Abstract
The clinical syndrome of heart failure is characterized by a systemic inflammatory response that contributes to end organ damage in the heart and circulation and can thus lead to worsening heart failure. The ensemble of inflammatory mediators that have been detected in heart failure patients include pro-inflammatory cytokines and their cognate receptors, as well as molecules secreted/released by macrophages (galectin-3 and pentraxin-3). Inflammatory biomarkers correlate with disease severity and prognosis across the broad spectrum of heart failure syndromes. Given the proliferation of new biomarkers that predict disease severity and prognosis in heart failure, it is reasonable to ask whether there is a current role for measuring inflammatory mediators in heart failure. This review will attempt to address this question, as well as review several novel approaches that have utilized inflammatory biomarkers to enhance risk stratification and prognosis in heart failure patients.
Keywords: Inflammation, Heart failure, Biomarkers, Pro-inflammatory cytokines, Cytokine receptors, Tumor necrosis factor, Interleukins, C-reactive protein (CRP), sST2, Galectin-3, Pentraxin-3
Introduction
Heart failure is as progressive disorder that is initiated after an index event that either damages the heart muscle, with a resultant loss of functioning cardiac myocytes, or alternatively disrupts the ability of the myocardium to contract and/or relax normally. Although there have been repeated attempts to define a unique pathophysiological mechanism that explains the progressive nature of heart failure, no single conceptual paradigm has withstood the test of time. This statement notwithstanding, research from a multiple laboratories has shown consistently that heart failure is accompanied by an inflammatory response, which is believed to occur as a response to the tissue injury that occurs in the failing heart. Since the original observation by Levine et al. [17], who reported that heart failure patients had elevated circulating levels of tumor necrosis factor (TNF), countless studies have confirmed and expanded upon this observation by demonstrating that ensembles of pro-inflammatory cytokines, pro-inflammatory cytokine receptors, cell adhesion molecules, and chemokines are elevated in patients with heart failure with a decreased ejection fraction (HFrEF). Moreover, elevated levels of inflammatory mediators have been identified in heart failure patients with a preserved ejection fraction (HFpEF), as well as in the setting of acute decompensated heart failure (ADHF). The observation that elevated levels of inflammatory mediators can be detected across the spectrum of heart failure syndromes raises important questions about whether elevated levels of these proteins can be used as clinical biomarkers that can be used to provide diagnostic, prognostic, and/or therapeutic information in heart failure patients. The following review will attempt to provide a current overview of what is known about inflammatory biomarkers in heart failure, as well as discuss several novel analytic approaches in which the serial measurement of inflammatory biomarkers has been used to increase the diagnostic accuracy of traditional heart failure risk models.
Overview of Inflammation in the Heart
Over the past two decades, research from multiple laboratories has shown that the heart possesses an intrinsic or “innate” stress response system that is activated in response to tissue injury. This intrinsic stress response system is mediated, at least in part, by a family of pattern recognition receptors (PRRs), most notably the Toll-like receptors (TLRs), which are responsible for coordinating the initial response to tissue injury. TLRs serve as PRRs that recognize the molecular patterns of endogenous host material that is released during cellular injury or death, the so-called damage-associated molecular patterns (DAMPs) [7, 10]. DAMPs can be derived from dying or injured cells, damaged extracellular matrix proteins, or circulating oxidized proteins. Pro-inflammatory cytokines serve as the downstream “effectors” of the innate immune system and are responsible for activating the cellular and molecular mechanisms that facilitate tissue repair in the heart [20, 22]. Although the innate immune system provides a short-term adaptive response to tissue injury, the beneficial effects of this phylogenetically ancient system are lost if innate immune signaling becomes sustained and/or excessive, in which case the salutary effects of the activation of these pathways is contravened by the known deleterious effects of inflammatory signaling.
The initial interest in understanding the role of inflammatory mediators in heart failure arose from the observation that many aspects of the syndrome of heart failure could be mimicked by the known biological effects of pro-inflammatory cytokines. Although the disappointing results with targeted anti-cytokine therapies in heart failure dampened the initial enthusiasm for studying the role of inflammation in heart failure [21], continued progress in the field has led to a deeper understanding of the role of inflammation in the failing heart, as well as the identification of new inflammatory biomarkers such as soluble ST2 (sST2), galectin-3, and pentraxin-3, which have provided exciting new information with respect to the diagnosis and prognosis of heart failure patients. In the sections that follow, we will review the families of inflammatory mediators that have been identified across the spectrum of clinical heart failure syndromes, with the intent of placing these observations in a more global perspective regarding the utility of measuring inflammatory mediators in heart failure.
Which Inflammatory Mediators are Best Suited for Use as Biomarkers?
Given the critical importance of innate immune responses to the host, it is not surprising that nature has developed a myriad of pro-inflammatory and anti-inflammatory molecules that are responsible for initiating and subsequently terminating inflammatory responses, respectively. Unfortunately, there is no consensus in the literature with regard to which inflammatory mediators are the most biologically relevant biomarkers to follow in different disease states. The families of molecules that have been evaluated in the setting of heart failure thus far include pro-inflammatory cytokines, anti-inflammatory cytokines, soluble pro-inflammatory cytokine receptors, cell adhesion molecules, chemokines, chemokine receptors, as well as molecules that are secreted/released by macrophages and/or granulocytes. In order to better focus this review, we will limit the present discussion to the literature on pro-inflammatory cytokines and their receptors, as well as molecules that are released by macrophages. For recent reviews on chemokines in heart failure, the reader is referred to recent publications [2, 9].
The pro-inflammatory cytokines that are elaborated in heart failure include members of the TNF superfamily (TNFSF), members of the interleukin (IL)-1 family (IL1-F), and IL-6 (see Table 1) [2]. Pro-inflammatory cytokines are expressed by all nucleated cell types residing in the myocardium, including the cardiac myocyte [36]. And indeed, there is evidence that the failing heart releases pro-inflammatory “cardiokines” (e.g., TNF) into the circulation [5, 37]. However, the relative contribution of cardiac myocytes, non-myocytes, and inflammatory cells to total cytokine production in the heart is not known.
Table 1.
Inflammatory biomarkers in heart failure
| HFrEF | HFpEF | ADHF |
|---|---|---|
| Pro-inflammatory cytokines | ||
| TNF (TNFSF2), TWEAK (TNFSF12), FasL (TNFSF6), LIGHT (TNFSF12), IL-1β (IL-1F2), IL-2,IL-6, IL-18 (IL-1F8), IL-33 (IL-1F11) |
TNF (TNFSF2), IL-6 (?) | TNF (TNFSF2), IL-6, IL-18 |
| Cytokine receptors | ||
| sTNFR1 (TNFRSF1A), sTNFR2 (TNFRSF1B), gp130 (IL6ST); IL-1ra (IL1F3), sST2 (IL-1RL1) |
sST2 (IL-1RL1) | sST2 (IL-1RL1) |
| Macrophage | ||
| Galectin-3, pentraxin-3 | Galectin-3, pentraxin-3 | Galectin-3, pentraxin-3 |
The parenthesis denotes the nomenclature for the TNF and IL-1 superfamily of cytokines and cytokine receptors
FasL Fas ligand, LIGHT homologous to lymphotoxins, inducible expression, competes with HSV glycoprotein D for HVEM, a receptor expressed on T lymphocytes, gp130 soluble gp130, IL-1β interleukin-1β, IL-2 interleukin-2, IL-6 interleukin-6, IL-18 interleukin-18, IL-33 interleukin 33, IL1-F interleukin-1 family, IL-1RL1 interleukin-1 receptor-like-1, sST2 soluble ST2 receptor, TNF tumor necrosis factor, sTNFR1 soluble TNF type 1 receptor, sTNFR2 soluble TNF receptor type 2, TNFSF tumor necrosis factor superfamily, TNFSFR tumor necrosis factor superfamily receptor, TWEAK TNF-like weak inducer of apoptosis, ? conflicting data
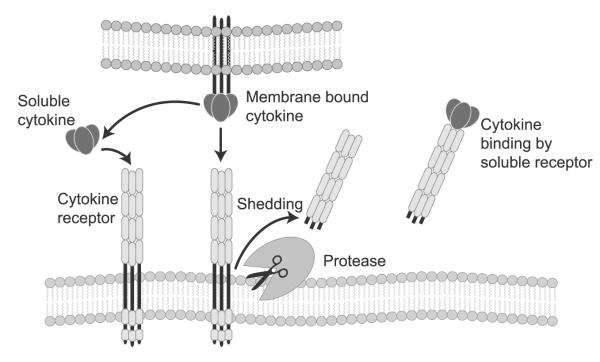
Cytokines exert their biological effects by binding to cognate cytokine receptors on cell membranes (Fig. 1). One interesting aspect of the biology of cytokine receptors is that they can be proteolytically cleaved from cell membranes (referred to as “shedding”), thereby releasing the soluble extracellular ectodomain of the cytokine receptor into the extracellular space and/or circulation (Fig. 1). Receptor shedding occurs in response to exposure to inflammatory cytokines, mechanical stretch, and/or lipopolysaccharide. Soluble cytokine receptors can also be generated through alternative splicing of mRNA transcripts, which deletes the transmembrane domain of membrane-associated receptors, or by distinct genes that encode secreted cytokine-binding proteins that can function as decoy proteins [18]. Cleaved and/or secreted “soluble” cytokine receptors are quite stable in the circulation and retain their ability to bind ligand and to inhibit the biological activities of cytokines by preventing cytokines from binding to cognate receptors on target cells (Fig. 1). Because of their larger molecular weight, shed/secreted cytokine receptors remain in the circulation longer than cytokines, which have a much lower molecular weight and a larger volume of distribution and, hence, are cleared from the circulation more rapidly. Accordingly, based on first principles, one would predict that circulating levels of pro-inflammatory cytokine receptors would integrate past and present inflammatory responses more precisely than pro-inflammatory cytokines would and, hence, would be more accurate biomarkers of inflammation.
Fig. 1.
The biology of cytokines and cytokine receptors. Pro-inflammatory cytokines are released by nucleated cell types residing in the heart, including cardiac myocytes. Cytokines exert their biological effects by binding to cognate cytokine receptors on cell membranes (left side of the diagram). Cytokine receptors can be proteolytically cleaved or “shed” from cell membranes, which releases the soluble extracellular ectodomain of the cytokine receptor into the extracellular space and/or circulation (middle of the diagram). Cytokine receptors retain their ability to bind cytokine and to inhibit the biological activities of cytokines by preventing cytokines from binding to cognate receptors on target cells (right side of the diagram)
In addition to cytokines and cytokine receptors, elevated circulating levels of a number of inflammatory mediators that were originally identified in immune cells, most notably macrophages, have also been observed in patients with heart failure. The inflammatory mediators in this group that have garnered the most attention include galectin-3 and pentraxin-3. Galectin-3, a member of the lectin family, is released by macrophages in response to tissue injury, as well as by damaged and/or dying cells. Galectin-3 has a number of important biological roles, including fibroblast activation leading to the formation of tissue fibrosis. Pentraxin-3, a novel inflammatory marker and member of the pentraxin superfamily of cytokines, has also recently been identified in patients with heart failure. Although the role of pentraxin-3 in heart failure is not known, this molecule is known to bind to C1q and initiate the classical pathway of complement activation and to facilitate pathogen recognition by macrophages. For want of a better description, in the present review, we will refer to pentraxin-3 and galectin-3 as macrophage biomarkers, recognizing that the precise source of these molecules in heart failure is not known and that multiple cell types in the heart are capable of producing/releasing these molecules. By way of review, C-reactive protein (CRP) is also a member of the pentraxin family that participates in the systemic response to inflammation [11]. Although CRP was thought to be exclusively produced in the liver, more recent studies have shown that CRP is produced by nonhepatic cells as well
The section that follows will review the literature which suggests that pro-inflammatory cytokines and pro-inflammatory cytokine receptors, as well as inflammatory mediators linked to immune activation can predict disease severity, the transition to symptomatic heart failure, as well as prognosis across the spectrum of heart failure syndromes, including patients with HFrEF, HFpEF, and ADHF. For more in depth coverage of these and other inflammatory mediators in heart failure, the interested reader is referred to several more extensive reviews [2, 9].
Inflammatory Mediators are Increased and Correlated with Disease Severity, Prognosis, and the Development of Symptomatic Heart Failure in Patients with a Depressed Ejection Fraction
Disease Severity
Circulating levels of TNF and members of the TNF superfamily (TNFSF) [38], IL-1β, IL-6, IL-18, and IL-33 are elevated in heart failure patients (see Table 1; reviewed in [2]). Pro-inflammatory cytokines are activated in asymptomatic left ventricular (LV) dysfunction [40] and continue to rise in direct relation to worsening New York Heart Association (NYHA) functional class regardless of the etiology of heart failure [35]. In addition to the pro-inflammatory cytokines, circulating levels of pro-inflammatory cytokine receptors are elevated in heart failure. These include the soluble type 1 and type 2 TNF receptors (sTNFR1 and sTNFR2, respectively) and soluble transmembrane glycoprotein 130 (gp130; one of the receptors for IL-6), which are increased in relation to worsening heart failure functional class [2]. Analogous to the findings with soluble TNF receptor levels, IL-1 receptor antagonist levels are also elevated in heart failure. Further, as discussed by Januzzi in this issue, there is growing interest in ST2, which is the receptor for IL-33, and is a member of the IL-1 superfamily (IL1-F) of cytokines. Of note, sST2 is secreted by cultured myocytes that are subjected to mechanical strain and is thus a marker of mechanical strain and inflammation [43]. In addition to pro-inflammatory cytokines and cytokine receptors, molecules that are released by macrophages/monocytes such as galectin-3 and pentraxin-3 also correlate with disease severity and predict worse outcomes in heart failure [34, 39].
Transition to Symptomatic Heart Failure
Elevated levels of pro-inflammatory cytokines predicted the development of symptomatic heart failure in previously asymptomatic older subjects in an analysis of Framingham data [40]. In this study, baseline levels of IL-6, as well as spontaneous production of TNF by peripheral blood mononuclear cells (PBMCs), predicted the development of heart failure over a 5-year period. After adjustment for traditional risk factors, the risk of developing heart failure increased 1.6-fold to 1.7-fold per tertile increment in PBMC TNF and IL-6 levels, respectively, whereas patients with a CRP≥5 mg/dl had a 2.8-fold increased risk of developing heart failure. Of note, subjects with elevated levels of all three inflammatory mediators had a 4.1-fold risk for developing heart failure. Unfortunately, baseline levels of LV function were not measured, so it is possible that this study may have identified patients with subclinical LV dysfunction. It is noteworthy in this regard that very similar findings with respect to the circulating levels of TNF, IL-6, and CRP and the development of incident heart failure were observed in older subjects enrolled in the Health, Aging, and Body Composition study [13].
Prognosis
In addition to correlating with disease severity, elevated circulating levels of pro-inflammatory cytokines also correlate with increased mortality in heart failure patients. Circulating levels of TNF, IL-6, sTNFR1, and sTNFR2 have been reported to predict poorer survival [4, 29]. As shown in Fig. 2a, there was a decline in survival as a function of increasing TNF levels in patients with moderate to severe heart failure in the Vesnarinone Trial (VEST), with the worst survival observed in patients with TNF levels >75th percentile [4]. Similar findings were observed with circulating levels of IL-6 (Fig. 2b) and levels of sTNFR1 and sTNFR2 (Fig. 2c, d). When each cytokine and/or cytokine receptor was separately entered into a multivariate Cox proportional hazards model that included age, sex, etiology of heart failure, NYHA class, ejection fraction, and serum sodium, TNF, IL-6, sTNFR1, and sTNFR2 remained significant independent predictors of mortality, along with NYHA class and ejection fraction. However, when all the cytokines and receptors were entered into the model together, only sTNFR2 remained a significant predictor of mortality [4]. In a smaller study of 152 heart failure patients, Rauchhaus et al. reported that sTNFR1 was the strongest and most accurate prognosticator [29]. More recent studies have shown that elevated levels of sST2 correlate with heart failure outcomes (see also the article by Januzzi in this issue). Weinberg and colleagues demonstrated that increased sST2 levels predicted increased mortality or cardiac transplantation, independent of circulating levels of BNP or ProANP [43]. Similar findings were reported by Ky et al., who demonstrated that elevated levels of sST2 predicted adverse outcomes in patients with chronic heart failure [15]. Elevated levels (fifth quintile) of soluble gp130 were associated with all-cause mortality, cardiovascular mortality, and death from worsening heart failure in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) trial, whereas elevated levels of IL-6 were not associated with adverse outcomes [1].
Fig. 2.
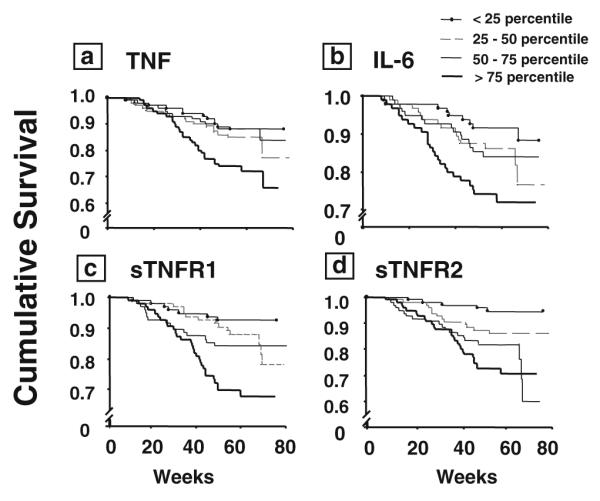
Kaplan–Meier survival analysis. The circulating levels of TNF (a), IL-6 (b), sTNFR1 (c), and sTNFR2 (d) were examined in relation to patient survival during follow-up (mean duration, 55 weeks; maximum duration, 78 weeks). For this analysis, the circulating levels of cytokines and cytokine receptors were arbitrarily divided into quartiles. Reproduced with permission from Deswal et al. [4]
In addition to pro-inflammatory cytokines and pro-inflammatory cytokine receptors, inflammatory molecules that are secreted by macrophages have been shown to predict untoward outcomes in HFrEF patients. In a substudy of the CORONA trial, elevated levels of galectin-3 were significantly associated with the primary end point of cardiovascular death, nonfatal myocardial infarction, or stroke, as well as all-cause and cardiovascular mortality. However, when NT-proBNP was added to the statistical model, the association of galectin-3 with the aforementioned clinical end points was no longer significant [8]. In contrast, elevated baseline levels of pentraxin-3 were associated with a higher risk of all-cause mortality, cardiovascular mortality, or hospitalization for worsening heart failure in the GISSI (Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico) Heart Failure and CORONA trials, even after adjusting for hsCRP or NT-proBNP levels [16].
Elevated Levels of Inflammatory Mediators Correlate with Disease Severity and Prognosis in Patients with Heart Failure with a Preserved Ejection Fraction
Disease Severity
Although most studies have focused on the role of inflammatory biomarkers in the setting of HFrEF, there is an emerging body of literature which suggests that elevated levels of inflammatory biomarkers are associated with increased risk of mortality in patients with HFpEF [6, 23]. Thus far, the extant literature suggests that TNF, sST2, galectin-3, and pentraxin-3 are elevated across the spectrum of heart failure syndromes, regardless of ejection fraction [24, 39]. Moreover, pentraxin-3 has been show to be produced within the heart of patients with HFrEF, as measured by differences in pentraxin-3 levels in the aorta and coronary sinus [24]. However, the data with respect to IL-6 and CRP in HFpEF patients are conflicting [24–26]. Whereas some studies found these markers to be elevated to a similar extent in all patients with heart failure, regardless of LV ejection fraction, other studies have reported lower levels in patients with HFpEF. These discrepant findings may be due, at least in part, to small numbers of patients with differing severities of illness.
Transition to Symptomatic Heart Failure
At the time of this writing, there are scant data to suggest that elevated levels of inflammatory mediators predict the transition to symptomatic heart failure in patients with HFpEF. Low-grade inflammation as evidenced by elevated urinary levels of TNF has been shown to identify a hypertensive population at higher risk of developing LV hypertrophy, which is a known risk factor for the development of symptomatic HFpEF [27]. Moreover, given that inflammatory mediators are elevated in patients with hypertension, as well as in older subjects, it is likely that elevated levels of inflammatory mediators will predict the transition to symptomatic heart failure in HFpEF patients [19].
Prognosis
Surprisingly, there are relatively few studies with respect to the prognostic importance of elevated pro-inflammatory cytokine levels in HFpEF patients. Thus far, only TNF levels have been shown to correlate with increased mortality in HFpEF [6, 23].
Elevated Levels of Inflammatory Mediators Correlate with Disease Severity and Prognosis in Acute Decompensated Heart Failure
Prognosis
Elevated levels of CRP, ST-2, galectin-3, and IL-6 have all been correlated with increased rates of mortality in patients diagnosed with ADHF [3, 12, 26, 28, 30, 32, 39, 41]. Of note, the majority of these studies compared the utility of inflammatory biomarkers to natriuretic peptides with respect to the predictive accuracy for diagnosing heart failure in dyspneic patients. The aggregate data suggest that, when compared to natriuretic peptides, inflammatory biomarkers are less accurate in terms of diagnosing heart failure in patients who present with acute dyspnea [12, 31]. The decreased diagnostic accuracy of inflammatory mediators in this setting is not at all surprising given the wide range of inflammatory diseases that present with dyspnea (e.g., asthma, chronic obstructive lung disease, and pneumonia). Importantly, all of the aforementioned inflammatory biomarkers independently predicted increased mortality in the setting of ADHF, even after adjusting for BNP levels. Interestingly, in pooled data sets when sST2 levels were included in the statistical model, NT-proBNP was not a predictor of death in patients with HFrEF [23]. Viewed together, the previously discussed findings have led to the notion that risk stratification may be best accomplished using models that incorporate multiple biomarkers, including both inflammatory cytokines and natriuretic peptides, as will be discussed in the next section.
Positioning of Inflammatory Biomarkers in the Heart Failure Landscape
Given the striking rise in the number of biomarkers currently available or in development, as well as the understanding that high-throughput proteomic approaches will likely contribute to proliferation of new biomarkers, it is reasonable to question whether measuring inflammatory biomarkers is of added value in heart failure patients. Since different biomarkers reflect different aspects of the many aspects of heart failure and since ongoing tissue injury contributes to the inexorable progression of heart failure, it is likely that inflammatory biomarkers will have the most immediate clinical utility in heart failure when they are incorporated into multimarker biomarker panels, rather than being used as a single biomarker. And indeed, incorporation of sST2 into a stepwise risk model with NT-proBNP resulted in improved risk stratification in the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. Remarkably, elevated levels of CRP were not meaningful prognostically when sST2 was added to the model, which may reflect the fact that CRP is an indirect marker of inflammation in heart failure, whereas sST2 integrates both inflammation and mechanical stress. Similar findings were observed with respect to galectin-3 in the PRIDE study, wherein galectin-3 was a better univariate predictor of 60-day mortality than NT-proBNP and was the best predictor of 60-day mortality in a multivariable model that included NT-proBNP and apelin [39]. A multimarker strategy that included sST2, troponin I, and growth differentiation factor-14 predicted increased risk of heart failure when added to traditional clinical factors in a study from the Framingham cohort [42]. Similarly, a multimarker panel that included sST2, along with biomarkers that detected myocyte injury (TnI), neurohormonal activation (BNP), vascular growth and remodeling (sFlt-134), inflammation (CRP), oxidative stress (uric acid, MPO), and renal dysfunction (creatinine), resulted in increased ability to predict 1 year risk that was superior to the Seattle Heart Failure Model [14]. Furthermore, adding the multimarker score to the Seattle Heart Failure Model risk score reclassified ~24% of the patients to a higher risk category [14]. Whether a more parsimonious multimarker strategy would have yielded similar results is unclear but is not likely, insofar as many of the biomarkers correlated with one another and likely provided similar and/or overlapping prognostic information.
More recently, serial measurement of inflammatory biomarkers has been shown to improve the predictive accuracy of 1 year mortality in heart failure patients, above and beyond what can be obtained through multimarker strategies performed at a single time point. As noted previously, inclusion of a multimarker approach to a clinical heart failure model resulted in a modest but significant improvement in the C statistic from 0.76 to 0.80 (P<0.001). In a recent analysis of the VESTcytokine database, a multivariate logistic regression model of mortality that employed baseline and serial measurements of cytokine and cytokine receptors levels predicted 1 year mortality that was significantly better than a logistic regression model of mortality without the serial measurements of cytokines and cytokine receptors (C statistic 0.81 versus 0.73, respectively; P=0.001) (Fig. 3). Thus, significant gains in predictive accuracy of 1 year mortality in chronic heart failure can be obtained by using serial measurements of inflammatory biomarkers rather than baseline values alone. Changes observed with serial measurements are more likely to lead to improved prognostic capability because they reflect both ongoing changes in the underlying disease process, as well as the individual response (i.e., responder or nonresponder) of a patient to a given form of therapy. Moreover, when the data from the VEST study were analyzed using a well-established method of statistical machine learning termed “ensemble modeling,” ensemble models performed significantly better when compared to standard logistic regression models that employed time series data alone (C statistic=0.84 versus 0.81, respectively; P=0.04). One potential reason for the significant increase in predictive accuracy with ensemble modeling is that this statistical method may better adjust for the biological variability inherent in clinical studies. The results of this study support the point of view that clinical models that predict mortality can be improved significantly by moving away from obtaining a large series of measurements at a single point in time and focusing instead on a smaller set of relevant measurements that are measured serially. Given that inflammatory biomarkers are surrogate markers for ongoing tissue injury, it is likely that these biomarkers will continue to contribute to prognostic assessments obtained using time series measurements.
Fig. 3.
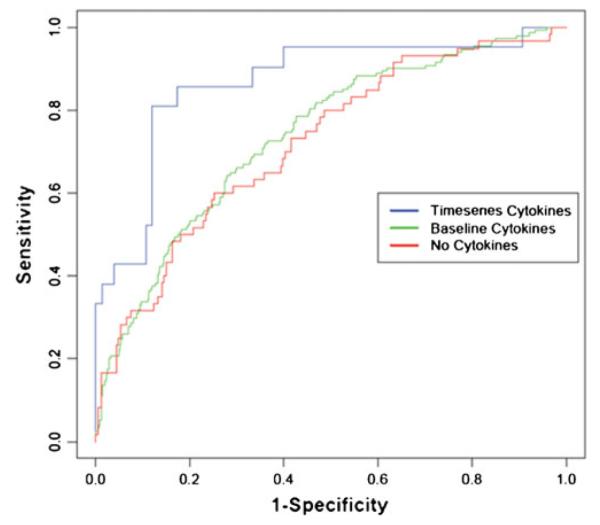
Serial measurements of pro-inflammatory cytokines and pro-inflammatory cytokine receptors in the VEST trial. Receiver operating curves are for three logistic regression models for 1 year mortality in the VEST trial. The three models use standard clinical variables only (no cytokines, C statistic of 0.73), standard variables and baseline cytokines only (C statistic of 0.74), and standard variables and serial cytokine measurements at baseline and 8, 16, and 24 weeks (C statistic of 0.81). Reproduced with permission from Subramanian et al. [33]
Conclusions
In the foregoing review, we have discussed the utility of measuring inflammatory biomarkers in the setting of HFrEF, HFpEF, and ADHF. As noted, the extant literature suggests that inflammatory biomarkers provide important diagnostic and prognostic information across the entire spectrum of heart failure syndromes. What is less clear at the time of this writing is whether these biomarkers are directly involved in the pathogenesis and/or progression of heart failure or whether the elevated levels of these inflammatory biomarkers reflect an intrinsic adaptive response to ongoing/incessant tissue damage in the failing heart. This distinction is important, insofar as the former possibility suggests that elevated levels of inflammatory biomarker may represent potential therapeutic targets, whereas the latter possibility suggests that not all inflammatory biomarkers that are elevated in heart failure are necessarily harmful and may, therefore, not represent appropriate therapeutic targets. With that said, the totality of the data reviewed herein suggests that the measurement of inflammatory biomarkers continues to provide important information with respect to prognosis and risk stratification across the entire spectrum of heart failure syndromes. Moreover, given that inflammatory mediators are surrogate markers for ongoing tissue injury and/or mechanical strain, it is likely that measurements of inflammatory biomarkers will continue to contribute to our understanding of the pathogenesis of heart failure now and for the foreseeable future.
References
- 1.Askevold ET, Nymo S, Ueland T, Gravning J, Wergeland R, Kjekshus J, et al. Soluble glycoprotein 130 predicts fatal outcomes in chronic heart failure: Analysis from the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) Circulation Heart Failure. 2013;6:91–98. doi: 10.1161/CIRCHEARTFAILURE.112.972653. [DOI] [PubMed] [Google Scholar]
- 2.Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Failure Reviews. 2010;15:331–341. doi: 10.1007/s10741-009-9140-3. [DOI] [PubMed] [Google Scholar]
- 3.Chin BS, Conway DS, Chung NA, Blann AD, Gibbs CR, Lip GY. Interleukin-6, tissue factor and von Willebrand factor in acute decompensated heart failure: Relationship to treatment and prognosis. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 2003;14:515–521. doi: 10.1097/00001721-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone Trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 5.Doroudgar S, Glembotski CC. The cardiokine story unfolds: Ischemic stress-induced protein secretion in the heart. Trends in Molecular Medicine. 2011;17:207–214. doi: 10.1016/j.molmed.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Tumor necrosis factor-alpha and mortality in heart failure: A community study. Circulation. 2008;118:625–631. doi: 10.1161/CIRCULATIONAHA.107.759191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Current Opinion in Immunology. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 8.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, et al. The predictive value of galectin-3 for mortality and cardiovascular events in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) American Heart Journal. 2012;164:878–883. doi: 10.1016/j.ahj.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: Mediators and markers. Cardiology. 2012;122:23–35. doi: 10.1159/000338166. [DOI] [PubMed] [Google Scholar]
- 10.Ionita MG, Arslan F, de Kleijn DP, Pasterkamp G. Endogenous inflammatory molecules engage Toll-like receptors in cardiovascular disease. Journal of Innate Immunity. 2010;2:307–315. doi: 10.1159/000314270. [DOI] [PubMed] [Google Scholar]
- 11.Jacobshagen C, Belardinelli L, Hasenfuss G, Maier LS. Ranolazine for the treatment of heart failure with preserved ejection fraction: Background, aims, and design of the RALI-DHF study. Clinical Cardiology. 2011;34:426–432. doi: 10.1002/clc.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Januzzi JL, Jr., Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: Results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. Journal of the American College of Cardiology. 2007;50:607–613. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, et al. Inflammatory markers and incident heart failure risk in older adults: The Health ABC (Health, Aging, and Body Composition) study. Journal of the American College of Cardiology. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ky B, French B, Levy WC, Sweitzer NK, Fang JC, Wu AH, et al. Multiple biomarkers for risk prediction in chronic heart failure. Circulation. Heart Failure. 2012;5:183–190. doi: 10.1161/CIRCHEARTFAILURE.111.965020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circulation. Heart Failure. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latini R, Gullestad L, Masson S, Nymo SH, Ueland T, Cuccovillo I, et al. Pentraxin-3 in chronic heart failure: The CORONA and GISSI-HF trials. European Journal of Heart Failure. 2012;14:992–999. doi: 10.1093/eurjhf/hfs092. [DOI] [PubMed] [Google Scholar]
- 17.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. The New England Journal of Medicine. 1990;223:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 18.Levine SJ. Molecular mechanisms of soluble cytokine receptor generation. The Journal of Biological Chemistry. 2008;283:14177–14181. doi: 10.1074/jbc.R700052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luft FC. Angiotensin, inflammation, hypertension, and cardiovascular disease. Current Hypertension Reports. 2001;3:61–67. doi: 10.1007/s11906-001-0082-y. [DOI] [PubMed] [Google Scholar]
- 20.Mann DL. Stress activated cytokines and the heart. Cytokine & Growth Factor Reviews. 1996;7:341–354. doi: 10.1016/s1359-6101(96)00043-3. [DOI] [PubMed] [Google Scholar]
- 21.Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circulation Research. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 22.Mann DL. The emerging role of innate immunity in the heart and vascular system: For whom the cell tolls. Circulation Research. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzano-Fernandez S, Mueller T, Pascual-Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. The American Journal of Cardiology. 2011;107:259–267. doi: 10.1016/j.amjcard.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. Journal of the American College of Cardiology. 2011;57:861–869. doi: 10.1016/j.jacc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto M, Tsujino T, Lee-Kawabata M, Naito Y, Sakoda T, Ohyanagi M, et al. Serum interleukin-6 and C-reactive protein are markedly elevated in acute decompensated heart failure patients with left ventricular systolic dysfunction. Cytokine. 2010;49:264–268. doi: 10.1016/j.cyto.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Mueller C, Laule-Kilian K, Christ A, Brunner-La Rocca HP, Perruchoud AP. Inflammation and long-term mortality in acute congestive heart failure. American Heart Journal. 2006;151:845–850. doi: 10.1016/j.ahj.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-Gonzalez JF, Mora C, Muros M, Jarque A, Herrera H, Garcia J. Association of tumor necrosis factor-alpha with early target organ damage in newly diagnosed patients with essential hypertension. Journal of Hypertension. 2008;26:2168–2175. doi: 10.1097/HJH.0b013e32830e2545. [DOI] [PubMed] [Google Scholar]
- 28.Pieske B, Maier LS, Bers DM, Hasenfuss G. Ca2+ handling and sarcoplasmic reticulum Ca2+ content in isolated failing and nonfailing human myocardium. Circulation Research. 1999;85:38–46. doi: 10.1161/01.res.85.1.38. [DOI] [PubMed] [Google Scholar]
- 29.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102:3060–3067. doi: 10.1161/01.cir.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 30.Rehman SU, Mueller T, Januzzi JL., Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. Journal of the American College of Cardiology. 2008;52:1458–1465. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Reichlin T, Socrates T, Egli P, Potocki M, Breidthardt T, Arenja N, et al. Use of myeloperoxidase for risk stratification in acute heart failure. Clinical Chemistry. 2010;56:944–951. doi: 10.1373/clinchem.2009.142257. [DOI] [PubMed] [Google Scholar]
- 32.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. European Journal of Heart Failure. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian D, Subramanian V, Deswal A, Mann D. New predictive models of heart failure mortality using time-series measurements and ensemble models. Circulation: Heart Failure. 2011;4:456–462. doi: 10.1161/CIRCHEARTFAILURE.110.958496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki S, Takeishi Y, Niizeki T, Koyama Y, Kitahara T, Sasaki T, et al. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. American Heart Journal. 2008;155:75–81. doi: 10.1016/j.ahj.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Torre-Amione G, Kapadia S, Benedict CR, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD) Journal of the American College of Cardiology. 1996;27:1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 36.Torre-Amione G, Kapadia S, Lee J, Bies RD, Lebovitz R, Mann DL. Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation. 1995;92:1487–1493. doi: 10.1161/01.cir.92.6.1487. [DOI] [PubMed] [Google Scholar]
- 37.Tsutamoto T, Wada A, Ohnishi M, Tsutsui T, Ishii C, Ohno K, et al. Transcardiac increase in tumor necrosis factor-alpha and left ventricular end-diastolic volume in patients with dilated cardiomyopathy. European Journal of Heart Failure. 2004;6:173–180. doi: 10.1016/j.ejheart.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Ueland T, Aukrust P, Damas JK, Gullestad L, Yndestad A. The tumor necrosis factor superfamily in heart failure. Future Cardiology. 2006;2:101–111. doi: 10.2217/14796678.2.1.101. [DOI] [PubMed] [Google Scholar]
- 39.van Kimmenade RR, Januzzi JL, Jr., Ellinor PT, Sharma UC, Bakker JA, Low AF, et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. Journal of the American College of Cardiology. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 40.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: The Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 41.Villacorta H, Masetto AC, Mesquita ET. C-reactive protein: An inflammatory marker with prognostic value in patients with decompensated heart failure. Arquivos Brasileiros de Cardiologia. 2007;88:585–589. doi: 10.1590/s0066-782x2007000500014. [DOI] [PubMed] [Google Scholar]
- 42.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, et al. Prognostic utility of novel biomarkers of cardiovascular stress: The Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]