Preoperative Management
Although many patients with pancreatic malignancies will have lost substantial body weight, there is no evidence that preoperative nutritional repletion with total parenteral nutrition reduces perioperative complications or mortality. In the now uncommon case in which high-grade jaundice is associated with coagulation defects, preoperative administration of vitamin K and intraoperative fresh-frozen plasma are used as needed. Bowel preparation is unnecessary unless colonic resection is anticipated. Broad-spectrum antibiotics germane to biliary flora are given prior to skin incision and continued for one dose postoperatively.
Pancreaticoduodenectomy: General Considerations
This operation, whether performed as a classic Whipple procedure or as a pylorus-preserving variation, should incur a 30-day in-hospital mortality rate of less than 5%. Although the majority of surgeons worldwide may, at present, favor the pylorus-preserving modification of pancreaticoduodenectomy, it has been our experience that the postoperative length of hospital stay is significantly longer with the pylorus-preserving operation because of a higher incidence of delayed gastric emptying in approximately one third of patients. Because there is neither a nutritional advantage conferred nor any difference in cure rates between the two operations, we have favored the traditional antrectomy.
There has been recent interest in extending the operation to include tissues outside the standard field of dissection, retroperitoneal lymph nodes, and the nerve plexuses along the superior mesenteric artery. Popularized in Japan, this extended dissection has shown no benefit in clinical trials in Europe and the United States. In addition, circumferential dissection of the nerve plexuses around the artery frequently leads to debilitating diarrhea and gastrointestinal dysfunction.
Lateral or segmental resection of the portal or superior mesenteric vein may allow completion of the pancreaticoduodenectomy but has not convincingly increased the rate of cure even if an R0 resection (negative margin) is accomplished.
Operative Technique
Step 1
Either a vertical midline incision, our preference, or a bilateral subcostal transverse incision can be used with equal access. The liver and peritoneal surfaces are examined for unexpected extrapancreatic metastases. Intraoperative ultrasonography of the liver may be used selectively when the findings are suspicious but indeterminate by palpation. Routine biopsy of apparently normal regional lymph nodes is unnecessary, but suspicious lesions and enlarged lymph nodes outside the planned field of dissection should be biopsied and examined by frozen section and the resection aborted if positive for metastatic cancer. Positive lymph nodes within the planned field of resection are not considered a contraindication to pancreaticoduodenectomy.
Step 2
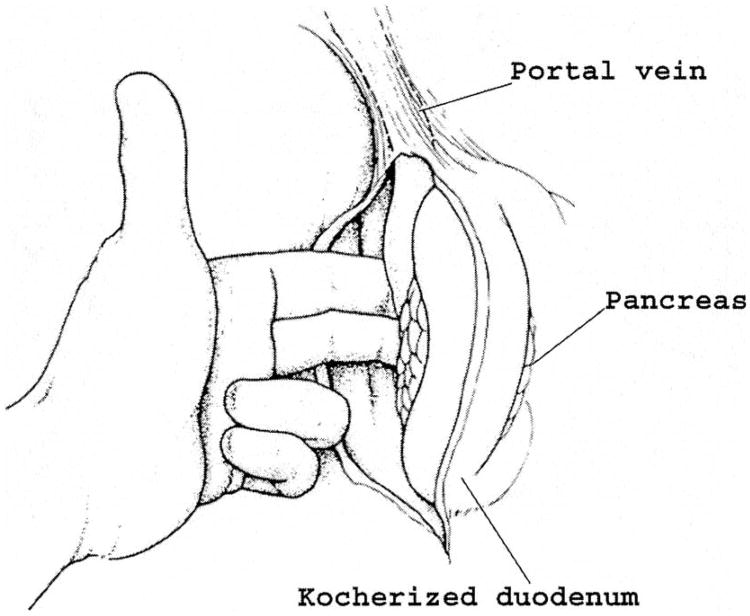
The hepatic flexure of the colon is mobilized from its retroperitoneal attachments to access the third and fourth portions of the duodenum. Extensive mobilization of the entire right colon and small bowel mesentery (Cattell-Braasch maneuver) is unnecessary except for lesions involving the fourth portion of the duodenum or for the approach to mobilization and resection of a segment of the superior mesenteric vein. The duodenum and head of the pancreas are separated from the retroperitoneal bed medially past the aorta and distally to the ligament of Treitz. It is now possible to palpate the superior mesenteric artery posteriorly as it originates from the aorta and to establish that the cleft between the artery and uncinate process of the pancreas is not obliterated by tumor (Fig. 1). A silk suture placed in the duodenum to mark the junction of the third and fourth portions is particularly helpful for identification of the proximal point of devascularization of the duodenum later when working back from the transected jejunum (step 7).
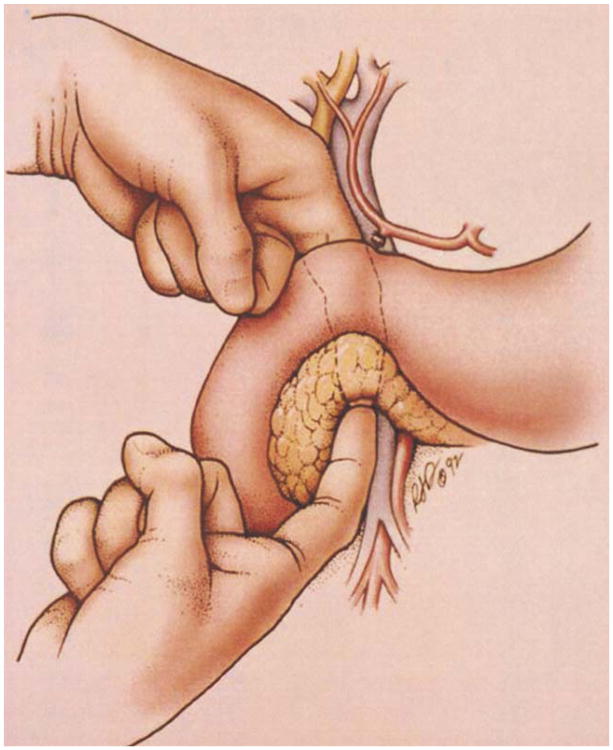
Fig. 1.
The duodenum and head of the pancreas are extensively mobilized and medially rotated away from the inferior vena cava and aorta. Posterior extension of the tumor is rare. The relationship between the tumor and the superior mesenteric artery is established by palpation.
Step 3
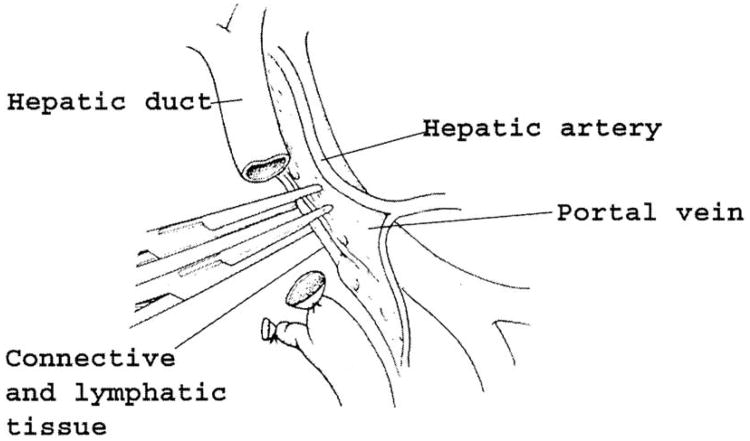
The gallbladder is removed if still present. The bile duct is dissected free from the adjacent portal structures and divided above the cystic duct entry across the common hepatic duct. This proximal point of division minimizes the risk of a positive biliary resection margin consequent to the tendency of periampullary cancers to infiltrate cephalad along the submucosal lymphatic channels of the bile duct. The proximal bile duct is left unclamped to avoid trauma to the duct, but the distal bile duct orifice is sutured to minimize spillage of tumor cells. If evidence of unresectable cancer is discovered subsequently, the proximal bile duct is used for palliative bypass. The tissues lateral to the portal vein are carefully separated, divided, and ligated with care taken that a replaced right hepatic artery off the superior mesenteric artery is not included (Fig. 2).
Fig. 2.
After removal of the gallbladder, the bile duct is transected above the cystic duct. The distal bile duct is sutured to limit cancer spillage. The proximal duct is left unclamped to avoid crush injury. The lymphatic vessels and connective tissue lateral to the portal vein are divided and swept caudally with the specimen. Care must be taken not to injure an aberrant right hepatic artery originating from the superior mesenteric artery that, if present, will be found in this area.
Step 4
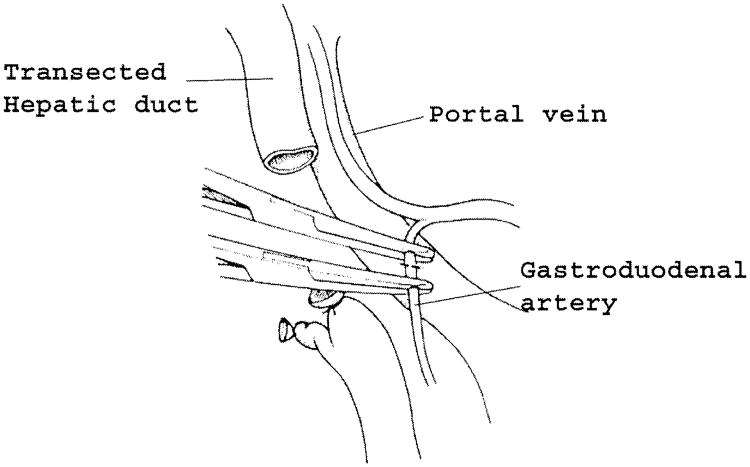
The portal dissection is continued down the anterior aspect of the portal vein, with beginning development of the tunnel in front of the vein and behind the neck of the pancreas. Division of the right gastric artery and gastroduodenal artery greatly enlarges this window and facilitates access to the portal vein behind the pancreas (Fig. 3). The gastroduodenal artery should be doubly ligated or suture ligated to minimize the chance of subsequent erosion and bleeding. Lymph nodes anterior to the proper hepatic artery are taken with the specimen. Although there are no direct anterior branches to the portal vein, there are significant branches at the upper and lower margins of the pancreas that enter the anteromedial aspect of the portal/mesenteric vein, and these can be easily damaged during dissection of the tunnel.
Fig. 3.
Division of the gastroduodenal artery. The gastroduodenal artery should be doubly ligated or suture ligated. Division of the gastroduodenal artery exposes the anterior surface of the portal vein and greatly facilitates the dissection of the portal vein behind the pancreatic neck.
The approach to the mesenteric vein below the neck of the pancreas is facilitated by dividing the gastrocolic omentum outside the gastroepiploic vessels down to the level of the head of the pancreas and further mobilizing the hepatic flexure caudally. The middle colic vein and gastroepiploic vein can be traced down to the superior mesenteric vein for rapid identification.
Step 5
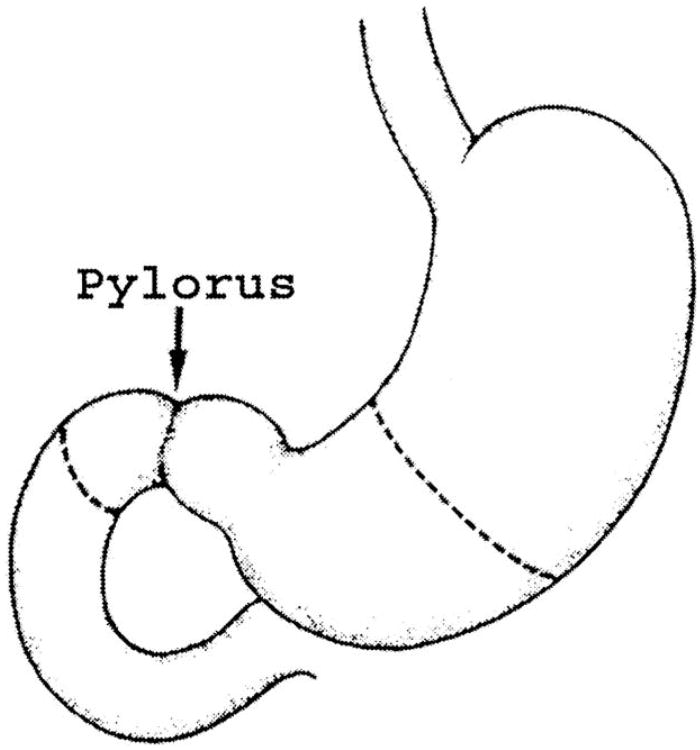
After the left gastric and gastroepiploic vessels are divided at the gastric wall, the stomach is divided across the proximal antrum with a stapler (Fig. 4). Alternately, the duodenum 2 cm past the pylorus may be divided with the stapler. The lesser curvature portion of the stomach is turned in with nonabsorbable sutures over the staple line in Hofmeister fashion, leaving a sufficient portion of the staple line for a 4 cm anastomosis.
Fig. 4.
Line of division of the stomach or duodenum. In a standard Whipple procedure, an antrectomy is performed. If pylorus preservation is desired, the duodenum is divided 2 cm distal to the pylorus.
Step 6
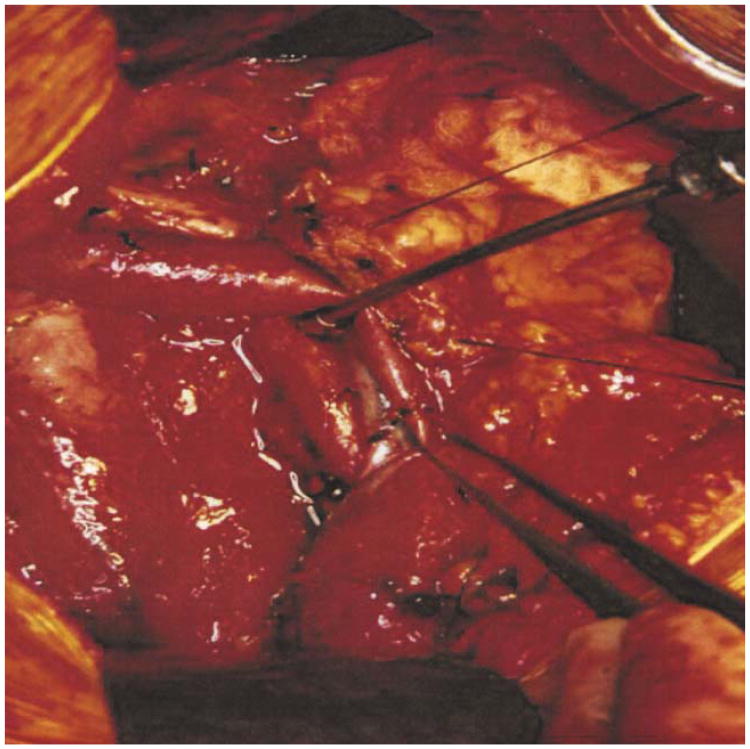
Transection of the pancreas in front of the portal vein is next. The dissection of the tunnel behind the pancreatic neck should be completed by blunt dissection under direct vision (Fig. 5). Although traditional descriptions cite this maneuver as critical to proving resectability, it is only relevant to cancers in the neck and body of the pancreas. Periampullary tumors are much more likely to involve the lateral and posterior aspects of the portal/mesenteric vein. Evaluation of this area can be uncertain at this point in the operation, and the disappointing finding of involvement of the vein may not be possible until much later in the operation, after division of the pancreas and attempted dissection of the uncinate process. A soft Penrose drain is passed behind the pancreas both for elevation of the pancreatic neck and protection of the portal vein. Sutures are placed at the four quadrants of the transection line for guidance and for ligation of the vascular arcades, which run along the cephalad and caudad margins of the pancreatic parenchyma (Fig. 6). The pancreas is divided with electrocautery, and additional bleeding points on the cut margins are controlled. Suture closure of the pancreatic duct on the side of the specimen may help to reduce spillage of tumor cells during subsequent manipulation.
Fig. 5.
The portal vein is identified above the pancreatic neck and the superior mesenteric vein is identified inferior to the pancreatic neck. The anterior surface of the vein is separated by blunt dissection from the pancreas, creating a tunnel behind the pancreatic neck.
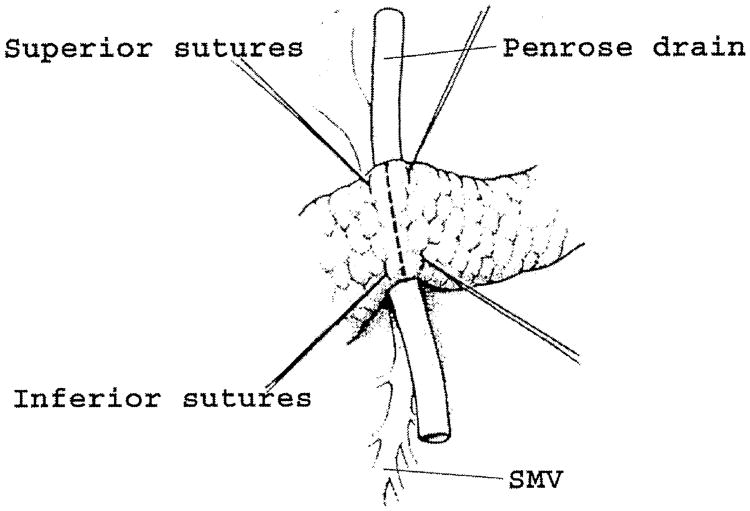
Fig. 6.
Transection of the pancreatic neck. After the pancreatic neck has been separated from the anterior surface of the portal mesenteric vein, a Penrose drain is passed behind the pancreatic neck to elevate and protect the vein during division. Four hemostatic sutures are placed on either side of the transection line. The pancreas is now ready to be divided with electrocautery. SMV - superior mesenteric vein
Step 7
The transverse colon and its mesentery are elevated cephalad, and the entire small bowel is eviscerated to facilitate exposure and the dissection of the distal duodenum proximal to the ligament of Treitz. The jejunum is divided with a stapler 6 to 10 cm past the ligament of Treitz at a point that will provide sufficient mobility of the distal jejunum to reach easily to the right upper quadrant for the biliary and pancreatic anastomoses (Fig. 7). The feeding vessels to the proximal jejunum and distal duodenum are divided at the enteric wall back to the previously placed marker suture. This suture is particularly valuable in order to ensure that the duodenal dissection is adequate to allow subsequent freedom to complete the resection of the uncinate process but that it is not carried too far proximal into the cancer field.
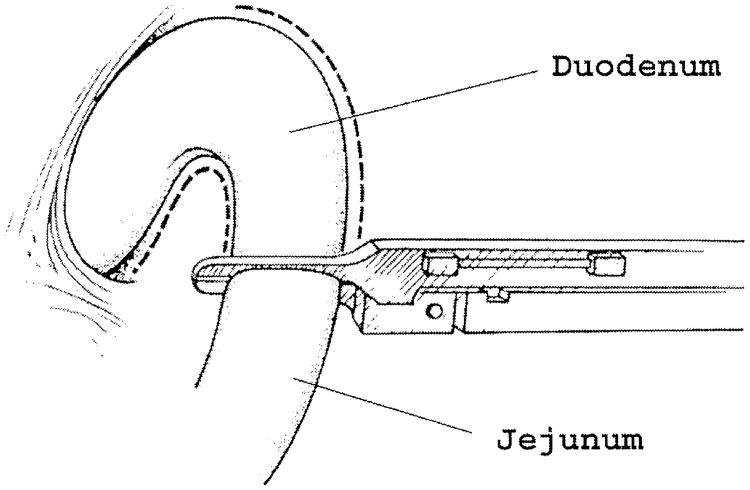
Fig. 7.
The ligament of Treitz is taken down, and the jejunum is divided with a stapler 6 to 10 cm past the ligament of Treitz. The jejunal vascular branches are divided at the bowel wall.
Step 8
The final step in removal of the specimen is to divide the venous tributaries of the uncinate process to the portal and mesenteric veins and to dissect along the lateral margin of the superior mesenteric artery, taking both the arterial branches and the anterolateral periarterial soft tissues, which include both lymphatics and nerve plexuses that can contain tumor (Fig. 8). The plane between the pancreas and the mesenteric vessels is opened by retracting the specimen to the right and the mesenteric vein to the left. The safety of this dissection may be increased by passing vessel loops around the portal vein and superior mesenteric vein for retraction. This is of particular value if there is an inflammatory reaction or tumor in the region of the dissection. In the event of laceration of the vein or the need for venous resection, an additional vessel loop must be placed around the splenic vein proximal to the splenoportal junction. Traction on the vessel loops and vascular clamps are used for venous control as necessary. Lateral venorrhaphy with a running fine nonabsorbable suture may suffice for repair or reconstruction, as long as the lumen is not unacceptably narrowed. Segmental resection and reconstruction, either end to end or with interposition of a graft (jugular or iliac vein, or polytetrafluoroethylene may be necessary in some cases, but it is debatable whether this contributes to cure. As noted previously, extensive mobilization of the right colon and small bowel mesentery may facilitate access to and mobilization of the superior mesenteric vein. Further mobility can be obtained by dividing the splenic vein proximal to the splenoportal junction.
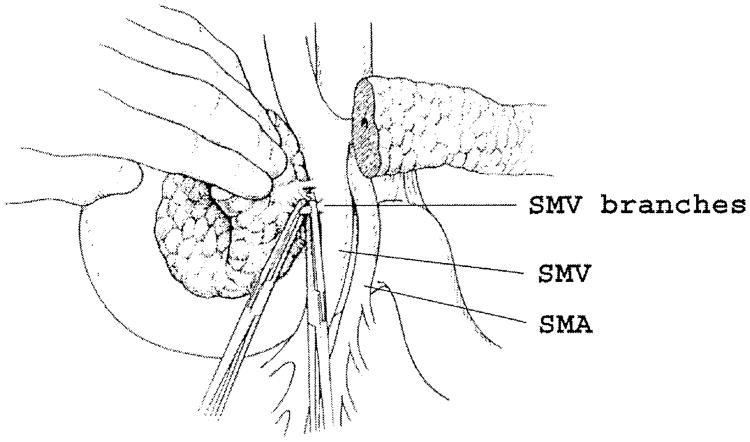
Fig. 8.
The final step of the dissection requires isolation, division, and ligation of the vascular supply and retroperitoneal attachments at the head of the pancreas. The venous tributaries to the superior mesenteric vein (SMV) are readily identified by retracting the pancreatic head gently to the right and the vein to the left. SMA = superior mesenteric artery.
Traction of the portal/mesenteric vein to the left exposes the groove lateral to the superior mesenteric artery. The tissues overlying the artery anteriorly are divided and the dissection is carefully carried down the lateral wall of the artery, skeletonizing it (Fig. 9). If there is a right hepatic artery originating at the superior mesenteric artery, care must be taken to avoid injuring it. The attachments of the uncinate process, including the arterial branches, are then taken sequentially along the lateral margin of the superior mesenteric artery. After removal of the specimen, metal clips are placed at the margins of the resection field to target postoperative radiation therapy.
Fig. 9.
After dividing venous tributaries from the uncinate process to the superior mesenteric vein portal vein, the vein is retracted to the left with instruments or vessel loops to identify and expose the superior mesenteric artery behind and to the left. The lymphatic, neural, and arterial branches are cleared from the anterolateral surface of the artery. If, in the rare instance it is necessary to retract the vein to the right to expose the artery, division of the splenic vein may also be required for adequate exposure.
It is helpful to the pathologist to orient the specimen before handing it off the field. We consistently place a safety pin in the uncinate margin and a long suture on the posterior soft tissue margin, and communicate this to the pathologist directly.
Step 9
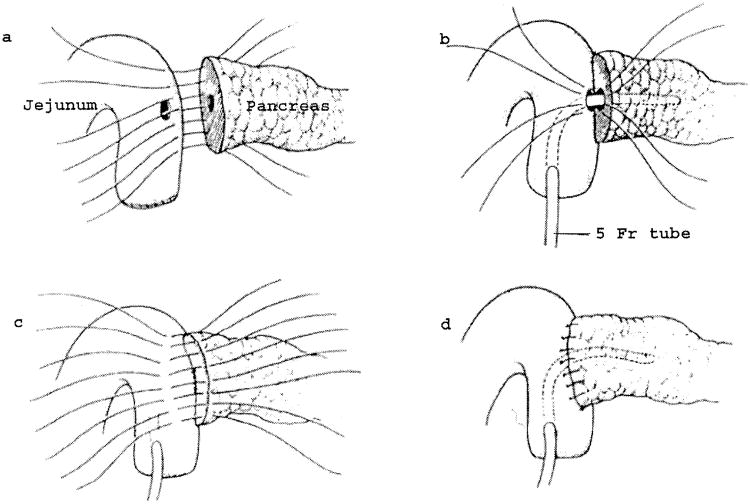
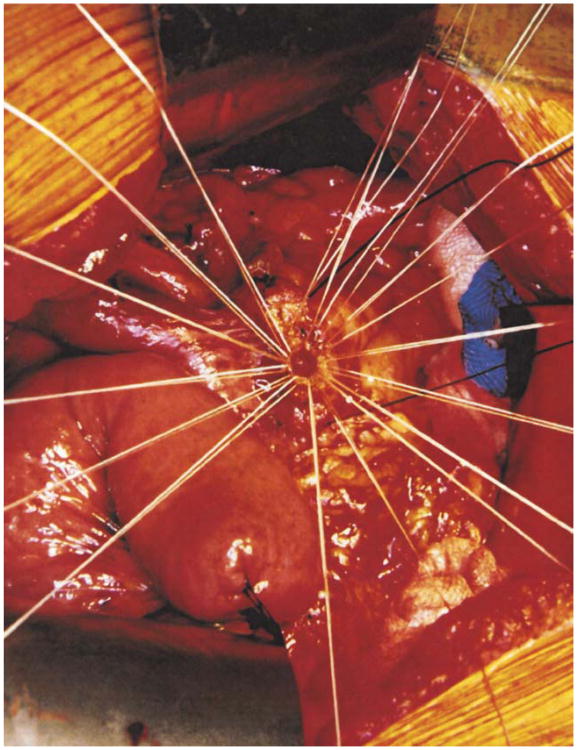
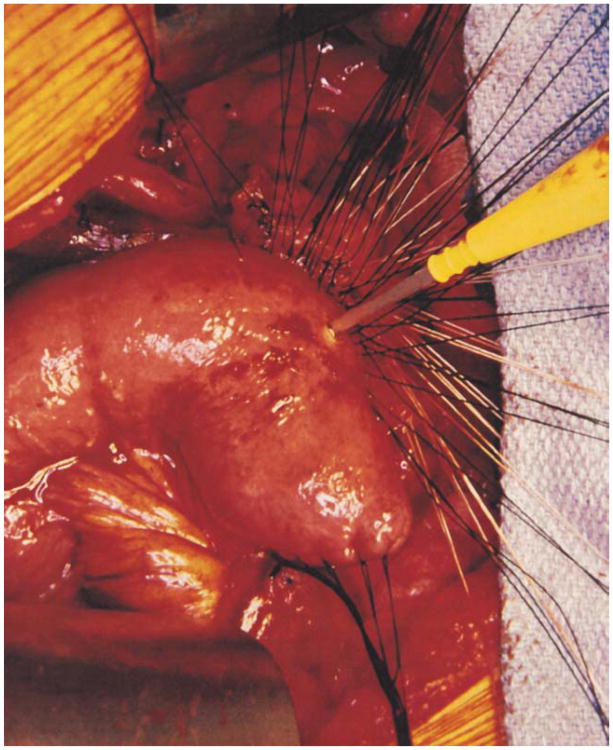
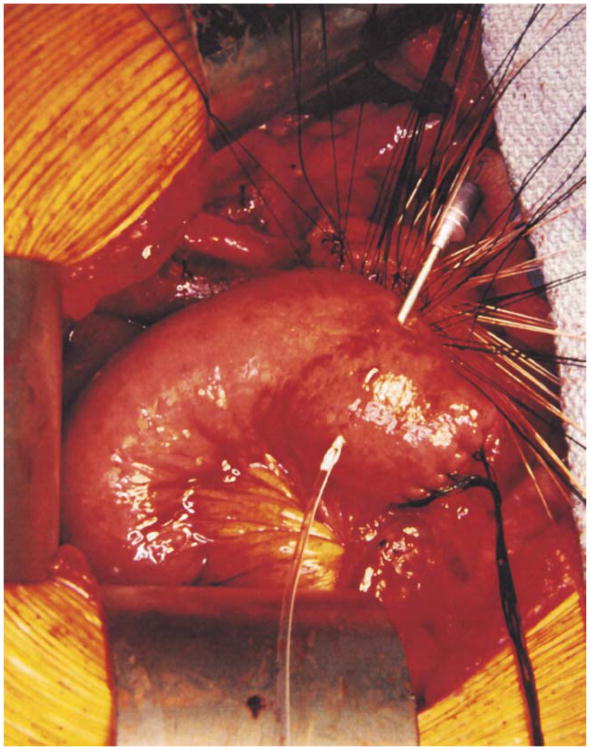
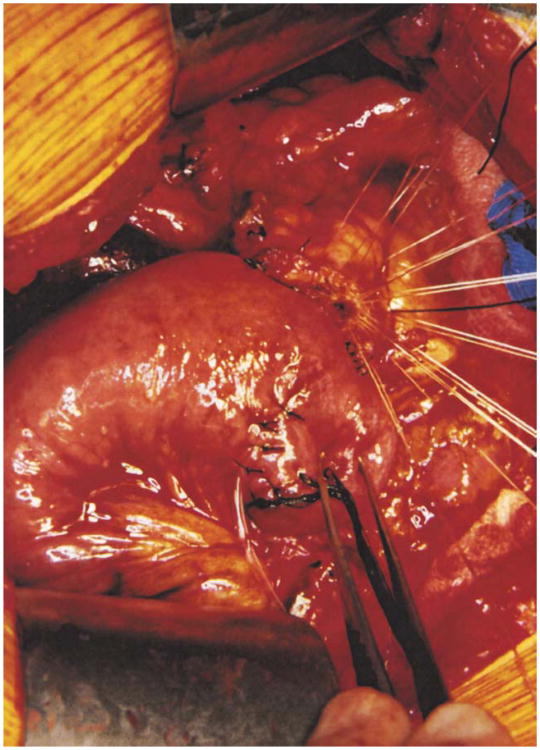
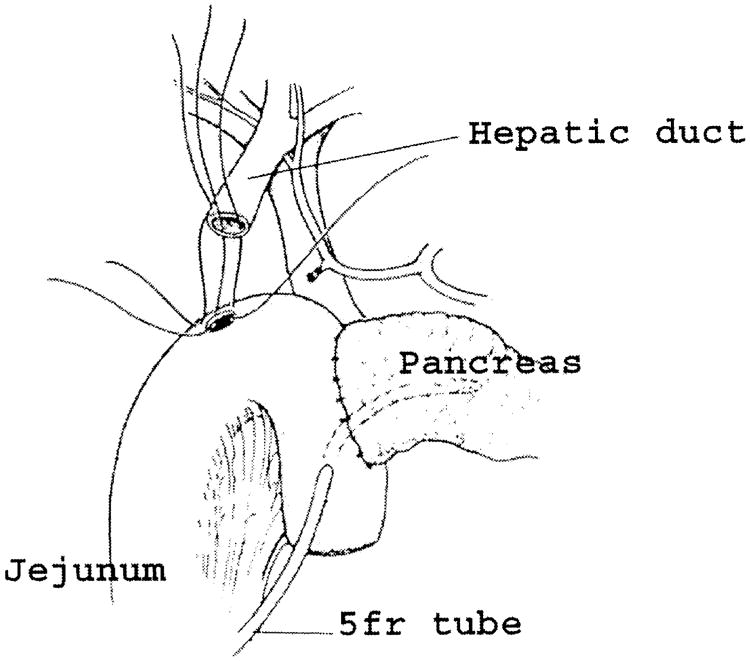
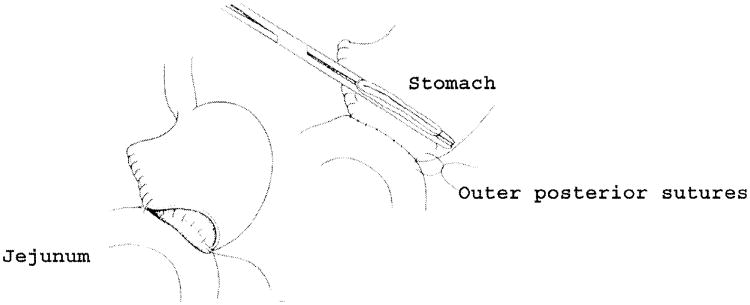
The colon and mesocolon are once again elevated, and the small bowel is eviscerated to facilitate closure of the ligament of Treitz with interrupted nonabsorbable sutures. The end of the jejunum is oversewn with nonabsorbable Lembert sutures over the staple line and it is brought through the right side of the transverse mesocolon. The pancreaticojejunostomy is performed first. Whenever possible, this anastomosis is created in two layers, end to side, using an outer row of interrupted nonabsorbable 3-0 sutures that includes most of the cut surface of the pancreas and an inner row of 4-0 interrupted synthetic absorbable sutures duct to mucosa (Fig. 10). It is almost always possible, even with a small/normal pancreatic duct, to perform the anastomosis with this technique. When the duct is very small, we place a no. 5 pediatric feeding tube into the lumen and place a minimum of eight sutures circumferentially through the cut edge of the duct. All of the inner layers of duct sutures, anterior and posterior rows, are placed first and arrayed carefully to avoid entanglement (Fig. 11). The outer posterior sutures are then laid in between the pancreas posterior to the duct and the seromuscular layer of the bowel. Before they are tied, a duct-sized incision is made in the jejunum opposite the pancreatic duct lumen (Fig. 12). The no. 5 pediatric feeding tube is then brought through the jejunal wall via a no. 14 needle (Fig. 13). The posterior row of sutures is tied, and the posterior row of duct sutures is placed through the full thickness of the jejunum. The feeding tube is then placed into the pancreatic duct, and its exit point in the jejunal wall is fixed with a purse-string chromic catgut suture and a Witzel tunnel of interrupted silk Lembert sutures (Fig. 14). The anterior row of duct sutures is then placed in the full thickness of the jejunal orifice and tied. The anastomosis is completed with an anterior row of silk sutures. It is desirable to have the outer anterior and posterior rows of sutures include 1 cm of the pancreas to create a wider surface of apposition. The pancreatic drainage tube will later be brought through the omentum and out through the right side of the abdominal wall. If there is a very dilated pancreatic duct, the pancreatic tube is not necessary.
Fig. 10.
Pancreaticojejunostomy. A two-layer end-to-side duct-to-mucosa pancreaticojejunostomy is preferred.
Fig. 11.
Interrupted synthetic absorbable sutures are placed circumferentially through the margins of the pancreatic duct and laid aside until after the posterior row of nonabsorbable sutures between the pancreas and jejunum are in place. This sequence allows precise suture placement even in small, normal pancreatic ducts without risk of lacerating injury to a soft gland.
Fig. 12.
Before fixation of the jejunal loop to the pancreas by tying the posterior rows of sutures, an opening is made in the jejunum to match the size and location of the pancreatic duct.
Fig. 13.
A no. 5 pediatric feeding tube is introduced into the lumen through a 14-gauge needle and laid aside until the outer and inner posterior rows of sutures have been tied.
Fig. 14.
After the feeding tube has been threaded into the pancreatic duct, it is fixed in position by a purse-string chrome catgut suture, and the jejunal entry is buried in a Wetzel tunnel to prevent leaking.
Step 10
Distal to the pancreatic anastomosis, an end-to-side hepaticojejunostomy is made with a single layer of interrupted closely spaced synthetic absorbable sutures (Fig. 15). Unless the bile duct is small or fragile, no transanastomotic drainage tube is necessary. In the event it is needed, a small (no. 8 pediatric feeding tube) catheter can be left through the anastomosis and brought out distally through the jejunum and through the abdominal wall. If there has been preoperative placement of a transhepatic drain, this can be left in place instead of a retrograde tube placed from the jejunum.
Fig. 15.
The hepaticojejunostomy is made with a single layer of interrupted closely placed 3-0 or 4-0 absorbable sutures and is placed several centimeters distal to the pancreaticojejunostomy. It is preferable that all duct sutures be placed first and carefully arranged to avoid entanglement. The posterior row is then sewn and tied prior to sewing the anterior row.
Step 11
After fixing the jejunal loop to the transverse mesocolon with interrupted nonabsorbable sutures, gastrointestinal continuity is restored with a retrocolic Hofmeister-type Billroth II gastrojejunostomy (Fig. 16). This anastomosis is made with running absorbable sutures as an inner layer and interrupted nonabsorbable sutures as an outer layer. The anastomosis is fixed below the mesocolon with interrupted silk sutures.
Fig. 16.
The gastrojejunostomy is preferably a retrocolic Hofmeister anastomosis with the efferent limb to the left, using an outer layer of silk Lembert stitches and an inner layer of running absorbable sutures and fixed below the mesocolon.
If a pylorus-preserving operation has been chosen, it has been suggested that placement of the duodenojejunostomy in an antecolic position may reduce the incidence of delayed gastric emptying.
Step 12
Soft closed-suction drains are placed in the right upper quadrant, anterior and posterior to the biliary and pancreatic anastomoses. These are brought out through separate incisions in the right side of the abdomen. Neither gastrostomy nor feeding jejunostomy tubes are necessary, but a gastrostomy may be advisable after pylorus preservation because of the problem of delayed gastric emptying. The abdominal wall is closed according to surgeon preference.
Postoperative Care
The nasogastric tube is typically discontinued on postoperative day 1, and clear liquids may be allowed on day 2. The diet is advanced to low-fat soft solids in frequent small feedings as tolerated. Blood glucose should be monitored and diabetes treated as appropriate. The concentration of amylase in the drainage is measured on day 5 or 6 when the patient is eating. If there is no indication of an anastomotic leak, the drains are removed individually on day 5, 6, or 7. The pancreatic (and biliary) stents are removed at one of the postoperative office visits, generally at 3 weeks.
Pancreatic fistula remains the most common serious complication of this operation. The mainstay of treatment is complete drainage, either by closed-suction drains placed at operation or by percutaneously placed catheters if necessary. The catheters should be left in place long enough to ensure formation of a secure tract and then withdrawn in segments to allow the tract to close behind as drainage diminishes. In the case of low-volume fistulas (<200 ml/day), patients may eat and be discharged to home. High-output fistulas may require a more aggressive approach with fasting, maintenance of fluid and electrolyte balance, and parenteral nutrition. Octreotide, 200 U subcutaneously 3 times a day, has been used an adjunct to reduce the volume of fistula output, but there is no conclusive evidence that closure is accelerated.
In the event of delayed gastric emptying for more than 7 to 10 days, a Gastrografin contrast upper gastrointestinal study should be performed to rule out mechanical obstruction. The condition resolves spontaneously, although the gastroparesis can persist as long as 3 to 4 weeks. Management consists of supportive measures. Prokinetic agents such as erythromycin (200 mg intraveneously 3 times daily, 30 minutes before meals) have been used with a modicum of purported success.