Effectively communicating information and engaging patients not only increases the likelihood of true shared decision making, but it also improves trust in the doctor-patient relationship and increases self-care skills.
Keywords: chronic kidney disease, decision-making, dialysis
Abstract
Background
Careful patient–clinician shared decision-making about dialysis initiation has been promoted, but few studies have addressed patient perspectives on the extent of information provided and how decisions to start dialysis are made.
Methods
Ninety-nine maintenance dialysis patients recruited from 15 outpatient dialysis centers in North Carolina completed semistructured interviews on information provision and communication about the initiation of dialysis. These data were examined with content analysis. In addition, informed decision-making (IDM) scores were created by summing patient responses (yes/no) to 10 questions about the decision-making.
Results
The mean IDM score was 4.4 (of 10; SD = 2.0); 67% scored 5 or lower. Age at the time of decision-making (r = −0.27, P = 0.006), years of education (r = 0.24, P = 0.02) and presence of a warning about progressing to end-stage kidney disease (t = 2.9, P = 0.005) were significantly associated with IDM scores. Nearly 70% said that the risks and burdens of dialysis were not mentioned at all, and only one patient recalled that the doctor offered the option of not starting dialysis. While a majority (67%) said that they felt they had no choice about starting dialysis (because the alternative would be death) or about dialysis modality, only 21.2% said that they had felt rushed to make a decision. About one-third of the patients perceived that the decision to start dialysis and modality was already made by the doctor.
Conclusions
A majority of patients felt unprepared and ill-informed about the initiation of dialysis. Improving the extent of IDM about dialysis may optimize patient preparation prior to starting treatment and their perceptions about the decision-making process.
INTRODUCTION
In the UK, nearly 7000 patients start renal replacement therapy each year [1]. In the USA, with a much higher incidence more than 110 000 patients with chronic kidney disease (CKD) progress to end-stage kidney disease (ESKD) every year [2]. Over 80% of these patients start maintenance dialysis to sustain life, but 1-, 3- and 5-year mortality rates are high: 25, 49 and 65%, respectively [2].
Given the significant burdens associated with dialysis [3] and its limited survival benefits [4], clinicians have an obligation to explain factors that may influence the patients' choice to initiate dialysis, such as the medical condition requiring dialysis, dialysis modality options (peritoneal or hemodialysis), the risks and benefits of dialysis, including prognosis, the possibility of a time-limited dialysis trial, the option of refusing dialysis and palliative care options [5]. Clinical practice guidelines [5, 6] endorse careful shared decision-making in the initiation and withdrawal of dialysis, and the Medicare Improvements for Patients and Providers Act of 2008 in the US provides reimbursement for up to six sessions of comprehensive CKD educational services for people with Stage IV CKD [7]. Nonetheless, little is known regarding the patients' perceptions of the decision-making process.
Studies have suggested that the patients' sense of the inevitability of dialysis and the notion of life-at-all-costs permeate the decision to start dialysis [8, 9]. Elderly patients with advanced CKD lack preparation for living with dialysis and rarely have discussions with nephrologists about prognosis and illness trajectories [10]. CKD patients generally perceive there to be a lack of choice in treatment decision-making [11], and if dialysis options are presented at all, they are not presented equally prior to the start of dialysis [12, 13].
However, it is not at all clear how patients perceive the extent of information on dialysis that has been provided to them. It is not known if they believe they have been informed of benefits and risks during decision-making to start dialysis and how they view their decision-making experience after starting dialysis. This study therefore examined patient perspectives on how decisions to start dialysis were made, with emphasis on the decision-making context, the information they received and their perceptions of communication with physicians prior to the decision to undergo dialysis.
MATERIALS AND METHODS
Design
This study used a qualitative descriptive design [14] nested in a multicenter randomized, controlled trial (RCT; NCT01259011) that is testing the effects of an end-of-life communication intervention on outcomes of dialysis patients and surrogate decision-makers. From September 2011 to September 2012, the baseline session of the parent RCT was expanded to include a semistructured interview focusing on perceptions about how the decision to start dialysis was made. Approval for the study was obtained from the Institutional Review Board of the University of North Carolina at Chapel Hill and the Office of Clinical Trials of the participating dialysis organizations.
Setting and sample
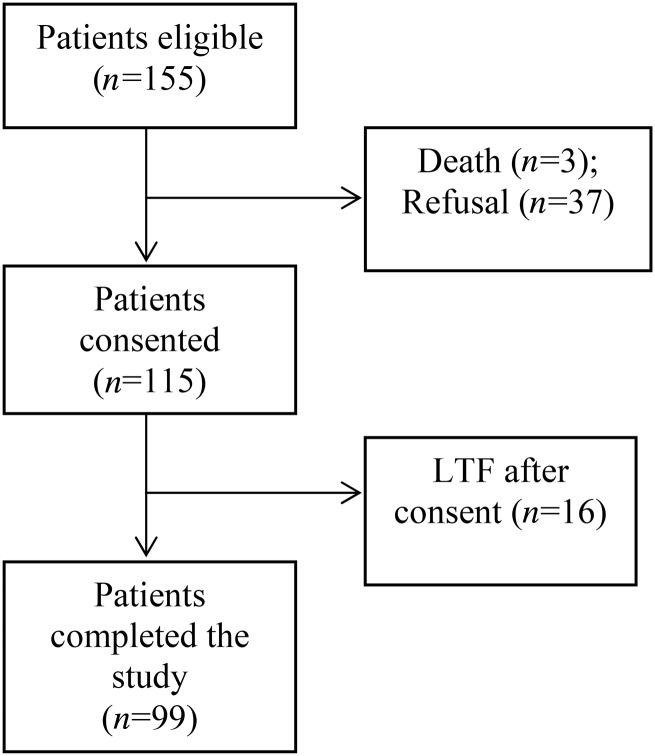
Patients were recruited from 15 outpatient dialysis centers in North Carolina. Patients were eligible for the parent RCT and the present study if they (i) were self-identified African-American or Caucasian, (ii) had been on dialysis for at least 6 months, (iii) scored ≥ 6 on the Charlson Comorbidity Index (CCI) or 5 if they had been hospitalized at least once in the last 6 months [15, 16] and (iv) were English speaking. The research staff approached eligible patients for informed consent and administered a cognitive screening test, the 10-item Short Portable Mental Status Questionnaire (SPMSQ) [17]. Patients with >2 errors on the SPMSQ were deemed ineligible. Of the 155 eligible patients, 99 participated in the study. Figure 1 gives a study flow diagram.
FIGURE 1:
Study flow diagram.
Data collection procedures
A research staff member conducted semistructured telephone interviews that were audio-recorded and transcribed. The initial questions were open-ended questions about context, followed by structured questions that addressed informed decision-making (IDM), and ending with questions about the decision-making experience including a question about what the patients would change if they could. The interviews lasted 15–45 min. A $25 check was mailed to each patient at completion of the interview.
Semistructured questions about the context of initiating dialysis
Patients were asked to describe their perceptions of decision-making about starting dialysis. Each interview began with a broad question, ‘Tell me how the decision to start dialysis came about’. Probing questions explored the context of decision-making, for example whether the patient had prior knowledge of progression to ESKD and the possible need for dialysis, or whether the need to start dialysis came up urgently, whether the decision-making occurred in the doctor's office or in the hospital, and whether the decision-making was initiated by a nephrologist or a non-nephrologist.
Informed decision-making
Patients then responded (yes/no) to 10 questions about their communication with the physician during the decision-making process. These investigator-developed items focused on the types of information and communication endorsed by clinical guidelines for initiation of dialysis [5] and recommended approaches to renal replacement therapy [18, 19]. The binary responses (1 versus 0) to the 10 items were summed to create a composite variable, the IDM score, with a range of 0–10 with higher scores indicating being better informed. Summing the responses was appropriate for creating the composite variable because each question carried roughly equal weight [20]. Internal consistency reliability was KR-20 = 0.70, which is considered acceptable [21].
Perceptions of the decision-making experience
Patients were also asked whether they felt decision-making was rushed (yes/no), whether they felt they had any choice about dialysis (yes/no), whether they felt the decision to start dialysis was made by the doctor, on their own or with their family or collaboratively with the family and the doctor and, lastly, what they would change about how the decision was made if they could.
Data analysis
Text data files verified by the interviewer for accuracy were transferred into ATLAS.ti version 7.0 (Scientific Software Development, Berlin, Germany) for data management and analysis. Using content analysis [22, 23], words and phrases capturing the patients' responses on what was communicated to the patient and how the decision to start dialysis was made were formulated into codes. A conventional content analysis involved interpretations of narrative responses on decision-making contexts, information provided and perceptions about how the decision about dialysis was made through a process of coding that generated categories or a typology of expressions [22]. The coding schema was created by team consensus on coding definitions. At monthly team meetings, we critiqued the credibility of the codes [24]. After the coding scheme was established, intercoder reliability (authors M.S. and C.G.) was evaluated with 10 randomly selected transcripts. Cohen's Kappa was deemed satisfactory (0.7–0.8) [25, 26].
Our sample size was designed to achieve at least 80% statistical power to detect a mean difference of 2.0 in IDM scores in at least one of the variables indicating the patients' perceptions about the decision-making process, e.g. differences between those who felt the decision-making rushed and those who did not.
To examine the relationships of IDM scores to patient characteristics, decision-making context and perceptions of the decision-making process, we used Pearson correlation coefficients, t-tests, ANOVA and general linear models. We used multivariable logistic regression to examine whether IDM scores were associated with the patients' perceptions of the decision-making process. All statistical analyses were performed using IBM SPSS Statistics 19 (SPSS, Inc., Chicago, IL).
RESULTS
Patient characteristics
Table 1 provides sociodemographic and clinical characteristics of the sample. The mean (SD) age of those joining the study was 60.9 (12.4) years, with a range of 28–89 years. Roughly half were male (n = 47, 47.5%), and 77% were African-Americans. Only two were receiving peritoneal dialysis. The median time on dialysis was 4 years (M [SD] = 4.77 [4.72]). In comparison, those who declined to join the study (n = 37) were 68.4 (11.2) years of age (range 33–83 years), more than half were male (n = 22, 59.5%) and African-American (n = 21, 56.8%), and all were receiving hemodialysis with a median time on dialysis of 3.3 years (M [SD] = 5.2 [6.0]). Their mean (SD) CCI score was 7.7 [1.9].
Table 1.
Sample characteristics (n = 99)a
| Characteristic | n (%) |
|---|---|
| Gender | |
| Male | 47 (47.5) |
| Female | 52 (52.5) |
| Race/ethnicity | |
| African-American or black | 76 (76.8) |
| Caucasian or white | 23 (23.2) |
| Years of formal education completed | |
| Less than high school | 20 (20.2) |
| High school or equivalence | 57 (57.6) |
| At least some college | 22 (22.2) |
| Employment status | |
| Disabled/unable to work | 60 (60.6) |
| Retired | 30 (30.3) |
| Part time | 4 (4.0) |
| Full time | 3 (3.0) |
| Unemployed | 2 (2.0) |
| Total gross annual income | |
| <$10 000 | 18 (18.2) |
| $10 000–$19 999 | 35 (35.4) |
| $20 000–$49 999 | 32 (32.3) |
| $50 000 or higher | 11 (11.1) |
| Refused to answer | 3 (3.0) |
| Religion | |
| Protestant | 71 (71.7) |
| Catholic | 2 (2.0) |
| Jewish | 2 (2.0) |
| Muslim | 2 (2.0) |
| Other (Jehovah's witness etc.) | 14 (14.1) |
| None | 8 (8.1) |
| Extent of following religious customs | |
| Never or sometimes | 24 (26.4) |
| Frequently | 24 (26.4) |
| Always | 43 (47.4) |
| Health insuranceb | |
| Medicare | 96 (97.0) |
| Medicaid | 39 (39.4) |
| Private | 48 (48.5) |
| Dialysis modality | |
| Hemodialysis | 97 (98.0) |
| Peritoneal | 2 (2.0) |
| Comorbid conditions | |
| Myocardial infarction | 32 (32.3) |
| Congestive heart failure | 61 (61.6) |
| Peripheral vascular disease | 37 (37.4) |
| CVA | 33 (33.3) |
| COPD | 34 (34.3) |
| Connective tissue disorder | 3 (3.0) |
| Pulmonary hypertension | 16 (16.2) |
| Ulcer disease | 10 (10.1) |
| Mild liver disease | 14 (14.1) |
| Diabetes | 88 (88.9) |
| HIV+ | 3 (3.0) |
| Tumor without metastasis | 5 (5.1) |
| Mean CCI score (SD) | 8.4 (1.9) |
aDue to rounding, some of the percentages may not add up to 100.
bMultiple responses.
Context of decision-making to start dialysis
Table 2 presents data on the decision-making contexts identified from the semistructured interviews. Sixty-three patients (63.6%) said that their doctors had told them about CKD progressing to ESKD. These 63 patients had been referred to a nephrologist by their primary care provider or another specialist at least 6 months before the decision to start dialysis. However, of those, 17 (27%) reported that they did not know ESKD would require dialysis. Thirty patients (30%) said that they had no prior knowledge of CKD progressing to ESKD although they had been seeing a physician for CKD, and 6 (6.1%) reported that the decision to start dialysis was made urgently with limited time for discussion.
Table 2.
Decision-making context
| Context | n (%)a |
|---|---|
| Had a prior knowledge of progression to ESKD | |
| Yes | 63 (63.6) |
| No | 36 (36.4) |
| Setting | |
| Doctor's office | 53 (53.5) |
| Hospital | 46 (46.5) |
| The need for dialysis told by | |
| Nephrologist | 86 (86.9) |
| Non-nephrologist | 13 (13.1) |
| With a family member when the need for dialysis was told | |
| Yes | 46 (46.5) |
| No | 53 (53.5) |
| Have/had a family or a friend on maintenance dialysis | |
| Yes | 36 (36.4) |
| No | 63 (63.6) |
| Decision made | |
| ≤4 years ago | 55 (55.6) |
| >4 years ago | 44 (44.4) |
| Patient's age at the time of decision-making (years) | |
| ≤60 | 56 (56.6) |
| 61–70 | 29 (29.3) |
| 71–80 | 8 (8.1) |
| >81 | 6 (6.1) |
aDue to rounding, some of the percentages may not add up to 100.
Eighty-six patients (86.9%) were informed by their nephrologist about the need to start dialysis. Fifty-three patients (53.5%) recalled that they were alone when their doctor told them about the need for dialysis initiation. Fifty-five patients (55.6%) made the decision to start dialysis after enactment of the 2008 Medicare Improvements for Patients and Providers Act.
Extent of IDM
The mean IDM score was 4.4 (SD = 2.0), with a range of 0–8 (of 10). Two-thirds of the patients (67%) scored 5 or lower. Table 3 shows the numbers of patients who responded ‘yes’ to each IDM item. Roughly half of the patients (53.5%) said that the underlying illness conditions leading to ESKD had been explained and a similar percentage said that dialysis options other than hemodialysis and a life-long need for dialysis had been explained. Nonetheless, only 20 patients (20.2%) said that the doctor asked them about their values and preferences for dialysis modality. Sixty-seven patients (67.7%) said that the risks and burdens of dialysis were not mentioned at all. Only one patient said that the doctor offered the option of not starting dialysis and suggested palliative care. Most patients (74.7%) felt their doctor was trying to make sure the patient understood the information, while 58.6% felt their doctor tried to understand what was important to the patient.
Table 3.
Number (%) of patients responding ‘Yes’ to each informed decision-making item
| Content of the item | n (%) |
|---|---|
| 1. Condition that led to kidney failure | 53 (53.5) |
| 2. How long you would live with or without dialysis | 45 (45.5) |
| 3. Dialysis options, such as peritoneal dialysis and hemodialysis | 59 (59.6) |
| 4. Benefits and burdens associated with each type of dialysis | 32 (32.3) |
| 5. Doctor asked your values and preferences for those dialysis options | 20 (20.2) |
| 6. How your daily life might change after starting dialysis | 44 (44.4) |
| 7. Need for dialysis for the rest of your life unless you receive kidney transplantation | 82 (82.8) |
| 8. Not starting dialysis could be an option | 1 (1.0) |
| 9. Doctor tried to make sure you understood what he/she told you | 74 (74.7) |
| 10. Doctor tried to understand what was important to you | 58 (58.6) |
Patient perceptions of the decision-making experience
A majority (n = 66, 67%) of patients said that they felt they had no choice about initiating dialysis (because the alternative would be death) or about dialysis modality, but only 21.2% said that they had felt rushed to make a decision. About one-third of the patients (n = 31, 31.3%) felt that the decision was made by the doctor and only 13% felt the decision was made collaboratively between themselves and the doctor.
When asked whether they would like to change anything about how the decision was made, 39 patients (39.3%) said that there was nothing they wished had been different. Their reasons for not wanting anything different were of two types. First, some patients felt informed and prepared for dialysis. They recalled being told about the possibility of progression to ESKD and felt they were kept informed over time. Second, some experienced a sense of fatalism. They said that they did not know any other way to make the decision to start dialysis and it just had to be done. The remaining 60 patients (60.6%) would have liked (i) to have received more information to get a full picture, or (ii) to have experienced better ways to break bad news or (iii) to have been powerful enough to resist or delay dialysis. Table 4 provides examples of the patients' responses that illustrate these three perspectives.
Table 4.
Selected patient statements on what they would have changed about the decision-making process
| Nothing that I wish differently |
| Being informed and prepared for dialysis over time |
| ‘I don't think she could have done anything differently. I think she did it well. She worked with me. She kept me informed.’ |
| ‘No. Everything was fine. From the beginning, it was explained step by step to me. So, I already knew what I was going into.’ |
| Sense of fatalism |
| ‘Nothing. You've got to make a decision and then you have to live with it.’ |
| ‘Nothing. I don't think he could've done anything differently. I had no choice in it.’ |
| I wish… |
| Desired more information to get a full picture |
| ‘I wish he would have discussed the chance or, I don't know what I'm trying to say, but the part about it, that you will not live a full life expectancy or some of the side effects of dialysis. I learned that as I, you know, as I went along on dialysis.’ |
| ‘I'd like to have known what was going on, and why I was needing this and how long it was going to last, you know, um it kind of helps you plan a little bit. Get your head wrapped around it before you are on it three days a week for the rest of your life…I would just like to have had the information, but not in a three inch thick book. Now, as far as I can tell, I'll be on dialysis, period. If I decide to stop, I'll die. It's just relatively straightforward. But I know now something else can happen and you die.’ |
| A better way to break bad news |
| ‘For him to come and say, “If you don't get on dialysis, you're gonna die.” I just thought that was the most despicable thing a doctor could ever have said to me. It's like telling, “You have cancer” and then just walking out. That's how I felt, like he didn't care. Like “Oh, well, you're just another patient.” So, I was very upset and started crying. And he said, “I'm gonna call a social worker” and then left.’ |
| ‘It would be to be more understanding and more compassionate towards me, the way it was announced to me. If he could've explained to me like, this is what happened to you, and this is the treatment or whatever. But I didn't see that. I didn't feel that. All he said was, “You are going to have to be dialyzed.”’ |
| Being powerful enough to resist or delay |
| ‘I should have found out more about it, read up on it, Googled it somewhere before you know, because I went straight from his office to having a shunt or whatever. So I wished I would've just waited. But I was tired, nervous and he was like, acted like I was gonna die tomorrow if I didn't, you know, go ahead with it. So I just went on with it. |
IDM associations with other study variables
Of the sociodemographic and clinical variables, including years on dialysis, only age at the time of decision-making, years of education and race were associated with IDM scores. There was a significant, but weak correlation between IDM scores and age at the time of decision-making (r = −0.27, P = 0.006) and years of education and IDM scores (r = 0.24, P = 0.02). With respect to race, the IDM scores of African-Americans were significantly higher than were those of Caucasians (M [SD] = 4.7 [2.2] versus 2.5 [1.9], t = 2.5, P = 0.015). This race difference in IDM score can be explained by two items: a higher percentage of African-Americans said that their doctors had explained how their daily life might change after starting dialysis (50 versus 26%, χ2 = 4.1, P = 0.043) and a higher percentage of African-Americans said that their doctor had explained the life-long need for dialysis (90 versus 61%, χ2 = 10.2, P = 0.001). However, as a higher percentage of African-Americans than Caucasians had a family member or friend receiving dialysis (42.1 versus 17.4%), we used multivariate analysis to control for this factor and for age and years of education. After controlling for these factors, the racial difference in IDM score nearly disappeared (P = 0.05).
With respect to the associations with the decision-making context variables, IDM scores did not differ depending on whether the decision to initiate dialysis was made before or after the enactment of Medicare Improvements for Patients and Providers Act of 2008, whether the decision occurred at the doctor's office or hospital, or whether it was initiated by a nephrologist or not. However, not surprisingly, patients who had had a warning about progressing to ESKD scored significantly higher on IDM than did those who initiated dialysis without prior knowledge of CKD progressing to ESKD (M [SD] = 4.8 [2.0] versus 3.7 [1.8], t = 2.9, P = 0.005).
Controlling for age, education, race and warning about the progression to ESKD, multivariate logistic regression analysis showed that patients with higher IDM scores were significantly more likely not to feel rushed (OR = 3.4, 95% CI = 1.7–6.8), to feel they had choices about dialysis (OR = 2.8, 95% CI = 1.6–4.8) and to perceive that the decision was made on their own or with family (OR = 1.7, 95% CI = 1.2–2.6) or collaboratively (OR = 2.0, 95% CI = 1.1–3.6), rather than by the doctor.
DISCUSSION
Taken together, the data in this study reveal that only a limited number of patients felt prepared for the initiation of dialysis. It is encouraging that a majority of patients felt they had been informed about the possibility of progression to ESKD and those who had been so warned had higher IDM scores than those who had not been warned. On the other hand, the patients' descriptions indicated that IDM about initiating dialysis was far less than ideal. These findings are similar to previous studies [10–13] but reveal the patients' perceptions about the decision-making process surrounding dialysis initiation that go beyond modality decisions, suggesting areas of significant communication gap and unbalanced information provision.
While the majority of patients acknowledged that starting dialysis was the ‘right’ decision because they would have not survived otherwise, they recalled that the matter of survival had overshadowed other important aspects of treatment decision-making such as side effects or the burdens of the therapy, complications and quality-of-life changes. These findings are consistent with previous research showing that patients often step into life on dialysis unprepared [10] and may regret the decision to start dialysis [27].
Patients reported that what was most consistently conveyed was the certainty of death without dialysis and the life-long need for dialysis. Complications and mortality after starting dialysis were rarely discussed. Only one patient reported being offered the option of not starting dialysis but rather initiating palliative care and he recalled that the way palliative care was presented made it too objectionable to consider as an option. Unlike other countries, such as UK, and Canada, conservative therapy is rarely discussed in the USA as a viable option [28–30], and both patients and providers fear abandonment if no renal replacement therapy is offered [31]. Some physicians may have emphasized survival benefits of dialysis to alleviate the patients' fears and to convince them to begin dialysis so that their lives could be extended. Or some physicians might have believed that conveying details about dialysis was not feasible or helpful to patients [32]. Yet a recent study underscores the importance of helping patients to understand the demands and outcomes of dialysis before deciding to start dialysis, because some patients are willing to trade survival time for quality of life [28].
Providing timely education about ESKD and dialysis to patients is challenging. Indeed, some patients said that they themselves were resistant to discussions until their conditions spiraled into an urgent need for dialysis. Also, uncertainty of progression of CKD to ESKD [33, 34] makes it difficult for physicians to prepare patients for dialysis. The prevalence of silent brain infarction and cognitive impairment among CKD patients [35–37] adds to the challenges since cognitive impairment compromises the patients' ability to comprehend and weigh the benefits and burdens of dialysis [38]. Although we screened out patients with gross impairments in cognitive functioning, the significantly lower IDM scores among elderly patients points to their need for extra attention with respect to comprehending information [39, 40].
Over half of the patients were alone when asked to decide about dialysis initiation, and some felt that the bad news was delivered without much sensitivity. Given the complexity and emotional charge of dialysis decision-making, patients would benefit from family involvement during the process, and clinicians should be sensitive to the magnitude of the decision to initiate dialysis [10].
Our finding that the racial difference in the IDM scores nearly disappeared after controlling for having a family member or friend on dialysis is interesting. Given that ESKD is four times more likely to occur among African-Americans than Caucasians in the USA [2], it is not surprising that a significantly higher number of African-Americans in the study had a family or friend on dialysis. Whether this unfavorable health disparity may lead African-Americans to better preparation for life on dialysis than Caucasians requires further research.
Our findings are based on the patients' recollections and interpretations of what happened at the time of decision-making; we cannot verify whether information was actually provided or how it was provided. Nonetheless, these patient accounts reflect their understandings of the decision to start dialysis. In fact, we explored the potential impact of poor recollections on IDM scores but did not find any association between the IDM scores and the years on dialysis. Although our consent rate was relatively high (75.7%) considering the well-documented challenges in recruiting patients with serious chronic illness [41–45], we recognize that the sample size was modest and might not be representative of the US dialysis patient population because it did not include a sufficient number of PD patients, patients receiving dialysis for <6 months and those who did not survive the first year on dialysis. Further, we adjusted for confounding variables that we measured, but unmeasured factors might have influenced relationships between IDM scores and the patients' perceptions of decision-making. For example, health literacy might have influenced patients understanding of the information presented and the way they felt about the decision-making process [46, 47]. Finally, the IDM was an investigator-developed tool that requires further testing and validation.
Despite these limitations, our findings raise concerns that some patients might not be receiving all the information they need about renal replacement therapy options for ESKD and the limited likelihood that dialysis has for restoring health or prolonging life. We found that many patients did not have much understanding of dialysis prior to their decision even in the absence of urgency. Interventions to promote effective communication to help patients understand that the decision to initiate dialysis is complex and requires significant trade-offs are solely needed. The effects of communication gaps on the patients' illness management, mental health, quality of life and survival need to be examined. Further, the likelihood that those who feel more positively about the decision-making process cope better with their life on dialysis warrants further research. Finally, more data on the physicians' perspectives on the decision to initiate dialysis are needed to complement the patients' perspectives.
FUNDING
This study was supported by a grant from the National Institutes of Health, the Office of the Director (R01NR011464-03S1) to M.S. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or part, except in abstract format. All authors have no financial conflict of interest to disclose. (See related article by Tamura and Periyakoil. The patient perspective and physician's role in making decisions on instituting dialysis. Nephrol Dial Transplant 2013; 28: 2663–2666.)
ACKNOWLEDGEMENTS
The authors thank Jason P. Fine, Department of Biostatistics, University of North Carolina at Chapel Hill, and Annette DeVito Dabbs, University of Pittsburgh, for their reviews and thoughtful comments on drafts of this manuscript.
REFERENCES
- 1.Gilg J, Castledine C, Fogarty D. Bristol, UK,: UK Renal Registry; 2011. Chapter 1 UK RRT incidence in 2010: National and centre-specific analyses. In The Renal Association ed. UK Renal Registry The 14th Annual Report. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System. Bethesda, MD: 2012. USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health. National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 3.Song MK, Gilet CA, Lin FC, et al. Characterizing daily life experience of patients on maintenance dialysis. Nephrol Dial Transplant. 2011;26:3671–3677. doi: 10.1093/ndt/gfr071. doi:10.1093/ndt/gfr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. doi:10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline. Rockville, MD: Renal Physicians Association; 2010. [Google Scholar]
- 6.Renal Physicians Association, American Society of Nephrology. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline No. 2. Washington, DC: Renal Physicians Association; 2000. [DOI] [PubMed] [Google Scholar]
- 7.Medicare Improvements for Patients and Providers Act of 2008. 2008. Public Law 110–275. United States Government.
- 8.Russ AJ, Shim JK, Kaufman SR. “Is there life on dialysis?”: time and aging in a clinically sustained existence. Med Anthropol. 2005;24:297–324. doi: 10.1080/01459740500330639. doi:10.1080/01459740500330639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russ AJ, Shim JK, Kaufman SR. The value of “life at any cost”: Talk about stopping kidney dialysis. Soc Sci Med. 2007;64:2236–2247. doi: 10.1016/j.socscimed.2007.02.016. doi:10.1016/j.socscimed.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schell JO, Patel UD, Steinhauser KE, et al. Discussions of the kidney disease trajectory by elderly patients and nephrologists: a qualitative study. Am J Kidney Dis. 2012;59:495–503. doi: 10.1053/j.ajkd.2011.11.023. doi:10.1053/j.ajkd.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morton RL, Tong A, Howard K, et al. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ. 2010;340:c112. doi: 10.1136/bmj.c112. doi:10.1136/bmj.c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrotra R, Marsh D, Vonesh E, et al. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int. 2005;68:378–390. doi: 10.1111/j.1523-1755.2005.00453.x. doi:10.1111/j.1523-1755.2005.00453. [DOI] [PubMed] [Google Scholar]
- 13.Fadem SZ, Walker DR, Abbott G, et al. Satisfaction with renal replacement therapy and education: the American Association of Kidney Patients survey. Clin J Am Soc Nephrol. 2011;6:605–612. doi: 10.2215/CJN.06970810. doi:10.2215/CJN.06970810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandelowski M. What's in a name? Qualitative description revisited. Res Nurs Health. 2010;33:77–84. doi: 10.1002/nur.20362. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. doi:10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 16.Fried L, Bernardini J, Piraino B. Comparison of the Charlson Comorbidity Index and the Davies score as a predictor of outcomes in PD patients. Perit Dial Int. 2003;23:568–573. [PubMed] [Google Scholar]
- 17.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 18.Schell JO, Germain MJ, Finkelstein FO, et al. An integrative approach to advanced kidney disease in the elderly. Adv Chronic Kidney Dis. 2010;17:368–377. doi: 10.1053/j.ackd.2010.03.004. doi:10.1053/j.ackd.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latos DL, Lucas J. Geriatric nephrology: a paradigm shift in the approach to renal replacement therapy. Adv Chronic Kidney Dis. 2011;18:412–419. doi: 10.1053/j.ackd.2011.09.008. doi:10.1053/j.ackd.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Song MK, Lin FC, Ward SE, et al. Composite variables: when and how. Nurs Res. 2013;62:45–49. doi: 10.1097/NNR.0b013e3182741948. doi:10.1097/NNR.0b013e3182741948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunnally JC, Bernstein IH. Psychometric Theory. New York, NY: McGraw-Hill; 1994. [Google Scholar]
- 22.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–1288. doi: 10.1177/1049732305276687. doi:10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 23.Marks DF, Yardley L. Research Methods for Clinical and Health Psychology. Thousand Oaks, CA: SAGE; 2004. [Google Scholar]
- 24.Onwuegbuzie AJ, Teddlie C. A framework for analyzing data in mixed methods research. In: Teddlie C, Tashakkori A, editors. Handbook of Mixed Methods in Social & Behavioral Research. Thousand Oaks, CA: Sage; 2003. pp. 351–383. [Google Scholar]
- 25.Burla L, Knierim B, Barth J, et al. From text to codings: intercoder reliability assessment in qualitative content analysis. Nurs Res. 2008;57:113–117. doi: 10.1097/01.NNR.0000313482.33917.7d. doi:10.1097/01.NNR.0000313482.33917.7d. [DOI] [PubMed] [Google Scholar]
- 26.Everitt B. Making Sense of Statistics in Psychology: A Second-Level Course. Oxford, UK: Oxford University Press; 1996. [Google Scholar]
- 27.Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5:195–204. doi: 10.2215/CJN.05960809. doi:10.2215/CJN.05960809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton RL, Turner RM, Howard K, et al. Patients who plan for conservative care rather than dialysis: a national observational study in Australia. Am J Kidney Dis. 2012;59:419–427. doi: 10.1053/j.ajkd.2011.08.024. doi:10.1053/j.ajkd.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Murtagh FE, Addington-Hall JM, Higginson IJ. End-stage renal disease: a new trajectory of functional decline in the last year of life. J Am Geriatr Soc. 2011;59:304–308. doi: 10.1111/j.1532-5415.2010.03248.x. doi:10.1111/j.1532-5415.2010.03248. [DOI] [PubMed] [Google Scholar]
- 30.Carson RC, Juszczak M, Davenport A, et al. Is maximum conservative management an equivalent treatment option to dialysis for elderly patients with significant comorbid disease? Clin J Am Soc Nephrol. 2009;4:1611–1619. doi: 10.2215/CJN.00510109. doi:10.2215/CJN.00510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moss AH. Too many patients who are too sick to benefit start chronic dialysis nephrologists need to learn to “just say no. Am J Kidney Dis. 2003;41:723–727. doi: 10.1016/s0272-6386(03)00131-8. doi:10.1016/S0272-6386(03)00131-8. [DOI] [PubMed] [Google Scholar]
- 32.Iezzoni LI, Rao SR, DesRoches CM, et al. Survey shows that at least some physicians are not always open or honest with patients. Health Aff (Millwood) 2012;31:383–391. doi: 10.1377/hlthaff.2010.1137. doi:10.1377/hlthaff.2010.1137. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. 2012;59:504–512. doi: 10.1053/j.ajkd.2011.12.009. doi:10.1053/j.ajkd.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. doi:10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 35.Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17:293–301. doi: 10.1053/j.ackd.2010.03.010. doi:10.1053/j.ackd.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Post JB, Jegede AB, Morin K, et al. Cognitive profile of chronic kidney disease and hemodialysis patients without dementia. Nephron Clin Pract. 2010;116:c247–c255. doi: 10.1159/000317206. doi:10.1159/000317206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai CF, Wang SJ, Fuh JL. Moderate chronic kidney disease is associated with reduced cognitive performance in midlife women. Kidney Int. 2010;78:605–610. doi: 10.1038/ki.2010.185. doi:10.1038/ki.2010.185. [DOI] [PubMed] [Google Scholar]
- 38.Kurella Tamura M, Wadley V, Yaffe K, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52:227–234. doi: 10.1053/j.ajkd.2008.05.004. doi:10.1053/j.ajkd.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavalli A, Del Vecchio L, Locatelli F. Geriatric nephrology. J Nephrol. 2010;23(Suppl. 15):S11–S15. [PubMed] [Google Scholar]
- 40.Nulsen RS, Yaqoob MM, Mahon A, et al. Prevalence of cognitive impairment in patients attending pre-dialysis clinic. J Ren Care. 2008;34:121–126. doi: 10.1111/j.1755-6686.2008.00028.x. doi:10.1111/j.1755-6686.2008.00028. [DOI] [PubMed] [Google Scholar]
- 41.Steinhauser KE, Clipp EC, Hays JC, et al. Identifying, recruiting, and retaining seriously-ill patients and their caregivers in longitudinal research. Palliat Med. 2006;20:745–754. doi: 10.1177/0269216306073112. doi:10.1177/0269216306073112. [DOI] [PubMed] [Google Scholar]
- 42.Kirchhoff KT, Kehl KA. Recruiting Participants in End-of-Life Research. Am J Hosp Palliat Care. 2008;24:515–521. doi: 10.1177/1049909107300551. doi:10.1177/1049909107300551. [DOI] [PubMed] [Google Scholar]
- 43.McMillan SC, Weitzner MA. Methodologic issues in collecting data from debilitated patients with cancer near the end of life. Oncol Nurs Forum. 2003;30:123–129. doi: 10.1188/03.ONF.123-129. doi:10.1188/03.ONF.123-129. [DOI] [PubMed] [Google Scholar]
- 44.Northouse LL, Rosset T, Phillips L, et al. Research with families facing cancer: the challenges of accrual and retention. Res Nurs Health. 2006;29:199–211. doi: 10.1002/nur.20128. doi:10.1002/nur.20128. [DOI] [PubMed] [Google Scholar]
- 45.Shields AM, Park M, Ward SE, et al. Subject recruitment and retention against quadruple challenges in an intervention trial of end-of-life communication. J Hosp Palliat Nurs. 2010;12:312–318. doi: 10.1097/NJH.0b013e3181ec9dd1. doi:10.1097/NJH.0b013e3181ec9dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.James BD, Boyle PA, Bennett JS, et al. The impact of health and financial literacy on decision making in community-based older adults. Gerontology. 2012;58:531–539. doi: 10.1159/000339094. doi:10.1159/000339094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marks R. Ethics and patient education: health literacy and cultural dilemmas. Health Promot Pract. 2009;10:328–332. doi: 10.1177/1524839909335657. doi:10.1177/1524839909335657. [DOI] [PubMed] [Google Scholar]