Abstract
Bortezomib selectively binds and inhibits the 20S proteasome enzyme’s active sites. This study was conducted to determine the side effects and maximum tolerated dose (MTD) of bortezomib in patients with recurrent malignant glioma. Separate dose escalations were conducted in patients taking or not taking enzyme-inducing anti-seizure drugs (+/−EIASD). The starting dose in both groups was 0.9 mg/m2 intravenously twice weekly for the first three of each 4 week cycle. Imaging assessment of response was carried out and Plasma 20S proteasome activity inhibition and imaging was conducted to monitor efficacy. The 66 patients enrolled had a median age of 51 years, median KPS of 90%, and 77% had glioblastoma multiforme. The MTD in the −EIASD group was 1.70 mg/m2 based on grade 3 thrombocytopenia, sensory neuropathy and fatigue. In the +EIASD group escalation was terminated at 2.5 mg/m2 without meeting meet the MTD criteria. However, proteasome inhibition in this group did not change at doses above 1.90 mg/m2 suggesting that further escalations would be unlikely to increase a biologic effect. Mean proteasome inhibition plateaued in +EIASD patients receiving 2.1 mg/m2 of bortezomib at 77 ± 12% and in −EIASD patients treated with a dose of 1.7 mg/m2 at 79 ± 6%. Two partial responses were observed. This study determined that EIASDs effect the MTD of bortezomib and the dose required for maximal inhibition of whole blood 20S proteasome. Some evidence of clinical activity was noted in this phase I study in patients with recurrent high grade gliomas.
Keywords: Bortezomib, Proteasome inhibitors, Cancer, Clinical trials, Malignant gliomas, Pharmacodynamics
Introduction
Bortezomib is a boronic acid peptide that selectively blocks the catalytic site of the 20S subunit of the 26S proteasome complex [1]. The ubiquitin–proteasome pathway has been well described. In eukaryotic cells it plays an essential role in the degradation of most short and long-lived intracellular proteins including regulatory proteins that govern the cell cycle, transcription factor activation, apoptosis and cell trafficking [2–4]. Modulating the catabolism of proteins such as cyclin dependent kinases, p53 and IκB-alpha, the inhibitor of NF-κB, by inhibiting the proteasome promotes the death of cancer cells by a number of potential pathways [1, 5].
Bortezomib has been shown to block NF-κB activation, and proliferation, and induce apoptosis in head and neck squamous cell carcinoma cell lines. It has also been shown to inhibit growth, radioresistance and angiogenesis in murine squamous cell carcinoma models and human head and neck squamous cell carcinoma xenograft models [6, 7]. Evaluation of the anticancer activity of bortezomib has been expanded to multiple tumor types even though these antiproliferative effects are multifactorial in nature and variable in degree based on the underlying unique tumor signaling pathways [8]. Models of ovarian, prostate and pancreatic cancer have demonstrated sensitivity to the agent on various levels [9, 10]. Ultimately, the most profound effects have been observed against cell lines derived from multiple myeloma and other hematologic malignancies [11–19]. Bortezomib was originally approved for the treatment of recurrent multiple myeloma in May 2003 and has been subsequently approved for treating newly diagnosed multiple myeloma and recurrent mantle cell lymphoma [20–25].
Bortezomib inhibits the proliferation of human malignant glioma cell lines and sensitizes primary astrocytoma and oligoastrocytoma cultures to TRAIL-induced apoptosis in vitro [26, 27]. Intratumoral administration of bortezomib improved the survival of mice bearing the intracranially implanted 9L gliosarcoma model [28]. Bortezomib does not appear to be distributed into the normal cerebral parenchyma, although the blood brain barrier in malignant gliomas is variably disrupted and there is evidence that it does reach the tumor in vivo [1, 28, 29]. This report describes the findings of a phase I trial undertaken to determine the dose-limiting toxicities (DLTs) and maximum tolerated dose (MTD) of bortezomib administered twice weekly for 2 weeks repeated every 21 days in patients with recurrent high-grade gliomas. Antiseizure drugs that induce the activity of hepatic drug metabolizing enzymes (EIASDs), such as the cytochrome P450 (CYP450) enzymes, are frequently used in patients with brain tumors [30, 31]. Hepatic oxidative metabolism mediated by several CYP450 isoenzymes appears to represent a significant route of elimination for bortezomib and several systemically circulating metabolites have been identified in cancer patients treated with the drug [32]. Accordingly, the dose of bortezomib was escalated independently in two groups of patients stratified by their pre-existing use of EIASDs in recognition of the potential for a pharmacokinetic or pharmacodynamic interaction when administering bortezomib in combination with EIASDs. A secondary objective of the study was to obtain a preliminary assessment of the activity of single agent bortezomib in patients with recurrent gliomas.
Patients and methods
This study was conducted by the New Approaches to Brain Tumor Therapy (NABTT) Consortium. Participating institutions included the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Winship Cancer Institute at Emory University, Wake Forest University, Henry Ford Hospital, the University of Alabama at Birmingham and the University of Pennsylvania. The protocol for this clinical trial was reviewed and approved by the Cancer Therapy Evaluation Program of the NCI and the institutional review boards of each participating institution. All patients provided written informed consent as a condition for participating in the study.
Patient selection
Adults (age ≥ 18 years) with histologically proven malignant glioma (anaplastic astrocytoma, anaplastic oligodendroglioma, glioblastoma multiforme) that was progressive or recurrent after radiation therapy, either alone or in combination with chemotherapy, were eligible for the study. Conditions required for entry into the study included: (a) measurable disease by contrast-enhanced MRI or CT; (b) not more than one prior regimen of chemotherapy (c) KPS ≥ 60% (d) full recovery from the effects of any earlier intervention and (e) a mini-mental status exam score ≥ 15. The minimum time intervals from prior treatments were 3 months for radiation, 6 weeks for chloroethylnitrosoureas, and 4 weeks for all other chemotherapeutic agents. Eligibility also required demonstrating acceptable hematologic parameters (absolute neutrophil count (ANC) ≥ 1,500/μl; platelet count ≥ 100,000/μl), renal function (serum creatinine ≤ 1.7 mg/dl), and hepatic function (total bilirubin ≤ 1.5 mg/dl; serum levels of aspartate and alanine aminotransferase ≤4 times the upper limit of normal). Exclusion criteria included: (a) a prior malignancy within 5 years other than curatively treated basal or squamous cell carcinoma or carcinoma of the cervix or breast in situ; (b) a serious concurrent infection, illness, or medical condition; (c) females who were pregnant or nursing; (d) any other condition that would compromise treatment with reasonable safety or result in noncompliance with prescribed medical care. Agreement to practice adequate birth control methods was required for fertile patients.
Drug administration and dose escalation
Patients were assigned to one of two treatment groups based on their preexisting use of EIASDs, which included phenytoin, carbamazepine, phenobarbital, primidone and oxcarbazepine. Patients assigned to the −EIASD group were either not being treated with an antiseizure drug or taking one that does not significantly induce hepatic enzymes, such as gabapentin, lamotrigine, valproic acid, levetiracetam, tiagabine, topiramate, zonisamide, and felbamate. Inclusion in the −EIASD group also required discontinuation of any EIASD for at least 10 days. For patients who required treatment with a corticosteroid, such as dexamethasone, the lowest clinically appropriate daily dose was determined before beginning the first cycle of therapy. Efforts were made to maintain the same dose until the radiographic tumor measurement was performed after completing the second cycle of therapy [33]. The corticosteroid dose could be reduced as clinically indicated for patients responding to therapy based upon serial tumor measurements.
Bortezomib was provided by Millennium Pharmaceuticals, Inc. (Cambridge, MA) and administered by rapid intravenous injection (3–5 s) twice weekly for 2 weeks (days 1, 4, 8 and 11) followed by 10 days without treatment. The use of antiemetics was permitted at the discretion of the treating physician. Treatment with any other approved or investigational chemotherapeutic agent was not permitted.
The starting dose of bortezomib was 0.9 mg/m2 for both treatment groups. The dose was independently escalated in each group, initially by 40% to 1.25 mg/m2 (dose level 2), followed by 20% to 1.50 mg/m2 (dose level 3), and then by a constant increment of 0.20 mg/m2 relative to the preceding dose level. The MTD was established by the occurrence of DLTs during the initial 21-day cycle of therapy. DLT was defined as any of the following treatment-related adverse events: (a) ANC ≤ 500/μl; (b) platelet count ≤ 25,000/μl; (c) febrile neutropenia; (d) any grade 3 or 4 nonhematologic toxicity, with the exception of nausea and vomiting; and (e) a delay in starting a subsequent course of treatment for more than 7 days because of incomplete recovery from toxicity. Cohorts of three patients were initially treated at each dose level and monitored for treatment-related toxicities, as described below. Escalation of the dose to the next level proceeded in the absence of DLT. An additional three patients were entered into a dose level if a DLT occurred in one of the initial three patients treated. Dose escalation continued if there were no DLTs in any of these additional patients. The MTD was considered to have been exceeded if more than one patient in a cohort of 3–6 experienced a DLT, thereby establishing the previous dose as the MTD. Once an MTD was defined an additional 10 patients were accrued to further assess safety.
Additional cycles of therapy with the same dose were repeated every 21 days in patients who did not experience a DLT or tumor progression if all eligibility requirements continued to be satisfied. Retreatment in the event of a DLT during any course of therapy was permitted with a reduction of the dose to the preceding level, or to 0.50, 0.26, and 0.13 mg/m2 for reductions below the starting dose, provided that the toxicity resolved within 2 weeks. This required recovery to either baseline values or grade ≤ 1 for hematological toxicities or grade ≤ 2 for nonhematological toxicities. Further decreases in the dose were allowed upon the occurrence of a DLT after treatment with a reduced dose. A maximum of three dose reductions were permitted before the patient was removed from the study. Patients were also removed from the study because of tumor progression, circumstances for which continued treatment could be detrimental to the health of a patient, noncompliance, or upon the decision of a patient to discontinue treatment for any reason.
Evaluations for toxicity and response
Evaluations performed within 14 days of initiating therapy included: a medical history; physical and neurological examinations; mini-mental status evaluation; KPS determination; electrocardiogram; chest X-ray; vital signs; complete blood count with differential and platelet counts; blood coagulation parameters; serum chemistry profile; urinalysis; and pregnancy test for women of child-bearing potential. Toxicities were evaluated during each cycle and graded according to the NCI Common Toxicity Criteria, version 2.0 (http://ctep/info.nih.gov). After initiating treatment, a complete blood count with differentials and platelet count was performed weekly or more often if significant myelosuppression was observed. All pretreatment evaluations were repeated before beginning every odd numbered cycle of therapy and within 7 days of the last treatment for patients removed from the study, except for the blood coagulation tests, urinalysis, and pregnancy test, which were performed only as needed.
Response to therapy was determined by MRI or CT imaging and neurologic examinations. The use of CT was restricted to patients who were unable to undergo MRI for physical or medical reasons. Imaging studies to provide tumor measurements were performed within 14 days of beginning treatment (baseline) and after every four cycles of therapy until relapse. A confirmatory scan was obtained 6 weeks (two cycles) after the initial detection of a complete or partial response. Standard NABTT response criteria were used as described previously [34]. The pathology and MRI scans for all patients responding to therapy were centrally reviewed. All patients were followed for survival. Survival time was calculated from start of treatment until death from any cause for overall survival and date of progression or death for progression free survival (PFS). Survival times were censored at date of the last follow-up. The survival distribution was estimated by the method of Kaplan and Meier. Confidence intervals (CI) were calculated using standard methods. Analyses were performed using SAS version 9.1.3 (SAS Institute, Inc., Cary, NC) and STATA (Version 8, College Station, TX). All P values reported are two sided.
Pharmacodynamic studies
Blood samples (5 ml) were collected in tubes containing sodium heparin before treatment and at 1 and 24 h after administering the first dose of bortezomib. The blood was transferred directly into a polypropylene cryovial and stored at ≤ −70°C until packaged in dry-ice for shipment to Millennium Pharmaceuticals, Inc. (Cambridge, MA) for analysis. Activity of the 20S proteasome and total protein content in whole blood lysates were determined as previously described [35]. The percentage inhibition of 20S proteasome activity relative to the pretreatment sample was calculated by the chymotryptic-to-tryptic activity ratio method and reported as the mean ± SD of the values for the individual patients at each dose level.
Results
Patient characteristics
Characteristics of the 66 patients enrolled into this study between May 2001 and March 2007 and treated with bortezomib are summarized in Table 1. The median age was 51 years, the majority were white, had a KPS of 90% or above and had a histology of glioblastoma. Two patients had no prior chemotherapy and 64 (97%) patients had one prior regimen. Fifteen individuals had not received temozolomide prior to enrollment.
Table 1.
Baseline demographic and clinical characteristics of all patients and stratified by enzyme inducing anti-seizure drug (EIASD) group
| Characteristic | All patients n = 66 |
+EIASD n = 33 |
−EIASD n = 33 |
|---|---|---|---|
| Age, years | 51 (26–85) | 50 (27–75) | 52 (26–85) |
| Gender, male | 46 (70) | 25 (76) | 21 (64) |
| Race | |||
| White | 60 (91) | 29 (88) | 31 (94) |
| African American | 5 (8) | 4 (12) | 1 (3) |
| Asian | 1 (2) | 0 (0) | 1 (3) |
| Karnofsky performance status | |||
| 60% | 5 (8) | 3 (9) | 2 (6) |
| 70–80% | 20 (30) | 8 (24) | 12 (36) |
| 90–100% | 41 (62) | 22 (67) | 19 (58) |
| Histological diagnosis | |||
| Glioblastoma multiforme | 51 (77) | 26 (79) | 25 (76) |
| Anaplastic astrocytoma | 8 (12) | 4 (12) | 4 (12) |
| Anaplastic oligodendroglioma | 3 (5) | 1 (3) | 2 (6) |
| Other | 4 (7) | 2 (6) | 2 (6) |
| Corticosteroid use | |||
| No | 24 (36) | 11 (33) | 13 (39) |
| Yes | 42 (64) | 22 (67) | 20 (61) |
Dose escalation and toxicities
All 66 patients were evaluable for toxicity. Initially, the duration of a cycle was defined as 6 weeks, with bortezomib given twice a week for four consecutive weeks, followed by 2 weeks without treatment. None of the patients in either treatment group experienced a DLT following treatment with daily doses of 0.9 mg/m2 (dose level 1) or 1.25 mg/m2 (dose level 2). Nevertheless, in response to concerns raised by the sponsor and monitors of the study at the NCI about the potential for toxicity resulting from administering the drug on four consecutive weeks, the administration schedule was revised by defining the duration of a cycle as 3 weeks with bortezomib given twice a week on weeks 1 and 2, and no treatment on week 3. This is the same as the administration schedule for the FDA approved indications of the drug. The two initial dose levels were reevaluated without the occurrence of a DLT in any patient. Dose escalation in the −EIASD group proceeded through dose level 4 (1.7 mg/m2) with no DLTs. One patient in dose level 5 (1.9 mg/m2) experienced a DLT, grade 3 thrombocytopenia that failed to recover within 2 weeks, requiring expansion of the cohort to evaluate an additional three patients. One of these patients experienced grade 3 neuropathy-sensory and grade 3 fatigue, which were DLTs. The preceding dose of 1.7 mg/m2 was declared the putative MTD. An additional 10 patients were accrued at this dose level, with only one patient experiencing DLTs of grade 3 neuropathy-sensory and headache. The median (range) of the number of cycles of treatment with bortezomib for the −EIASD group was 3 (1–12).
Accrual to the +EIASD arm of the study was completed through dose level 7 (2.3 mg/m2) with no DLTs. A single patient treated with 2.5 mg/m2 (dose level 8) experienced grade 3 thrombocytopenia, mandating expansion of the cohort. Although none of the additional three patients receiving 2.5 mg/m2 of bortezomib experienced a DLT, the decision was made not to escalate the dose further because the dose was already close to twofold higher than the approved dose for the treatment of multiple myeloma and the results of the pharmacodynamic studies did not support further dose escalation, as described below. The median (range) of the number of cycles of treatment with bortezomib for the +EIASD group was 2 (1–6).
Treatment related grade 3 or higher toxicities by group and dose level are presented in Table 2. In the +EIASD group at the 2.5 mg/m2 dose level four cases of grade 3 or higher thrombocytopenia were observed. One case of grade 3 ANC depression was observed. At the same level one case each of similar intensity arthralgia, motor neuropathy, sensory neuropathy, and ALT elevation was noted. At the 1.9 mg/m2 dose one case each of grade 3 rash and ANC depression was observed. At 1.7 mg/m2 one case of grade 3 leukopenia was observed. In the −EIASD group thrombocytopenia was the most commonly observed toxicity occurring in four individuals in the 1.9 mg/m2 dose group, five individuals receiving the 1.7 mg/m2 dose, two individuals in the 1.5 mg/m2 dose group and one each in the 0.9 and 1.25 mg/m2 dose for the 6 week cycle groups and again in the 1.25 mg/m2 dose group in the 4 week cycle regimen. Also, in the −EIASD group at the 1.9 mg/m2 dose one case each of grade 3 or 4 clinical toxicity manifest as diarrhea, hypotension, hypoxia, infection, and sensory neuropathy was observed. Grade 3 and 4 laboratory toxicity included acidosis, CPK elevation, low bicarbonate, hypokalemia and ALT elevation. At the 1.7 mg/m2 dose grade 3 toxicity manifest as sensory neuropathy in three cases, constitutional symptoms in two cases and abdominal pain, constipation, depressed level of consciousness, fatigue, headache, and pain were seen in one case each. Grade 3 laboratory toxicity included one episode of hypokalemia. Isolated toxicities observed at 1.5 mg/m2 and below included laboratory observations of AST elevation, PT elevation, low hemoglobin, and clinical observation of petechiae.
Table 2.
Grade 3 and 4 toxicities observed during all cycles of therapy possibly related to bortezomib by dose level and enzyme inducing anti-seizure drug (EIASD) group
| Toxicity | Dose level (mg/m2 6-week cycle)
|
Dose level (mg/m2 3-week cycle)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.9 | 1.25 | 0.9 | 1.25 | 1.5 | 1.7 | 1.9 | 2.1 | 2.3 | 2.5 | |
| +EIASD group (patients) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 6 |
| Non-hematologic | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 |
| Hematologic | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 5 |
| −EIASD group (patients) | 3 | 2 | 3 | 3 | 3 | 13 | 6 | 0 | 0 | 0 |
| Non-hematologic | 0 | 1 | 0 | 0 | 1 | 12 | 11 | |||
| Hematologic | 2 | 1 | 1 | 1 | 2 | 5 | 5 | |||
Anti-tumor activity
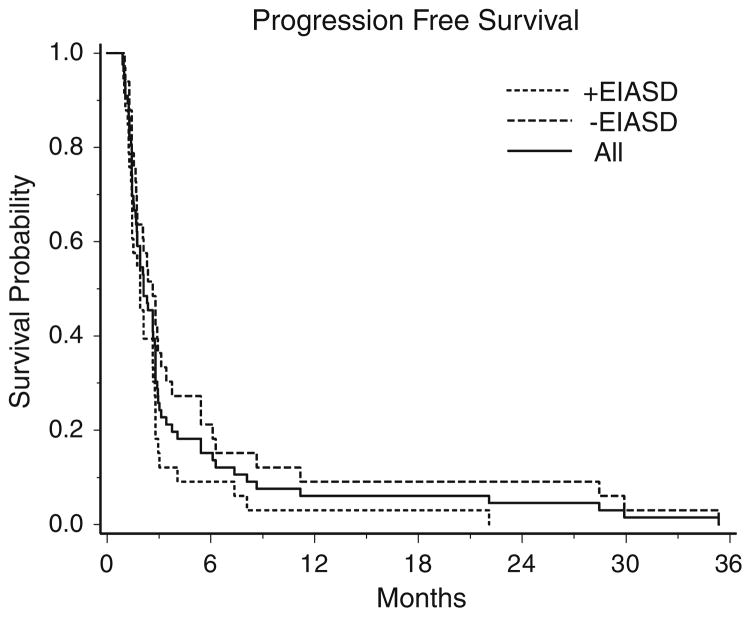
Patients that did not have follow-up imaging (n = 4) or readable imaging (n = 1) were not considered evaluable for response. Among the 61 cases that could be evaluated for response there were two partial responses, no complete responses, and 14 patients had stable disease. One patient with a partial response was in the −EIASD group treated with 1.25 mg/m2 dose and progressed 6 months later. The other patient was in the −EIASD group treated at 1.9 mg/m2 and was stable for 3 months and then taken off study for toxicity. The overall objective response rate was 3% (exact binomial 95% CI: 0.4–11%). The 6-month PFS rate for +EIASD group was 9% (3 out of 33 patients, 95% CI: 2–24%) and in the −EIASD group it was 21% (7 out of 33 patients 95% CI: 9–39%). For all patients the 6 month PFS was 15% (10 out of 66 patients, 95% CI: 8–26%). Median PFS survival time for the +EIASD group was 1.9 months (95% CI: 1.4–2.6 months) and for the −EIASD group it was 2.6 months (95% CI: 1.7–3.1 months). For all patients the median PFS was 2.1 months (95% CI: 1.7–2.8 months, Fig. 1).
Fig. 1.
Kaplan–Meier survival curves of progression free survival of all patients, patients taking enzyme-inducing antiseizure drugs (EIASDs and patients not taking EIASDs
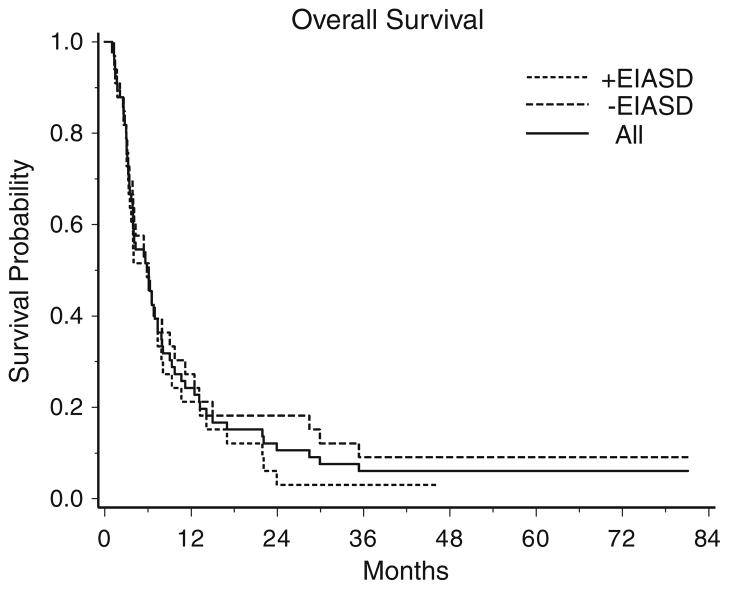
Median overall survival time for patients in the +EIASD group was 5.8 months (95% CI: 3.5–7.4 months) and 6.1 months (95% CI: 3.9–9.0 months) for those in the −EIASD arm. For all patients the median overall survival was 6.0 months (95% CI: 3.9–7.4 months, Fig. 2). One of 33 patients in the +EIASD group and 3 of the 33 patients in the −EIASD group are still alive at the time of this report. All 66 patients have either progressed or died.
Fig. 2.
Kaplan–Meier survival curves of overall survival of all patients, patients taking enzyme-inducing antiseizure drugs (EIASDs) and patients not taking EIASDs
20S proteasome inhibition
The average percentage inhibition of 20S proteasome activity in whole blood lysates at 1 and 24 h after treatment with the initial dose of bortezomib for each dose level evaluated in this clinical trial are presented in Table 3. As found in previous phase I trials of the drug in patients with solid tumors and hematological malignancies, proteasome inhibition at 1 h was dose-dependent at lower doses and independent of the dose at higher doses of bortezomib, with maximum inhibition near 70% [36, 37]. Proteasome inhibition at 1 h was very similar at the starting dose of 0.9 mg/m2 in both treatment groups, being 55.8 ± 5.8% for −EIASD patients and 54.4 ± 1.8% for those in the +EIASD arm. Maximum proteasome inhibition at 1 h was achieved at the third dose level (1.5 mg/m2) of the −EIASD arm, but not until the fifth dose level (1.9 mg/m2) of the +EIASD arm. The magnitude of maximum proteasome inhibition was essentially the same for both treatment groups, ranging from 70.3 to 73.8% for −EIASD patients receiving doses of 1.5–1.9 mg/m2 and 69.8–76.7% in patients treated with doses of 1.9–2.5 mg/m2.
Table 3.
Mean (±SD) percentage proteasome inhibition in whole blood lysates
| Dose (mg/m2) | +EIASD arm
|
−EIASD arm
|
||||
|---|---|---|---|---|---|---|
| Na | 1 h | 24 h | Na | 1 h | 24 h | |
| 0.90 | 6 | 54.4 ± 1.8 | 24.3 ± 3.4 | 4 | 55.8 ± 5.8 | 26.5 ± 3.0 |
| 1.25 | 6 | 59.8 ± 4.8 | 25.8 ± 3.9 | 5 | 57.2 ± 6.5 | 24.7 ± 8.9 |
| 1.50 | 3 | 62.6 ± 13.6 | 26.5 ± 6.8 | 3 | 71.7 ± 11.8 | 37.8 ± 3.4 |
| 1.70 | 3 | 64.3 ± 3.3 | 19.6 ± 4.7 | 10 | 73.8 ± 6.9 | 41.1 ± 13.9 |
| 1.90 | 3 | 72.5 ± 1.7 | 39.9 ± 9.0 | 6 | 70.3 ± 13.3 | 40.6 ± 14.8 |
| 2.10 | 3 | 76.7 ± 11.7 | 57.1 ± 9.6 | |||
| 2.30 | 3 | 69.8 ± 7.0 | 43.0 ± 8.3 | |||
| 2.50 | 5 | 75.5 ± 5.9 | 42.3 ± 14.8 | |||
Number of patients
Discussion
The MTD of bortezomib for patients in the −EIASD dose escalation arm of the study was 1.7 mg/m2, which is similar to the 1.56 mg/m2 MTD of the drug when given by the same dosing schedule to patients with advanced solid tumors other than CNS malignancies [36, 38–40]. Escalation of the dose in the +EIASD arm was terminated at the 2.5 mg/m2 dose level, even though only 1 of 6 patients experienced a DLT. Thus, the MTD was not established for patients who were concurrently receiving EIASDs. The toleration of relatively high doses of the bortezomib was not unexpected in the +EIASD group based on prior experience with other systemic chemotherapeutics in this population [41]. A large majority (76%) of the patients enrolled for treatment in the +EIASD dose escalation arm of this clinical trial were receiving phenytoin. Phenytoin induces CYP3A4 in human hepatocytes, which was found to be the predominant CYP450 isoform responsible for the metabolism of bortezomib in human liver microsomes [42–44]. As in other early clinical trials of bortezomib, the pharmacokinetics of bortezomib were not characterized during the present study because concentrations of the drug in plasma decay rapidly and approach the detection limit of the analytical method available at the time of this study’s initiation within minutes after its administration by i.v. injection [35]. Subsequently, an assay with sufficient sensitivity to more adequately define the time course of the drug in plasma, based upon high performance liquid chromatography with tandem mass spectrometric detection, has been developed and applied to clinical pharmacokinetic studies [38, 45, 46]. Nevertheless, information on the pharmacokinetic behavior of bortezomib in humans remains limited.
As an alternative to obtaining pharmacokinetic data, an accurate pharmacodynamic assay to measure proteasome inhibition in whole blood was developed and used extensively during the preclinical and clinical development of bortezomib [35–38, 47, 48]. Maximum proteasome inhibition in whole blood occurs 1 h after the administration of bortezomib by rapid i.v. injection [38]. The extent of proteasome inhibition at 1 h achieved with the approved dose of 1.3 mg/m2 is approximately 65% [36]. Proteasome inhibition at 1 h is dose-dependent at lower doses and approaches a plateau with a maximum level of inhibition of 70–75%, at doses of approximately 1.5 mg/m2 and greater, suggestive of saturable inhibition of enzyme activity [38]. This was very similar to the behavior observed in the present study for patients in the −EIASD arm, with maximum proteasome inhibition ranging from 70 to 74% 1 h after giving doses of 1.5–1.9 mg/m2. In comparison, the plateau in the extent of proteasome inhibition (range, 70–76%) occurred at higher doses (≥1.9 mg/m2) in patients who were concurrently receiving EIASDs, consistent with enhanced clearance of the drug resulting from the induction of hepatic CYP450 enzymes. Further increases of the dose did not result in a significantly greater degree of enzyme inhibition; however, it cannot be concluded that the plateau observed in peripheral blood would reflect a plateau in brain or tumor tissue proteasome inhibition.
The toxicity profile in this set of gliomas is different than reports in multiple myeloma. The most commonly reported drug-related grade 3 or 4 toxicities of bortezomib in hematologic malignancies are peripheral neuropathy, thrombocytopenia and neutropenia [49, 50]. As was summarized in Table 2 thrombocytopenia was clearly the most commonly observed toxicity in −EIASD and +EIASD groups in the current report. Though peripheral motor and sensory neuropathy was observed, it was less frequent. Thrombocytopenia was three-and-a-half times more common than peripheral neuropathy in the −EIASD group. No grade 3 or 4 neutropenia was observed in the −EIASD group. Thrombocytopenia was twice as common as peripheral neuropathies and four times as common as neutropenia in the +EIASD. In a phase 1 study of refractory solid tumors it was reported that diarrhea, syncope and hypotension were the most common DLTs [38]. Though grade 3 and 4 diarrhea and hypotension were observed in individual instances in the current report, they were not dominating problems. The toxicities in patients with recurrent malignant gliomas are not the same as individuals with hematologic malignancies or with refractory solid tumors outside the CNS. This may possibly be attributed to differences in the profiles of concomitant drugs being administered or to the relatively good integrity of the hepatic, renal and cardiopulmonary systems in patients with brain tumors as they do not usually metastasize or alter systemic parameters. Most individuals in this study had already been exposed to temozolomide and its propensity for induction anemia, neutropenia and thrombocytopenia may explain why this group was more sensitive to this effect of bortezomib than patients with tumors from other sites [51].
The phase I nature of this study does not allow a meaningful assessment of the efficacy of bortezomib in this population of patients. Under this caveat, the median overall survival was found to be 5.8 months with a 6-month PFS rate of 9% for patients in the +EIASD group and 6.0 months for the −EIASD group with a 21% 6-month PFS rate. The 6-month PFS was 15% for all patients. This is comparable with previous cytotoxic chemotherapy studies and indicative of minimal antitumor activity despite a small response rate of 3% [52, 53]. DLT was observed only in −EIASD group at 1.9 mg/m2, which is higher than 1.3 mg/m2 dose approved by the United States FDA for the treatment of relapsed or refractory multiple myeloma and mantle cell lymphoma. These brain tumor patients experienced a lesser frequency of side effects of sensory peripheral neuropathy (6%) and fatigue (3%) than patients with multiple myeloma. In terms of overall hematotoxicity, there was a 26% incidence of thrombocytopenia, a 3% incidence of neutropenia and only a 2% incidence of anemia associated with dose levels of 1.7 mg/m2 or higher. This may be unique in this group of patients because of the effect of EIASDs on the pharmacodynamics of bortezomib and similar to previously reported studies [34, 41, 54]. This group of patients was not heavily treated with chemotherapy in comparison to patients with multiple myeloma and other solid tumors.
In view of the multiple mechanisms by which this class of tumors is able to grow, it is likely that bortezomib will not be the sole answer to this disease process [55–57]. This is supported, but not proven, by the low response rate observed. In a phase 2 study of advanced stage non-small cell lung cancer, the study was terminated early after only 14 patients were accrued because no objective responses were observed [58]. In other studies of bortezomib in combination with lenaliomide and dexamethazone for Non-Hodgkin’s lymphoma and multiple myeloma, more activity was observed than with bortezomib as a single agent [59]. There is also an on-going study of bortezomib in combination with temozolamide in malignant melanoma and glioma (personal communication from Millennium Pharmaceuticals, Inc). Further development of bortezomib in recurrent malignant gliomas should be considered in combination with cytotoxic chemotherapy and/or other targeted agents in patients who are not receiving EIASDs. Ideally bortezomib will serve as a part of the treatment in selected brain tumor patients found to be most likely to respond based upon the molecular profile of their tumor.
Acknowledgments
This study was supported in part by a grant UO1 CA-62475 from the National Cancer Institute and Millennium Pharmaceuticals, Inc. Surasak Phuphanich was also supported in part as a Georgia Cancer Coalition Distinguished Cancer Scholar.
Footnotes
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, Atlanta, USA, June 2–5, 2006 and the 10th Annual Meeting of the Society for Neuro-Oncology, Orlando, Florida, November 16–19, 2006.
Contributor Information
Surasak Phuphanich, Email: phuphanich@cshs.org, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA. Johnnie Cochran Brain Tumor Center, Department of Neurosurgery and Neurology, Cedars-Sinai Medical Center, 8631 W, 3rd Street, Suite 410 E, Los Angeles, CA 90048, USA.
Jeffrey G. Supko, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA
Kathryn A. Carson, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA
Stuart A. Grossman, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA
L. Burt Nabors, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA.
Tom Mikkelsen, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA.
Glenn Lesser, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA.
Steve Rosenfeld, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA.
Serena Desideri, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA.
Jeffrey J. Olson, The New Approaches to Brain Tumor Therapy (NABTT) Consortium, NABTT Central Office, 1550 Orleans Street, Suite 1M-16, Baltimore, MD 21231, USA
References
- 1.Adams J, Palombella VJ, Elliott PJ. Proteasome inhibition: a new strategy in cancer treatment. Invest New Drugs. 2000;18:109–121. doi: 10.1023/a:1006321828515. [DOI] [PubMed] [Google Scholar]
- 2.King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 3.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 4.Read MA, Neish AS, Luscinskas FW, Palombella VJ, Maniatis T, Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 1995;2:493–506. doi: 10.1016/1074-7613(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 5.Tariman JD. Current therapies for multiple myeloma. J Infus Nurs. 2007;30:113–118. doi: 10.1097/01.NAN.0000264715.28005.ea. quiz 121. [DOI] [PubMed] [Google Scholar]
- 6.Sunwoo JB, Chen Z, Dong G, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7:1419–1428. [PubMed] [Google Scholar]
- 7.Lun M, Zhang PL, Pellitteri PK, Law A, Kennedy TL, Brown RE. Nuclear factor-kappaB pathway as a therapeutic target in head and neck squamous cell carcinoma: Pharmaceutical and molecular validation in human cell lines using velcade and siR-NA/NF-kappaB. Ann Clin Lab Sci. 2005;35:251–258. [PubMed] [Google Scholar]
- 8.Allen C, Saigal K, Nottingham L, Arun P, Chen Z, Van Waes C. Bortezomib-induced apoptosis with limited clinical response is accompanied by inhibition of canonical but not alternative nuclear factor-{kappa}B subunits in head and neck cancer. Clin Cancer Res. 2008;14:4175–4185. doi: 10.1158/1078-0432.CCR-07-4470. [DOI] [PubMed] [Google Scholar]
- 9.Frankel A, Man S, Elliott P, Adams J, Kerbel RS. Lack of multicellular drug resistance observed in human ovarian and prostate carcinoma treated with the proteasome inhibitor PS-341. Clin Cancer Res. 2000;6:3719–3728. [PubMed] [Google Scholar]
- 10.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 11.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 12.LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 13.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 14.Schenkein D. Proteasome inhibitors in the treatment of B-cell malignancies. Clin Lymphoma. 2002;3:49–55. doi: 10.3816/clm.2002.n.011. [DOI] [PubMed] [Google Scholar]
- 15.Orlowski RZ, Eswara JR, Lafond-Walker A, Grever MR, Orlowski M, Dang CV. Tumor growth inhibition induced in a murine model of human burkitt’s lymphoma by a proteasome inhibitor. Cancer Res. 1998;58:4342–4348. [PubMed] [Google Scholar]
- 16.Giles F. New drugs in acute myeloid leukemia. Curr Oncol Rep. 2002;4:369–374. doi: 10.1007/s11912-002-0029-8. [DOI] [PubMed] [Google Scholar]
- 17.Yu C, Rahmani M, Conrad D, Subler M, Dent P, Grant S. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood. 2003;102:3765–3774. doi: 10.1182/blood-2003-03-0737. [DOI] [PubMed] [Google Scholar]
- 18.Sayers TJ, Brooks AD, Koh CY, et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102:303–310. doi: 10.1182/blood-2002-09-2975. [DOI] [PubMed] [Google Scholar]
- 19.Tan C, Waldmann TA. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Res. 2002;62:1083–1086. [PubMed] [Google Scholar]
- 20.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 21.Richardson PG, Barlogie B, Berenson J, et al. Clinical factors predictive of outcome with bortezomib in patients with relapsed, refractory multiple myeloma. Blood. 2005;106:2977–2981. doi: 10.1182/blood-2005-02-0691. [DOI] [PubMed] [Google Scholar]
- 22.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127:165–172. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 23.Richardson PG, Sonneveld P, Schuster MW, et al. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br J Haematol. 2007;137:429–435. doi: 10.1111/j.1365-2141.2007.06585.x. [DOI] [PubMed] [Google Scholar]
- 24.Anderson K, Richardson P, Chanan-Khan A. Single-agent bortezomib in previously untreated multiple myeloma (MM): results of a phase II multicenter study. J Clin Oncol. 2006;24(18S):7504. [Google Scholar]
- 25.Raab MS, Breitkreutz I, Anderson KC. Targeted treatments to improve stem cell outcome: Old and new drugs. Bone Marrow Transplant. 2007;40:1129–1137. doi: 10.1038/sj.bmt.1705829. [DOI] [PubMed] [Google Scholar]
- 26.Strzelczyk J, Safadi R. Radiation safety considerations in GliaSite 125I brain implant procedures. Health Phys. 2004;86:S120–S123. [PubMed] [Google Scholar]
- 27.Koschny R, Holland H, Sykora J, et al. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res. 2007;13:3403–3412. doi: 10.1158/1078-0432.CCR-07-0251. [DOI] [PubMed] [Google Scholar]
- 28.Olson JJ, Bowers G, Zhang Z. Protease inhibitors in a brain tumor model. In: Adams J, editor. Cancer drug discovery and development: proteasome inhibitors in cancer therapy. Humana Press Inc; Totowa: 2004. [Google Scholar]
- 29.Zunkeler B, Carson RE, Olson J, et al. Quantification and pharmacokinetics of blood-brain barrier disruption in humans. J Neurosurg. 1996;85:1056–1065. doi: 10.3171/jns.1996.85.6.1056. [DOI] [PubMed] [Google Scholar]
- 30.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the quality standards subcommittee of the American academy of neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 31.Vecht CJ, Wagner GL, Wilms EB. Interactions between antiepileptic and chemotherapeutic drugs. Lancet Neurol. 2003;2:404–409. doi: 10.1016/s1474-4422(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 32.Pekol T, Daniels JS, Labutti J, et al. Human metabolism of the proteasome inhibitor bortezomib: identification of circulating metabolites. Drug Metab Dispos. 2005;33:771–777. doi: 10.1124/dmd.104.002956. [DOI] [PubMed] [Google Scholar]
- 33.Watling CJ, Lee DH, Macdonald DR, Cairncross JG. Corticosteroid-induced magnetic resonance imaging changes in patients with recurrent malignant glioma. J Clin Oncol. 1994;12:1886–1889. doi: 10.1200/JCO.1994.12.9.1886. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert MR, Supko JG, Batchelor T, et al. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–2949. [PubMed] [Google Scholar]
- 35.Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Proteasome inhibition measurements: clinical application. Clin Chem. 2000;46:673–683. [PubMed] [Google Scholar]
- 36.Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 37.Orlowski RZ, Stinchcombe TE, Mitchell BS, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- 38.Papandreou CN, Daliani DD, Nix D, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 39.Cortes J, Thomas D, Koller C, et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10:3371–3376. doi: 10.1158/1078-0432.CCR-03-0508. [DOI] [PubMed] [Google Scholar]
- 40.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: Final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 41.Fetell MR, Grossman SA, Fisher JD, et al. Preirradiation paclitaxel in glioblastoma multiforme: efficacy, pharmacology, and drug interactions. New approaches to brain tumor therapy central nervous system consortium. J Clin Oncol. 1997;15:3121–3128. doi: 10.1200/JCO.1997.15.9.3121. [DOI] [PubMed] [Google Scholar]
- 42.Faucette SR, Wang H, Hamilton GA, et al. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32:348–358. doi: 10.1124/dmd.32.3.348. [DOI] [PubMed] [Google Scholar]
- 43.Raucy JL. Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab Dispos. 2003;31:533–539. doi: 10.1124/dmd.31.5.533. [DOI] [PubMed] [Google Scholar]
- 44.Uttamsingh V, Lu C, Miwa G, Gan LS. Relative contributions of the five major human cytochromes P450, 1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the proteasome inhibitor bortezomib. Drug Metab Dispos. 2005;33:1723–1728. doi: 10.1124/dmd.105.005710. [DOI] [PubMed] [Google Scholar]
- 45.Horton TM, Pati D, Plon SE, et al. A phase 1 study of the proteasome inhibitor bortezomib in pediatric patients with refractory leukemia: a children’s oncology group study. Clin Cancer Res. 2007;13:1516–1522. doi: 10.1158/1078-0432.CCR-06-2173. [DOI] [PubMed] [Google Scholar]
- 46.Attar EC, De Angelo DJ, Supko JG, et al. Phase I and pharmacokinetic study of bortezomib in combination with idarubicin and cytarabine in patients with acute myelogenous leukemia. Clin Cancer Res. 2008;14:1446–1454. doi: 10.1158/1078-0432.CCR-07-4626. [DOI] [PubMed] [Google Scholar]
- 47.Stanford BL, Zondor SD. Bortezomib treatment for multiple myeloma. Ann Pharmacother. 2003;37:1825–1830. doi: 10.1345/aph.1D262. [DOI] [PubMed] [Google Scholar]
- 48.Davis NB, Taber DA, Ansari RH, et al. Phase II trial of PS-341 in patients with renal cell cancer: a university of Chicago phase II consortium study. J Clin Oncol. 2004;22:115–119. doi: 10.1200/JCO.2004.07.165. [DOI] [PubMed] [Google Scholar]
- 49.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 50.Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777–3784. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balmaceda C, Peereboom D, Pannullo S, Cheung YK, Fisher PG, Alavi J, Sisti M, Chen J, Fine RL. Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas. Cancer. 2008;112:1139–1146. doi: 10.1002/cncr.23167. [DOI] [PubMed] [Google Scholar]
- 52.Carson KA, Grossman SA, Fisher JD, Shaw EG. Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol. 2007;25:2601–2606. doi: 10.1200/JCO.2006.08.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 54.Grossman SA, Carson KA, Phuphanich S, et al. Phase I and pharmacokinetic study of karenitecin in patients with recurrent malignant gliomas. Neuro Oncol. 2008;10:608–616. doi: 10.1215/15228517-2008-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shapiro WR, Shapiro JR. A changing paradigm of glioma biology. Hematol Oncol Clin North Am. 2006;20:1171–1191. doi: 10.1016/j.hoc.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Chi A, Norden AD, Wen PY. Inhibition of angiogenesis and invasion in malignant gliomas. Expert Rev Anticancer Ther. 2007;7:1537–1560. doi: 10.1586/14737140.7.11.1537. [DOI] [PubMed] [Google Scholar]
- 57.de Groot JF, Gilbert MR. New molecular targets in malignant giomas. Curr Opin Neurol. 2007;20:712–718. doi: 10.1097/WCO.0b013e3282f15650. [DOI] [PubMed] [Google Scholar]
- 58.Anderson KC, Jagannath S, Jakubowiak SA, Lonial S, Raje N, Schlossman R, Munshi N, Knight Esseltine RD, Richardson PG. Phase II study to evaluate the efficacy and safety of bortezomib (PS-341) in chemotherapy-naive patients with advanced stage non-small cell lung cancer. J Clin Oncol. 2008;26(15S):8545. [Google Scholar]
- 59.Ho L, Li T, Piperdi B, Macapinlac M, Rigas JR, Camacho F, Perez-Soler R, Gucalp R. Phase II study of lenalidomide, bortezomib, and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma. J Clinc Oncol. 2008;26(15S):19096. [Google Scholar]