Abstract
Recent work has demonstrated the importance of macroautophagy in dendritic cell (DC) maturation and innate cytokine production upon viral infection through delivery of cytoplasmic viral components to intracellular toll-like receptors. To study the functional consequences of impaired autophagosome formation during a Respiratory Syncytial Virus (RSV) infection, mice harboring significant autophagy defects due to Beclin-1 haploinsufficiency (Beclin-1+/−) were used. Upon RSV infection in vivo, lungs of Beclin-1+/− mice showed increased Th2 cytokine production, mucus secretion, and lung infiltration of eosinophils and inflammatory DCs. While isolated airway epithelial cells from Beclin-1+/− mice demonstrated little change compared to wildtype, Beclin-1+/− pulmonary and bone marrow-derived DCs (BMDCs) showed decreased expression of MHC-II and innate cytokine production upon RSV infection. Further examination indicated that Beclin-1+/− DC stimulated less IFNγ and IL-17 production by co-cultured CD4+ T cells and increased Th2 cytokine production in comparison to wild-type controls. Finally, adoptive transfer of RSV-infected Beclin-1+/− DCs into the airways of wild-type mice produced severe lung pathology and increased Th2 cytokine production upon subsequent RSV challenge compared to wild-type DC transfer controls. These results indicate a critical role of autophagy in dendritic cells during pulmonary viral infection, facilitating appropriate antiviral adaptive immune responses.
Introduction
Respiratory viral infections are associated with significant morbidity and mortality in susceptible patient populations, outcomes primarily linked to inappropriate inflammatory and immune responses that compromise lung function (1, 2). Respiratory syncytial virus (RSV) is a single-stranded RNA virus of the paramyxoviridae family, with a double-stranded RNA intermediate (3). RSV is a ubiquitous human pathogen that predominantly causes mild respiratory tract infection, yet it remains one of the leading causes of respiratory infection-related hospitalization worldwide (4–6). Vulnerable individuals such as infants, the elderly, or the immunosuppressed often develop severe symptoms such as bronchiolitis and pneumonia, characterized by mucus secretion and pulmonary infiltration of monocytes and granulocytes (3). In addition, hospitalization due to RSV in infancy is associated with an increased risk of developing allergic asthma and recurrent wheezing later in life (7, 8). The epidemiological evidence of subsequent immune alteration after RSV infection, combined with the complex nature of host and viral factors contributing to disease pathogenesis, underscore the need to understand the host response to RSV and its contribution to viral clearance, as well as to immune-mediated lung pathology.
Within the lung environment, dendritic cells (DCs) direct innate and adaptive immune responses to viral pathogens through secretion of pro-inflammatory cytokines and type I interferon (IFN), as well as through migration and antigen presentation to T cells in lung-draining lymph nodes. DC activation is accomplished through detection of viral antigens by pattern-recognition receptors (PRRs) such as PKR, RIG-I, and both MyD88-dependent and TRIF-dependent toll-like receptors (TLRs) (9). Activation of RNA-sensing intracellular TLRs such as TLR3 and TLR7 is required for robust production of type I IFN and APC function in virally infected plasmacytoid and myeloid DC (10, 11). Acquisition of viral antigens may be accomplished through phagocytosis of virally-infected cells, yet recent work conducted by ourselves and others suggests that the intracellular process of macroautophagy (autophagy) within virally-infected DCs functions as an important driver of DC maturation and pro-inflammatory cytokine production (12, 13). This process may be especially important during RSV infection, as RSV directly enters the cytoplasm via membrane fusion (14), thus requiring delivery of cytosolic viral nucleic acid to endosomal TLRs (14, 15).
Autophagy is a highly conserved process through which cytoplasmic contents are enveloped in a double-walled membrane and degraded upon fusion with lysosomes. Autophagosome formation is initiated in mammalian cells by release of ATG6/Beclin-1 from Bcl-2, enabling formation of the Beclin-1-containing VPS34-PI3K complex that is required for generation of pre-autophagosome structures (16). Beclin-1 is a frequent target of viral subversion, attesting to the importance of autophagy in clearance of intracellular pathogens from infected host cells (17). In addition, autophagy modulates several important functions within professional antigen-presenting cells (APCs) by enabling cytoplasmic antigen capture and MHC-mediated presentation to T cells (18, 19), by regulating inflammasome activity and IL-1β secretion (20, 21), and by promoting TLR-dependent DC maturation and type I IFN production through delivery of TLR ligands to endosomes (22). Furthermore, TLR ligation upregulates autophagosome formation through TRAF6-dependent ubiquitination and release of Beclin-1 from BCL-2, potentially serving as a positive regulation mechanism of TLR signaling (23). While the functions of autophagy in APCs infected in vitro have been examined, studies of autophagy in vivo have largely focused on host protection during bacterial infection, or host protection from encephalitis upon infection with neuropathogenic viruses (24–26). While viral subversion of autophagy and decreased CD4+ T cell responses to neurovirulent HSV-1 were recently elucidated (18, 24, 26), the role of autophagy in promoting DC maturation during pulmonary viral infection and the impact on CD4+ T cell responses is poorly understood.
Our laboratory recently reported that TLR-dependent innate cytokine production and maturation of RSV-infected DCs was attenuated upon blockade of autophagy, resulting in decreased production of IFNγ and IL-17a in co-cultured CD4+ T cells (13). In order to examine the importance of autophagosome formation during RSV infection in vivo, we used Beclin-1+/− mice that harbor defects in the upregulation of autophagosome formation upon stimulation (22). Altogether, these studies demonstrated the role of autophagy within DCs to facilitate priming of a robust antiviral adaptive immune response to RSV, as well as in the limitation of excessive pathology during pulmonary infection. The impact of these findings may have significant therapeutic implications for severe clinical disease and may contribute strategies for viral vaccine development.
Materials and Methods
Mice
Female C57Bl/6J, BALB/cJ, and B6.Cg-Tg(TcraTcrb)425Cbn/J (OT-II) transgenic mice were purchased at 6–7 weeks of age from The Jackson Laboratory (Bar Harbor, ME). Beclin-1+/− mice were originally obtained from Z. Yue (22), and a breeding colony was subsequently established at the University of Michigan (Ann Arbor, MI). D011.10 mice were bred in-house at the University of Michigan. All work involving animals was conducted in compliance with University of Michigan Committee on Use and Care of Animals policy.
RSV
Our laboratory uses antigenic subgroup A, Line 19 RSV, originally obtained from a sick infant at the University of Michigan Hospital System. This isolate has been shown in animal models to mimic human infection by eliciting airway mucus production upon inoculation with 1 × 105 pfu RSV (27). Mice were infected intratracheally with 1 × 105 pfu RSV.
Quantitative PCR
RNA was isolated from cell cultures and lung tissue using TRIzol, according to manufacturer’s instructions (Invitrogen). 5µg of RNA was then reverse-transcribed to determine cytokine gene expression using pre-developed TaqMan Gene Expression Assay primer/probe sets and analyzed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA). Transcription levels of muc5ac, gob5, ifnb, and RSV-G, -F, and –N proteins were assessed using custom primers as previously described (28). Gene expression was normalized using GAPDH expression as an internal control, and fold change values were calculated relative to an uninfected or wild-type control group assigned an arbitrary value of 1.
Dendritic cell culture
BMDC were cultured from whole bone marrow, obtained from WT C57Bl/6 mice, Beclin-1+/− mice, or WT littermates as indicated. Bone marrow cells were seeded into tissue culture flasks containing RPMI 1640-based complete media supplemented with 20ng/ml GM-CSF (R&D systems, Minneapolis, MN). Cells were fed on day 3 and 5, and harvested on day 7. On day 7, cells were ≥85% CD11b+ CD11c+ BMDC by flow cytometric analysis.
Pulmonary CD103+ and CD11b+ DC were obtained from lungs and bronchi of C57Bl/6 mice by enzymatic digestion, through modification of previously-published methods (29). Minced tissue was incubated in RPMI-1640 with 200µg/ml Liberase TM (Roche Applied Science, Indianapolis, IN) and 200 U/ml DNase I (Sigma-Aldrich) for 45 minutes at 37°C, drawn through an 18-gauge needle/10cc syringe, and filtered through 40µm nylon mesh. The cell suspension was enriched for CD11c+ cells using anti-mouse CD11c microbeads and magnetic column separation (Miltenyi Biotec, Auburn, CA), then stained with PE-conjugated anti-CD11b and APC-conjugated anti-CD103 antibodies (eBioscience, San Diego, CA). DC subsets were sorted using an iCyt Synergy 3200 fluorescence-activated cell sorter (iCyt, Champaign, IL).
Alveolar Epithelial Cell culture
Whole lungs of naïve mice were digested in Dispase (BD Biosiences), filtered through 25µm mesh, and depleted of immune cells through labeling with biotinylated antibodies to CD16/32 and CD45 (BD Pharmingen), followed by labeling with anti-biotin microbeads and passage through a MACS column (Miltenyi Biotec). Depleted cell suspensions were adherence-purified overnight in DMEM-based complete media, and non-adherent cells cultured for 4 days in complete media within fibronectin-coated wells, yielding ≥90% e-cadherin positive cells. Cultures were RSV-infected at 1:1 MOI.
CD4+ T cell isolation and DC-T cell co-culture
RSV-responsive CD4+ T cells were isolated from mediastinal and cervical lymph nodes of C57Bl/6 mice infected 8 days previously with 1 × 105 pfu RSV. Ovalbumin-responsive T lymphocytes were isolated from minced spleens from OT-II or D011.10 transgenic mice as indicated. Lymph nodes or minced spleens were forced through a 40µm nylon strainer, then CD4+ T cells were isolated using magnetic bead selection, using a negative selection protocol yielding >95% pure CD4+ T cells (Miltenyi Biotec, Auburn, CA). T cells were subsequently plated at 5 × 105 cells per well in 96-well cell culture plates, on top of 5 × 104 DC treated two hours previously with 1:1 MOI RSV. Experiments conducted with OT-II or D011.10 T cells were treated with 200µg/ml whole ovalbumin protein as indicated. Co-cultures were incubated for 24 hours for mRNA analysis, or 48 hours for cell supernatant cytokine analysis on the BioRad Bioplex 200 system, according to manufacturer’s protocol. Custom kits containing Ab-coated beads for mouse IL-4, IL-5, IL-13, IL-17a, and IFNγ were used to assay cytokine concentration (BioRad).
Flow Cytometry
Right lungs of control and RSV-infected mice were digested enzymatically in RPMI-1640 complete media containing 1mg/ml Collagenase A (Roche Applied Science, Indianapolis, IN) and 30µg/ml DNase I (Sigma). LDLN were forced through a 40µm nylon strainer. Cells were stained with Live/Dead Fixable Yellow (Invitrogen) followed by appropriate antibodies as indicated. Analysis was performed using FlowJo software (Treestar Inc, Ashland, OR).
Confocal Microscopy
BMDCs were cultured as described, then plated in Labtek chamber slides (Thermo Fisher Scientific). Cells were treated as indicated, then fixed in 4% paraformaldehyde for 20 minutes. Cells were blocked for one hour at room temperature in PBS containing 5% normal goat serum and 0.1% Tween-20, and stained with DyLight 550-conjugated anti-ATG5 antibodies (Novus Biologicals, Littleton, CO). Pro-long Gold anti-fade reagent plus DAPI was added (Invitrogen), and cells were imaged on a Nikon A1 Confocal Laser Microscope system under 60× oil immersion, using NIS Elements acquisition software (Nikon Instruments Inc.). Maximum intensity projection images were created from Z-stack images using ImageJ software (NIH).
Statistics
Data was analyzed and graphs generated using GraphPad Prism software. Statistical significance was assessed by one-way ANOVA, followed by Bonferroni post-test to obtain p values. Significant differences were regarded as p≤ 0.05.
Results
Beclin-1+/− mice show increased lung pathology upon RSV infection
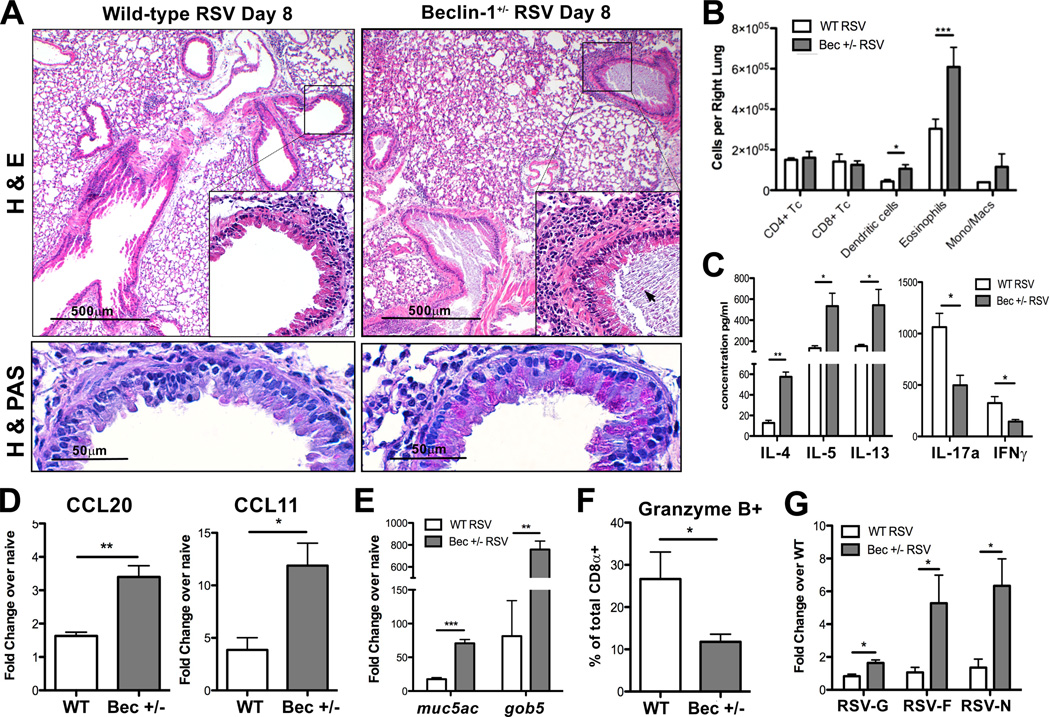
Homozygous deletion of many autophagy genes, including Beclin-1, results in early embryonic or neonatal lethality in mice (22, 30). In order to assess the importance of autophagosome formation during RSV infection in vivo, we chose to use Beclin-1+/− mice, which are viable but show defects in autophagosome formation (22). Beclin-1+/− and wild-type (WT) littermate mice were infected with RSV and sacrificed at day 8 post-infection to assess lung pathology. Histological examination of paraffin-embedded lung sections revealed increased peribronchial inflammation, goblet cell metaplasia, and occlusion of airways by mucus and cellular debris in RSV-infected Beclin-1+/− mice (Fig. 1A). Flow cytometric analysis of single-cell suspensions obtained from collagenase-digested lung tissue revealed increased numbers of eosinophils and DCs within the lungs of Beclin-1+/− mice (Fig. 1B). Additionally, lung-draining lymph node (LDLN) cultures prepared from RSV-infected Beclin-1+/− mice secreted significantly greater amounts of Th2 cytokines, and significantly less IFNγ and IL-17a production upon restimulation with RSV ex vivo when compared to LDLN from WT mice (Fig. 1C). As lung recruitment of eosinophils and DCs during RSV infection was shown to be respectively dependent on secretion of CCL11/eotaxin (31) and CCL20 (32), these chemokines were assessed by qPCR and were found to be significantly increased in lungs of RSV-infected Beclin-1+/− mice (Fig. 1D). Increased mucus secretion within the lungs was observed by periodic acid schiff (PAS) staining of lung sections, while mRNA transcript levels of mucus-associated genes muc5ac and gob5 were significantly elevated in lungs of Beclin-1+/− mice (Fig. 1A, 1E). Interestingly, overall numbers of CD8a+ T cells did not differ at day 8 post-infection (Fig. 1B), yet fewer CD8a+ T cells from Beclin-1+/− lungs expressed the cytotoxic protease Granzyme B when compared to lungs from WT mice (Fig. 1F). Finally, qPCR measurement of RSV-G, -F, and -N mRNA within lung tissue showed significantly increased viral mRNA expression in infected Beclin-1+/− mice in comparison to RSV-infected WT littermates, suggesting increased viral replication (Fig. 1G). These data suggest that Beclin-1+/− mice are impaired in their ability to mount an effective antiviral adaptive immune response upon RSV infection, instead producing increased Th2 cytokine-associated lung pathology in vivo.
Fig. 1. Beclin-1+/− mice show increased pulmonary immune infiltration and pathology upon RSV infection.
(A) Lung sections from RSV-infected Beclin-1+/− mice or WT littermate controls 8 days post-infection, stained with either haematoxylin and eosin (H&E, top panels) or haematoxylin and periodic acid schiff (H&PAS, bottom panels) stain. Arrow indicates apoptotic cell debris within airway mucus plug (B) Total numbers of lineage-positive immune cells obtained from collagenase-digested lungs of Beclin-1+/− or WT mice, 8 days post-RSV infection. (C) Lung-draining lymph nodes of Beclin+/− or WT mice, 8 days post-RSV infection, were dissociated into a single cell suspension and restimulated in culture with RSV. Cytokine concentrations in culture supernatants were assayed using a Bioplex system. Lung mRNA expression of CCL20 and CCL11/eotaxin (D) 6 days post-RSV infection, and (E) the mucus-associated genes muc5ac and gob5 8 days post-infection, was obtained using quantitative real-time PCR (qPCR) and compared to naïve controls. (F) Percentage of total lung CD8α+ T cells expressing Granzyme B by intracellular staining and flow cytometry, obtained from collagenase-digested lungs 8 days post-RSV infection. (G) Lung mRNA expression values of RSV-G, -F, and –N transcript was obtained by qPCR 8 days post-infection. Fold change was calculated relative to WT, RSV-infected lungs. Data are representative of three independent experiments, with four to six mice per group. Error bars represent SEM. *p<0.05, **p<0.01, ***p<0.001.
Beclin-1+/− pulmonary DCs show impaired maturation upon RSV infection
Autophagosome formation and maturation is known to be critical in mediating innate viral recognition and presentation of cytoplasmic viral antigens by DCs to T cells (17). In agreement, previous studies conducted by our laboratory found that blockade of autophagosome formation within DCs resulted in impaired surface expression of MHC class II and costimulatory molecules CD80/CD86 upon RSV infection in vitro (13). We therefore examined the numbers and maturation status of DCs that were present in the lungs and LDLN of RSV infected Beclin-1+/− mice and compared them to control tissues from RSV-infected WT mice. Pulmonary DC subsets in enzyme-digested lungs and LDLN were identified and quantified by flow cytometry (Supp. Fig. S1, (33)), and revealed sharply increased numbers of CD11c+ MHC-IIhigh CD11bhigh DCs (CD11b+ DCs) in lungs of infected Beclin-1+/− mice, with no significant differences detected in numbers of CD11c+ MHC-IIhigh CD11blow CD103+ intraepithelial DCs (CD103+ DCs) (Fig. 2A, 2B). Due to increased cellularity, greater numbers of both CD11b+ DCs and CD103+ DCs were recovered from the LDLN of infected Beclin-1+/− mice, although relative percentages did not reach significance (Fig. 2C, 2D). The lungs of RSV-infected Beclin-1+/− mice contained a significantly greater number of CD45+ cells (Fig 2E), while the mediastinal lymph nodes recovered from infected Beclin-1+/− mice appeared larger than those from WT littermates. This was reflected in significantly higher total numbers of lymph node cells recovered per mouse (Fig. 2F). Further examination of surface molecule expression of CD11b+ DCs in the lungs revealed that a large percentage were FcεRIα+ Ly6C+ inflammatory DCs, and were present in greater numbers in lungs of RSV-infected Beclin-1+/− mice (Fig. 2G). Finally, assessment of DCs within the LDLNs revealed decreased MHC-II expression on both CD11b+ DCs and CD103+ DCs (Fig. 2H), with no differences in expression of co-stimulatory molecules CD80, CD86, or CD40 in comparison to infected WT mice (data not shown).
Fig. 2. Beclin-1+/− mice recruit greater numbers of DCs displaying decreased MHC class II expression upon RSV infection.
Lungs and lung-draining lymph nodes (LDLNs) were harvested 8 days post-RSV infection, and tissues from Beclin-1+/− mice were compared to those from WT mice using flow cytometry. (A) Representative flow plots of CD11b+ and CD103+ DCs from collagenase-digested lungs of Beclin-1+/− or WT littermate mice. Cells were gated on CD11c+ MHC-IIhigh according to the scheme in Suppl. Fig. S1. Total numbers of CD11b+ and CD103+ DCs (B) were calculated using percentages and total cell counts within lungs. (C) Representative flow plots of CD11b+ and CD103+ DCs in single-cell preparations of LDLNs of Beclin-1+/− or WT littermate mice. Total numbers of CD11b+ and CD103+ DCs (D) were calculated using percentages and total cell counts within LDLNs. (E) Total CD45+ cell counts from lungs, (F) LDLNs, and (G) lung inflammatory CD11b+ DCs co-staining with FcεRIα+ (clone MAR-1) and Ly6C+ were calculated by flow cytometry. (H) Median fluorescence intensity (MFI) of MHC-II surface staining on CD11b+ and CD103+ DCs from LDLNs. Data are representative of at least three independent experiments, with four to six mice per group. Error bars represent SEM. *p<0.05, **p<0.01, ***p<0.001.
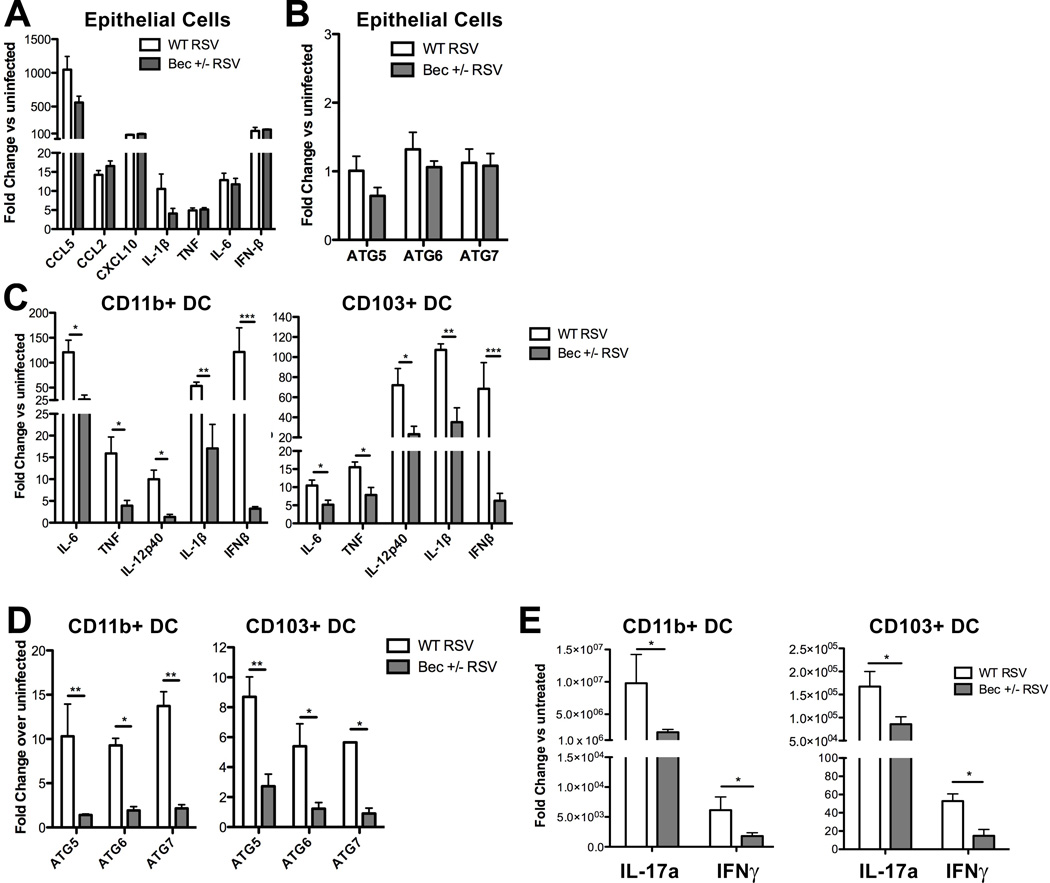
In addition to modulating DC maturation, autophagy proteins have been shown to regulate cytokine production in virally-infected non-hematopoietic cells through delivery of viral antigens to intracellular TLRs, as well as through antagonism of signaling by cytosolic pattern recognition receptors such as RIG-I (34). As RSV predominantly infects the respiratory epithelium and intraepithelial DCs (35), we investigated innate cytokine responses of both RSV-infected epithelial cells and pulmonary DCs from Beclin-1+/− mice. Pro-inflammatory cytokine production by RSV-infected Beclin-1+/− primary airway epithelial cell cultures (AECs) revealed no significant differences in comparison to WT cultures when assessed by qPCR 24 hours post-infection (Fig. 3A). Similarly, both WT and Beclin-1+/− RSV-infected AECs showed no significant upregulation of key autophagy genes 24 hours post-RSV infection (Fig. 3B). Further examination of autophagy induction through ATG5 punctate staining and confocal microscopy, as well as through accumulation of membrane-bound LC3-II by immunoblotting, revealed no significant changes in RSV-infected AECs compared to uninfected controls (data not shown).
Fig. 3. Beclin-1+/− lung epithelial cells are competent in innate cytokine responses to RSV infection, while pulmonary DCs are impaired in innate cytokine production, autophagy gene expression, and antigen presentation upon RSV infection.
(A) Cytokine production and (B) autophagy gene expression by Beclin-1+/− or wild-type mouse primary alveolar epithelial cells was assessed by qPCR 24 hours post-RSV infection. (C–E) Pulmonary DCs were fluorescently labeled and flow-sorted from collagenase-digested lungs of naïve Beclin-1+/− or wild-type mice (see Supp. Fig. S1 for gating strategy), and infected with RSV at 1 DC: 1 pfu (1:1 MOI). Innate cytokine production and autophagy gene expression by CD11b+ lung DCs or CD103+ DCs (C) was assessed at 24 hours post-RSV infection by qPCR. Autophagy gene expression by CD11b+ DCs or CD103+ DCs (D) was measured by qPCR at 24 hours post-infection. Cytokine production by purified splenic CD4+ OT-II T cells, co-cultured with CD11b+ lung DCs or CD103+ DCs (E) treated concurrently with RSV and 200µg/ml whole ovalbumin protein, was assessed by qPCR at 24 hours. Data are representative of at least two independent experiments, with at least four replicates per group. Error bars represent SEM. *p<0.05, **p<0.01.
Controlled regulation of Beclin-1 expression has been documented during T cell development and activation, although the functional significance of this observation is not known (36). To ensure that Beclin-1+/− CD4+ T cells do not possess an intrinsic cytokine production deficiency or Th2 bias upon activation, we stimulated purified splenic CD4+ T cells from naïve Beclin-1+/− or WT littermate control mice with antibodies to CD3 and CD28. Measurement of cytokine secretion in culture supernatants across a five-day time course revealed no significant differences in production of IL-5, IL-13, IL-17a, or IFNγ by Beclin-1+/− T cells (Supp. Fig S2).
In contrast, both CD11b+ and CD103+ pulmonary DCs from naïve Beclin-1+/− lungs showed reduced innate cytokine production upon ex vivo RSV infection (Fig. 3C). Pulmonary DCs from WT and Beclin-1+/− mice showed differential upregulation of autophagy genes upon infection, as RSV-infected Beclin-1+/− DCs failed to upregulate expression of ATG5, ATG6, and ATG7 in comparison to RSV-infected WT DCs (Fig. 3D). Importantly, as a measure of antigen presentation function, RSV-infected Beclin-1+/− DCs co-cultured with CD4+ OT-II T cells elicited less IFNγ and IL-17a production in comparison to WT controls (Fig. 3E). These results suggest that while Beclin-1+/− mice appear to have no innate defect in cytokine production by epithelial cells or CD4+ T cells, Beclin-1+/− pulmonary DCs are impaired in innate cytokine production and antigen presentation in response to RSV infection.
Beclin-1+/− BMDCs are deficient in innate cytokine production and fail to mature upon RSV infection
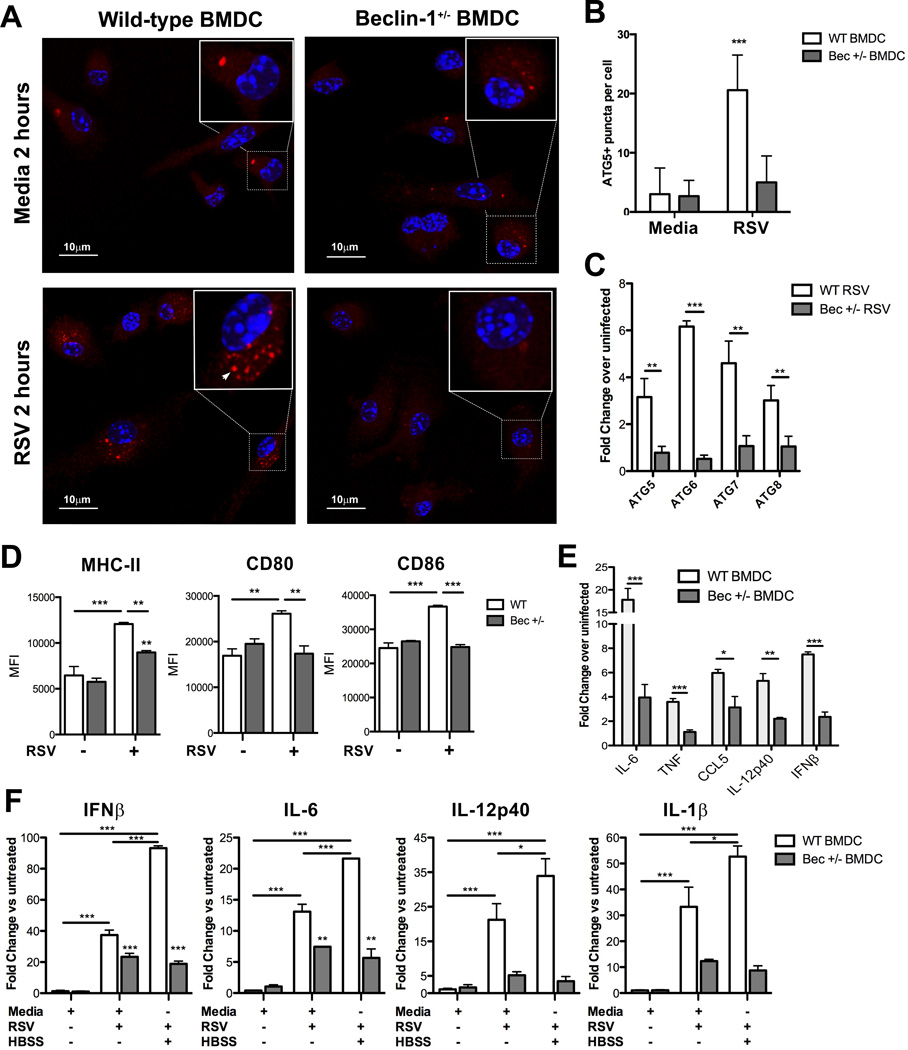
Previous work conducted by our laboratory demonstrated that TLR-dependent DC maturation and innate cytokine production in response to RSV is dependent on autophagy (13). Having obtained similar results for lung-derived Beclin-1+/− DCs infected with RSV ex vivo, we utilized bone marrow-derived DCs (BMDCs) cultured from Beclin-1+/− mice to further study the impact of Beclin-1 insufficiency on the upregulation of autophagy and on DC maturation. Examination of punctate ATG5 staining by confocal microscopy, which identifies nascent autophagosomes (16), confirmed a defect in autophagosome formation within RSV-infected Beclin-1+/− BMDCs (Fig. 4A, 4B). Beclin-1+/− BMDCs similarly failed to upregulate autophagy gene mRNA expression in response to RSV (Fig. 4C).
Fig. 4. Altered autophagosome formation, autophagy-dependent innate cytokine production, and maturation in response to RSV infection by Beclin-1+/− DCs.
(A) Autophagosome formation by Beclin-1+/− or WT bone marrow-derived DCs two hours post-RSV infection (1:1 MOI) was measured by punctate ATG5 staining and confocal microscopy, quantified in (B). (C) Autophagy gene expression by Beclin-1+/− or WT BMDCs was assessed 24 hours post-RSV infection. (D) Surface costimulatory marker expression by Beclin-1+/− or WT BMDCs was measured by flow cytometry, 24 hours post-RSV infection. (E) Innate cytokine production by Beclin-1+/− or WT BMDCs, measured by qPCR 24 hours post-RSV infection. (F) Innate cytokine production by Beclin-1+/− or WT BMDCs, incubated in media or HBSS for two hours followed by 24 hour RSV infection, was assessed by qPCR. Data are representative of at least two independent experiments, with at least four replicates per group. Error bars represent SEM. *p<0.05, **p<0.01, ***p<0.001.
Beclin-1+/− DC maturation was next examined by assessing costimulatory molecule expression and innate cytokine production upon RSV infection. Analysis of surface staining of RSV-infected BMDCs by flow cytometry revealed that while WT BMDCs upregulated surface expression of MHC-II and CD80/86 co-stimulatory molecule expression in response to RSV infection, Beclin-1+/− BMDCs only weakly upregulated MHC-II expression and did not increase expression of CD80 or CD86 above the levels of uninfected controls (Fig. 4D). In addition, qPCR of RSV-infected Beclin-1+/− BMDCs revealed significantly less type I interferon and pro-inflammatory cytokine production in comparison to WT controls (Fig. 4E). These results confirm our previous findings that support the role of autophagy in promoting DC maturation, as Beclin-1+/− DCs fail to upregulate autophagosome formation, MHC-II expression, and produce innate cytokines upon RSV infection.
We next sought to verify the modulatory role of autophagy in altered cytokine production by Beclin-1+/− DCs. In agreement with our previous findings (13), induction of autophagy in WT BMDCs through amino acid starvation prior to RSV infection synergistically increased IFNβ and IL-6 production in comparison to RSV infection alone, as well as IL-12p40 and IL-1β to a lesser extent (Fig. 4F). In contrast, Beclin-1+/− BMDCs produced significantly less of these cytokines in response to either RSV infection or starvation-induced autophagy prior to RSV infection, with no synergistic increase in cytokine production observed with starvation (Fig. 4F).
Beclin-1+/− DCs fail to stimulate antiviral cytokine production by CD4+ T cells in vitro
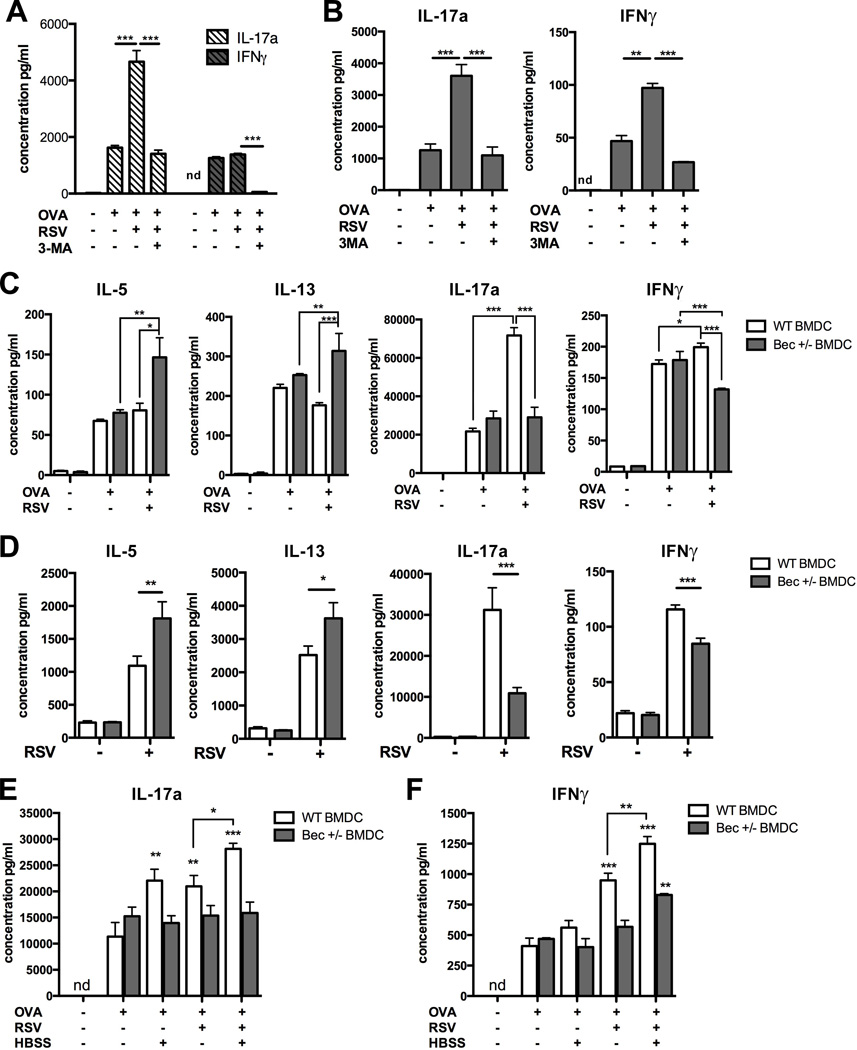
Innate cytokine production by DCs during antigen presentation is critical to the induction of Th1 adaptive immune responses, and data obtained thus far suggested that deficient autophagy-dependent maturation and pro-inflammatory cytokine production by Beclin-1+/− DCs may result in impaired antigen presentation capacity. We first verified our previously published finding that blockade of autophagy in RSV-infected BMDCs leads to attenuated IL-17a and IFNγ production by co-cultured CD4+ T cells (13). Treatment of BMDCs with the autophagy inhibitor 3-methyladenine (3-MA) prior to ovalbumin treatment and RSV infection blocked IFNγ and RSV-associated IL-17a production by ovalbumin peptide-recognizing CD4+ T cells (Fig. 5A). Similar results were obtained using CD11b+ pulmonary DCs, flow-sorted from lungs of naïve C57Bl/6 mice and co-cultured with purified CD4+ OT-II T cells (Fig. 5B).
Fig. 5. Elicitation of CD4+ T cell cytokine production by RSV-infected DCs is dependent on autophagy.
(A) WT BMDCs cultured from BALB/cJ mice were treated with saline or 5µM 3-methyladenine (3-MA), 30 minutes prior to the addition of 1:1 MOI RSV and 200µg/ml whole ovalbumin protein. Cells were co-cultured with purified splenic D011.10 CD4+ T cells, and culture supernatants tested at 48 hours by Bioplex assay. (B) CD11b+ DCs were flow-sorted from lungs of naïve C57Bl/6 mice and co-cultured with purified splenic OT-II CD4+ T cells (C) Cytokine production by CD4+ OT-II T cells, co-cultured with Beclin-1+/− or wild-type BMDCs infected with RSV and treated with 200µg/ml whole ovalbumin protein, was measured at 48 hours by Bioplex assay. (D)Cytokine production by CD4+ T cells purified from LDLNs of RSV-infected C57Bl/6 mice, 8 days post-infection. Cells were co-cultured with RSV-infected Beclin-1+/− or WT BMDCs for 48 hours, was measured in culture supernatants by Bioplex assay. (E,F) Beclin-1+/− or WT BMDCs were incubated in media or HBSS for 2 hours, placed in fresh media and treated with RSV and 200µg/ml whole ovalbumin protein as indicated. Production of IL-17a (E) and IFNγ (F) by co-cultured CD4+ OT-II T cells was measured in culture supernatants at 48 hours by Bioplex assay. Data are representative of at least two independent experiments, with at least four replicates per group. Error bars represent SEM. *p<0.05, **p<0.01, ***p<0.001.
Surprisingly, we found no baseline defect in the ability of Beclin-1+/− BMDCs treated with ovalbumin alone to elicit cytokine production from co-cultured CD4+ OT-II T cells (Fig. 5C). We also found no significant differences in the upregulation of MHC-II expression, costimulatory molecule expression, or cytokine production by Beclin-1+/− BMDCs treated with LPS, IFNβ, or ovalbumin protein, in comparison to WT controls (Supp. Fig. S3A–C). Finally, confocal microscopy revealed no evidence of increased autophagy in WT BMDCs treated with ovalbumin alone, suggesting that our ovalbumin preparation does not induce autophagy (Supp. Fig. S3D).
As observed previously with OT-II T cells co-cultured with pulmonary CD11b+ DCs, RSV-infected WT BMDCs elicited greater production of IL-17a and IFNγ from co-cultured OT-II T cells in comparison to ovalbumin treatment alone (Fig. 5C). In contrast, RSV-infected Beclin-1+/− BMDCs failed to stimulate greater production of IL-17a by co-cultured T cells, and elicited significantly less IFNγ production in comparison to both uninfected BMDCs and RSV-infected WT BMDCs (Fig. 5C). Moreover, Beclin-1+/− BMDCs infected with RSV prior to treatment with ovalbumin stimulated increased production of the Th2 cytokines IL-5 and IL-13 from co-cultured OT-II T cells, in comparison to ovalbumin treatment alone (Fig. 5C). These results were similar to those obtained through co-culture of WT or Beclin-1+/− BMDCs with CD4+ T cells purified from the LDLNs of RSV-infected C57Bl/6 mice, 8 days post-infection. In response to co-culture with RSV-infected Beclin-1+/− BMDCs, CD4+ lymph node T cells produced significantly greater quantities of Th2 cytokines and less IL-17a and IFNγ than T cells co-cultured with WT BMDCs (Fig 5D).
In order to confirm that DC elicitation of robust IL-17a and IFNγ production by co-cultured CD4+ T cells is indeed autophagy-dependent, we used starvation by incubation in HBSS as a non-viral autophagy stimulus. In agreement with our earlier finding that autophagy induction prior to RSV infection synergistically increases innate cytokine production by WT BMDCs (Fig. 4F), CD4+ OT-II T cells co-cultured with either starved or RSV-infected WT BMDCs produced greater quantities of IL-17a in comparison to co-cultures with BMDCs treated with ovalbumin alone (Fig. 5E). Furthermore, autophagy induction prior to RSV infection in WT BMDCs augmented production of both IL-17a and IFNγ by co-cultured T cells in comparison to either treatment alone (Fig. 5E, 5F). In contrast, we previously found that innate cytokine production by starved or RSV-infected Beclin-1+/− BMDCs was muted or attenuated in comparison to WT BMDCs, with no observed augmentation of cytokine production in response to starvation prior to infection (Fig. 4F). Accordingly, no increase in production of IL-17a or IFNγ by CD4+ OT-II T cells was observed upon co-culture with starved or RSV-infected Beclin-1+/− BMDCs, while only a slight increase in IFNγ production and no change in IL-17a production was observed in co-cultures with Beclin-1+/− BMDCs that were both starved and RSV-infected (Fig. 5E, 5F).
These results suggest that the autophagy-dependent production of innate cytokines by RSV-infected DCs is necessary for robust production of IL-17a and IFNγ by co-cultured CD4+ T cells, and that T cell cytokine elicitation is increased by autophagy induction prior to RSV infection of WT BMDCs. In contrast, while Beclin-1+/− BMDCs appear to be capable of normal presentation of ovalbumin protein in the absence of an autophagic stimulus, the lack of increased production of IL-17a and IFNγ by CD4+ T cells co-cultured with either RSV-infected or starved Beclin-1+/− BMDCs suggests that these results are due to the failure of Beclin-1+/− BMDCs to upregulate autophagy.
Adoptive transfer of RSV-infected Beclin-1+/− DCs into wild-type mice produces increased lung pathology upon subsequent RSV challenge
Data generated in the current study suggested that the primary defect leading to increased pathology during RSV infection in Beclin-1+/− mice was associated with altered DC activation, resulting in deficient induction of antiviral cytokine production and an altered CD4+ T cell response. In order to further address this mechanism, an adoptive transfer system was employed where WT C57Bl/6 mice were administered RSV-infected WT or Beclin-1+/− BMDCs into the trachea, followed by a live RSV challenge 28 days later (Fig. 6A). Since neither WT nor Beclin-1+/− BMDCs propagate RSV (Supp. Fig S4), the use of this model allows the examination of airway sensitization with an autophagy defect in DCs alone, and assessment of the elicited anti-viral immune response through direct RSV challenge. Analysis of immune cell infiltrates in enzymatically digested lungs showed that C57Bl/6 mice administered RSV-infected Beclin-1+/− BMDCs had increased lung infiltration of DCs and macrophages 8 days post-RSV challenge (Fig. 6B). Lung histology from mice sacrificed 8 days post-RSV challenge revealed peribronchial inflammation within the lungs of all mice airway-sensitized with RSV-infected BMDCs; however this inflammation was much more extensive in animals given RSV-infected Beclin-1+/− BMDCs (Fig. 6C). In agreement with our findings on primary infection of Beclin-1+/− mice, only RSV-infected Beclin-1+/− BMDC recipients showed mucus plugging and granulocyte infiltration into the airways after challenge (Fig. 6C). Additionally, mRNA levels of the mucus production-associated genes muc5ac and gob5 were significantly elevated in lungs of RSV-infected Beclin-1+/− DC recipient mice in comparison to controls (Fig. 6D).
Fig. 6. Adoptive transfer of RSV-infected Beclin-1+/− dendritic cells into C57Bl/6 mice produces severe lung pathology upon subsequent RSV challenge.
(A) DC adoptive transfer setup, with C57Bl/6 mice receiving media- or RSV-pulsed Beclin-1+/− or WT BMDCs i.t., followed by a live RSV challenge i.t. 28 days later. (B) Total numbers of lineage-positive immune cells obtained from collagenase-digested lungs of DC-sensitized mice, 8 days post-RSV challenge. (C) Lung sections from DC-sensitized mice 8 days post-RSV challenge, stained with either haematoxylin and eosin (H&E, top panel) or haematoxylin and periodic acid schiff (H&PAS, bottom panel) stain. (D) Lung expression of the mucus-associated genes muc5ac and gob5 8 days post-RSV challenge was obtained using quantitative real-time PCR (qPCR) in comparison to naïve controls. (E) Single-cell suspensions prepared from lung-draining lymph nodes of DC-sensitized mice, 8 days post-RSV challenge, were restimulated in culture with RSV. Cytokine concentrations in culture supernatants were assayed at 48 hours using a Bioplex system. (F) Intracellular expression of Granzyme B by CD8a+ T cells within lung digests was assessed using flow cytometry. Data are representative of three independent experiments, with four to six mice per group. Error bars represent SEM. *p<0.05, **p<0.01, ***p<0.001.
In further concurrence with our findings in RSV-infected Beclin-1+/− mice, examination of T cell cytokine production from restimulated LDLNs of RSV-infected Beclin-1+/− BMDC recipient mice revealed increased production of IL-4, IL-5 and IL-13. Similarly, restimulated LDLNs produced less IL-17a in comparison to RSV-infected WT BMDC recipients, although IFNγ production was not significantly different (Fig. 6E). Finally, intracellular staining and flow cytometry revealed decreased expression of Granzyme B in CD8+ T cells from lung digests of RSV-infected Beclin-1+/− BMDC recipients (Fig. 6F). These results provide additional evidence that the defect in the Beclin-1+/− response to RSV resides within the DC population, leading to the observed lung pathology and Th2 cytokine skewed responses in fully heterozygous mice.
Discussion
Host inflammatory responses must balance pathogen clearance with minimal damage to healthy tissue, and this is particularly true in the respiratory tract. In the current study, we provide several pieces of evidence that autophagy is critical to DC-mediated induction of an effective antiviral adaptive immune response upon infection with RSV: 1) RSV-infected Beclin-1+/− mice showed increased lung pathology characterized by increased mucus production and infiltration of eosinophils and DCs into the lungs; 2) Pulmonary DCs from RSV-infected Beclin-1+/− mice show incomplete maturation in response to RSV, resulting in significantly less IFNγ and IL-17a production by co-cultured CD4+ T cells; 3) RSV-infected Beclin-1+/− BMDCs fail to upregulate innate cytokine production upon viral and non-viral autophagy stimulus; and 4) immunization of WT mice through adoptive transfer of RSV-infected Beclin-1+/− BMDC resulted in severe Th2-associated lung pathology upon subsequent challenge with RSV. Investigation of primary airway epithelial cells indicated that there was no induction of autophagy by RSV infection alone and no alteration in cytokine responses observed in the epithelial cells from Beclin-1+/− mice. Thus, the altered responses appeared to be focused upon DC activation during RSV infection. DCs are uniquely specialized for surveillance and rapid detection of invading pathogens, as well as for initiating and directing both innate and adaptive immune responses through antigen presentation and pro-inflammatory cytokine production. Histological staining of infected human lung tissue, as well as in vitro experiments conducted with human and mouse DCs, suggest that lung-residing intraepithelial DCs are capable of being directly infected with RSV (35, 37, 38). Infection of pulmonary DCs may be particularly important to the rapid acquisition of RSV antigens, as RSV infects through pH-independent cell membrane fusion and cytoplasmic entry (14), making autophagy mechanisms critical for rapid activation and antigen processing in DCs. In agreement with recent in vivo studies implicating immunological autophagy in the clearance of intracellular bacteria (26), and the limitation of harmful inflammation while enhancing viral clearance within infected neurons (24), this study identifies a role for autophagy within pulmonary DCs in promoting virally-induced maturation, and the priming of an effective antiviral adaptive immune response during RSV infection.
Within antigen-presenting cells, autophagy modulates both pro- and anti-inflammatory events upon activation of pattern-recognition receptors (34). One mechanism whereby autophagy may promote DC maturation and innate cytokine production is through expedited delivery of cytoplasmic material to TLR-containing endosomes (12). Previous studies conducted by our laboratory support this, as autophagy induction within BMDCs prior to RSV infection synergistically increased innate cytokine production, while this effect was attenuated in BMDCs treated with autophagy inhibitors or cultured from TRIF- or MyD88-deficient mice (13). In agreement, the current findings of Th2-associated cytokine secretion, pulmonary eosinophilia, and mucus production in RSV-infected Beclin-1+/− mice are consistent with the RSV-induced phenotype observed in mice genetically deficient in TLR3 or MyD88 (39, 40). RSV-derived dsRNA and ssRNA, ligands specific to TLR3 and TLR7 respectively, are likely responsible for the induction of autophagy in infected DCs, as synthetic ligands to TLR3 and TLR7 induce autophagosome formation in murine macrophages and DCs (41, 42). Autophagy induction may therefore function as a positive feedback mechanism by increasing delivery of cytosolic viral components to endosomal TLRs, while simultaneously regulating other pro-inflammatory signals through inhibition of cytosolic pattern-recognition receptors (21, 43). Alternately, autophagy may indirectly promote TLR-dependent cytokine production by removing the non-structural RSV proteins NS1 and NS2 from the cytoplasm, as they disrupt TLR and IFN receptor signaling by decreasing intracellular expression of TRAF3 and STAT2 (44, 45). Further studies will be needed to address the mechanistic details of potential interaction of the autophagic pathway with other PRR pathways, as well as with virally-encoded proteins during in vitro and in vivo RSV infection.
In the current study, increased lung pathology in both RSV-infected Beclin-1+/− mice and RSV-infected Beclin-1+/− BMDC recipient mice was characterized by infiltration of inflammatory DCs. Similarly, RSV-infected Beclin-1+/− BMDCs failed to mature upon RSV infection in vitro, and subsequently promoted increased Th2 cytokine synthesis from co-cultured CD4+ T cells. Inflammatory DCs, which are phenotypically similar to BMDCs (46) and are derived in vivo from blood monocytes (47, 48), are recruited in large numbers to the respiratory tract in both infected humans and mice (38, 49), and have been shown to drive Th2-associated lung pathology in mice during infection with the paramyxovirus Sendai virus (50) and in a mouse model of allergic asthma (46). Previous studies also noted that addition of BMDCs to the respiratory tract at the time of RSV infection increased lung pathology (40), while blockade of inflammatory DC emigration into lungs by genetic CCR6 deficiency or CCL20 neutralization increased viral clearance and reduced lung pathology upon RSV infection (32). In agreement, RSV-infected Beclin-1+/− mice showed elevated production of CCL20 and increased lung infiltration by inflammatory DCs. Furthermore, transfer of RSV-infected Beclin-1+/− BMDCs into WT host mice induced greater Th2 cytokine production and lung pathology upon subsequent challenge with RSV, ultimately suggesting that impaired maturation in inflammatory DCs may enhance Th2-associated pathology. In contrast, we found that while Beclin-1+/− CD103+ and CD11b+ lung DCs stimulated significantly less IFNγ and IL-17a production from co-cultured OT-II T cells upon infection with RSV, both WT and Beclin-1+/− RSV-infected lung-derived DCs elicited substantially less Th2 cytokine production in comparison to RSV-infected BMDCs of either genotype. Kinetic studies of DC migration upon RSV infection suggest that inflammatory DCs as well as lung-resident CD11b+ and CD103+ DCs transport viral RNA to the LDLNs (38), suggesting that the observed phenotype in RSV-infected Beclin+/− mice is due to impaired autophagy-dependent maturation in both lung-resident and inflammatory DCs. The impaired activation and maturation of lung-resident DCs may therefore result in insufficient induction of an RSV-specific Th1 response, enabling Th2 cytokine-driven pathology in its absence. The role of autophagy in various lung DC subsets and the associated contribution to CD4+ T cell activation during RSV infection remains to be explored.
While potential non-autophagic functions of Beclin-1 in DC activation and function cannot be ruled out at the present time, this study provides supportive evidence for the critical role of autophagy in DCs in the induction of antiviral CD4+ T cell responses to RSV infection. This is supported not only by the current findings in beclin-1 mice and in vitro studies of DC function, but more importantly through adoptive transfer of RSV-infected Beclin DCs into fully wild-type mice. The recapitulation of increased Th2 cytokine production and occlusion of the airways with mucus and cellular debris in Beclin-1 BMDC recipients emphasizes that impaired autophagy within RSV-infected DCs alone is sufficient to induce much of the lung pathology observed in fully heterozygous mice. However, additional studies utilizing other models of autophagy deficiency will be necessary to further elucidate the exact mechanism by which autophagy in DCs contributes to host defense against RSV.
Genome-wide association studies recently identified autophagy gene polymorphisms associated with increased susceptibility to disease, including tuberculosis infection in human populations (51), as well as the development of Crohns disease (52). Interestingly, one recent study showed that viral infection interacting with underlying genetic susceptibility produces autoimmune illness in mice harboring the human ATG16L1 variant associated with increased Crohns disease risk (53). As severe RSV and other viral infections in infancy is associated with an increased risk of developing atopic asthma later in life (8, 54), it seems plausible that polymorphisms in autophagy genes may lead to pathological responses to respiratory viral infection early in life and the establishment of a Th2-skewed lung environment. Future studies addressing the importance of autophagy in different DC subsets may provide valuable information regarding respiratory viral infections and novel vaccination strategies.
Supplementary Material
Acknowledgements
This research was supported by NIH Grant HL114858 (NWL).
We would like to acknowledge Judith Connet for her editing expertise in assembling this manuscript.
Abbreviation used in text
- LDLN
lung-draining lymph node
References
- 1.Tan WC. Viruses in asthma exacerbations. Curr Opin Pulm Med. 2005;11:21–26. doi: 10.1097/01.mcp.0000146781.11092.0d. [DOI] [PubMed] [Google Scholar]
- 2.Wedzicha JA. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 3.Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EAF, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 6.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997–2006. Pediatr. Infect. Dis. J. 2012;31:5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 7.Openshaw PJ, Dean GS, Culley FJ. Links between respiratory syncytial virus bronchiolitis and childhood asthma: clinical and research approaches. Pediatr. Infect. Dis. J. 2003;22:S58–S64. doi: 10.1097/01.inf.0000053887.26571.eb. discussion S64–65. [DOI] [PubMed] [Google Scholar]
- 8.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit Care Med. 2005;171:137–141. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S-Y, Jouanguy E, Sancho-Shimizu V, Bernuth Hvon, Yang K, Abel L, Picard C, Puel A, Casanova J-L. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts C, West MA, Zaru R. TLR signalling regulated antigen presentation in dendritic cells. Curr. Opin Immunol. 2010;22:124–130. doi: 10.1016/j.coi.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 13.Morris S, Swanson MS, Lieberman A, Reed M, Yue Z, Lindell DM, Lukacs NW. Autophagy-mediated dendritic cell activation is essential for innate cytokine production and APC function with respiratory syncytial virus responses. J Immunol. 2011;187:3953–3961. doi: 10.4049/jimmunol.1100524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasakumar N, Ogra PL, Flanagan TD. Characteristics of fusion of respiratory syncytial virus with HEp-2 cells as measured by R18 fluorescence dequenching assay. J Virol. 1991;65:4063–4069. doi: 10.1128/jvi.65.8.4063-4069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudchodkar SB, Levine B. Viruses and autophagy. Rev. Med. Virol. 2009;19:359–378. doi: 10.1002/rmv.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English L, Chemali M, Duron J, Rondeau C, Laplante A, Gingras D, Alexander D, Leib D, Norbury C, Lippé R, Desjardins M. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid D, Pypaert M, Münz C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity. 2007;26:79–92. doi: 10.1016/j.immuni.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakahira K, Haspel JA, Rathinam VAK, Lee S-J, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AMK. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi C-S, Shenderov K, Huang N-N, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci U.S.A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi C-S, Kehrl JH. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 2008;283:33175–33182. doi: 10.1074/jbc.M804478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leib DA, Alexander DE, Cox D, Yin J, Ferguson TA. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J. Virol. 2009;83:12164–12171. doi: 10.1128/JVI.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HK, Mattei LM, Steinberg BE, Alberts P, Lee YH, Chervonsky A, Mizushima N, Grinstein S, Iwasaki A. In vivo requirement for Atg5 in antigen presentation by dendritic cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, Schmidt RE, Mizushima N, Deretic V, Sibley LD, Virgin HW. Autophagosome-Independent Essential Function for the Autophagy Protein Atg5 in Cellular Immunity to Intracellular Pathogens. Cell Host & Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, Ho SB, Peebles SR., Jr Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am. J. Pathol. 2006;169:977–986. doi: 10.2353/ajpath.2006.051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J. Infect. Dis. 2004;189:1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- 29.Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K, Cook DN. Pulmonary CD103(+) dendritic cells prime Th2 responses to inhaled allergens. Mucosal Immunol. 2012;5:53–65. doi: 10.1038/mi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 31.Matthews SP, Tregoning JS, Coyle AJ, Hussell T, Openshaw PJM. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J. Virol. 2005;79:2050–2057. doi: 10.1128/JVI.79.4.2050-2057.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallal LE, Schaller MA, Lindell DM, Lira SA, Lukacs NW. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur. J. Immunol. 2010;40:1042–1052. doi: 10.1002/eji.200939778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu. Rev. Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 34.Yordy B, Iwasaki A. Autophagy in the control and pathogenesis of viral infection. Curr Opin Virol. 2011;1:196–203. doi: 10.1016/j.coviro.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007;20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 36.Arsov I, Li X, Matthews G, Coradin J, Hartmann B, Simon AK, Sealfon SC, Yue Z. BAC-mediated transgenic expression of fluorescent autophagic protein Beclin 1 reveals a role for Beclin 1 in lymphocyte development. Cell Death Differ. 2008;15:1385–1395. doi: 10.1038/cdd.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson TR, Johnson CN, Corbett KS, Edwards GC, Graham BS. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS ONE. 2011;6:e16458. doi: 10.1371/journal.pone.0016458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukens MV, Kruijsen D, Coenjaerts FEJ, Kimpen JLL, van Bleek GM. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J. Virol. 2009;83:7235–7243. doi: 10.1128/JVI.00452-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, Berlin AA, Lukacs NW. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 40.Rudd BD, Schaller MA, Smit JJ, Kunkel SL, Neupane R, Kelley L, Berlin AA, Lukacs NW. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J. Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 41.Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJP, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 2010;16:90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 43.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci U.S.A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres J-P, Mejias A, Gomez AM, Jafri H, Ramilo O, Chen ZJ. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc. Natl. Acad. Sci U.S.A. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swedan S, Andrews J, Majumdar T, Musiyenko A, Barik S. Multiple functional domains and complexes of the two nonstructural proteins of human respiratory syncytial virus contribute to interferon suppression and cellular location. Journal of virology. 2011;85:10090–10100. doi: 10.1128/JVI.00413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MAM, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J. Exp. Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J. Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Peters N, Laza-Stanca V, Nawroly N, Johnston SL, Schwarze J. Local CD11c+ MHC class II- precursors generate lung dendritic cells during respiratory viral infection, but are depleted in the process. J. Immunol. 2006;177:2536–2542. doi: 10.4049/jimmunol.177.4.2536. [DOI] [PubMed] [Google Scholar]
- 49.Gill MA, Long K, Kwon T, Muniz L, Mejias A, Connolly J, Roy L, Banchereau J, Ramilo O. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. J. Infect. Dis. 2008;198:1667–1676. doi: 10.1086/593018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, Battaile JT, Alevy Y, Yan L, Agapov E. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. The Journal of experimental medicine. 2007;204:2759–2769. doi: 10.1084/jem.20070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Intemann CD, Thye T, Niemann S, Browne ENL, Amanua Chinbuah M, Enimil A, Gyapong J, Osei I, Owusu-Dabo E, Helm S, Rüsch-Gerdes S, Horstmann RD, Meyer CG. Autophagy gene variant IRGM −261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains. PLoS Pathog. 2009;5:e1000577. doi: 10.1371/journal.ppat.1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Massey DCO, Parkes M. Genome-wide association scanning highlights two autophagy genes, ATG16L1 and IRGM, as being significantly associated with Crohn’s disease. Autophagy. 2007;3:649–651. doi: 10.4161/auto.5075. [DOI] [PubMed] [Google Scholar]
- 53.Cadwell K, Patel KK, Maloney NS, Liu T-C, Ng ACY, Storer CE, Head RD, Xavier R, Stappenbeck TS, Virgin HW. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee W-M, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.