Abstract
Background
Sensitization to human leukocyte antigen (HLA) from red blood cell (RBC) transfusion is poorly quantified and is based on outdated, insensitive methods. The objective was to evaluate the effect of transfusion on the breadth, magnitude and specificity of HLA antibody formation using sensitive and specific methods.
Methods
Transfusion, demographic and clinical data from the US Renal Data System were obtained for patients on dialysis awaiting primary kidney transplant who had ≥2 HLA antibody measurements using the Luminex single-antigen bead assay. One cohort included patients with a transfusion (n = 50) between two antibody measurements matched with up to four nontransfused patients (n = 155) by age, sex, race and vintage (time on dialysis). A second crossover cohort (n = 25) included patients with multiple antibody measurements before and after transfusion. We studied changes in HLA antibody mean fluorescence intensity (MFI) and calculated panel reactive antibody (cPRA).
Results
In the matched cohort, 10 of 50 (20%) transfused versus 6 of 155 (4%) nontransfused patients had a ≥10 HLA antibodies increase of >3000 MFI (P = 0.0006); 6 of 50 (12%) transfused patients had a ≥30 antibodies increase (P = 0.0007). In the crossover cohort, the number of HLA antibodies increasing >1000 and >3000 MFI was higher in the transfused versus the control period, P = 0.03 and P = 0.008, respectively. Using a ≥3000 MFI threshold, cPRA significantly increased in both matched (P = 0.01) and crossover (P = 0.002) transfused patients.
Conclusions
Among prospective primary kidney transplant recipients, RBC transfusion results in clinically significant increases in HLA antibody strength and breadth, which adversely affect the opportunity for future transplant.
Keywords: calculated panel reactive antibody, HLA antibody, kidney transplantation, sensitization, transfusion
INTRODUCTION
Kidney transplantation is the most effective form of therapy for end-stage renal disease (ESRD) in terms of survival, health-related quality of life and costs [1–3]. Human leukocyte antigen (HLA) sensitization is a major barrier to successful kidney transplantation. In the USA, roughly 400 000 persons require maintenance dialysis to sustain life with ∼30% ultimately listed for kidney transplantation [4]. Of the >94 000 candidates currently listed for kidney transplant, about one-third are sensitized [5]. Previous transplants, pregnancies and blood transfusions are the major causes of sensitization. With average waiting times for deceased donor kidney transplantation in the USA exceeding the life expectancy of most middle-aged and older patients on dialysis and with pediatric patients likely to need repeat transplant in their lifetimes, few can afford barriers such as sensitization that can delay or prevent receipt of a compatible organ [5]. Of the three major causes of sensitization, blood transfusion is the only widespread, medically discretionary, ‘preventable’ one.
Anemia is an important complication of chronic kidney disease (CKD) and is particularly severe in patients on dialysis [6, 7]. In addition to erythropoietin deficiency, anemia can be due to blood loss from the vascular access, tubing and dialyzer (artificial kidney), chronic inflammation, iron deficiency and gastrointestinal blood loss [8]. Before the development of erythropoiesis-stimulating agents (ESAs), patients on dialysis were frequently transfused, typically 6–8 units of packed red blood cells (RBCs) per year [9]. After ESAs were introduced, RBC transfusion declined precipitously [10]. However, with concerns regarding the safety of ESAs in patients with CKD [11–13] and a major recent change in ESA labeling, ESA use has declined and, in turn, transfusion use has increased among patients on dialysis [4].
In past years, the combination of insensitive methods of characterizing HLA antibodies and underreporting of transfusions likely underestimated the degree of transfusion-related sensitization [10]. In an effort to inform clinical decision-making for patients awaiting transplantation, we sought to quantify the strength and breadth of HLA sensitization due to transfusion in prospective primary kidney transplant recipients using highly sensitive and specific HLA antibody assays.
MATERIALS AND METHODS
Data sources
After obtaining approval from the Stanford University Institutional Review Board and the US Renal Data System (USRDS), we merged the Stanford HLA antibody database with data from the USRDS, including demographic factors, cause of ESRD, waitlist time, vintage (time since initiation of dialysis), comorbid conditions (Supplementary Table S1), prior surgeries and reasons for acute hospitalization. Comorbidities were obtained from inpatient and outpatient Medicare claims (based on ‘International Classification of Diseases, Ninth Revision, Clinical Modification’, ICD-9-CM) in the 1-year period preceding the first HLA antibody measurement used for the analyses [14]. We identified RBC transfusions using billing claims with corresponding ICD-9-CM codes, documented transfusions from medical records and also queried for history of pregnancies. We identified proinflammatory events, including major surgeries and infections, before the first and between HLA antibody measurements (Supplementary Table S2) and used limited data sets to perform analyses in compliance with the provisions of the Health Insurance Portability and Accountability Act.
HLA antibody measurements
Luminex HLA Class I and II single-antigen beads and Fusion analysis software (LabScreen, One Lambda, Canoga Park, CA, USA) were used to determine HLA antibody specificities. All specificities with ≥1000 normalized mean fluorescence intensity (MFI) at baseline measurement were considered positive. We did not distinguish between antibody to intact and ‘denatured’ antigen. Calculated panel reactive antibody (cPRA) was determined using HLA frequencies from United Network of Organ Sharing (UNOS) deceased donors procured from January 1999 through October 2008 (n = 42 671), fully typed for HLA-A, B, C, DRB1, DQB1 and, unlike UNOS, included C locus frequencies.
Study population
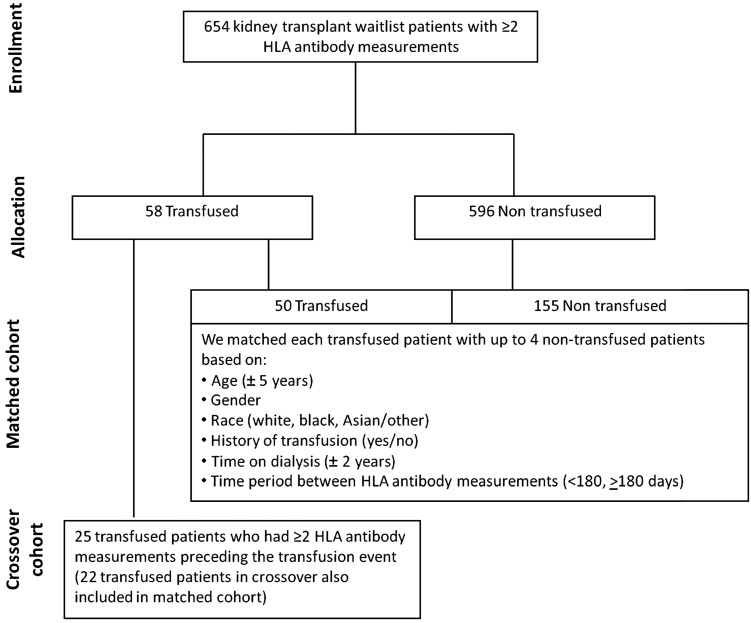
We included all adult patients who were (i) listed on the Stanford kidney transplant waiting list from 1999 to 2009 for a primary kidney transplant, (ii) on dialysis and (iii) who had at least two measurements for HLA antibodies (median 74 days, interquartile range 61–134 days). Six hundred and fifty-four patients were identified. Of these, 58 patients received one or more RBC transfusions between two HLA antibody measurements.
Matched reference cohort
The schema for the selection of patients into the matched and crossover cohorts is shown in Figure 1. We matched 50 of 58 transfused patients with 155 patients who did not receive a transfusion between two HLA measurements. We matched each transfused patient with up to four nontransfused patients based on age (±5 years), sex, race (white, black and Asian/other), history of transfusion (yes/no), vintage (±2 years) and time period between HLA antibody measurements (<180 and ≥180 days). Using all six criteria, we were able to match each transfused patient with up to four nontransfused subjects. Eight transfused patients had no matches. The distribution of our matching ratio was 1:4 (56%), 1:3 (16%), 1:2 (10%) and 1:1 (18%). For transfused patients, the index HLA antibody was defined as the most proximal HLA antibody measurement before the first transfusion event. The HLA antibody measurement following a transfusion was the maximum MFI value throughout the follow-up period (median 71 days, interquartile range 44–153 days). For nontransfused controls, the index HLA antibody was defined as the first available HLA antibody value during the study period; the second HLA measurement was defined as the maximum MFI value after the first HLA measurement. The mean number of MFI determinations was 2.0 ± 1.3 in the transfused group and 2.0 ± 1.0 in the nontransfused group, with the maximum number of determinations 6 and 5, respectively.
FIGURE 1:
Study population and schema for matched and crossover cohorts according to transfused versus nontransfused groups.
Crossover cohort
We assembled a crossover cohort from individuals (25 of the 58 transfused patients) who had two HLA measurements and an intervening transfusion event, but also had multiple HLA antibody measurements preceding the transfusion event. We compared the change in HLA antibodies between successive HLA antibody measurements before the transfusion (control period) with that in HLA antibodies by MFI following a transfusion event (transfusion period). The time periods between successive HLA antibody measurements were <180 days for both the control and transfused periods.
Statistical analysis
Baseline patient characteristics and Class I and Class II HLA antibody MFI values were compared using Pearson χ2 analysis and Student's t-test, as appropriate. In the matched cohort, we used Pearson χ2 analysis to compare the number of patients with 10 or more (versus fewer than 10) MFI changes of >1000 and, separately, >3000 among the transfused and nontransfused patients. In the crossover cohort, we used the Sign test to assess differences in the number of antibodies with an MFI change of >1000 and >3000 before and after a RBC transfusion. We used Pearson χ2 analysis to compare change in cPRA in the matched cohort and the Sign test in the crossover cohort. We conducted various subgroup analyses where we examined change in MFI stratified by demographic characteristics: age (above and below the median 52 years), gender (male/female), parity (nulliparous/parous) and race (white/Asian, black and other). Since proinflammatory events have been postulated as potential sensitizing events, we conducted an additional analysis excluding patients with any proinflammatory event occurring between the index and follow-up measurements. We considered a P-value of <0.05 statistically significant. We conducted all analyses using SAS, version 9.2 (Cary, NC, USA).
RESULTS
Table 1 summarizes the demographic and clinical characteristics of the two study cohorts.
Table 1.
Demographic characteristics of study population and matched cohorts
| Patient characteristicsa | Matched cohortb |
Crossover cohort(N = 25) | ||
|---|---|---|---|---|
| Nontransfused (N = 155) | Transfused (N = 50) | P-valuec | ||
| Demographics | ||||
| Age (year), n (%)a | 49.6 (11.7) | 49.4 (12.3) | 0.9 | 54.0 (11) |
| Female (%) | 51.6 | 51.6 | 1.0 | 56.0 |
| Race (%) | 1.0 | |||
| White | 50.3 | 50.3 | 48.0 | |
| Black | 4.5 | 4.5 | 8.0 | |
| Asian/Hispanic/Native American | 45.2 | 45.2 | 44.0 | |
| Cause of ESRD (%) | ||||
| Diabetes | 28.4 | 35.5 | 0.3 | 36.0 |
| Hypertension | 21.3 | 21.3 | 20.0 | |
| Glomerulonephritis | 19.4 | 10.3 | 20.0 | |
| Others | 30.9 | 32.9 | 24.0 | |
| Length of time on dialysis (years)a | 2.1 (0.7, 3.6) | 3.0 (1.5, 4.4) | 0.009 | 4.3 (3.2, 6.2) |
| Potential sensitizing events (%) | ||||
| History of transfusion | 3.9 | 3.9 | 1.0 | 8.0 |
| History of pregnancy | 41.3 | 42.0 | 0.4 | 64.0 |
| Proinflammatory eventsd | 5.2 | 52.0 | <.0001 | 36.0 |
| Comorbidities (%, yes) | ||||
| Heart failure | 3.2 | 7.7 | 0.08 | 16.0 |
| Atherosclerotic heart disease | 3.9 | 6.5 | 0.3 | 12.0 |
| Cancer | 0.0 | 2.6 | 0.04 | 0.0 |
| Cerebrovascular disease | 0.6 | 0.6 | 1.0 | 0.0 |
| Baseline level of allosensitization, n (%) | ||||
| cPRAe | 20.3 (31.4) | 21.9 (34.3) | 0.5 | 23.8 (33.7) |
aEstimates of central tendency are reported as means (standard deviation [SD]) or median (25th/75th percentiles), as appropriate, or percentages (%).
bPercentage contribution within nontransfused patients weighted based on the proportion of matches out of a total of 4.
cP-values were calculated using the Pearson χ2 analysis for categorical variables and Student's t-test for continuous variables.
dPercentage of patients with major surgeries or infections requiring hospitalization between the two HLA antibody measurements.
ecPRA: calculated panel reactive antibody level using an MFI threshold of 1000 or greater for defining unacceptable antigens.
Changes in antibody strength (MFI)
Overall, index Class I and Class II HLA antibody MFI values were not significantly different between the transfused and nontransfused groups. Significant increases of Class I B locus (matched cohort) and A and B locus (crossover cohort) antibodies were observed in the posttransfusion measurements without any increases in Class II antibodies (Supplementary Table S3).
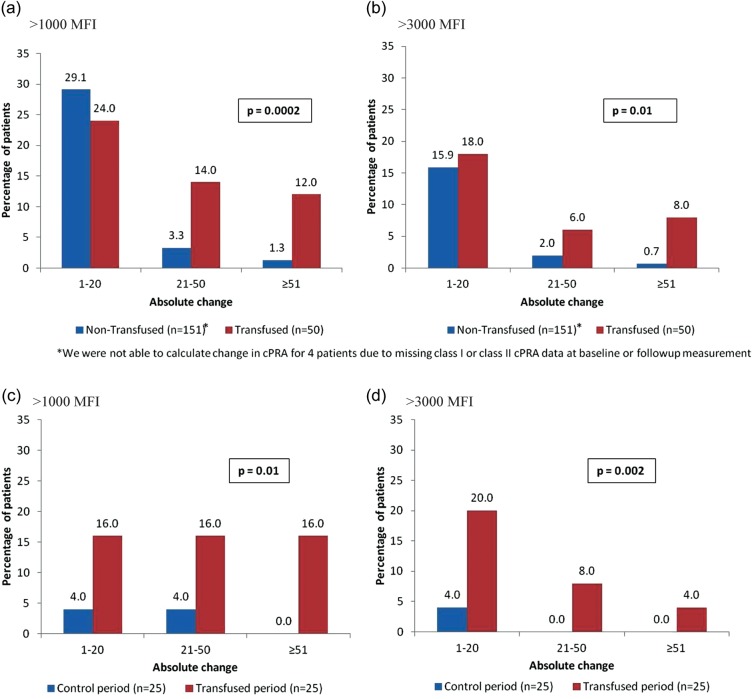
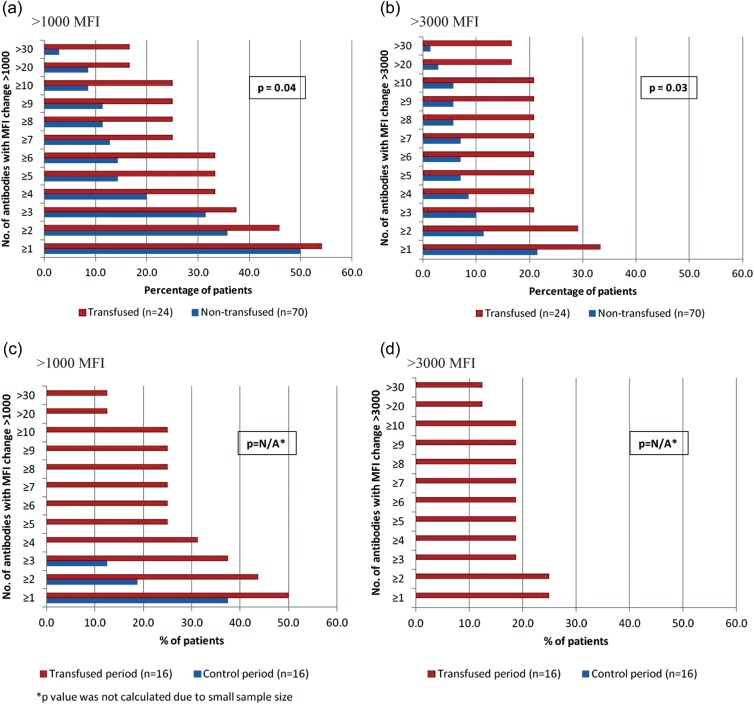
The proportion of patients with a change in MFI of >1000 and >3000 for 10 or more HLA antibodies was significantly higher for the transfused group compared with the matched nontransfused group (Figure 2a and b) and the transfused period in the crossover cohort (Figure 2c and d). Twenty-two percent of the transfused and 8% of the matched nontransfused groups had a 10 or more HLA antibodies increase of >1000 MFI (P = 0.006); corresponding proportions with increases of >3000 MFI were 20 versus 4% (P = 0.0006). In the crossover cohort, the number of HLA antibodies increasing >1000 and >3000 MFI was substantively higher during the transfused period versus the control period, P = 0.03 and P = 0.008, respectively; 24% of patients had a 10+ HLA antibodies increase of >1000 MFI during the transfused period compared with 4% in the control period. Strikingly, some patients had a >30 antibodies increase of >3000 MFI after transfusion compared with nontransfused matched controls (6 of 50 versus 2 of 155; P = 0.0007) or their own control (nontransfused) period (4 of 25 versus 0 of 25; P not calculated) (Figure 2).
FIGURE 2:
Change in MFI for each unique HLA antibody identified in all patients included in both transfused and matched nontransfused groups (a and b) and crossover cohorts (c and d). The P-values presented in a and b are based on Pearson χ2 analyses comparing the proportion of patients in the transfused and nontransfused groups with >10 (versus <10) HLA antibodies increasing >1000 MFI (a) and >3000 MFI (b). The P-values presented in c and d are based on Sign tests comparing the number of HLA antibodies increasing >1000 MFI (c) and >3000 MFI (d) during the transfused and control periods.
Changes in antibody breadth (cPRA)
The proportion of patients with an increase in cPRA was significantly higher among transfused compared with nontransfused patients using either ≥1000 or ≥3000 MFI as the threshold for defining unacceptable antigens (Table 2). Transfused patients with nonzero cPRA at baseline were especially susceptible to increases in cPRA (Table 2). These differences were more pronounced in the crossover cohort owing to the low level of change during the control period. There were large absolute increases in cPRA when comparing the transfused with the matched nontransfused group using MFI of ≥1000 (P = 0.0002) or ≥3000 (P = 0.01) as thresholds (Figure 3a and b) and during the transfused period compared with the control period in the crossover cohort for MFI of ≥1000 (P = 0.01) and ≥3000 (P = 0.002) thresholds, respectively (Figure 3c and d).
Table 2.
Incidence of increases in cPRA for transfused and nontransfused (matched cohort) and transfused and control periods (crossover cohort)
| Matched cohort |
Crossover cohort |
||||
|---|---|---|---|---|---|
| Nontransfused (N = 151)a | Transfused (N = 50) | P-valueb | Control period (N = 25) | Transfused period (N = 25) | |
| MFI threshold of 1000 for defining unacceptable antigens in cPRA calculation | |||||
| Patients with cPRA = 0% at baseline | 74 | 25 | 12 | 12c | |
| Patients (%) with a positive change in cPRA among those with cPRA = 0% at baseline | 18 (24) | 7 (28) | 0.7 | 1 (8.3) | 3 (25) |
| Patients with cPRA >0% at baseline | 77 | 25 | 13 | 13 | |
| Patients (%) with a positive change in cPRA among those with cPRA >0% at baseline | 33 (42.9) | 18 (72) | 0.01 | 1 (7.7) | 9 (69.2) |
| Total percentage of patients with a positive change in cPRA over baseline value | 34% | 50% | 0.04 | 8% | 48% |
| Mean (SD) cPRA at follow-up | 21 (32) | 34 (41) | 0.04 | 17 (31) | 33 (39) |
| MFI threshold of 3000 for defining unacceptable antigens in cPRA calculation | |||||
| Patients with cPRA = 0% at baseline | 109 | 36 | 17 | 19d | |
| Patients (%) with a positive change in cPRA among those with cPRA = 0% at baseline | 14 (12.7) | 5 (13.9) | 0.87 | 0 (0) | 4 (21.1) |
| Patients with cPRA >0% at baseline | 42 | 14 | 8 | 6 | |
| Patients (%) with a positive change in cPRA among those with cPRA >0% at baseline | 14 (33.3) | 11 (78.6) | 0.0032 | 1 (12.5) | 4 (66.7) |
| Total percentage of patients with a positive change in cPRA over baseline value | 18% | 32% | 0.05 | 4% | 32% |
| Mean (SD) cPRA at follow-up | 14 (29) | 26 (40) | 0.06 | 12 (28) | 20 (37) |
aWe were not able to calculate cPRA for four patients due to missing Class I or Class II cPRA data at baseline or the follow-up measurement.
bP-values were calculated using Pearson χ2 analysis for categorical variables and Student's t-test for continuous variables.
cThe baseline cPRA for one patient decreased from cPRA of >0% at baseline to cPRA of 0% for the transfused period.
dThe baseline cPRA for two patients decreased from cPRA of >0% at baseline to cPRA of 0% for the transfused period.
FIGURE 3:
Absolute change in cPRA levels for all patients in transfused and matched nontransfused groups (a and b) and crossover cohorts (c and d). The P-values presented in a and b are based on Pearson χ2 analyses comparing the distribution of cPRA changes in the transfused and nontransfused groups using >1000 MFI (a) and >3000 MFI (b) as the threshold for defining an unacceptable antigen. The P-values presented in c and d are based on Sign tests comparing the change in cPRA during the transfused and control periods using >1000 MFI (c) and >3000 MFI (d) as the threshold for defining an unacceptable antigen.
Subgroup analyses
In a companion analysis, we excluded patients (80 nontransfused and 26 transfused; 53%) with any major proinflammatory events between the two measurements (Figure 4). In the matched cohort, 21% of transfused and 6% of nontransfused patients had 10 or more MFI changes of >3000 (P = 0.03). In the crossover cohort, 19% of patients during the transfused period and 0% during the control period had 10 or more MFI changes of >3000. Results were comparable across age groups and by race/ethnicity (comparing white versus nonwhite). Transfusion effects on changes in HLA antibody formation by MFI and cPRA were more pronounced among women compared with men and among parous compared with nulliparous women (Supplementary Figure S1).
FIGURE 4:
Change in MFI for each unique HLA antibody identified in all patients included in transfused and matched nontransfused groups (a and b) and crossover cohorts (c and d) excluding patients with proinflammatory events. The P-values presented in a and b are based on Pearson χ2 analyses comparing the proportion of patients in the transfused and nontransfused groups with >10 (versus <10) HLA antibodies increasing >1000 MFI (a) and >3000 MFI (b). The P-values presented in c and d are based on Sign tests comparing the number of HLA antibodies increasing >1000 MFI (c) and >3000 MFI (d) during the transfused and control periods.
DISCUSSION
Understanding the effect of transfusion on HLA sensitization is of importance to all prospective recipients of solid organ and bone marrow transplants. Patients with CKD are particularly prone to anemia due to erythropoietin deficiency [15]. Although existing data suggest that a substantial fraction of transfused patients become sensitized [16–20], the effect of RBC transfusions on sensitization in prospective kidney transplant recipients has not been quantified.
In the current study, we used sensitive and specific antibody assays to determine the strength (MFI) and breadth (cPRA) of HLA antibodies in transfused patients using both a matched reference cohort and a crossover (pre- and posttransfusion) cohort. As MFI strength increases and preset thresholds are exceeded, more incompatible antigens are defined with a concomitant increase in cPRA. We found that transfusion significantly increased sensitization irrespective of whether ≥1000 or ≥3000 MFI was used to define unacceptable antigens, and that the effect was independent of proinflammatory events. These changes could not be attributed to increases in antibody to denatured antigen due to their increased breadth and their higher frequency in the transfused cohort. Because cPRA is calculated based on the identified HLA antibody specificities combined with the frequency of those HLA antigens in the donor population, the cPRA essentially provides an estimate of the probability of receiving a compatible transplant [21]. The increase in MFI and cPRA was sufficient to significantly diminish the number of potentially compatible donors for transfused patients. These data comport with previous studies as well as UNOS and USRDS data, showing that sensitized patients wait for a kidney longer, are more likely to die while awaiting a transplant [22, 23] and, if transplanted, suffer a significantly foreshortened graft survival [24–27].
Dausset [28] observed in 1954 that blood transfusions resulted in antibodies reactive with leukocytes. Subsequent methods for HLA antibody detection employed complement-dependent cytotoxicity assays on random panels of 20–50 cells and were insensitive and nonspecific [29]. Results were necessarily dependent on the composition of the panel, which could not represent the antigenic diversity we now know exists [29]. Until recently, molecular tools and solid-phase antibody detection methods to fully define HLA specificities were nonexistent. Consequently, assessment of PRA and HLA sensitization from transfusion was very likely underestimated. The use of ‘leuko-poor’ or ‘leuko-reduced’ blood products gained popularity, but failed to abrogate or substantially reduce the risk of sensitization [30]. Fisher et al. [31] showed that the volume of 15 × 106 residual leukocytes (equivalent of 2–3 mL of whole blood) in a single unit of leukocyte-depleted platelets was sufficient to result in sensitization. Everett et al. [32] showed that HLA was evident on RBCs at levels corresponding to roughly 1014 HLA molecules per transfused unit. These authors concluded that leukocyte or platelet depletion did not significantly mitigate the risk of transfusion-associated sensitization [31, 32].
Potential causes of allosensitization other than transplantation, pregnancy and transfusions are thought to be secondary to exposure to non-HLA antigens that are cross-reactive with specific HLA epitopes [33, 34]. Therefore, events that do not involve exposure to HLA, such as infections or surgical trauma, can possibly lead to, or amplify, apparent sensitization to HLA. There is a high prevalence of infections in patients on dialysis due in part to the effects of uremia, complications of dialysis access (hemo- and peritoneal dialysis) and the frequent need for procedures and hospitalizations [4, 35]. Locke et al. [36] reported a strong association between infection and increases in HLA antibodies. To address confounding by these factors, we excluded patients who experienced proinflammatory events between the two HLA antibody measurements and found similar results.
Previously sensitized patients may acquire a wider spectrum of antibody formation after a subsequent antigenic challenge, such as transfusion [37]. Although we excluded patients with a prior transplant, our study does provide evidence regarding the risk of transfusion-induced allosensitization in other previously sensitized populations. In our cohorts, the sensitizing effects of blood were significantly more pronounced among patients with pre-existing sensitization at baseline (cPRA >0) and among parous females. Previous studies report that pregnancy is a strong risk factor for transfusion-associated allosensitization [37], and in our population, among parous women, 33% of those transfused exhibited a significant antibody response (10+ antibodies increased) compared with only 17% among nulliparous women. This study has several strengths. We included a well-characterized cohort of patients on dialysis awaiting primary kidney transplantation monitored on a regular schedule for HLA antibodies. We used Luminex single-antigen bead assays performed in a single laboratory, thereby decreasing variability that results from testing at different laboratories. We provided a detailed analysis of changes in frequency, specificity and strength of HLA antibodies following a blood transfusion using increases in MFI to define sensitization, and did so in two cohorts—one matched and one crossover.
This study has several important limitations. First, while we attempted to address important confounding factors such as pregnancies and transplants in addition to using matched controls, the potential for residual confounding cannot be ruled out. A randomized clinical trial (e.g. comparing transfusion versus no transfusion) could balance residual confounding, but is not considered feasible. Secondly, while we benefitted from performing all testing in a single laboratory, some modest run-to-run variability can occur in the Luminex-based HLA antibody assay over time. We monitor for this daily, but have attempted to address this issue for the purposes of this study by analyzing two different MFI thresholds. Thirdly, we drew our patients from a single center. While diverse in age, sex and race, the proportion of Blacks was lower and the proportion of Asian and Pacific Islanders higher than that in the US population. As such, we may have under or overestimated the effects of transfusion on HLA antibody formation and/or cPRA that might be seen in other regions or nationally. Finally, we captured transfusion events from claims data. We expect claims data to have high specificity yet moderate sensitivity, which we would expect to result in an underestimation of the true effect of transfusion on sensitization. While we would anticipate excellent accuracy for transfusion per se, we cannot be certain that the overall exposure to HLA antigens in transfused blood products was accurately captured (e.g. the number of units of packed RBCs, exposure to plasma, cryoprecipitate or other blood products, or whether irradiation or filtering was employed).
The current study offers additional evidence that RBC transfusions are not a benign therapy. In addition to causing allosensitization and compromising transplantation [38], there are well-known transfusion-related risks including transmission of blood-borne diseases, the potential for iron, volume and/or potassium overload as well as transfusion-related acute lung injury (TRALI) [39–42] along with unknown or yet to be identified risks [43]. Blood transfusion should be reserved for patients with, or at risk for, severe anemia, in keeping with recommendations from published clinical practice guidelines [44]. Strategies to reduce blood transfusion [45] should include education regarding transfusion-related risks (directed to nontransplant physicians, surgeons, emergency department personnel and other providers involved in the care of patients with CKD), efforts to reduce unnecessary blood loss (e.g. reducing the use of tunneled catheters in hemodialysis, which obligates blood wastage), vigilance for non-CKD-related causes of anemia, such as gastrointestinal bleeding, chronic infection or malignancy, and the judicious use of IV iron and ESAs.
In summary, the current study using highly sensitive and specific HLA detection methods provides contemporary evidence that blood transfusion results in clinically meaningful increases in HLA antibody strength and breadth, which, translated into cPRA, can have important negative downstream clinical consequences. Our data suggest that previously sensitized patients and parous women may be at particularly high risk for transfusion-related sensitization. Thus, transfusion can jointly reduce the likelihood of receiving a kidney transplant (owing to more donors becoming incompatible) and result in accelerated allograft loss should such a transplant occur. The sensitizing effect of blood combined with the other known transfusion-related risks adds further evidence in favor of transfusion-minimizing strategies. In an environment where deceased donor organs are scarce, waiting times long and lengthening, and dialysis-related outcomes poor, we should attempt to limit blood exposure to all persons who may eventually require kidney transplantation.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
J.M.Y. reports having received grant support to her institution from Amgen and to her institution from the National Institutes of Health; M.W.A. reports having received grant support to his institution from Amgen and payment to him for travel expenses from One Lambda, Inc.; D.K. and B.D.B. are employees in the Center for Observational Research at Amgen, Inc. and own stock in the company; C.D.L. reports having received grant support to his institution from Amgen; J.P. and J.R. are employees in the Division of Global Clinical Development at Amgen, Inc. and own stock in the company; G.M.C. reports having received payment to him for consultancy/lecture fees and travel expenses from Fresenius Medical Care, payment to him for board membership fees from Satellite Healthcare, payment to him for expert testimony from Amgen, payment to him for stock/stock options from Ardelyx, Allocure, PuraCath and Thrasos and payment to his institution from the National Institutes of Health and D.B.T. reports having received grant support to her institution from Amgen, payment to her for consultancy from Amgen, royalties to her and to her institution from One Lambda, Inc., payment to her for travel expenses from One Lambda, Inc. and owns stock in Amgen. No other disclosures were reported.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank Fangfei Chen and Corina Bigham for their assistance with the statistical programming for this study. This research was supported by a grant from Amgen with no restrictions on publications. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number K23AI104401 (J.M.Y.) and the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K24DK54488 (G.M.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the United States Renal Data System.
REFERENCES
- 1.Port FK, Wolfe RA, Mauger EA, et al. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA. 1993;270:1339–1343. [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3.Lee AJ, Morgan CL, Conway P, et al. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin. 2005;21:1777–1783. doi: 10.1185/030079905X65277. [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012. USRDS 2012 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Vol. 2, Chapter 10. [Google Scholar]
- 5.Network OPTN. Scientific Registry of Transplant Recipients: OPTN: Data 2011. http://optn.transplant.hrsa.gov/ (September 2012, date last accessed) [Google Scholar]
- 6.Locatelli F, Pisoni RL, Combe C, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004;19:121–132. doi: 10.1093/ndt/gfg458. [DOI] [PubMed] [Google Scholar]
- 7.McClellan WM, Flanders WD, Langston RD, et al. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J Am Soc Nephrol. 2002;13:1928–1936. doi: 10.1097/01.asn.0000018409.45834.fa. [DOI] [PubMed] [Google Scholar]
- 8.Eschbach JW. Current concepts of anemia management in chronic renal failure: impact of NKF-DOQI. Semin Nephrol. 2000;20:320–329. [PubMed] [Google Scholar]
- 9.Churchill DN, Taylor DW, Cook RJ, et al. Canadian hemodialysis morbidity study. Am J Kidney Dis. 1992;19:214–234. doi: 10.1016/s0272-6386(13)80002-9. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim HN, Ishani A, Foley RN, et al. Temporal trends in red blood transfusion among US dialysis patients, 1992–2005. Am J Kidney Dis. 2008;52:1115–1121. doi: 10.1053/j.ajkd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 12.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 13.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 14.Hebert PL, Geiss LS, Tierney EF, et al. Identifying persons with diabetes using medicare claims data. Am J Med Qual. 1999;14:270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 15.Eschbach JW, Haley NR, Adamson JW. The anemia of chronic renal failure: pathophysiology and effects of recombinant erythropoietin. Contrib Nephrol. 1990;78:24–36. discussion 7. [PubMed] [Google Scholar]
- 16.Opelz G, Graver B, Mickey MR, et al. Lymphocytotoxic antibody responses to transfusions in potential kidney transplant recipients. Transplantation. 1981;32:177–183. doi: 10.1097/00007890-198109000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Scornik JC, Pfaff WW, Howard RJ, et al. Increased antibody responsiveness to blood transfusions in pediatric patients. Transplantation. 1994;58:1361–1365. [PubMed] [Google Scholar]
- 18.Karpinski M, Pochinco D, Dembinski I, et al. Leukocyte reduction of red blood cell transfusions does not decrease allosensitization rates in potential kidney transplant candidates. J Am Soc Nephrol. 2004;15:818–824. doi: 10.1097/01.asn.0000115399.80913.b1. [DOI] [PubMed] [Google Scholar]
- 19.Scornik JC, Meier-Kriesche HU. Blood transfusions in organ transplant patients: mechanisms of sensitization and implications for prevention. Am J Transplant. 2011;11:1785–1791. doi: 10.1111/j.1600-6143.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- 20.Balasubramaniam GS, Morris M, Gupta A, et al. Allosensitization rate of male patients awaiting first kidney grafts after leuko-depleted blood transfusion. Transplantation. 2012;93:418–422. doi: 10.1097/TP.0b013e3182419864. [DOI] [PubMed] [Google Scholar]
- 21.Cecka JM. Calculated PRA (CPRA): the new measure of sensitization for transplant candidates. Am J Transplant. 2010;10:26–29. doi: 10.1111/j.1600-6143.2009.02927.x. [DOI] [PubMed] [Google Scholar]
- 22.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipi-ents (SRTR) Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2011. OPTN/SRTR 2010 Annual Data Report. [Google Scholar]
- 23.USRDS. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. [Google Scholar]
- 24.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4:438–443. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 25.Terasaki PI, Ozawa M. Predictive value of HLA antibodies and serum creatinine in chronic rejection: results of a 2-year prospective trial. Transplantation. 2005;80:1194–1197. doi: 10.1097/01.tp.0000174338.97313.5a. [DOI] [PubMed] [Google Scholar]
- 26.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim HN, Skeans MA, Li Q, et al. Blood transfusions in kidney transplant candidates are common and associated with adverse outcomes. Clin Transplant. 2011;25:653–659. doi: 10.1111/j.1399-0012.2011.01397.x. [DOI] [PubMed] [Google Scholar]
- 28.Dausset J. Indications for transfusion with concentrated or washed erythrocytes. Concours Med. 1954;76:2975–2977. [PubMed] [Google Scholar]
- 29.Murphey CL, Forsthuber TG. Trends in HLA antibody screening and identification and their role in transplantation. Expert Rev Clin Immunol. 2008;4:391–399. doi: 10.1586/1744666X.4.3.391. [DOI] [PubMed] [Google Scholar]
- 30.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337:1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 31.Fisher M, Chapman JR, Ting A, et al. Alloimmunisation to HLA antigens following transfusion with leucocyte-poor and purified platelet suspensions. Vox Sang. 1985;49:331–335. doi: 10.1111/j.1423-0410.1985.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 32.Everett ET, Kao KJ, Scornik JC. Class I HLA molecules on human erythrocytes. Quantitation and Transfusion Effects. Transplantation. 1987;44:123–129. doi: 10.1097/00007890-198707000-00025. [DOI] [PubMed] [Google Scholar]
- 33.Morales-Buenrostro LE, Terasaki PI, Marino-Vazquez LA, et al. "Natural" human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86:1111–1115. doi: 10.1097/TP.0b013e318186d87b. [DOI] [PubMed] [Google Scholar]
- 34.Alberu J, Morales-Buenrostro LE, de Leo C, et al. A non-allogeneic stimulus triggers the production of de novo HLA antibodies in healthy adults. Transpl Immunol. 2007;18:166–171. doi: 10.1016/j.trim.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Troidle L, Eisen T, Pacelli L, et al. Complications associated with the development of bacteremia with staphylococcus aureus. Hemodial Int. 2007;11:72–75. doi: 10.1111/j.1542-4758.2007.00156.x. [DOI] [PubMed] [Google Scholar]
- 36.Locke JE, Zachary AA, Warren DS, et al. Proinflammatory events are associated with significant increases in breadth and strength of HLA-specific antibody. Am J Transplant. 2009;9:2136–2139. doi: 10.1111/j.1600-6143.2009.02764.x. [DOI] [PubMed] [Google Scholar]
- 37.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49:1825–1835. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Organ Procurement and Transplantation Network (OPTN) Proposal to Substantially Revise the National Kidney Allocation System. 2012 http://optn.transplant.hrsa.gov/PublicComment/pubcommentPropSub_311.pdf. 17 June 2013, date last accessed. [Google Scholar]
- 39.Despotis GJ, Zhang L, Lublin DM. Transfusion risks and transfusion-related pro-inflammatory responses. Hematol Oncol Clin North Am. 2007;21:147–161. doi: 10.1016/j.hoc.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carson JL, Altman DG, Duff A, et al. Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair. Transfusion. 1999;39:694–700. doi: 10.1046/j.1537-2995.1999.39070694.x. [DOI] [PubMed] [Google Scholar]
- 41.Epstein JS, Holmberg JA. Progress in monitoring blood safety. Transfusion. 2010;50:1408–1412. doi: 10.1111/j.1537-2995.2010.02728.x. [DOI] [PubMed] [Google Scholar]
- 42.Zou S, Dorsey KA, Notari EP, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion. 2010;50:1495–1504. doi: 10.1111/j.1537-2995.2010.02622.x. [DOI] [PubMed] [Google Scholar]
- 43.Stramer SL, Hollinger FB, Katz LM, et al. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49(Suppl 2):1s–29s. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 44.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012;2(Suppl 2):279–335. [Google Scholar]
- 45.2009. Circular of Information for the Use of Human Blood and Blood Components. http://www.aabb.org/resources/bct/pages/aabb_coi.aspx. July 2013, date last accessed. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.