Summary
We performed this study to evaluate how age and device affect the systemic exposure of inhaled fluticasone propionate (FP) in children. The findings indicate an anti-static valved holding chamber significantly increases systemic exposure of FP.
Keywords: valved holding chamber, lung bioavailability, fluticasone propionate, asthma
INTRODUCTION
Previous studies in adults have shown that a valved holding chamber (VHC) increases drug delivery to the airways [1–3]. However, in children, guidelines state that less drug is delivered with a spacer [4]. As a consequence, we performed this study to evaluate how age and device affect the systemic exposure of inhaled fluticasone propionate (FP) in children.
METHODS
All subjects were studied in the Asthma Research Lab at the University of Florida. The study was approved by the University of Florida Institutional Review Board, and registered with clinicaltrials.gov (NCT00308932).
Study Design
This study was a single center, unblinded, cross-sectional, observational study of one hour steady-state FP plasma concentrations. 61 children with well controlled persistent asthma were enrolled in the study. Subjects 12–18 years used the actuator alone with optimal technique. Subjects 5–9 years of age were divided into 3 groups based on the device they could optimally use: actuator alone, a VHC with mouthpiece, or a VHC with mask. Children 1–4 years used a VHC with mask. Both VHCs were made from an anti-static polymer (AeroChamber MAX®, Monaghan Medical Corporation, Plattsburgh, NY).
All subjects were converted from their regular inhaled corticosteroid regimen (80.4% on ≥ 440μg/day) to hydrofluoroalkane fluticasone propionate MDI (Flovent® HFA), 110μg/actuation, 2 actuations BID for at least 3 days with the optimal delivery device as described above. This duration of treatment ensured that the fluticasone plasma concentrations would be at steady state in all subjects since the mean half life in children is about 6–8 hours[5].
Adherence to the twice daily regimen was documented by an electronic monitor (Doser®, Meditrack Products, Easton, MA). Four actuations of albuterol MDI (90μg/actuation) were administered 20 minutes before the FP test dose on each study day to minimize airway obstruction. Subsequently, the study coordinator observed the administration of the test dose and if it was optimal, a single 5mL blood sample was collected 1 hour later.
Measurements
FP plasma concentrations were measured by a liquid chromatography-mass spectrometry assay[6].
Statistical Analysis
An Analysis of Variance was used to assess the means of the one hour FP concentrations for each group. The differences between each group and the reference group were tested using the two-sided Dunnett Multiple Comparison Procedure[7].
RESULTS
In total, 88 subjects were screened. Of these subjects, 61 completed the study, with at least 12 in each group. The most common cause of withdrawal (11/27) was a documented adherence of less than 100% during the study. Additionally, nine subjects could not be taught proper inhalation technique and seven withdrew because of other reasons (e.g. child refused venipuncture). However, all subjects who completed the study had 100% adherence for at least three days before the test day.
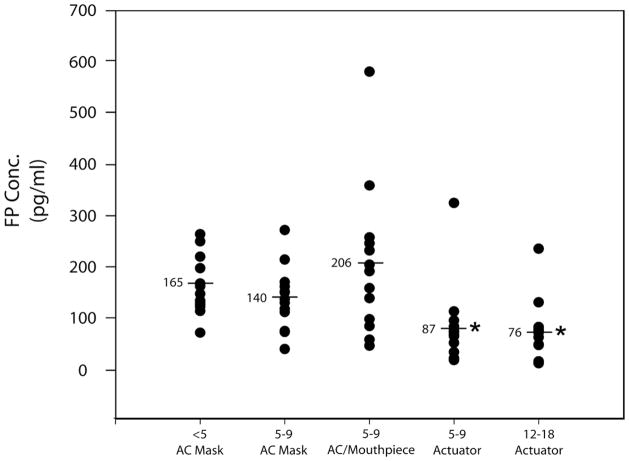
Individual Cmax values are depicted in Figure 3. The mean concentration in the 12–18 year MDI alone group was significantly lower (p<0.003) than all of the groups using a chamber but was not significantly different from the 5–9 year group who was able to use the actuator alone (Table).
TABLE 1.
Demographics and Results of Subjects who Completed the Study
| Age (years) | Gender Male: Female | Race Caucasian: African American: Hispanic | BMI (kg/m2) | Duration of FP treatment (days) | 1-hr FP plasma conc pg/mL Geometric mean (95% CI) | |
|---|---|---|---|---|---|---|
| 1 to 4 yrs VHC/mask (n=12) | 2.8 ± 0.8 | 10:2 | 9:3:0 | 16.76 | 5.2 ± 1.7 | 155 (124:196) |
| 5–9 yrs VHC/mask (n=13) | 6.2 ± 0.8 | 8:5 | 8:5:0 | 18.73 | 6.2 ± 3.2 | 127 (95:170) |
| 5–9 yrs VHC/mouthpiece (n=12) | 6.5 ± 1.0 | 8:4 | 5:7:0 | 19.49 | 6.4 ± 2.6 | 164 (103:259) |
| 5–9 yrs Actuator alone (n=12) | 8.1 ± 1.1 | 7:5 | 7:5:0 | 18.67 | 6.8 ± 6.4 | 67* (43:105) |
| 12–18 yrs Actuator alone (n=12) | 15.1 ± 1.6 | 8:4 | 7:4:1 | 27.20 | 6.5 ± 3.8 | 57 (33:95)* |
Significantly lower than all groups using a VHC.
There were no adverse events observed during this study.
DISCUSSION
The results of this study indicate that systemic exposure was significantly higher in all groups receiving FP through a VHC compared to the two groups who effectively used an MDI through the actuator alone. For the 5–9 age groups we can assume that the average volumes of distribution and clearances of the three groups were similar since there was no significant difference in mean body mass index (Table). The lack of a statistical difference between the VHC with mask and the VHC with mouthpiece groups indicates that passive inhalation does not significantly decrease lung bioavailability. We recognize that this dose of FP is listed as high-dose in the NIH guidelines. However, this dose is routinely prescribed at our tertiary pediatric pulmonary center and that is why we chose to use it. The FP package insert states that the dose-related increase in systemic exposure occurred using a VHC in all age groups ≥4 years. Specifically, they stated that in patients ≥ 12 years receiving 220μg of FP twice a day with a chamber there was increased systemic exposure with a Cmax of 47.3pg/mL[8]. In our study systemic exposure using a VHC was much higher with averages ranging between 140–207pg/mL in all age groups when an anti-static VHC was used. These disparities may require clinical trials assessing the safety of using the FP HFA with antistatic VHCs.
The clinical relevance of our finding of increased systemic exposure with the use of an anti-static VHC is unclear. In adults, the plasma concentration of FP producing a 50 % decrease in maximal cortisol secretion (EC50) was 100 pg/mL in one study[9] and 130 pg/ML in another[10]. The corresponding value for children is unknown. However, Eid et al[11] reported a dose-dependent decrease in 8 am cortisol concentrations in children receiving long term therapy with FP through a conventional VHC. In those receiving 440 μg/d, the medium dose employed in our study, 35 % had an abnormal cortisol concentration that subsequently normalized after dose reduction. In contrast, Lipworth et al [12] did not find a difference in overnight urinary cortisol/creatinine excretion between 200 and 400 μg/d of FP delivered by large volume spacer to children, but the duration of treatment was only 4 days with each dose and urinary cortisol excretion is less sensitive than plasma cortisol concentrations[13]. We, therefore, recommend initiating therapy with a lower dose of FP (176 μg/d) or reducing the dose as soon as asthma control is achieved if therapy is initiated with 440 μg/d.
Supplementary Material
Figure 1.
Individual one-hour post-dose steady state fluticasone propionate plasma concentrations. The mean±SD in pg/mL were as follows: 12–18 yr reference group, 76±61; 5–9 yr actuation alone, 87±80 (p=0.75); 5–9 yr VHC/mouthpiece, 207±149 (p=0.0006); 5–9 yr VHC/mask, 140±61 (p=0.07); and 1–4 yr VHC/mask, 165±58 (p=0.016). *Significantly lower than groups receiving FP through VHC (p=0.003).
Acknowledgments
Source of Funding: Supported, in part, by an investigator-initiated grant from GlaxoSmithKline and NIH Research Facilities Construction Program C06, grant RR17568. Dr. Shuster is supported by NIH General Clinical Research Center grants M01RR00082 and U54RR025208.
ABBREVIATIONS
- Cmax
the peak plasma concentration
- EC50
the concentration of FP suppressing the maximum cortisol excretion by 50%
- FEV1
forced expiratory volume in the first second
- FP
fluticasone propionate
- HFA
hydrofluoroalkane
- MDI
metered-dose inhaler
- Vd
volume of distribution
- VHC
valved holding chamber
Footnotes
Clinicaltrials.gov #NCT00308932
Conflicts of Interest: None
Contributor Information
Mai K. ElMallah, University of Florida.
Yasmeen Khan, University of Miami.
Guenther Hochhaus, Department of Pharmaceutics, University of Florida.
Jonathan J. Shuster, Department of Health Outcomes and Policy, University of Florida.
Leslie Hendeles, Pharmacotherapy and Translational Research, and Professor of Pediatrics (Pulmonary), University of Florida.
References
- 1.Nair A, et al. Respirable dose delivery of fluticasone propionate from a small valved holding chamber, a compact breath actuated integrated vortex device and a metered dose inhaler. British Journal of Clinical Pharmacology. 2008;66(1):20–26. doi: 10.1111/j.1365-2125.2008.03153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey OJ, et al. Evaluation of the effect of a large volume spacer on the systemic bioactivity of fluticasone propionate metered-dose inhaler. Chest. 1999;116(4):935–940. doi: 10.1378/chest.116.4.935. [DOI] [PubMed] [Google Scholar]
- 3.Nair A, et al. In vivo comparison of the relative systemic bioavailability of fluticasone propionate from three anti-static spacers and a metered dose inhaler. Br J Clin Pharmacol. 2009;67(2):191–8. doi: 10.1111/j.1365-2125.2008.03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH, National Asthma Education and Prevention Program. Expert Panel Report III: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; National Heart, Lung, and Blood Institute; 2007. NIH Publication. No. 07-4051. [Google Scholar]
- 5.Xu J, et al. Population Pharmacokinetics and Pharmacodynamics of Inhaled Ciclesonide and Fluticasone Propionate in Patients With Persistent Asthma. J Clin Pharmacol. doi: 10.1177/0091270009354994. [DOI] [PubMed] [Google Scholar]
- 6.Krishnaswami S, et al. A sensitive LC-MS/MS method for the quantification of fluticasone propionate in human plasma. J Pharm Biomed Anal. 2000;22(1):123–9. doi: 10.1016/s0731-7085(99)00246-0. [DOI] [PubMed] [Google Scholar]
- 7.Steel RGD, Torrie JH. Principles and Procedures of Statistics. New York: McGraw-Hill; 1960. [Google Scholar]
- 8.http://us.gsk.com/products/assets/us_flovent_hfa.pdf.
- 9.Meibohm B, et al. A pharmacokinetic/pharmacodynamic approach to predict the cumulative cortisol suppression of inhaled corticosteroids. J Pharmacokinet Biopharm. 1999;27(2):127–47. doi: 10.1023/a:1020670421957. [DOI] [PubMed] [Google Scholar]
- 10.Krishnaswami S, Hochhaus G, Derendorf H. An interactive algorithm for the assessment of cumulative cortisol suppression during inhaled corticosteroid therapy. AAPS PharmSci. 2000;2(3):E22. doi: 10.1208/ps020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eid N, et al. Decreased morning serum cortisol levels in children with asthma treated with inhaled fluticasone propionate. Pediatrics. 2002;109(2):217–21. doi: 10.1542/peds.109.2.217. [DOI] [PubMed] [Google Scholar]
- 12.Lipworth BJ, Clark DJ, McFarlane LC. Adrenocortical activity with repeated twice daily dosing of fluticasone propionate and budesonide given via a large volume spacer to asthmatic school children. Thorax. 1997;52(8):686–9. doi: 10.1136/thx.52.8.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson HS, et al. A comparison of methods for assessing hypothalamic-pituitary-adrenal (HPA) axis activity in asthma patients treated with inhaled corticosteroids. J Clin Pharmacol. 2002;42(3):319–26. doi: 10.1177/00912700222011355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.