new vasculature in a number of different animal models and wound types[1, 5, 13]. Further, polymer systems that provide sustained and temporally controlled release of pro-angiogenic and pro-maturation growth factors[15, 36, 40, 57, 58] have been demonstrated. In particular, polymeric scaffolds capable of delivering VEGF and PFGF with distinct kinetics from a single, structural polymer scaffold led to a substantial increase in both vascular density and vessel maturation[12, 15, 36].
This report addresses the hypothesis that proper temporal presentation of Ang1 and Ang2 can enhance VEGF-mediated vascular network growth and PDGF-mediated vessel maturation to collectively improve vascularization. The administration of Ang2 during the early stages of angiogenesis was anticipated to enhance angiogenesis by destabilizing existing vessels and promoting endothelial sprouting. Release of Ang1 was anticipated to improve vessel maturation by stabilizing newly formed vessels via enhanced EC-pericyte cell adhesion and antagonizing Ang2 activity. The effects of these factors in combination with VEGF and PDGF was first studied in an in vitro system, capable of capturing both angiogenic sprouting and pericyte detachment, two indicators of early angiogenesis. In vitro insights were then applied to a mouse in vivo model of vascularization and vessel maturation using a macroporous polymer system designed to ensure the sustained and localized release of multiple growth factors with temporal control[36, 58].
Materials and Methods
Cell Culture
Pooled Human Umbilical Vascular Endothelial Cells (HUVECs) (Lonza CC-2519) (passage 3) were used for all experiments and cultured in 2% FBS EGM-2 (Lonza, CC-3162). Newborn, placental pericytes (Promocell C-12980) were grown in 2% FBS Pericyte Medium (ScienCell 1201) and used at passage 6.
Animal Experiment
All animal work was performed in compliance with NIH and institutional guidelines. C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Other supplies were obtained from Sigma (St. Louis, MO).
Sprouting assay / pericyte detachment assay
HUVECs (passage 3) were incubated with octadecyl rhodamine B chloride, (R18, Molecular Probes O-246) at a concentration of 1ug/ml for 1–3 hours. Pericytes (passage 6) were incubated with 1uM CMFDA (Invitrogen C7025) for 1–3 hours. Cells were trypsinized prior to seeding onto microcarriers.
Cytodex 3 microcarriers (Amersham Biosciences, Piscataway, NJ, USA) were hydrated in PBS at room temperature (0.2 mL/ mg of dry Cytodex 3) and after 3h, the supernatant was decanted and replaced with fresh PBS followed by sterilization by autoclaving. HUVECs (passage 3) (1–2e6 cells) and pericytes (1–200,000 cells) in EGM-2 were combined with 50 mg of microcarriers at a 10:1 (cell/microcarrier) ratio in a spinner vessel (Bellco Glass Inc., Vineland, NJ, USA) and subjected to repeated stirring (2 mins) and no stirring (28 mins) cycles. After 3 h, microcarriers with cells transferred to tissue culture T25 flasks, and cultured for 1–2 days on an orbital shaker. To perform the sprouting assay, 250uL bead solution containing cell-seeded beads (450 beads / mL) in suspension, fibrinogen (EMD 341576, 2.72 mg/mL), and aprotinin (Sigma #A4528, 34.1 ug/mL) was mixed with 200uL thrombin solution containing thrombin (Sigma #T4265, 2.1U/mL) and incubated at 37 °C for 20 min, allowing gel formation. Cultures were fed every day with 0.8 mL of EGM-2 without BlueKit growth factors, or EGM-2 without BlueKit factors, but containing VEGF, Ang2, Ang1 or PDGF at stated concentrations. For in vitro studies vascular endothelial growth factor (rhVEGF, R&D 293-VE) and platelet-derived growth factor-BB (PDGF-BB, R&D 220-8B) were used, angiopoietin–1 (ANG1) and angiopoietin–2 were provided by R&D (R&D 923-AN/CF and 623-AN/CF, respectively). After three days (figure 2) or five days (figure 3) gels were washed twice with PBS, and incubated with 4% formaldehyde overnight at 4 °C. The formaldehyde solution was then aspirated and the gels were washed twice with PBS and stained with Hoechst solution (1ug/mL). Sprouts or migrated pericytes were quantified on a fluorescence microscope (> 100 beads analyzed per condition). A sprout was defined as an elongated structure extending from the bead with the participation of two or more endothelial cells. A detached pericyte was defined as one whose CMFDA-labeled cytoplasmic signal did not overlap with endothelial R18 signal.
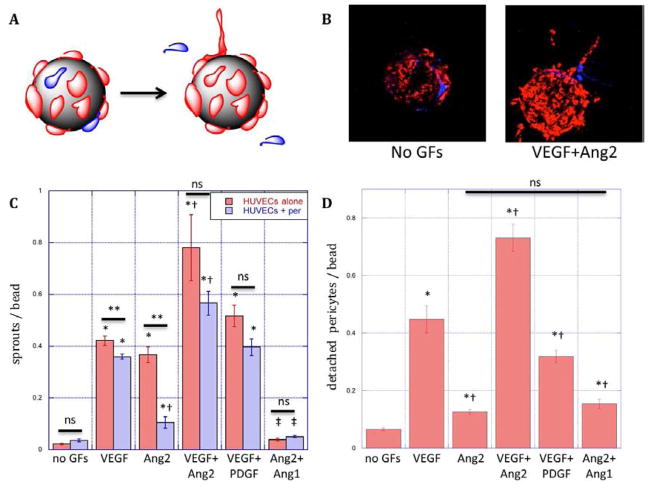
Figure 2.
Schematic (A) and microscopy (B) images of R18-labeled endothelial cell (red) sprouting and CMFDA-labeled pericyte (blue) detachment from microcarriers in response to growth factor signals. (C) Endothelial cell sprouting from microcarriers over three days with EC (red bars) or EC/pericytes (blue bars) cultures in fibrin in response to VEGF (50ng/mL), Ang2 (250ng/mL), combined VEGF(50ng/mL) and Ang2(250ng/mL, VA2), combined VEGF and PDGF(both 50ng/mL, VP) or combined Ang2 and Ang1 (both 250ng/mL, Ang1). (D) Pericyte migration away from endothelium on microcarriers with EC/pericyte co-cultures in response to conditions specified in C. *: statistically significant relative to no GF control. †: statistically significant relative to VEGF; ‡: statistically significant relative to Ang2; **: statistically significant between presence and absence of pericytes; ns: not significant by Student’s T-test. Data represent mean and S.E.M.
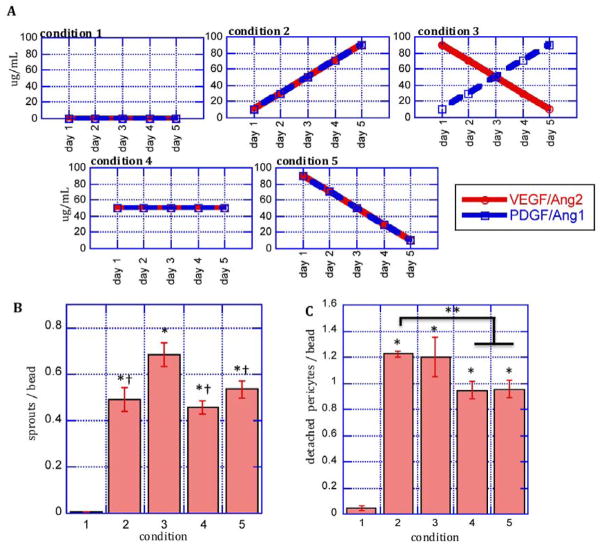
Figure 3.
Early angiogenic responses to temporally regulated growth factor presentation. A: EC/pericyte co-cultured on microcarriers in fibrin gels were subjected to different growth factor conditions over five days. B: Quantification of endothelial cell sprouting and C: quantification pericyte detachment from the endothelium in response to growth factor conditions in A. *: statistically significant relative to condition 1; †: statistically significant relative to condition 3; **: statistically significant relative to condition 2 by Student’s T-test. Data represent mean and S.E.M.
Microspheres and Scaffold Preparation
Microspheres (particle size 5–50μm) were fabricated using 75:25 PLG (Resomer RG755 (intrinsic viscosity = 0.59 L/g, MW 63kDa) Boehringer Ingelheim; Petersburg, VA), and were formed to incorporate 0.75 μg/mg of either Human Platelet-Derived Growth Factor (rhPDGF) (Pepro Tech; Rocky Hill, NJ) or Recombinant Human Angiopoeitin-1 (rhAng-1) (R&D Systems; Minneapolis, MN), using a double emulsion (water/oil/water) process as previously described[36].
Scaffolds were prepared from particulate PLG and PLG microspheres using a gas foaming/particulate leaching process as previously described[36]. Scaffolds were fabricated from a mixture of particulate PLG (85:15, ground to an average diameter of 125μm, ± VEGF, PDGF, Ang1, and/or Ang2 [direct protein loading], 3μg final loading of each growth factor per implanted scaffold) and 30% of total polymer mass of PLG microspheres (± PDGF and/or Ang1 [indirect protein loading], 3μg growth factor total loaded per implanted scaffold). The mixture of polymer in the form of particles and microspheres was combined with NaCl particles (diameter 250–425μm) and a 1% (w/v) alginate solution. This mixture was lyophilized and pressed into a pellet using a Carver Press. The scaffolds were placed under high pressure CO2 gas and allowed to equilibrate. The pressure was rapidly returned to ambient conditions leading to a thermodynamic instability and causing the polymer, whether in the form of particulate or microspheres, to foam and create an interconnected structure around the NaCl. Both types of particles foam and fuse together to create the scaffold, and no distinct particles or microspheres are present in the scaffolds after this processing. The NaCl was leached in a CaCl2 solution to create a macroporous structure, while mediating gelation of alginate. Scaffolds were prepared 13mm in diameter and 3mm thick (40mg total polymer and 760mg NaCl) and divided into quarters for subcutaneous implants.
Vessel Formation
For in vivo studies vascular endothelial growth factor (VEGF) was provided by the National Cancer Institute (NCI) Biological Resources Branch (Frederick, MD), PDGF-BB was obtained from Pepro Tech Incorporated, angiopoietin–1 (Ang1) and angiopoietin–2 (Ang2) from R&D (R&D 923-AN/CF and 623-AN/CF, respectively).
Scaffolds containing no growth factor and various combinations of factors were subcutaneously implanted in C57Bl/6J mice (n=3 per animal per condition), as previously described[36, 58]. Scaffolds were retrieved from subcutaneous pockets after two weeks, fixed in formalin, and stored in ethanol. Tissues were embedded in paraffin, sectioned (~5μm thick), and placed on glass slides at the histology core in the School of Dentistry at the University of Michigan. The sections were stained for CD31 (antigen found on endothelial cells) at the University of Michigan Cancer Center Histology Core, to identify blood vessels as previously described[36, 58]. The density of blood vessels was determined as previously described[36, 58]. In brief, CD31 stained sections were viewed at 200X magnification using a light microscope (Nikon; Indianapolis, IN). Blood vessels in three different tissue samples from each condition (n=3 per condition) were manually counted. The matrix area was determined using Image Pro Plus software to calculate blood vessel density from the number and area measurements. These sections were also analyzed to determine blood vessel size distribution. For each condition, 4–8 images and at least 50 vessels were measured and the area of these vessels was determined using Image Pro Plus software.
Histological sections were also stained for α-smooth muscle actin (α-SMA) (marker found on pericytes and smooth muscle cells associating with endothelial cells) to identify the number of blood vessels that contained interacting mural cells, as a measure of vessel maturation. In brief, α-SMA stained sections were viewed at 200X magnification using a light microscope (Nikon; Indianapolis, IN). Blood vessels in three different tissue samples from each condition (n=3 per condition) were manually counted, and the percentage that had associated smooth muscle cells calculated.
Statistical Analysis
Statistical analysis was performed by Student’s T-tests (two-tail comparisons) and statistically significant differences were defined as p<0.05. Values in graphs are expressed as averages with standard error from the mean.
Results
Impact of growth factor presentation on endothelial sprouting and pericyte detachment in vitro
A three-dimensional in vitro model of angiogenesis was first developing by co-culturing endothelial cells and pericytes in a 3D fibrin gel, and pericyte detachment and endothelial sprouting were quantified as a function of factor stimulation (Fig. 2A). EC-seeded microcarriers with and without pericytes were incubated in fibrin gels with growth factors for 3 days (Fig. 2B). VEGF (50ng/mL) and Ang2 (250ng/mL) significantly promoted endothelial sprouting from microcarriers, as compared to the control, and VEGF and Ang2-promotion of sprouting was additive when added in combination (Fig. 2C). The influence of pro-maturation factors in this model was assayed by the addition of PDGF or Ang1 to culture medium. While PDGF (50ng/mL) did not significantly inhibit VEGF-mediated EC sprouting, Ang1 (250ng/mL) completely abolished Ang2-mediated EC sprouting (Fig. 2C). The presence of pericytes in co-culture with ECs generally had an inhibitory effect on EC sprouting (Fig. 2C, red vs. blue bars). The inhibitory effect of pericytes was particularly striking under Ang2-induced endothelial sprouting (Fig. 2C, Ang2), where pericytes reduced endothelial sprouting by 70%.
Co-culture of pericytes in contact with ECs in the three-dimensional model of EC sprouting allowed the study of pericyte detachment in response to different growth factors, as a model of pericyte detachment from blood vessels in the early stages of angiogenesis. Both VEGF and Ang2 promoted pericyte detachment away from EC-covered beads, and the combination of VEGF and Ang2 had an additive effect (Fig. 2D). The addition of PDGF inhibited VEGF-mediated pericyte detachment (Fig. 2D), while Ang1 did not have an effect on the modest level of pericyte detachment promoted by Ang2 (Fig. 2D).
In view of the observation that PDGF and Ang1 interfered with the action of VEGF and Ang2 to promote early angiogenesis, endothelial sprouting and pericyte migration were next assessed as the time-frame over which GFs were presented was varied. Due to their additive roles in early angiogenesis, VEGF and Ang2 were always used in combination in these studies. Similarly PDGF and Ang1 were combined because of their known functions in late-stage vessel maturation. Five different growth factor regiments of VEGF/Ang2 and PDGF/Ang1 were studied over five days (Fig. 3A), and the total dose of each factor was maintained constant over all conditions (excepting the no factor condition). Very little EC sprouting was observed in the absence of exogenous growth factors, as expected (Fig. 3B). The presentation of all growth factors regardless of the temporal profile of their presentation led to significantly more EC sprouting and pericyte detachment than the blank control (conditions 2–5). Early exposure to high initial concentrations of VEGF/Ang2 that decreased over time, combined with increasing concentrations of PDGF/Ang1 (condition 3) led to significantly enhanced endothelial sprouting compared to conditions in which the four factor concentrations increased over time (condition 2), all four factors had constant concentrations (condition 4), or the four factors decreased over time (condition 5, Figure 3B).
Pericyte detachment was also assessed with temporal control over GF presentation in the culture medium under the same conditions (Fig. 3C). Very little pericyte detachment was observed in the condition without exogenous growth factors, as expected (condition 1). Initial low concentrations of PDGF/Ang1 led to the greatest extent of pericyte detachment away from endothelium, whether coupled with increasing (condition 2) or decreasing (condition 3) VEGF/Ang2 concentrations. A constant concentration of VEGF/Ang2 and PDGF/Ang1 (condition 4) or high initial concentrations of both (condition 5) led to less pericyte detachment, as compared to conditions with initially low concentrations of PDGF and Ang1.
These results suggest that a delayed presentation of pro-maturation factors act cooperatively to increase the effects of early presentation of pro-angiogenic factors, and that growth factor formulations supplying an early burst of pro-angiogenic factors and delayed release of promaturation factors may have an improved ability to stimulate angiogenesis in vivo.
Effect of temporal control over growth factor presentation on vascularization in vivo
As the in vitro results suggest that a delayed presentation of pro-maturation factors act cooperatively to increase the effects of early presentation of pro-angiogenic factors, blood vessel formation in response to the four growth factors (VEGF, PDGF, Ang1 and Ang2) was next assessed in vivo in a subcutaneous implant model in mice. A macroporous polymer system with kinetic control over growth factor release [36, 58] was used to allow for sustained and localized release of the four growth factors of interests. Scaffolds were fabricated that rapidly release VEGF (3μg), Ang2 (3μg), PDGF (3μg), and/or Ang1 (3μg) or alternatively, that combine rapid release of VEGF/Ang2 with a delayed release of PDGF/Ang1.
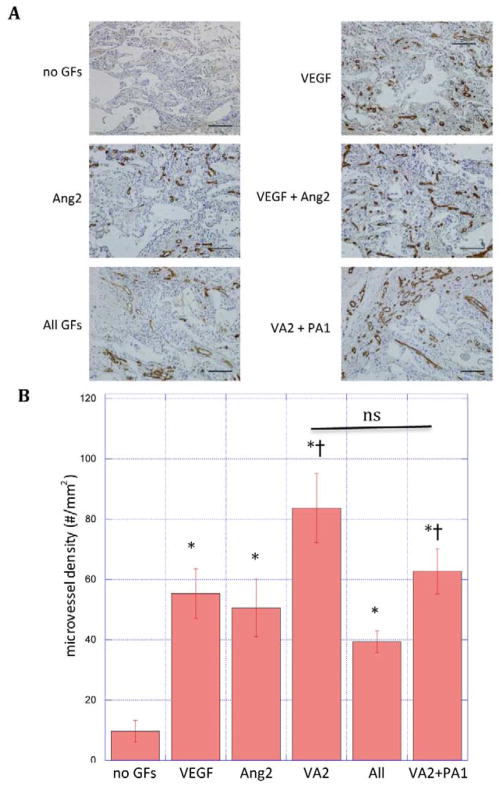
The blood vessel density in scaffolds implanted in the subcutaneous tissue of C57Bl6 mice was analyzed at two weeks (Fig. 4A). Both VEGF and Ang2 alone increased the density of blood vessels, and their combined delivery resulted in a more pronounced, roughly additive, effect relative to blank scaffolds (Fig. 4B). To determine whether combining delivery of PDGF and Ang1 with VEGF and Ang2 would further affect vascularization, an experiment with scaffolds also releasing these factors was performed. Simultaneous release of all four factors, Ang1/PDGF and VEGF/Ang2 decreased blood vessel density as compared to release of VEGF/Ang2 only (All vs. VA2). In contrast, scaffolds that rapidly released VEGF/Ang2 followed by delayed release of PDGF and Ang1 did not inhibit blood vessel formation in a statistically significant way, compared to delivery of VEGF/Ang2 only (Fig. 4B, VA2 vs. VA2 + PA1).
Figure 4.
(A) Photomicrographs of hematoxylin and CD31 stained section of scaffolds and (B) quantification of microvessel density in PLG scaffolds subcutaneously implanted in mice for two weeks. Scaffolds (n = 3) containing no growth factors (no GFs), rapidly releasing VEGF, Ang2, VEGF and Ang2 simultaneously (VA2), VEGF, Ang2, PDGF and Ang1 all simultaneously (all GF), or rapidly releasing VEGF and Ang2 followed by delayed release of PDGF and Ang1 (VA2+PA1). Data represent mean and S.E.M.
*: statistically significant relative to no GFs; † statistically significant relative to All GFs; ns: not significant by Student’s T-test. scale bar: 100μm
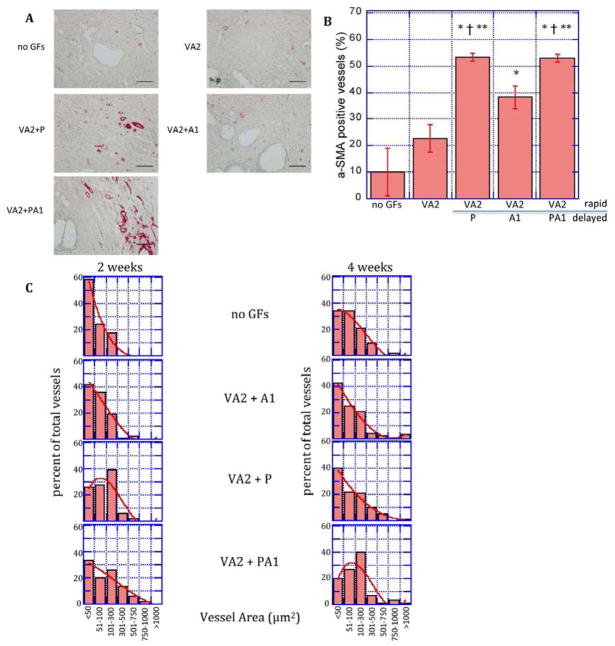
The maturity of blood vessels formed in response to VEGF/Ang2 administration alone or with VEGF/Ang2 followed by PDGF, Ang1, or simultaneous delivery of PDGF and Ang1 was next analyzed. Scaffolds that rapidly released VEGF and Ang2 and slowly released PDGF, Ang1, or both molecules were implanted subcutaneously in C57Bl/6J mice. Vessel maturity was measured by the presence of α-SMA-positive mural (pericytes/smooth muscle) cells associated with vessels[59] (Fig. 5A). Scaffolds rapidly releasing VEGF/Ang2 did not significantly increase the percentage of vessels associated with mural cells, as compared to blank scaffolds (Fig. 5B). Scaffolds presenting Ang1 or PDGF sequentially after VEGF/Ang2 presentation had a significantly higher percentage of vessels with associated mural cells relative to blank controls or scaffolds presenting VEGF/Ang2 only. Scaffolds releasing a combination of VEGF/Ang2 with later release of PDGF and Ang1 led to higher mural cell association, as compared to scaffolds presenting Ang1 alone after VEGF/Ang2, but a similar maturation relative to scaffolds releasing PDGF after VEGF/Ang2 (Fig. 5B).
Figure 5.
Delayed release of pro-maturation factors increases mural cell association and vascular remodeling. A: Photomicrographs of α-SMA-stained sections of scaffolds implanted subcutaneously releasing no growth factors (no GFs), VEGF/Ang2 (VA2) alone or followed by a delayed release of PDGF (P), Ang1 (A1), or both (PA1) at two weeks. B: Quantification of the percentage of blood vessels associating with smooth muscle cells determined by α-smooth muscle actin staining of tissue sections from subcutaneously-implanted scaffolds C: Cross-sectional area of vessels formed by rapid delivery of VEGF/Ang2 (VA2) followed by delayed release of PDGF (P), Ang1 (A1), or both (PA1) at two and four weeks. Values represent mean and S.E.M.
*, †, ** Denote p<0.05 relative to blank, VA2, and VA2+A1, respectively by Student’s T-test. Scale bar = 100μm
The changes in blood vessel size distribution over two weeks was also measured as a metric of vascular remodeling (Fig. 5C). Rapid release of VEGF/Ang2 with delayed release of either PDGF or Ang1, or their combination, resulted in increased vessel size, as compared to the control condition at two weeks (Figure 5C). At four weeks, scaffolds releasing both PDGF and Ang1 after rapid release of VEGF/Ang2 resulted in larger vessels, as compared to either blank scaffolds or scaffolds with delayed release of Ang1 or PDGF alone after VEGF/Ang2.
Discussion
The results of this study demonstrate that sequential presentation of multiple pro-angiogenic and pro-maturation factors promotes angiogenic responses and increases vascular maturation and remodeling. The pro-angiogenic factors VEGF and Ang2 additively promoted early angiogenesis through increasing endothelial sprouting and pericyte detachment. While pro-maturation factors PDGF and Ang1 inhibited these early stages of VEGF- and Ang2-mediated angiogenesis if present too early, the two factors independently promoted vessel maturation if released sequentially after VEGF and Ang2 administration. PDGF and Ang1 in combination further promoted vascular remodeling. Taken together, these results suggest that proper temporal delivery of these factors will improve therapeutic interventions, in which both a robust initial angiogenic response and a subsequent mature vasculature is desired.
VEGF and Ang2 alone and in combination increased angiogenesis in vitro and in vivo in an additive manner. Previous work has shown that EC sprouting is supported by an early burst of VEGF[5]. VEGF, with its ability to initiate EC proliferation and sprouting, and Ang2, with its ability to weaken the vessel-stabilizing interactions between ECs, and between pericytes and ECs[60, 61] have key, but different, roles in angiogenesis. Additive effects between VEGF and Ang2 have been reported in several previous studies of cancer progression and angiogenesis[16, 62], with the prevailing view suggesting that Ang2 enhances VEGF-mediated angiogenesis, but causes sprout regression when delivered alone. However, in both the in vitro and in vivo models of this study, Ang2 alone significantly increased endothelial sprouting and microvessel formation, suggesting that either Ang2 alone can stimulate angiogenesis or that endogenously derived VEGF is sufficient to provide the necessary stimuli. Interestingly, Ang2 had significantly less effect in HUVEC-pericyte co-cultures. This variable impact of Ang2 may stem from the secretion of Ang1 by pericytes[63, 64]. This possibility is supported by the observation that addition of exogenous Ang1 to both HUVEC and pericyte-HUVEC in vitro models completely abrogated the effects of Ang2 (Fig. 2C). The significant reduction in EC sprouting when co-cultured with pericyte cells in vitro (Fig 2C) recapitulates one suggested role for pericytes as inhibitors of EC sprouting and migration[22, 23, 28]. Increased pericyte migration away from endothelium over five days (Fig 3C) as compared to three days (Fig 2C) in culture could suggest a diminished effect on EC sprouting over time. However, much work remains to be done in order to understand the precise role of pericytes in this co-culture system and their effects on cell-cell contacts, secretion and degradation of extracellular matrix and paracrine growth factor signaling.
The pro-maturation factors PDGF and Ang1 demonstrated a dual role in in vitro and in vivo angiogenesis. The simultaneous delivery of PDGF and Ang1 with VEGF and Ang2 retarded nascent vessel formation and inhibited pericyte detachment. This suppression of early angiogenesis is likely due to the specific roles of these two factors. In vitro, Ang1 inhibited Ang2-mediated endothelial sprouting (Fig. 2C), in agreement with previous work suggesting that Ang2 antagonizes the Ang1 receptor Tie-2[61]. Additionally, PDGF inhibited VEGF-mediated pericyte detachment from the endothelium-like EC monolayer in vitro (Fig. 2D), also in agreement with previous studies[49]. A temporally regulated regime in which EC-pericyte co-cultures were exposed to an early burst of VEGF and Ang2 and a delayed exposure to PDGF and Ang1 provided the best conditions for endothelial sprouting and pericyte detachment.
Rapid release of a combination of VEGF and Ang2, followed by the delayed release of PDGF and Ang1 in vivo dramatically altered local vascularization by both initiating vessel formation and promoting maturation through mural cell association and vessel remodeling. PDGF activates mural cells in the microenvironment and promotes their interaction with nascent vessels, as evident from the increase in the percentage of vessels positively stained for α-SMA in PDGF-releasing scaffolds, as compared to scaffolds delivering no growth factors or those delivering only VEGF and Ang2 (Fig. 5B). Delivery of Ang1 singly after VEGF/Ang2 also enhanced recruitment of mural cells, though to a lesser degree than PDGF. Ang1 proved important in promoting vascular remodeling as reflected by vessel size at 4 weeks (Fig. 5C). Ang1 potentially plays a role in remodeling newly forming vessels to more highly ordered structures[65], thus generating larger diameter vessels that are critical in restoring tissue perfusion and preventing necrosis in an ischemic environment. Future work will be required to further characterize the maturation of the vasculature through measures of perfusion, microCT analysis of vascular architecture and/or an assessment of vascular permeability. Altogether, these observations suggest that reciprocal interactions between Ang1 and PDGF stimulate blood vessel maturation.
Conclusion
In summary, the approach taken in this study recognizes that the angiogenic cascade is a complex process with different growth factors functioning at different times in a concerted manner to drive blood vessel formation. The use of polymeric scaffolds capable of combined and sequential release of multiple growth factors moves the field closer to simulating and augmenting native repair conditions. This multi-factor and time-controlled response may aid in the creation of functional vasculature in tissue-engineered constructs, and promote healing after acute wounds and in chronic conditions such as diabetic ulcers and peripheral artery disease.
Acknowledgments
We are grateful to the assistance of Des White for help with PLG microsphere and scaffold fabrication. This work was supported by the National Institutes of Health R01 HL069957 and by the Wyss Institute for Biologically Inspired Engineering at Harvard University. Additionally, we thank the Biological Resources Branch of the National Cancer Institute for supplying the VEGF used in the experiments described in this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Attanasio S, Snell J. Therapeutic angiogenesis in the management of critical limb ischemia: current concepts and review. Cardiol Rev. 2009;17:115–20. doi: 10.1097/CRD.0b013e318199e9b7. [DOI] [PubMed] [Google Scholar]
- 2.Freedman S, Isner J. Therapeutic angiogenesis for ischemic cardiovascular disease. J Mol Cell Cardiol. 2001;33:379–93. doi: 10.1006/jmcc.2000.1329. [DOI] [PubMed] [Google Scholar]
- 3.Losordo D, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109:2692–7. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 4.Silva E, Mooney D. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 5.Silva E, Mooney D. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials. 2010;31:1235–41. doi: 10.1016/j.biomaterials.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–95. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 7.Jain R. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 8.Chu H, Wang Y. Therapeutic angiogenesis: controlled delivery of angiogenic factors. Ther Deliv. 2012;3:693–714. doi: 10.4155/tde.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conway E, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–21. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 10.Jain R. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 11.Vailhé B, Vittet D, Feige J. In vitro models of vasculogenesis and angiogenesis. Lab Invest. 2001;81:439–52. doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- 12.Cao L, Arany P, Kim J, Rivera-Feliciano J, Wang Y-S, He Z, et al. Modulating Notch signaling to enhance neovascularization and reperfusion in diabetic mice. Biomaterials. 2010;31:9048–56. doi: 10.1016/j.biomaterials.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao L, Arany P, Wang Y-S, Mooney D. Promoting angiogenesis via manipulation of VEGF responsiveness with notch signaling. Biomaterials. 2009;30:4085–93. doi: 10.1016/j.biomaterials.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao L, Mooney D. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv Drug Deliv Rev. 2007;59:1340–50. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, Silva E, Yuen W, Mooney D. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res. 2007;24:258–64. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 16.Yung Y, Chae J, Buehler M, Hunter C, Mooney D. Cyclic tensile strain triggers a sequence of autocrine and paracrine signaling to regulate angiogenic sprouting in human vascular cells. Proc Natl Acad Sci U S A. 2009;106:15279–84. doi: 10.1073/pnas.0905891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tremblay P-L, Berthod F, Germain L, Auger F. In vitro evaluation of the angiostatic potential of drugs using an endothelialized tissue-engineered connective tissue. J Pharmacol Exp Ther. 2005;315:510–6. doi: 10.1124/jpet.105.089524. [DOI] [PubMed] [Google Scholar]
- 18.Clapp C, Martial J, Guzman R, Rentier-Delure F, Weiner R. The 16-kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology. 1993;133:1292–9. doi: 10.1210/endo.133.3.7689950. [DOI] [PubMed] [Google Scholar]
- 19.Yuen W, Du N, Chan C, Silva E, Mooney D. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc Natl Acad Sci U S A. 2010;107:17933–8. doi: 10.1073/pnas.1001192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelletier L, Regnard J, Fellmann D, Charbord P. An in vitro model for the study of human bone marrow angiogenesis: role of hematopoietic cytokines. Lab Invest. 2000;80:501–11. doi: 10.1038/labinvest.3780056. [DOI] [PubMed] [Google Scholar]
- 21.Korff T, Kimmina S, Martiny-Baron G, Augustin H. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447–57. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- 22.Orlidge A, D’Amore P. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987;105:1455–62. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–53. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman I, D’Amore P. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985;101:43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peppiatt C, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–4. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–7. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 27.Kutcher M, Herman I. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res. 2009;77:235–46. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonelli-Orlidge A, Smith S, D’Amore P. Influence of pericytes on capillary endothelial cell growth. Am Rev Respir Dis. 1989;140:1129–31. doi: 10.1164/ajrccm/140.4.1129. [DOI] [PubMed] [Google Scholar]
- 29.Darland D, Massingham L, Smith S, Piek E, Saint-Geniez M, D’Amore P. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Song S, Ewald A, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 32.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman J, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287–92. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borselli C, Cezar C, Shvartsman D, Vandenburgh H, Mooney D. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32:8905–14. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutton C, Young Y, Williams R, Meedeniya A, Mackay-Sim A, Goss B. Combined VEGF and PDGF treatment reduces secondary degeneration after spinal cord injury. J Neurotrauma. 2012;29:957–70. doi: 10.1089/neu.2010.1423. [DOI] [PubMed] [Google Scholar]
- 35.Reyes R, De la Riva B, Delgado A, Hernández A, Sánchez E, Évora C. Effect of triple growth factor controlled delivery by a brushite-PLGA system on a bone defect. Injury. 2012;43:334–42. doi: 10.1016/j.injury.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Richardson T, Peters M, Ennett A, Mooney D. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 37.Cao R, Bråkenhielm E, Pawliuk R, Wariaro D, Post M, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 38.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999;237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–24. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 40.Davies N, Schmidt C, Bezuidenhout D, Zilla P. Sustaining neovascularization of a scaffold through staged release of vascular endothelial growth factor-A and platelet-derived growth factor-BB. Tissue Eng Part A. 2012;18:26–34. doi: 10.1089/ten.tea.2011.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goel S, Duda D, Xu L, Munn L, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darland D, D’Amore P. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157–8. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–51. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song N, Huang Y, Shi H, Yuan S, Ding Y, Song X, et al. Overexpression of platelet-derived growth factor-BB increases tumor pericyte content via stromal-derived factor-1alpha/CXCR4 axis. Cancer Res. 2009;69:6057–64. doi: 10.1158/0008-5472.CAN-08-2007. [DOI] [PubMed] [Google Scholar]
- 45.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 46.Davis S, Yancopoulos G. The angiopoietins: Yin and Yang in angiogenesis. Curr Top Microbiol Immunol. 1999;237:173–85. doi: 10.1007/978-3-642-59953-8_9. [DOI] [PubMed] [Google Scholar]
- 47.Gale N, Yancopoulos G. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–66. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- 48.Maisonpierre P, Suri C, Jones P, Bartunkova S, Wiegand S, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg J, Shields D, Barillas S, Acevedo L, Murphy E, Huang J, et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature. 2008;456:809–13. doi: 10.1038/nature07424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edelman E, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12:619–26. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 51.Gombotz W, Pettit D. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem. 1995;6:332–51. doi: 10.1021/bc00034a002. [DOI] [PubMed] [Google Scholar]
- 52.Kuo P, Saltzman W. Novel systems for controlled delivery of macromolecules. Crit Rev Eukaryot Gene Expr. 1996;6:59–73. doi: 10.1615/critreveukargeneexpr.v6.i1.40. [DOI] [PubMed] [Google Scholar]
- 53.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 54.Lee K, Peters M, Anderson K, Mooney D. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 55.Mahoney M, Saltzman W. Millimeter-scale positioning of a nerve-growth-factor source and biological activity in the brain. Proc Natl Acad Sci U S A. 1999;96:4536–9. doi: 10.1073/pnas.96.8.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray J, Brown L, Langer R. Controlled release of microquantities of macromolecules. Cancer Drug Deliv. 1984;1:119–23. doi: 10.1089/cdd.1984.1.119. [DOI] [PubMed] [Google Scholar]
- 57.Peirce S, Price R, Skalak T. Spatial and temporal control of angiogenesis and arterialization using focal applications of VEGF164 and Ang-1. Am J Physiol Heart Circ Physiol. 2004;286:25. doi: 10.1152/ajpheart.00833.2003. [DOI] [PubMed] [Google Scholar]
- 58.Ennett A, Kaigler D, Mooney D. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79:176–84. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 59.Benjamin L, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 60.Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–44. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 61.Scharpfenecker M, Fiedler U, Reiss Y, Augustin H. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–80. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 62.Yoshiji H, Kuriyama S, Noguchi R, Yoshii J, Ikenaka Y, Yanase K, et al. Angiopoietin 2 displays a vascular endothelial growth factor dependent synergistic effect in hepatocellular carcinoma development in mice. Gut. 2005;54:1768–75. doi: 10.1136/gut.2005.067900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hori S, Ohtsuki S, Hosoya K-i, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–13. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 64.Sundberg C, Kowanetz M, Brown L, Detmar M, Dvorak H. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 65.Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, et al. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619–28. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]