Abstract
Rationale
An increasingly compelling literature points to a major role for the glutamate system in mediating the effects of alcohol on behavior and the pathophysiology of alcoholism. Preclinical studies indicate that glutamate signaling mediates certain aspects of ethanol’s intoxicating and rewarding effects, and undergoes adaptations following chronic alcohol exposure that may contribute to the withdrawal, craving and compulsive drug-seeking that drive alcohol abuse and alcoholism.
Objectives
We discuss the potential for targeting the glutamate system as a novel pharmacotherapeutic approach to treating alcohol use disorders, focusing on five major components of the glutamate system: the N-methyl-D-aspartate (NMDA) receptor and specific NMDA subunits, the glycineB site on the NMDA receptors (NMDAR), L-alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid ionotropic (AMPA) and kainate (KAR) receptors, metabotropic receptors (mGluR), and glutamate transporters.
Results
Chronic alcohol abuse produces a hyperglutamatergic state, characterized by elevated extracellular glutamate and altered glutamate receptors and transporters. Pharmacologically manipulating glutamatergic neurotransmission alters alcohol-related behaviors including intoxication, withdrawal, and alcohol-seeking, in rodents and human subjects. Blocking NMDA and AMPA receptors reduces alcohol consumption in rodents, but side-effects may limit this as a therapeutic approach. Selectively targeting NMDA and AMPA receptor subunits (e.g., GluN2B, GluA3), or the NMDAR glycineB site offers an alternative approach. Blocking mGluR5 potently affects various alcohol-related behaviors in rodents, and mGluR2/3 agonism also suppresses alcohol consumption. Finally, glutamate transporter upregulation may mitigate behavioral and neurotoxic sequelae of excess glutamate caused by alcohol.
Conclusions
Despite the many challenges that remain, targeting the glutamate system offers genuine promise for developing new treatments for alcoholism.
Keywords: Ethanol, Craving, NMDA, GluN2A, GluN2B, mGluR2/3, mGluR5, Memantine, Addiction, Glycine
The burden and costs of alcohol abuse
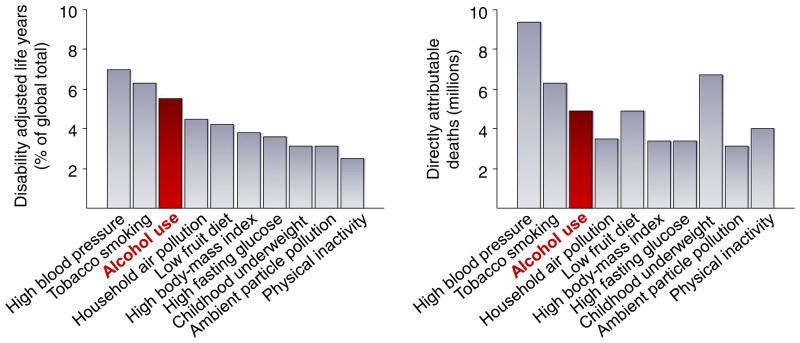
Problems that arise from excessive drinking, bingeing and the long-term chronic abuse of alcohol represent a major public health issue. In the USA, it is estimated that more than 10 % of the population have an alcohol use disorder (NIAAA 2000) while, worldwide, more than 2 billion people consume alcohol and around 6 % of adults have an alcohol use disorder (Rehm et al. 2009). Almost 4 % of deaths worldwide are attributed to alcohol; accounting for one in every ten European male deaths (Rehm et al. 2009). Alcohol abuse accounts for nearly 5 % of premature death and disability caused by disease and injury, and 30–40 % of the death and disability that is specifically attributable to neuropsychiatric disease (Rehm et al. 2009). Of the risk factors for global death and disability, alcohol use was found to rank third, behind only hypertension and tobacco smoking (Lim et al. 2012) (Fig. 1). Correspondingly, health care costs associated with alcohol abuse are on the order of $30 billion per year in the USA alone (Rehm et al. 2009). Adding in other factors indirectly linked to alcohol abuse, such as loss of economic productivity due to days off work, the annual cost to the United States economy was estimated to be $185 billion in the year 2000 and has likely grown further since then (NIAAA 2000).
Fig. 1.
The global burden of alcohol use. Alcohol use ranked third in 2010 risk factors for global disease burden, as measured by disability adjusted life years (DALYs) (left panel), and accounted for 5.5 million deaths worldwide (right panel). Adapted from Lim et al. 2012
Glutamate signaling as a target for new alcoholism treatments
While the problem of alcohol abuse continues to grow, the options currently available to treat these conditions remain inadequate and are prescribed at low rates (Iheanacho et al. 2013). Recent meta-analyses have concluded that both the opioid receptor blocker naltrexone (Revia, Vivitrol) and the mixed-pharmacology compound acamprosate (Campral) can significantly reduce heavy drinking and promote abstinence relative to placebo, with better outcomes typically seen for acamprosate (Maisel et al. 2013). However, despite the promise of early clinical data (Garbutt et al. 1999), the long-term efficacy of naltrexone has proven modest (Fuller et al. 1986; Krystal et al. 2001). In large-scale clinical trials and multicenter studies including Project COMBINE, the largest alcoholism pharmacotherapy trial conducted to date, naltrexone was ineffective when combined with cognitive–behavioral therapy and only had a small effect size (0.2) when combined with medication management therapy, while acamprosate was completely ineffective (Anton et al. 2006). Acamprosate also failed to meet its primary efficacy endpoint in its initial large multicenter trial in the United States (Mason et al. 2006a). The effect size for long-acting injectable naltrexone was marginally higher (Garbutt et al. 2005), but it is not clear whether this reflects greater medication efficacy or the selection of patients willing to accept injectable medications.
In the face of compelling need, the field of alcohol research must explore new directions to identify novel therapeutics. There are a variety of putatively therapeutic targets in several neurotransmitter and neuropeptide systems (Vengeliene et al. 2008a). Of these, there has been growing interest in developing pharmacotherapies for alcoholism around the glutamate system (Spanagel and Vengeliene 2013), as there has been for anxiety disorders (Conn and Jones 2009), depression (Skolnick et al. 2009) and schizophrenia (Coyle 2006).
Currently, two medications that have been studied extensively as alcoholism pharmacotherapies, acamprosate and the anticonvulsant topiramate, appear to have direct effects on glutamatergic neurotransmission. However, although acamprosate was initially thought to act in part by inhibiting N-methyl-D-aspartate receptors (NMDAR) and metabotropic glutamate (mGluR5) receptors (Dahchour and De Witte 2003; Popp and Lovinger 2000), actions on these receptors remain unclear (Brasser et al. 2004; Spanagel et al. 1996), especially at the plasma levels typically achieved in humans with oral administration (Johnson et al. 2003b) (c.f. Burattini et al. 2008). Topiramate is often used, off-label, as a treatment for alcoholism, and inhibits glutamate release and blocks L-alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid ionotropic (AMPAR)/ kainate receptors (KAR) as part of a complex pharmacological profile. In rodents, topiramate promotes alcohol intoxication and reduces alcohol drinking and withdrawal, but does not disrupt alcohol place preference (Breslin et al. 2010; Cagetti et al. 2004; Chen and Holmes 2009; Farook et al. 2007; Gremel et al. 2006; Hargreaves and McGregor 2007; Knapp et al. 2007; Lynch et al. 2011; Nguyen et al. 2007; Zalewska-Kaszubska et al. 2013). Topiramate also reduces craving, withdrawal and drinking in alcoholics (Baltieri et al. 2008; Florez et al. 2008; Johnson et al. 2004; Johnson et al. 2003a; Johnson et al. 2007; Komanduri 2003; Krupitsky et al. 2007b; Miranda et al. 2008; Paparrigopoulos et al. 2011; Rubio et al. 2004; Rustembegovic et al. 2002).
The example of topiramate supports the potential of developing other glutamate-targeting drugs for alcoholism. However, the complexity of glutamate neurotransmission makes designing safe, selective and efficacious drugs that target the system a very difficult task. On the other hand, because there are numerous ways to modulate this system, it affords many potential targets. In addition, the glutamatergic system might be a particularly attractive target for anti-alcohol medications due to its involvement in various aspects not only of alcohol’s behavioral effects, but also in the profound neuroadaptations occurring with chronic alcohol exposure (Tsai and Coyle 1998).
Here, we synthesize the preclinical evidence, along with any salient clinical data, addressing the potential of targeting glutamate neurotransmission for the treatment of alcohol use disorders. We first summarize the major effects of alcohol on glutamate neurotransmission, and then focus on preclinical and clinical studies that have investigated five major components of the glutamate system: NMDAR, the glycineB site on the NMDAR, AMPAR and KAR, metabotropic receptors (mGluR), and glutamate transporters (Table 1).
Table 1.
Effects of glutamatergic drugs on alcohol-related behaviors in rodents and clinical populations
| Target mechanism | Example compounds | Intoxication | Withdrawal | Consumption | Reinstatement |
|---|---|---|---|---|---|
| Blocking NMDAR | MK-801, memantine | ↑ [↑*] | ↓ [↓] | ↓ [↓] | ↓ |
| Activating glycineB site | Org25935, D-cycloserine | ↓ [↑*] | – | ↓ | – |
| Blocking glycineB site | L-701,324 | ↑ | – | ↓ | ↓ |
| Activating AMPAR | LY404187, LY451395 | – | – | ↑ | – |
| Blocking AMPAR | CNQX, GYKI 52466 | – | ↓ | ↓ | – |
| Blocking mGluR5 | MPEP, MTEP | ↑ | ↓ | ↓ | ↓ |
| Activating mGluR2/3 | LY379268, LY404039 | – | – | – | ↓ |
| GLT-1 upregulation | GPI-1046 | – | – | ↓ | – |
↑ increase in ethanol-related effect, ↓ decrease in ethanol-related effect, – unclear or not extensively studied, [] clinical data, ↑* mimics subjective feeling of alcohol intoxication
Alcohol-induced glutamatergic neuroadaptations
As with other drug addictions, alcohol use disorders progress from initial alcohol sampling to social drinking to habitual or compulsive use (Kalivas and O’Brien 2008). For some, alcohol use disorders are chronic relapsing syndromes. In these cases, alcohol use disorders may be associated with progressive functional impairment, medical and neuropsychiatric complications, and accrual of negative social consequences including divorce, unemployment, and legal problems. The progressive nature of alcoholism implies that brain mechanisms of plasticity are recruited and impaired over the course of the disease.
Glutamate-related neuroplasticity has been implicated in many steps of the progression from social drinking to chronic compulsive alcohol use, including Pavlovian conditioning, the development of alcohol-related habits, sensitization to the effects of alcohol, and “kindling” of alcohol withdrawal symptoms (Krystal et al. 2003). Glutamatergic agents might play several roles in the treatment of alcoholism including reducing alcohol consumption, suppressing alcohol withdrawal, and reducing neuroplasticity associated with intoxication and withdrawal (Krystal et al. 2003). Thus, these drugs might attenuate the progression of alcohol use disorders in addition to treating symptoms associated with particular states or phases of these disorders.
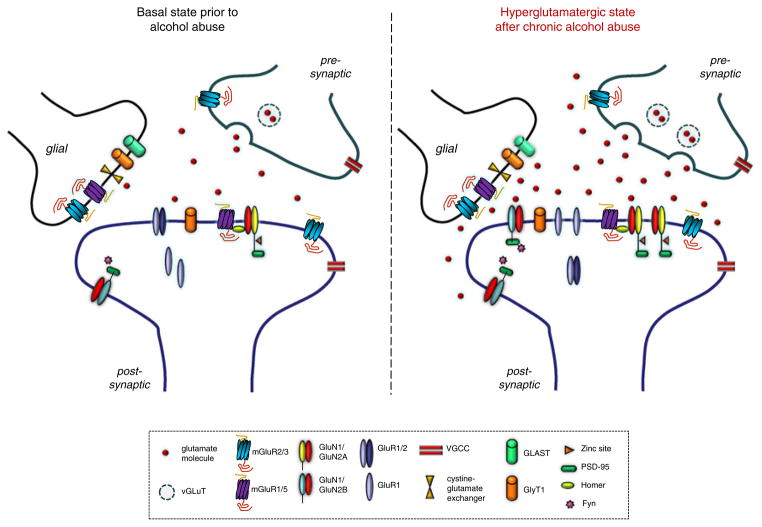
There is evidence that the neuroadaptations resulting from chronic alcohol abuse aggregate into a hyperglutamatergic state, typified by elevated extracellular glutamate levels and alterations in glutamate receptors and transporters (Krystal et al. 2003; Tsai and Coyle 1998) (summarized in Fig. 2). Elevated levels of excitatory amino acids are reported in the cerebrospinal fluid (CSF) of alcohol-dependent patients (Tsai et al. 1998) and severity of alcohol dependence, as measured by the Alcohol Dependence Severity Scale, positively correlates with CSF glutamate (Umhau et al. 2010). Magnetic resonance imaging studies have found that glutamate levels in the hippocampus and anterior cingulate cortex are increased during early withdrawal, but return to normal within 3 days post-withdrawal (Hermann et al. 2012; Mason et al. 2006b; Mon et al. 2012). This suggests that enhanced glutamate levels might occur during early conditioned withdrawal in abstinent alcohol-dependent patients—a hypothesis supported by evidence in animals showing increases in extracellular glutamate in rodent forebrain regions during acute alcohol withdrawal (Rossetti and Carboni 1995). A recent meta-analysis of rodent microdialysis studies confirmed elevated extracellular concentrations of glutamate in several brain regions and found that these increases, particularly within the nucleus accumbens, strongly correlated with the severity of alcohol withdrawal (Fliegel et al. 2013). Collectively, these findings illustrate a central role for glutamate in inducing and maintaining some of the adverse long-term behavioral effects of alcohol abuse. By extension, this predicts that drugs modulating glutamate activity may be useful as treatments for alcoholism. Here, we review the available evidence on the potential for developing drugs that target specific glutamate receptors, subunits, and transporters as novel medications for alcoholism.
Fig. 2.
Adaptations of the glutamate system as a result of chronic alcohol abuse. Chronic alcohol abuse produces a “hyperglutamatergic” state during abstinence. This state is characterized by elevated levels of extracellular glutamate and alterations in the expression and localization of various glutamate receptors, including upregulation of NMDARs, and possibly a decrease in calcium-restricting GluA1/2 heteromers in favor of calcium-permeable GluA1 homomers. Key to acronyms/abbreviations: vGLUT vesicular glutamate transporter; mGluR metabotropic glutamate receptor; GluN1, GluN2A, GluN2B NMDAR subunits; GluA1/2, GluA1 AMPAR subunits; VGCC voltage-gated calcium channel; GLAST glutamate transporter subtype (EAAT1); GlyT1 glutamate transporter 1, PSD-95 postsynaptic density 95
Glutamatergic targets
NMDA receptors
Acute administration of alcohol inhibits NMDAR function. The manner in which alcohol antagonizes NMDARs is not fully understood, but may be due to direct occupancy, actions on gating and phosphorylation (Lovinger et al. 1989; Woodward 2000). Notwithstanding, the importance of NMDARs to alcohol’s pharmacodynamic actions are evidenced by the effect of NMDAR antagonists to mimic the subjective feelings of intoxication in humans (Krupitsky et al. 2007b) and substitute for the discriminative stimulus effects of alcohol in rodents (Gass and Olive 2008; Hundt et al. 1998).
NMDAR ligand binding in human post-mortem brains is increased in cortical and limbic areas of alcoholics in some (Freund and Anderson 1999), but not other studies (Villegas et al. 2011)—perhaps reflecting differences in the extent of alcohol exposure, ante-mortem duration of abstinence, and cumulative effects of alcohol-related neurotoxicity. Abstinent alcoholics have also been shown to have an attenuated response to the perceptual and cognitive effects of the NMDAR antagonist ketamine (Krystal et al. 2003). Studies using distinct pharmacologic probes of NMDAR function have yielded evidence that alcohol dependence and the familial risk for alcoholism are associated with tolerance to the effects of NMDAR antagonists (Petrakis et al. 2004), suggestive of persisting upregulation of NMDAR function.
Rodents chronically exposed to alcohol also exhibit alterations in the expression of NMDARs in various brain regions. Typically, there is an upregulation of NMDARs early in alcohol abstinence that often rapidly normalizes, within days of the last alcohol exposure (for reviews see, Gass and Olive 2008; Kroener et al. 2012; Kumari and Ticku 2000; Ron 2004). However, decreased NMDAR expression and synaptic function, with associated behavioral alterations has also been found after chronic alcohol exposure (Abrahao et al. 2013; Holmes et al. 2012; Meinhardt et al. 2013). Indeed, the net effect of ethanol–NMDAR inhibition on neural excitability is complex and likely differs across doses, brain regions, and circuits.
In some circuits, inhibition of NMDARs depresses cortical excitability (Thomson et al. 1985), but in others NMDAR inhibition impairs the recruitment of GABA neurons, resulting in glutamatergic disinhibition and enhanced stimulation of non-NMDA glutamate receptors (Grunze et al. 1996; Moghaddam et al. 1997). Alcohol shows this pattern of effect, disinhibiting glutamate release in some circuits at doses associated with human alcohol intoxication, but more uniformly suppressing cortical excitability at higher doses (Moghaddam and Bolinao 1994). However, the facilitatory effects of ethanol on inhibitory neurotransmission may suppress some behavioral effects of potent NMDAR antagonists, such as their psychotomimetic effects (Krystal et al. 2003). Thus, while the effects of potent NMDAR antagonists fully generalize to those of ethanol, ethanol effects only partially generalize to those of NMDAR antagonists (Grant and Colombo 1993). Further, in animals and humans, NMDAR antagonists are more similar to the effects of “heavy” drinking (five or more drinks in men, four or more drinks in women) than lighter forms of drinking (Krystal et al. 1998).
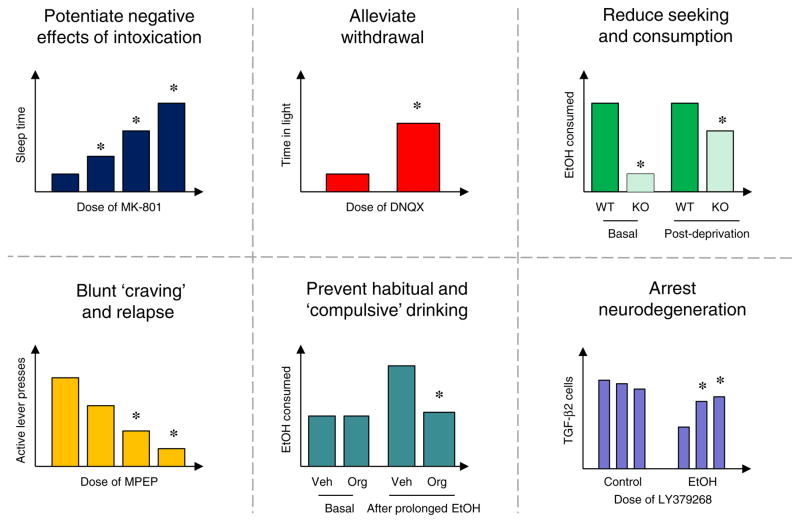
A large body of data demonstrates profound effects of NMDAR blockers on a variety of alcohol behaviors in rodents. For example, treatment with NMDAR channel blockers, such as MK-801 and memantine, potentiate the acute locomotor and sedative effects of alcohol administration (Boyce-Rustay and Cunningham 2004; Chen and Holmes 2009; Kuribara 1994; Meyer and Phillips 2003; Palachick et al. 2008; Shen and Phillips 1998; Vanover 1999; Wilson et al. 1990) (Fig. 3). These drugs can also reduce alcohol self-administration and cue-induced alcohol-seeking (Backstrom and Hyytia 2006; 2007; Rassnick et al. 1992), disrupt reconsolidation of alcohol-related memories (von der Goltz et al. 2009), dampen elevations in drinking produced by periods of forced abstinence (e.g., Vengeliene et al. 2005), and alleviate behavioral and neurotoxic effects of alcohol withdrawal (e.g., Grant et al. 1990; Stepanyan et al. 2008). These findings suggest that NMDAR antagonism could exert a multi-pronged therapeutic profile; partially substituting alcohol to lessen withdrawal and craving, while simultaneously suppressing withdrawal severity and discouraging drinking by reversing tolerance and increasing negative subjective effects of intoxication (Krystal et al. 2003). Neuroprotective effects of blocking NMDARs could also potentially mitigate neurotoxic the effects of alcohol abuse caused by hyperglutamatergia (Crews and Nixon 2009; Sullivan and Pfefferbaum 2005).
Fig. 3.
Preclinical examples of glutamate-based approaches to therapeutically targeting various elements of alcoholism. Potentiating alcohol intoxication: mice administered the NMDAR antagonist, MK-801, show markedly prolonged sedative/hypnotic responses to ethanol (Palachick et al. 2008). Alleviating alcohol withdrawal: rats infused with the AMPAR antagonist DNQX directly into amygdala exhibited less ethanol-induced withdrawal anxiety (Lack et al. 2007). Blunting alcohol craving: mice with deletion of the NMDAR anchoring protein PSD-95 consumed less ethanol at baseline and after deprivation (Camp et al. 2011). Reducing alcohol-seeking and consumption: mice with deletion of the NMDAR anchoring protein PSD-95 consumed less ethanol at baseline and after deprivation (Camp et al. 2011). Blunting alcohol craving: rats administered the mGluR5 antagonist, MPEP, reduced ethanol-seeking (black bars active lever presses) during cue-induced reinstatement (Cowen et al. 2003). Preventing habitual and compulsive alcohol consumption: rats administered the GlyT1 transporter blocker, Org25935, decreased “compulsive-like” alcohol consumption after a history of chronic ethanol treatment/deprivation (Vengeliene et al. 2010). Arresting alcohol-induced neurodegeneration: rats treated with the mGluR2/3 agonist, LY379268, were protected against binge ethanol-induced increased in TGF-β2-immunoreactivity, a measure of neurodegeneration. Adapted from Cippitelli et al. 2010
The predicted effects of NMDAR antagonists from preclinical studies have been explored to some extent in the clinic with memantine. Memantine has rapid on/off kinetics at the NMDAR, which may account for the drug being safe and well-tolerated with relatively minor abuse and psychotomimetic potential. In alcohol-dependent patients, memantine has been found to reduce withdrawal (Krupitsky et al. 2007b) and cue-induced alcohol craving (Krupitsky et al. 2007a), and to reduce alcohol consumption in moderate drinkers (Bisaga and Evans 2004). Disappointingly, however, neither memantine (in a double-blind study) (Evans et al. 2007) nor the chemically similar compound, neramexane, reduced drinking in rats or prevented relapse in alcohol-dependent patients (Spanagel and Vengeliene 2013). One interpretation of these various negative findings is that NMDAR antagonism may only be sufficient to reduce alcohol abuse in moderate cases, but may be of limited efficacy in preventing drinking in chronic alcoholism. Higher doses of memantine may be needed are needed to produce NMDAR-like substitution effects and thereby reduce craving, drinking, and relapse in such patients. Alternatively, the alcohol-like subjective effects and executive dysfunction produced by these drugs may have obscured their therapeutic effects (Krupitsky et al. 2007a). More clinical work will be needed to clarify these issues.
NMDA receptor subunits
A caveat to developing drugs for alcoholism that target NMDAR is the potential for poor tolerability due to the likelihood of cognitive and perceptual side-effects. Some of these unwanted effects might be avoided by targeting modulatory sites on the NMDAR. NMDARs are heteromeric complexes consisting of two obligatory GluN1 subunits and various combinations of GluN2 (A-D) (and sometimes GluN3) subunits. Subunit composition determines the pharmacological, biophysical and behavioral properties of the receptor (Brigman et al. 2013; Brigman et al. 2010; Woodward 2000). While the direct site of alcohol binding is probably on GluN1 (Ronald et al. 2001), the presence of the GluN2A and GluN2B subunits, in particular, have been shown to determine NMDAR sensitivity to alcohol (Woodward 2000). GluN2A and GluN2B also have intracellular tails with binding sites modulating NMDAR function (e.g., via phosphorylation state) and downstream signaling pathways implicated in alcohol behaviors (reviewed in Chandler 2003; Newton and Messing 2006).
The expression GluN2A and/or GluN2B subunits is altered in the brain after chronic alcohol (Carpenter-Hyland et al. 2004; Kroener et al. 2012; Qiang et al. 2007; Roberto et al. 2004), with some exceptions. For example, prolonged ethanol exposure increased GluN2B gene expression but reduced GluN2A mRNA in rat prefrontal cortex (Meinhardt et al. 2013), and did not alter mRNA for either subunit in postmortem brain or neuronal cultures from alcohol-dependent patients (Lieberman et al. 2012; Ridge et al. 2008). Varying changes in both mRNA and protein levels of these subunits have also been observed in striatal and amygdala subregions following chronic alcohol exposure in rodents (e.g., Falco et al. 2009; Floyd et al. 2003; Lack et al. 2005; Obara et al. 2009) and it remains unclear whether GluN2A and GluN2B expression may be differentially affected by chronic alcohol in vivo.
Parsing the role of GluN2A is hampered by the absence of pharmacological probes selective for this subunit (Kash and Winder 2007; Neyton and Paoletti 2006). However, independent genetic association studies have found a significant relationship between human GluN2A gene variation and alcoholism, an association that appeared to be especially strong in individuals scoring high on stress traits (Domart et al. 2012; Schumann et al. 2008). In an interesting parallel, mouse GluN2A gene deletion increases alcohol-induced ataxia and impairs alcohol conditioned place preference, and also attenuates stress- and anxiety-related behaviors (Boyce-Rustay and Holmes 2006a, b).
Regarding GluN2B, this subunit regulates alcohol-induced synaptic plasticity in the bed nucleus of the stria terminalis (Kash et al. 2008; Wills et al. 2012). Moreover, treatment with the selective GluN2B antagonists ifenprodil and Ro 25-6981 lessens deprivation-induced alcohol drinking (Vengeliene et al. 2005). In contrast to NMDAR channel blockers, however, GluN2B antagonists have minimal effects on acute alcohol intoxication or alcohol conditioned place preference (Boyce-Rustay and Cunningham 2004; Boyce-Rustay and Holmes 2005; Palachick et al. 2008). Interestingly, more selective GluN2B deletion on forebrain pyramidal neurons potentiates some measures of alcohol intoxication (stimulant) while attenuating others (depressant) (Badanich et al. 2011). These preliminary findings warrant follow-up, which will be facilitated by access to brain-penetrant bioavailable GluN2B antagonists, such as AZD 6765, that are currently being investigated for neuropsychiatric conditions such as major depression (Zarate et al. 2012).
Finally, the potential for targeting NMDAR interacting molecules should not be discounted. Examples include mammalian target of rapamycin complex 1 which mediates the NMDAR mRNA translation and encodes alcohol-related memories (Barak et al. 2013), and GluN2A/GluN2B trafficking regulator Homer2, which is upregulated by chronic alcohol (Cozzoli et al. 2009; Obara et al. 2009) and is associated with genetic liability to consume alcohol in mice (Goulding et al. 2011). Another example is the NMDAR anchoring protein, PSD-95, which increasingly clusters with NMDARs after chronic alcohol and, when deleted, significantly markedly potentiates alcohol intoxication and attenuates alcohol drinking (Camp et al. 2011; Mulholland and Chandler 2007) (Fig. 3).
NMDAR glycineB binding site
Another regulatory site that might be targeted in the treatment of alcohol abuse is the strychnine-insensitive (glycineB) co-agonist site (Clements and Westbrook 1991). GlycineB binding allosterically modulates the NMDAR, for example by enhancing the receptor’s affinity for glutamate and retarding receptor desensitization (Fadda et al. 1988; Parsons et al. 1998; Vyklicky et al. 1990). By altering NMDAR function, pharmacologically manipulating the glycineB site could modify NMDAR-mediated effects of alcohol in various ways. Blocking the site would remove a positive allosteric action on the NMDAR and thereby exert NMDAR antagonist-like effects with attendant changes in alcohol’s effects on intoxication (increased), withdrawal (decreased), and alcohol-seeking and consumption (decreased). On the other hand, stimulating the glycineB site would augment NMDAR function and potentially have the opposite effects on alcohol behaviors. The modulatory nature of the glycineB site also raises the possibility that targeting the site for its anti-alcohol actions may produce fewer side-effects (e.g., cognitive, psychotomimetic) than, for example, NMDAR antagonists (Parsons et al. 1998).
A role for the glycineB site in alcohol behaviors is supported by preclinical findings. One approach has been to pharmacologically raise endogenous glycine levels by blocking the glycine reuptake. In rats, treatment with the GlyT1 transporter blocker, Org25935, decreased alcohol drinking under non-dependent conditions (Lido et al. 2012; Molander et al. 2007) and produced a long-lasting reduction in “compulsive-like” alcohol consumption after a history of chronic alcohol treatment/deprivation (Vengeliene et al. 2010) (Fig. 3). The GlyT1 inhibitors ALX-5407 and NFPS have been shown to significantly potentiate acute intoxication (Debrouse et al. 2013) but these effects are likely due to actions at strychnine-sensitive glycine receptors (Adermark et al. 2011; Molander and Soderpalm 2005).
A more direct approach to targeting the glycineB site has been to directly activate or antagonize the site. The glycineB agonist, D-serine, or the partial agonist D-cycloserine, has been found to block the anxiolytic-like effects of alcohol (Moraes Ferreira and Morato 1997), promote tolerance to alcohol-induced ataxia (Khanna et al. 1993; Khanna et al. 1995), facilitate extinction of conditioned reward-related effects of alcohol (Vengeliene et al. 2008b) (but see Groblewski et al. 2009), reduce alcohol drinking (Lockridge et al. 2012), and attenuate acute intoxication in some but not all studies (Debrouse et al. 2013; Lockridge et al. 2012). On the other hand, the glycineB partial antagonist, L-701,324, substitutes for alcohol (Kotlinska and Liljequist 1997) (but see Bienkowski et al. 1997), potentiates acute intoxication (Debrouse et al. 2013), blocks alcohol conditioned place preference (Biala and Kotlinska 1999) and reduces both cue-induced reinstatement (Backstrom and Hyytia 2004; 2006) and deprivation-induced alcohol drinking (Alen et al. 2009; Vengeliene et al. 2005)—all effects predicted from an NMDAR antagonist-like action of the drug.
There has only been limited clinical investigation of glycineB-acting compounds to date. Administration of high dose D-cycloserine, or co-administration of D-cycloserine and alcohol, mimics/augments the subjective feelings of alcohol intoxication in healthy subjects, but does so in a blunted manner in alcohol-dependent individuals (Krystal et al. 2011; Trevisan et al. 2008). That D-cycloserine produced mild effects similar to NMDAR antagonism in these studies could be due to the known NMDAR antagonist activity of the drug at higher doses or high endogenous glycine levels (Emmett et al. 1991). In this context, raising glycine levels via glycine administration increased the alcohol-related effects of D-cycloserine in controls, but again less so in those with alcohol dependence (Krystal et al. 2011). The insensitivity of alcohol-dependent patients to D-cycloserine under these conditions may be explained by a relative failure to oppose a state of hyperglutamatergia and NMDAR upregulation in these patients. In turn, this suggests that more potent glycineB blockade may be necessary to produce therapeutic effects in patients suffering from an alcohol use disorder.
AMPA and kainate receptors
Ionotropic AMPAR and KAR function is sensitive to alcohol, but to a lesser and less direct extent than is NMDAR function (Costa et al. 2000; Dildy-Mayfield and Harris 1992; Lovinger et al. 1989; Moykkynen et al. 2003). In rodents, chronic alcohol enhances AMPAR-mediated currents in the basolateral amygdala and ventral tegmental area, and increases expression and synaptic localization of the GluA1 and GluA2 subunits in cortical, nucleus accumbens and dorsal striatal tissue or cultures (Ary et al. 2012; Chandler et al. 1999; Chen et al. 1999; Christian et al. 2012; Heikkinen et al. 2009; Lack et al. 2007; Neasta et al. 2010; Stuber et al. 2008; Wang et al. 2012). Also of note, GluA1 and GluA3 immunoreactivity is increased in the post-mortem alcohol brain (Breese et al. 1995), and variation in coding for the multi-PDZ gene, which is involved in NMDAR-mediated AMPAR trafficking, has been associated with risk for alcohol dependence (Czachowski et al. 2012). These adaptive alterations are intriguing in the context of evidence that other drugs of abuse, such as cocaine, produce profound changes in AMPAR-mediated plasticity in some of these same brain regions (Bellone and Luscher 2012; Wolf and Tseng 2012).
Although AMPARs are unlikely to directly mediate alcohol’s effects at self-administered doses, the receptors are well placed to modulate the function of alcohol-mediating brain circuits. Indeed, the importance of AMPARs to alcohol-related behaviors has been borne out by a number of published reports. Preclinical studies have found reduced alcohol-seeking behaviors after injection of the mixed AMPAR/ KAR antagonists CNQX and NBQX, either systemically or directly into the nucleus accumbens or ventral tegmental area, while the more specific AMPAR blocker, GYKI 52466, did so in some but not all studies (Backstrom and Hyytia 2004; 2006; 2007; Czachowski et al. 2012; Sanchis-Segura et al. 2006; Stephens and Brown 1999). GYKI 52466 has also been shown to reduce locomotor stimulant effects and the expression of sensitized locomotor responses to alcohol (Broadbent et al. 2003). In addition, DNQX injected directly into amygdala attenuates alcohol withdrawal (Lack et al. 2007) (Fig. 3), while NBQX into the dorsal striatum reduces alcohol consumption (Wang et al. 2012). With regards to the specific AMPAR subunits that may mediate these drug effects, gene deletion of the GluA1 or GluA3 subunits has minimal effects on alcohol intoxication, tolerance, or drinking (Cowen et al. 2003; Palachick et al. 2008), whereas GluA3 deletion attenuates cue-driven alcohol-seeking and deprivation-induced drinking (Sanchis-Segura et al. 2006). Thus, although AMPAR subunit contributions remain to be fully clarified, GluA3 in particular appears to play a prominent role.
Pharmacologically enhancing AMPAR function produces some effects opposite to antagonism. Treatment with the positive allosteric AMPAR modulator, aniracetam, promotes alcohol-seeking behaviors in a DNQX-dependent manner (Cannady et al. 2013). Additionally, the “AMPAkines,” LY404187 and LY451395, were found to reverse some of the disruptions in locomotor function caused by alcohol, possibly by exerting opposite (i.e., facilitating) effects on these behaviors (Jones et al. 2008). These data suggest that pharmacologically manipulating AMPARs can bidirectionally modulate alcohol behaviors, with AMPAR inhibition predicted to be of therapeutic benefit for alcoholism. However, with the exception of the aforementioned example of topiramate, which has AMPAR/KAR inhibiting properties and off-label efficacy for alcoholism, this hypothesis remains to be investigated clinically.
Also of uncertain clinical relevance are the alcohol-related effects of manipulating KAR. This is in part due to a relative paucity of pharmacological tools with selectivity for KAR over AMPAR. However, it has been shown that acute alcohol potently inhibits KAR in the amygdala and hippocampus and that there is an upregulation of amygdala KAR function associated with increased anxiety during withdrawal from chronic alcohol (Carta et al. 2003; Lack et al. 2008; Weiner et al. 1999) (but see Chandler et al. 1999; Ferreira et al. 2001). Taken together with evidence that amygdala KAR modulate anxiety-related behaviors in alcohol-naïve subjects (Lack et al. 2007; Mozhui et al. 2010), these initial data raise the possibility that inhibiting KAR could be a strategy for alleviating alcohol withdrawal.
Metabotropic glutamate receptors
mGluRs are G-protein-coupled receptors localized both pre-and post-synaptically throughout brain regions important for alcohol-related behaviors. Postsynaptic Group I mGluRs (mGluR1, mGluR5) have been intensively studied for their effect on rodent alcohol behaviors. Interestingly, gene variation in mGluR5 shows an association with risk for alcoholism (Schumann et al. 2008). In rodent studies, gene deletion or pharmacological blockade (via MPEP) of mGluR5 increases sensitivity to acute alcohol administration (Bird et al. 2008; Blednov and Harris 2008; Downing et al. 2010; Sharko and Hodge 2008). In addition, multiple studies have now shown that the mGluR5 deletion or antagonist treatment with MPEP and MTEP attenuates alcohol drinking, withdrawal, place preference and cue-induced alcohol-seeking (Adams et al. 2008; Backstrom et al. 2004; Backstrom and Hyytia 2006; 2007; Besheer et al. 2008; Besheer et al. 2010; Bird et al. 2008; Blednov and Harris 2008; Cowen et al. 2005; Cowen et al. 2007; Gupta et al. 2008; Hodge et al. 2006; Kotlinska et al. 2011; Lominac et al. 2006; Olive et al. 2005; Schroeder et al. 2005; Schroeder et al. 2008; Sidhpura et al. 2010) (but see Olive and Becker 2008) (Fig. 3).
The effects of mGluR5 inhibition on consumption and ethanol-seeking have been linked to mGluR5 activation specifically within the nucleus accumbens and basolateral amygdala (Besheer et al. 2010; Cozzoli et al. 2012; Cozzoli et al. 2009; Gass and Olive 2009; Sinclair et al. 2012). Recent work has also attributed deprivation-induced alcohol drinking to a population of mGluR5 receptors expressed on dopamine D1 receptor neurons (Parkitna et al. 2013). These findings support the clinical potential of mGluR5 antagonists as treatments for alcoholism. There do, however, remain issues with regards to potential unwanted effects of these drugs on other certain behaviors, including cognitive impairment (Simonyi et al. 2010), appetite suppression (Watterson et al. 2013), and even abuse liability (van der Kam et al. 2009) (c.f., Herzig et al. 2005), that remain to be fully clarified.
Of the other mGluRs, the presynaptic, glutamate inhibiting properties of the mGluR Group II family (mGluR2, mGluR3) are of significant interest to various neuropsychiatric disorders characterized by glutamatergic disturbances (Schoepp 2001). A new generation of mGluR2/3 selective agonists has attracted significant interest for their potential utility in schizophrenia (Karlsson et al. 2008; Patil et al. 2007) and has been studied in preclinical models of alcohol abuse. Treatment with mGluR2/3 agonists (LY379268, LY404039) reduces cue- and stress-induced alcohol-seeking in rats, and appears to do so most robustly in alcohol-preferring and alcohol-dependent subjects (Backstrom and Hyytia 2005; Kufahl et al. 2011; Rodd et al. 2006; Sidhpura et al. 2010; Zhao et al. 2006).
Extending these data, prolonged chronic ethanol exposure has been found to increase nucleus accumbens and central amygdala mGluR1 protein (Obara et al. 2009), reduce prefrontal cortical gene expression of mGluR2, but not mGluR3, and attenuate mGluR2 agonist inhibition of glutamate release in the nucleus accumbens (Meinhardt et al. 2013) (but see Kufahl et al. 2011). Post-mortem analysis revealed a similar decrease in mGluR2 gene expression in the frontal cortex of human alcohol-dependent patients (Meinhardt et al. 2013). Virally re-expressing mGluR2 in the infralimbic cortex of alcohol-exposed rats was sufficient to reduce cue-induced reinstatement (Meinhardt et al. 2013). These effects could conceivably reflect presynaptic inhibition of glutamate release and the attenuation of a hyperglutamatergic state. A similar mechanism might also account for the effect of LY379268 treatment to prevent alcohol-induced neurodegeneration in the entorhinal cortex (Cippitelli et al. 2010) (Fig. 3). Thus, mGluR2/3 agonists could serve a dual role—suppressing alcohol drinking while at the same time protect against the neural damage cause by chronic abuse. Studies in human subjects are awaited to test these hypotheses.
Glutamate transporters
Various approaches to reducing excess extracellular glutamate induced by chronic alcohol have been explored (e.g., via treatment with the cysteine pro-drug, N-acetylcysteine, Ferreira Seiva et al. 2009). Glutamate transporters remove glutamate from the synaptic and greater extracellular space. Expression of the glutamate transporters, GLAST and GLT-1, is reduced in post-mortem brains of those with alcohol dependence (Kryger and Wilce 2010). While this reduction likely reflects broader neurodegenerative changes, loss of transporter-mediated glutamate reuptake could augment the already elevated extracellular levels of glutamate resulting from chronic alcohol exposure. An animal model of deficient glutamate transport would therefore be predicted to produce a hyperglutamatergic state analogous with alcohol withdrawal, and thereby promote excessive alcohol drinking.
Two mutant mouse models have been generated that allow for the testing of this hypothesis. The first is a constitutive gene deletion of GLAST. These GLAST-deficient mutants exhibit reduced alcohol consumption and a loss of alcohol conditioned place preference, a seemingly paradoxical phenotype that may be due to the compensatory downregulation of (“pro-alcohol”) striatal endocannabinoid signaling observed in these mice (Karlsson et al. 2012). The second model involves gene deletion of the circadian clock gene, Per2. Per 2 knockout mice were found to have reduced GLAST expression and a concomitant increase in striatal extracellular glutamate (Spanagel et al. 2005). These mutants showed excessive alcohol drinking, which was reversed by acamprosate treatment (Brager et al. 2011; Spanagel et al. 2005). These data have been extended to humans by the finding that genetic variation in the human PER2 gene is associated with relative risk for alcoholism (Lee et al. 2011; Spanagel et al. 2005).
What do these findings mean for designing medications for alcoholism that target glutamate transport? One scheme posits that drugs that boost transport could “mop up” excessive glutamate in the alcohol brain and not only work to alleviate withdrawal and craving, but also possibly lessen some of the neuroadaptive and neurotoxic effects caused by a hyperglutamatergic state. Interestingly, recent studies have shown that ceftriaxone and GPI-1046 (another drug known to upregulate GLT-1) can reduce alcohol drinking in alcohol-preferring rats, in addition to blocking psychostimulant reinstatement (Abulseoud et al. 2012; Sari et al. 2011; Sari and Sreemantula 2012). These encouraging preliminary findings warrant further study.
Summary and conclusions
There is an ever growing understanding of how pharmacologically manipulating glutamatergic neurotransmission can alter alcohol behaviors, including intoxication, withdrawal, craving and alcohol-seeking, in both rodent and human subjects. Of the five major components (NMDAR, the NMDAR glycineB site, AMPAR, mGluR, and glutamate transporters) of the glutamate system considered here, all have promise as targets to alleviate symptoms of alcohol use disorders.
Both the NMDAR and AMPAR clearly have an important role in mediating alcohol consumption in rodents, but blocking these receptors might be therapeutically difficult due to associated side-effects. Selectively targeting individual subunits, for example GluN2B or GluA3, may be one way to circumvent this problem. Another approach is to target the glycineB site on the NMDAR. A consistent literature has emerged in support of the ability of mGluR5 antagonists to reduce a range of alcohol-related behaviors in rodents, but the issue of possible side-effects remains to be addressed. Alternatively, drugs that stimulate mGluR2/3 have potential to suppress alcohol drinking and even mitigate neurotoxic effects associated with elevations in glutamate by presynaptically inhibiting neurotransmitter release. Similar effects could be achieved by increasing glutamate transporter function to actively remove glutamate from the synaptic space.
To build upon this foundation and develop safe, well-tolerated pharmacotherapeutics that target a transmitter system as ubiquitous as glutamate is still a major challenge for drug development. However, we believe that by combining basic mechanistic discoveries with well-designed clinical investigations real progress can be made in the coming years to provide new, effective treatments for alcoholism.
Acknowledgments
AH is supported by the NIAAA intramural research program. RS is supported by the Bundesministerium für Bildung und Forschung (NGFN Plus; FKZ: 01GS08152, see under Spanagel et al., 2010 and www.ngfn-alkohol.de), the Deutsche Forschungsgemeinschaft (DFG): SFB 636 (B1) and Reinhart-Koselleck Award SP 383/5-1, and the MWK in Baden-Württemberg. JHK is supported by the U.S. National Institute on Alcohol Abuse and Alcoholism (2P50AA012870), the National Center on Advancing Translational Science (CTSA Grant Number UL1 RR024139), the U.S. Department of Veterans Affairs through its support for the VA National Center for PTSD, and Yale–New Haven Hospital.
Contributor Information
Andrew Holmes, Email: holmesan@mail.nih.gov, Laboratory of Behavioral and Genomic Neuroscience, National Institute on Alcohol Abuse and Alcoholism, NIH, Bethesda, MD, USA. 5625 Fishers Lane Room 2N09, Rockville, MD 20852-9411, USA.
Rainer Spanagel, Institute of Psychopharmacology, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Heidelberg, Germany.
John H. Krystal, NIAAA Center for the Translational Neuroscience of Alcoholism, Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA
References
- Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, Carter E, Alexander NP, McCool BA, Souza-Formigoni ML, Weiner JL. Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J Neurosci. 2013;33:4834–4842. doi: 10.1523/JNEUROSCI.5839-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CL, Cowen MS, Short JL, Lawrence AJ. Combined antagonism of glutamate mGlu5 and adenosine A2A receptors interact to regulate alcohol-seeking in rats. Int J Neuropsychopharmacol. 2008;11:229–241. doi: 10.1017/S1461145707007845. [DOI] [PubMed] [Google Scholar]
- Adermark L, Clarke RB, Olsson T, Hansson E, Soderpalm B, Ericson M. Implications for glycine receptors and astrocytes in ethanol-induced elevation of dopamine levels in the nucleus accumbens. Addict Biol. 2011;16:43–54. doi: 10.1111/j.1369-1600.2010.00206.x. [DOI] [PubMed] [Google Scholar]
- Alen F, Santos A, Moreno-Sanz G, Gonzalez-Cuevas G, Gine E, Franco-Ruiz L, Navarro M, Lopez-Moreno JA. Cannabinoid-induced increase in relapse-like drinking is prevented by the blockade of the glycine-binding site of N-methyl-D-aspartate receptors. Neuroscience. 2009;158:465–473. doi: 10.1016/j.neuroscience.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. Jam. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Ary AW, Cozzoli DK, Finn DA, Crabbe JC, Dehoff MH, Worley PF, Szumlinski KK. Ethanol up-regulates nucleus accumbens neuronal activity dependent pentraxin (Narp): implications for alcohol-induced behavioral plasticity. Alcohol. 2012;46:377–387. doi: 10.1016/j.alcohol.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–565. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Doremus-Fitzwater TL, Mulholland PJ, Randall PK, Delpire E, Becker HC. NR2B-deficient mice are more sensitive to the locomotor stimulant and depressant effects of ethanol. Genes Brain Behav. 2011;10:805–816. doi: 10.1111/j.1601-183X.2011.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltieri DA, Daro FR, Ribeiro PL, de Andrade AG. Comparing topiramate with naltrexone in the treatment of alcohol dependence. Addiction. 2008;103:2035–2044. doi: 10.1111/j.1360-0443.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- Barak S, Liu F, Hamida SB, Yowell QV, Neasta J, Kharazia V, Janak PH, Ron D. Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci. 2013 doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Drug-evoked plasticity: do addictive drugs reopen a critical period of postnatal synaptic development? Front Mol Neurosci. 2012;5:75. doi: 10.3389/fnmol.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Kotlinska J. Blockade of the acquisition of ethanol-induced conditioned place preference by N-methyl-D-aspartate receptor antagonists. Alcohol Alcohol. 1999;34:175–182. doi: 10.1093/alcalc/34.2.175. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Stefanski R, Kostowski W. Discriminative stimulus effects of ethanol: lack of antagonism with N-methyl-D-aspartate and D-cycloserine. Alcohol. 1997;14:345–350. doi: 10.1016/s0741-8329(96)00181-4. [DOI] [PubMed] [Google Scholar]
- Bird MK, Kirchhoff J, Djouma E, Lawrence AJ. Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol. 2008;11:765–774. doi: 10.1017/S1461145708008572. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology (Berl) 2004;172:16–24. doi: 10.1007/s00213-003-1617-5. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci. 2004;118:822–834. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Functional roles of NMDA receptor NR2A and NR2B subunits in the acute intoxicating effects of ethanol in mice. Synapse. 2005;56:222–225. doi: 10.1002/syn.20143. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006a;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006b;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Prosser RA, Glass JD. Circadian and acamprosate modulation of elevated ethanol drinking in mPer2 clock gene mutant mice. Chronobiol Int. 2011;28:664–672. doi: 10.3109/07420528.2011.601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasser SM, McCaul ME, Houtsmuller EJ. Alcohol effects during acamprosate treatment: a dose–response study in humans. Alcohol Clin Exp Res. 2004;28:1074–1083. doi: 10.1097/01.alc.0000130802.07692.29. [DOI] [PubMed] [Google Scholar]
- Breese CR, Freedman R, Leonard SS. Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Res. 1995;674:82–90. doi: 10.1016/0006-8993(94)01384-t. [DOI] [PubMed] [Google Scholar]
- Breslin FJ, Johnson BA, Lynch WJ. Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology (Berl) 2010;207:529–534. doi: 10.1007/s00213-009-1683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Daut RA, Wright T, Gunduz-Cinar O, Graybeal C, Davis MI, Jiang Z, Saksida LM, Jinde S, Pease M, Bussey TJ, Lovinger DM, Nakazawa K, Holmes A. GluN2B in corticostriatal circuits governs choice learning and choice shifting. Nat Neurosci. 2013 doi: 10.1038/nn.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30:4590–4600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Kampmueller KM, Koonse SA. Expression of behavioral sensitization to ethanol by DBA/2J mice: the role of NMDA and non-NMDA glutamate receptors. Psychopharmacology (Berl) 2003;167:225–234. doi: 10.1007/s00213-003-1404-3. [DOI] [PubMed] [Google Scholar]
- Burattini C, McGeehan AJ, Griffin WC, 3rd, Gass JT, Kinder JR, Janak PH, Olive MF. A microdialysis study of extracellular levels of acamprosate and naltrexone in the rat brain following acute and repeated administration. Addict Biol. 2008;13:70–79. doi: 10.1111/j.1369-1600.2008.00097.x. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Baicy KJ, Olsen RW. Topiramate attenuates withdrawal signs after chronic intermittent ethanol in rats. Neuroreport. 2004;15:207–210. doi: 10.1097/00001756-200401190-00040. [DOI] [PubMed] [Google Scholar]
- Camp MC, Feyder M, Ihne J, Palachick B, Hurd B, Karlsson RM, Noronha B, Chen YC, Coba MP, Grant SG, Holmes A. A novel role for PSD-95 in mediating ethanol intoxication, drinking and place preference. Addict Biol. 2011;16(3):428–439. doi: 10.1111/j.1369-1600.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Fisher KR, Durant B, Besheer J, Hodge CW. Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement. Addict Biol. 2013;18:54–65. doi: 10.1111/adb.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci U S A. 2003;100:6813–6818. doi: 10.1073/pnas.1137276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–370. [PubMed] [Google Scholar]
- Chen F, Jarrott B, Lawrence AJ. Upregulation of cortical AMPA receptor binding in the fawn-hooded rat following ethanol withdrawal. Eur J Pharmacol. 1999;384:139–146. doi: 10.1016/s0014-2999(99)00675-5. [DOI] [PubMed] [Google Scholar]
- Chen YC, Holmes A. Effects of topiramate and other anti-glutamatergic drugs on the acute intoxicating actions of ethanol in mice: modulation by genetic strain and stress. Neuropsychopharmacology. 2009;34:1454–1466. doi: 10.1038/npp.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Frankola K, Goldstein A, Thorsell A, Singley E, Eskay RL, Heilig M. Alcohol-induced neurodegeneration, suppression of transforming growth factor-beta, and cognitive impairment in rats: prevention by group II metabotropic glutamate receptor activation. Biol Psychiatry. 2010;67(9):823–830. doi: 10.1016/j.biopsych.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Jones CK. Promise of mGluR2/3 activators in psychiatry. Neuropsychopharmacology. 2009;34:248–249. doi: 10.1038/npp.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ET, Soto EE, Cardoso RA, Olivera DS, Valenzuela CF. Acute effects of ethanol on kainate receptors in cultured hippocampal neurons. Alcohol Clin Exp Res. 2000;24:220–225. [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Krstew E, Lawrence AJ. Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology (Berl) 2007;190:21–29. doi: 10.1007/s00213-006-0583-0. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Schroff KC, Gass P, Sprengel R, Spanagel R. Neurobehavioral effects of alcohol in AMPA receptor subunit (GluR1) deficient mice. Neuropharmacology. 2003;45(3):325–333. doi: 10.1016/s0028-3908(03)00174-6. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, Wroten MG, Zhang PW, Xiao B, Hu JH, Klugmann M, Metten P, Worley PF, Crabbe JC, Szumlinski KK. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012;36:1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ, Pope JD. Behavioral and neurotransmitter specific roles for the ventral tegmental area in reinforcer-seeking and intake. Alcohol Clin Exp Res. 2012;36:1659–1668. doi: 10.1111/j.1530-0277.2012.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effects of acamprosate on excitatory amino acids during multiple ethanol withdrawal periods. Alcohol Clin Exp Res. 2003;27:465–470. doi: 10.1097/01.ALC.0000056617.68874.18. [DOI] [PubMed] [Google Scholar]
- Debrouse L, Hurd B, Kiselycznyk C, Plitt A, Todaro A, Mishina M, Grant SG, Camp M, Gunduz-Cinar O, Holmes A. Probing the modulation of acute ethanol intoxication by pharmacological manipulation of the NMDAR glycine co-agonist site. Alcohol Clin Exp Res. 2013;37:223–233. doi: 10.1111/j.1530-0277.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Harris RA. Comparison of ethanol sensitivity of rat brain kainate, DL-alpha-amino-3-hydroxy-5-methyl-4-isoxalone proprionic acid and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1992;262:487–494. [PubMed] [Google Scholar]
- Domart MC, Benyamina A, Lemoine A, Bourgain C, Blecha L, Debuire B, Reynaud M, Saffroy R. Association between a polymorphism in the promoter of a glutamate receptor subunit gene (GRIN2A) and alcoholism. Addict Biol. 2012;17:783–785. doi: 10.1111/j.1369-1600.2011.00321.x. [DOI] [PubMed] [Google Scholar]
- Downing C, Marks MJ, Larson C, Johnson TE. The metabotropic glutamate receptor subtype 5 mediates sensitivity to the sedative properties of ethanol. Pharmacogenet Genomics. 2010;20:553–564. doi: 10.1097/FPC.0b013e32833d8c20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett MR, Mick SJ, Cler JA, Rao TS, Iyengar S, Wood PL. Actions of D-cycloserine at the N-methyl-D-aspartate-associated glycine receptor site in vivo. Neuropharmacology. 1991;30:1167–1171. doi: 10.1016/0028-3908(91)90161-4. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin Exp Res. 2007;31:775–782. doi: 10.1111/j.1530-0277.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Fadda E, Danysz W, Wroblewski JT, Costa E. Glycine and D-serine increase the affinity of N-methyl-D-aspartate sensitive glutamate binding sites in rat brain synaptic membranes. Neuropharmacology. 1988;27:1183–1185. doi: 10.1016/0028-3908(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Falco AM, Bergstrom HC, Bachus SE, Smith RF. Persisting changes in basolateral amygdala mRNAs after chronic ethanol consumption. Physiol Behav. 2009;96:169–173. doi: 10.1016/j.physbeh.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Farook JM, Morrell DJ, Lewis B, Littleton JM, Barron S. Topiramate (Topamax) reduces conditioned abstinence behaviours and handling-induced convulsions (HIC) after chronic administration of alcohol in Swiss-Webster mice. Alcohol Alcohol. 2007;42:296–300. doi: 10.1093/alcalc/agm047. [DOI] [PubMed] [Google Scholar]
- Ferreira Seiva FR, Amauchi JF, Ribeiro Rocha KK, Souza GA, Ebaid GX, Burneiko RM, Novelli EL. Effects of N-acetylcysteine on alcohol abstinence and alcohol-induced adverse effects in rats. Alcohol. 2009;43:127–135. doi: 10.1016/j.alcohol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Ferreira VM, Frausto S, Browning MD, Savage DD, Morato And GS, Valenzuela CF. Ionotropic glutamate receptor subunit expression in the rat hippocampus: lack of an effect of a long-term ethanol exposure paradigm. Alcohol Clin Exp Res. 2001;25:1536–1541. [PubMed] [Google Scholar]
- Fliegel S, Brand I, Spanagel R, Noori HR. Ethanol-induced alterations of amino acids measured by in vivo microdialysis in rats: a meta-analysis. In Silicon Pharmacol. 2013;1:7. doi: 10.1186/2193-9616-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez G, Garcia-Portilla P, Alvarez S, Saiz PA, Nogueiras L, Bobes J. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32:1251–1259. doi: 10.1111/j.1530-0277.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Jung KY, McCool BA. Chronic ethanol ingestion facilitates N-methyl-D-aspartate receptor function and expression in rat lateral/basolateral amygdala neurons. J Pharmacol Exp Ther. 2003;307:1020–1029. doi: 10.1124/jpet.103.057505. [DOI] [PubMed] [Google Scholar]
- Freund G, Anderson KJ. Glutamate receptors in the cingulate cortex, hippocampus, and cerebellar vermis of alcoholics. Alcohol Clin Exp Res. 1999;23:1–6. [PubMed] [Google Scholar]
- Fuller RK, Branchey L, Brightwell DR, Derman RM, Emrick CD, Iber FL, James KE, Lacoursiere RB, Lee KK, Lowenstam I, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. Jama. 1986;256:1449–1455. [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. Jam. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, West SL, Carey TS, Lohr KN, Crews FT. Pharmacological treatment of alcohol dependence: a review of the evidence. Jam. 1999;281:1318–1325. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 2009;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, Szumlinski KK. Accumbens Homer2-mediated signaling: a factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav. 2011;10:111–126. doi: 10.1111/j.1601-183X.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993;264:1241–1247. [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Gremel CM, Gabriel KI, Cunningham CL. Topiramate does not affect the acquisition or expression of ethanol conditioned place preference in DBA/2J or C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:783–790. doi: 10.1111/j.1530-0277.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Lattal KM, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol Clin Exp Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol drinking-in-the-dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, McGregor IS. Topiramate moderately reduces the motivation to consume alcohol and has a marked antidepressant effect in rats. Alcohol Clin Exp Res. 2007;31:1900–1907. doi: 10.1111/j.1530-0277.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Heikkinen AE, Moykkynen TP, Korpi ER. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology. 2009;34:290–298. doi: 10.1038/npp.2008.89. [DOI] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Herzig V, Capuani EM, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA- or amphetamine-conditioned place preference in rats. Addict Biol. 2005;10:243–249. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, Macpherson KP, Debrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundt W, Danysz W, Holter SM, Spanagel R. Ethanol and N-methyl-D-aspartate receptor complex interactions: a detailed drug discrimination study in the rat. Psychopharmacology (Berl) 1998;135:44–51. doi: 10.1007/s002130050484. [DOI] [PubMed] [Google Scholar]
- Iheanacho T, Issa M, Marienfeld C, Rosenheck R. Use of naltrexone for alcohol use disorders in the Veterans’ Health Administration: A national study. Drug Alcohol Depend. 2013 doi: 10.1016/j.drugalcdep.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Akhtar FZ, Ma JZ. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61:905–912. doi: 10.1001/archpsyc.61.9.905. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003a;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, O’Malley SS, Ciraulo DA, Roache JD, Chambers RA, Sarid-Segal O, Couper D. Dose-ranging kinetics and behavioral pharmacology of naltrexone and acamprosate, both alone and combined, in alcohol-dependent subjects. J Clin Psychopharmacol. 2003b;23:281–293. doi: 10.1097/01.jcp.0000084029.22282.bb. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM. Topiramate for treating alcohol dependence: a randomized controlled trial. Jam. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Jones N, Messenger MJ, O’Neill MJ, Oldershaw A, Gilmour G, Simmons RM, Iyengar S, Libri V, Tricklebank M, Williams SC. AMPA receptor potentiation can prevent ethanol-induced intoxication. Neuropsychopharmacology. 2008;33:1713–1723. doi: 10.1038/sj.npp.1301562. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Adermark L, Molander A, Perreau-Lenz S, Singley E, Solomon M, Holmes A, Tanaka K, Lovinger DM, Spanagel R, Heilig M. Reduced alcohol intake and reward associated with impaired endocannabinoid signaling in mice with a deletion of the glutamate transporter GLAST. Neuropharmacology. 2012;63:181–189. doi: 10.1016/j.neuropharm.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Heilig M, Holmes A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol Psychiatry. 2008;64:810–814. doi: 10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash T, Winder DG. NMDAR LTP and LTD induction: 2B or not 2B…is that the question? Debates Neurosci. 2007;1:79–84. [Google Scholar]
- Kash TL, Matthews RT, Winder DG. Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2008;33:1379–1390. doi: 10.1038/sj.npp.1301504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Shah G, Chau A. Effect of D-cycloserine on rapid tolerance to ethanol. Pharmacol Biochem Behav. 1993;45:983–986. doi: 10.1016/0091-3057(93)90152-j. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Morato GS, Chau A, Shah G. D-cycloserine enhances rapid tolerance to ethanol motor incoordination. Pharmacol Biochem Behav. 1995;52:609–614. doi: 10.1016/0091-3057(95)00149-q. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Mercado M, Markley TL, Crosby S, Ciraulo DA, Kornetsky C. Zonisamide decreases ethanol intake in rats and mice. Pharmacol Biochem Behav. 2007;87:65–72. doi: 10.1016/j.pbb.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komanduri R. Two cases of alcohol craving curbed by topiramate. J Clin Psychiatry. 2003;64:612. doi: 10.4088/jcp.v64n0518d. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Liljequist S. The NMDA/glycine receptor antagonist, L-701,324, produces discriminative stimuli similar to those of ethanol. Eur J Pharmacol. 1997;332:1–8. doi: 10.1016/s0014-2999(97)01069-8. [DOI] [PubMed] [Google Scholar]
- Kotlinska JH, Bochenski M, Danysz W. The role of group I mGlu receptors in the expression of ethanol-induced conditioned place preference and ethanol withdrawal seizures in rats. Eur J Pharmacol. 2011;670:154–161. doi: 10.1016/j.ejphar.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007a;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Rudenko AA, Burakov AM, Slavina TY, Grinenko AA, Pittman B, Gueorguieva R, Petrakis IL, Zvartau EE, Krystal JH. Antiglutamatergic strategies for ethanol detoxification: comparison with placebo and diazepam. Alcohol Clin Exp Res. 2007b;31:604–611. doi: 10.1111/j.1530-0277.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- Kryger R, Wilce PA. The effects of alcoholism on the human basolateral amygdala. Neuroscience. 2010;167:361–371. doi: 10.1016/j.neuroscience.2010.01.061. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Nappi SK, Trevisan L, Pittman B, D’Souza DC, Suckow RF. Characterization of the interactive effects of glycine and D-cycloserine in men: further evidence for enhanced NMDA receptor function associated with human alcohol dependence. Neuropsychopharmacology. 2011;36:701–710. doi: 10.1038/npp.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, Stetson P, Trevisan LA, Charney DS. Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry. 1998;55:354–360. doi: 10.1001/archpsyc.55.4.354. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, Zautra N, Foster Olive M. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2011;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Ticku MK. Regulation of NMDA receptors by ethanol. Prog Drug Res. 2000;54:152–189. [PubMed] [Google Scholar]
- Kuribara H. Potentiation of the ambulation-increasing effect induced by combined administration of MK-801 with ethanol in mice. Psychopharmacology (Berl) 1994;113:453–456. doi: 10.1007/BF02245222. [DOI] [PubMed] [Google Scholar]
- Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98(6):3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Floyd DW, McCool BA. Chronic ethanol ingestion modulates proanxiety factors expressed in rat central amygdala. Alcohol. 2005;36:83–90. doi: 10.1016/j.alcohol.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim L, Kang SG, Yoon HK, Choi JE, Park YM, Kim SJ, Kripke DF. PER2 variation is associated with diurnal preference in a Korean young population. Behav Genet. 2011;41:273–277. doi: 10.1007/s10519-010-9396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lido HH, Marston H, Ericson M, Soderpalm B. The glycine reuptake inhibitor Org24598 and acamprosate reduce ethanol intake in the rat; tolerance development to acamprosate but not to Org24598. Addict Biol. 2012;17:897–907. doi: 10.1111/j.1369-1600.2011.00367.x. [DOI] [PubMed] [Google Scholar]
- Lieberman R, Levine ES, Kranzler HR, Abreu C, Covault J. Pilot study of iPS-derived neural cells to examine biologic effects of alcohol on human neurons in vitro. Alcohol Clin Exp Res. 2012;36:1678–1687. doi: 10.1111/j.1530-0277.2012.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990– 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge A, Romero G, Harrington J, Newland B, Gong Z, Cameron A, Yuan LL. Timing-dependent reduction in ethanol sedation and drinking preference by NMDA receptor co-agonist d-serine. Alcohol. 2012;46:389–400. doi: 10.1016/j.alcohol.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Bond C, Breslin FJ, Johnson BA. Severity of drinking as a predictor of efficacy of the combination of ondansetron and topiramate in rat models of ethanol consumption and relapse. Psychopharmacology (Berl) 2011;217:3–12. doi: 10.1007/s00213-011-2253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: the role of patient motivation. J Psychiatr Res. 2006a;40:383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006b;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33:2794–2806. doi: 10.1523/JNEUROSCI.4062-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav Neurosci. 2003;117:641–649. doi: 10.1037/0735-7044.117.3.641. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML. Biphasic effect of ethanol on extracellular accumulation of glutamate in the hippocampus and the nucleus accumbens. Neurosci Lett. 1994;178:99–102. doi: 10.1016/0304-3940(94)90299-2. [DOI] [PubMed] [Google Scholar]
- Molander A, Lido HH, Lof E, Ericson M, Soderpalm B. The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol. 2007;42:11–18. doi: 10.1093/alcalc/agl085. [DOI] [PubMed] [Google Scholar]
- Molander A, Soderpalm B. Glycine receptors regulate dopamine release in the rat nucleus accumbens. Alcohol Clin Exp Res. 2005;29:17–26. doi: 10.1097/01.alc.0000150006.17168.f7. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes Ferreira VM, Morato GS. D-cycloserine blocks the effects of ethanol and HA-966 in rats tested in the elevated plus-maze. Alcohol Clin Exp Res. 1997;21:1638–1642. [PubMed] [Google Scholar]
- Moykkynen T, Korpi ER, Lovinger DM. Ethanol inhibits alpha-amino-3-hydyroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor function in central nervous system neurons by stabilizing desensitization. J Pharmacol Exp Ther. 2003;306:546–555. doi: 10.1124/jpet.103.050666. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ. The thorny side of addiction: adaptive plasticity and dendritic spines. Sci World J. 2007;7:9–21. doi: 10.1100/tsw.2007.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen SA, Malcolm R, Middaugh LD. Topiramate reduces ethanol consumption by C57BL/6 mice. Synapse. 2007;61:150–156. doi: 10.1002/syn.20350. [DOI] [PubMed] [Google Scholar]
- NIAAA NIoAaAA; Services HaH, editor Tenth Special Report on Alcohol and Health to the US Congress on Alcohol and Health. 2000. [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Becker HC. Effects of the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MPEP on handling-induced convulsions during ethanol withdrawal in mice. Alcohol. 2008;42:191–197. doi: 10.1016/j.alcohol.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, Messing RO. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- Palachick B, Chen YC, Enoch AJ, Karlsson RM, Mishina M, Holmes A. Role of major NMDA or AMPA receptor subunits in MK-801 potentiation of ethanol intoxication. Alcohol Clin Exp Res. 2008;32(8):1479–1492. doi: 10.1111/j.1530-0277.2008.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paparrigopoulos T, Tzavellas E, Karaiskos D, Kourlaba G, Liappas I. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi: 10.1186/1471-244X-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkitna JR, Sikora M, Golda S, Golembiowska K, Bystrowska B, Engblom D, Bilbao A, Przewlocki R. Novelty-seeking behaviors and the escalation of alcohol drinking after abstinence in mice are controlled by metabotropic glutamate receptor 5 on neurons expressing dopamine d1 receptors. Biol Psychiatry. 2013;73:263–270. doi: 10.1016/j.biopsych.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Hesselink M, Hartmann S, Lorenz B, Wollenburg C, Quack G. Modulation of NMDA receptors by glycine—introduction to some basic aspects and recent developments. Amino Acids. 1998;14:207–216. doi: 10.1007/BF01345264. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Popp RL, Lovinger DM. Interaction of acamprosate with ethanol and spermine on NMDA receptors in primary cultured neurons. Eur J Pharmacol. 2000;394:221–231. doi: 10.1016/s0014-2999(00)00195-3. [DOI] [PubMed] [Google Scholar]
- Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Mol Pharmacol. 2007;72:95–102. doi: 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Ho AM, Innes DJ, Dodd PR. The expression of NMDA receptor subunit mRNA in human chronic alcoholics. Ann N Y Acad Sci. 2008;1139:10–19. doi: 10.1196/annals.1432.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Ron D. Signaling cascades regulating NMDA receptor sensitivity to ethanol. Neuroscientist. 2004;10:325–336. doi: 10.1177/1073858404263516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem. 2001;276:44729–44735. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Jimenez-Arriero MA, Palomo T, Manzanares J, Ferre F. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37:37–40. doi: 10.1055/s-2004-815473. [DOI] [PubMed] [Google Scholar]
- Rustembegovic A, Sofic E, Kroyer G. A pilot study of topiramate (Topamax) in the treatment of tonic–clonic seizures of alcohol withdrawal syndromes. Med Arh. 2002;56:211–212. [PubMed] [Google Scholar]
- Sanchis-Segura C, Borchardt T, Vengeliene V, Zghoul T, Bachteler D, Gass P, Sprengel R, Spanagel R. Involvement of the AMPA receptor GluR-C subunit in alcohol-seeking behavior and relapse. J Neurosci. 2006;26:1231–1238. doi: 10.1523/JNEUROSCI.4237-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–246. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, Rietschel M, Rujescu D, Lourdusamy A, Clarke TK, Krause K, Dyer A, Depner M, Wellek S, Treutlein J, Szegedi A, Giegling I, Cichon S, Blomeyer D, Heinz A, Heath S, Lathrop M, Wodarz N, Soyka M, Spanagel R, Mann K. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- Sharko AC, Hodge CW. Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:67–76. doi: 10.1111/j.1530-0277.2007.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EH, Phillips TJ. MK-801 potentiates ethanol’s effects on locomotor activity in mice. Pharmacol Biochem Behav. 1998;59:135–143. doi: 10.1016/s0091-3057(97)00389-4. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Schachtman TR, Christoffersen GR. Metabotropic glutamate receptor subtype 5 antagonism in learning and memory. Eur J Pharmacol. 2010;639:17–25. doi: 10.1016/j.ejphar.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, Gass JT. mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav. 2012;101:329–335. doi: 10.1016/j.pbb.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Vengeliene V. New pharmacological treatment strategies for relapse prevention. Curr Top Behav Neurosci. 2013;13:583–609. doi: 10.1007/7854_2012_205. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W, Hundt W. Acamprosate and alcohol: III. Effects on alcohol discrimination in the rat. Eur J Pharmacol. 1996;305:51–56. doi: 10.1016/0014-2999(96)00176-8. [DOI] [PubMed] [Google Scholar]
- Stepanyan TD, Farook JM, Kowalski A, Kaplan E, Barron S, Littleton JM. Alcohol withdrawal-induced hippocampal neurotoxicity in vitro and seizures in vivo are both reduced by memantine. Alcohol Clin Exp Res. 2008;32:2128–2135. doi: 10.1111/j.1530-0277.2008.00801.x. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Brown G. Disruption of operant oral self-administration of ethanol, sucrose, and saccharin by the AMPA/ kainate antagonist, NBQX, but not the AMPA antagonist, GYKI 52466. Alcohol Clin Exp Res. 1999;23:1914–1920. [PubMed] [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Lodge D. An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature. 1985;313:479–481. doi: 10.1038/313479a0. [DOI] [PubMed] [Google Scholar]
- Trevisan L, Petrakis IL, Pittman B, Gueorguieva R, D’Souza DC, Perry E, Limoncelli D, Krystal JH. Absence of significant interactive effects of high-dose D-cycloserine and ethanol in healthy human subjects: preliminary insights into ethanol actions at the glycine B site of NMDA glutamate receptors. Alcohol Clin Exp Res. 2008;32:36–42. doi: 10.1111/j.1530-0277.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT. Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry. 1998;155:726–732. doi: 10.1176/ajp.155.6.726. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kam EL, De Vry J, Tzschentke TM. The mGlu5 receptor antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) supports intravenous self-administration and induces conditioned place preference in the rat. Eur J Pharmacol. 2009;607:114–120. doi: 10.1016/j.ejphar.2009.01.049. [DOI] [PubMed] [Google Scholar]
- Vanover KE. Interaction of ethanol with excitatory amino acid receptor antagonists in mice. Eur J Pharmacol. 1999;368:137–142. doi: 10.1016/s0014-2999(99)00030-8. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008a;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Kiefer F, Spanagel R. D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol. 2008b;43:626–629. doi: 10.1093/alcalc/agn067. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R. Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol Psychiatry. 2010;68:704–711. doi: 10.1016/j.biopsych.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Villegas E, Estruch R, Mengod G, Cortes R. NMDA receptors in frontal cortex and hippocampus of alcohol consumers. Addict Biol. 2011;16:163–165. doi: 10.1111/j.1369-1600.2009.00201.x. [DOI] [PubMed] [Google Scholar]
- von der Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, Spanagel R. Cue-induced alcohol-seeking behaviour is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology (Berl) 2009;205:389–397. doi: 10.1007/s00213-009-1544-1. [DOI] [PubMed] [Google Scholar]
- Vyklicky L, Jr, Benveniste M, Mayer ML. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci. 2012;32:15124–15132. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Hood LE, Olive MF. Attenuation of reinstatement of methamphetamine-, sucrose-, and food-seeking behavior in rats by fenobam, a metabotropic glutamate receptor 5 negative allosteric modulator. Psychopharmacology (Berl) 2013;225:151–159. doi: 10.1007/s00213-012-2804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Dunwiddie TV, Valenzuela CF. Ethanol inhibition of synaptically evoked kainate responses in rat hippocampal CA3 pyramidal neurons. Mol Pharmacol. 1999;56:85–90. doi: 10.1124/mol.56.1.85. [DOI] [PubMed] [Google Scholar]
- Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, Delpire E, Winder DG. GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci U S A. 2012;109:E278–E287. doi: 10.1073/pnas.1113820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WR, Bosy TZ, Ruth JA. NMDA agonists and antagonists alter the hypnotic response to ethanol in LS and SS mice. Alcohol. 1990;7:389–395. doi: 10.1016/0741-8329(90)90021-4. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Bajer B, Gorska D, Andrzejczak D, Dyr W, Bienkowski P. Effect of repeated treatment with topiramate on voluntary alcohol intake and beta-endorphin plasma level in Warsaw alcohol high-preferring rats. Psychopharmacology (Berl) 2013;225:275–281. doi: 10.1007/s00213-012-2812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA. A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]