Abstract
Background
Anemia is less prominent in patients with polycystic kidney disease (PKD). Such iron indices as ferritin and transferrin saturation (TSAT) values are used to guide management of anemia in individuals on maintenance hemodialysis (MHD). Optimal levels of correction of anemia and optimal levels of TSAT and ferritin are unclear in chronic kidney disease patients and have not been studied specifically in PKD.
Methods
We studied 2969 MHD patients with and 128 054 patients without PKD from 580 outpatient hemodialysis facilities between July 2001 and June 2006. Using baseline, time-dependent and time-averaged values with unadjusted and multivariable adjusted analysis models, the survival predictabilities of TSAT and ferritin were studied.
Results
PKD patients were 58 ± 13 years old and included 46% women, whereas non-PKD patients were 62 ± 15 years old and 45% women. In both PKD and non-PKD patients, a time-averaged TSAT between 30 and 40% was associated with the lowest mortality. Time-averaged ferritin between 100 and <800 ng/mL was associated with the lowest mortality in PKD patients, although this range was 500 to <800 ng/mL in non-PKD patients.
Conclusions
In MHD patients with and without PKD, there was a U-shaped relationship between the average TSAT and mortality, and a TSAT of 30–40% was associated with the best survival. However, an average ferritin of 100–800 ng/mL was associated with the best survival in PKD patients, whereas that of non-PKD patients was 500–800 ng/mL. Further studies in PKD and non-PKD patients are necessary to determine whether or not therapeutic attempts to keep TSAT and ferritin levels in these ranges will improve survival.
Keywords: ferritin, hemodialysis, iron, polycystic kidney disease, transferrin saturation
INTRODUCTION
Chronic kidney disease (CKD), particularly in its advanced stages, is associated with anemia and iron metabolism dysregulation. An association of anemia with increased mortality and morbidity has been shown in patients with CKD on maintenance hemodialysis (MHD) and those who are not yet on dialysis [1, 2]. Therefore, correction of anemia, replenishment of iron stores and regulating erythropoiesis and iron metabolism has been an integral part of the management of CKD.
However, several studies have shown that complete correction of anemia in CKD patients is associated with increased morbidity and mortality [3–6]. It is not clear whether increased hemoglobin levels per se affect the mortality and morbidity or attempted therapies to achieve higher hemoglobin levels, such as administration of erythropoiesis-stimulating agents (ESA) and iron, contribute to these adverse outcomes.
The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend using serum transferrin saturation (TSAT) and ferritin target levels as indicators to guide administration of iron supplements in CKD patients, even though these indices may not be able to demonstrate the optimal iron administration for each individual patient [7]. The 2006 National Kidney Foundation Disease Outcomes Quality Initiative (KDOQI) guidelines also recommended using the target levels of the same indices to guide administration of iron supplements in CKD patients [8].
Polycystic kidney disease (PKD) is the most common genetic disease that can cause CKD [9, 10]. Based on the 2010 annual report of the United States Renal Data System (USRDS), 4.6% of the prevalent cases of end-stage renal disease (ESRD) were patients with adult PKD, which represents ∼26 000 patients [11].
Although not unanimously, many studies have shown that PKD patients generally have higher hemoglobin and hematocrit levels in different stages of CKD compared with CKD patients due to other etiologies [12–15]. This may be at least in part owing to the fact that PKD patients have elevated levels of serum erythropoietin compared with non-PKD patients with CKD [13, 16]. PKD patients also have a better survival than other CKD patients [12, 15, 17]. Higher levels of hemoglobin may contribute to this survival advantage [18]. However, in a study by Abott and coworkers, this survival advantage persisted even after adjustment for differences in hematocrit levels [12]. This might be due to higher levels of serum erythropoietin in PKD patients and therefore, less need for exogenous ESA to achieve equal levels of hemoglobin and hematocrit in PKD patients compared with CKD patients without PKD. A recent study of PKD patients demonstrated an association between higher hemoglobin levels and better survival rates only in those patients who were not receiving frequent ESA administrations [19].
Given all these differences between CKD patients with and without PKD, we hypothesized that optimal levels of indices of iron status, namely TSAT and ferritin, might be different in MHD patients with PKD as compared with MHD patients without PKD. This study was performed to evaluate potential associations of various levels of serum ferritin and TSAT with mortality in MHD patients with PKD and its comparison to non-PKD patients.
METHODS
Patients
We extracted, refined and examined data from all individuals with ESRD who underwent dialysis treatment from July 2001 through June 2006 in any one of the 580 outpatient dialysis facilities of DaVita, a large dialysis organization in the United States (prior to its acquisition of units owned by Gambro). Of the 164 789 cumulative patients treated in all DaVita units over the 5-year period, we excluded 13 312 patients without quarterly based data, 19 652 patients who were on peritoneal dialysis or in whom method of renal replacement therapy was unknown, and 802 patients aged >99 or <18 years. Of our 131 023 MHD study population, 2969 were PKD patients and 128 054 were non-PKD patients. The study was approved by relevant Institutional Review Committees.
Clinical and demographic measures
The creation of the cohort has been described previously [20–27]. To minimize measurement variability, all repeated measures for each patient during any given calendar quarter, i.e. over a 13-week interval, were averaged and the summary estimate was used in all models. Average values were obtained from up to 20 calendar quarters (q1 through q20). The first (baseline) studied quarter for each patient was the calendar quarter in which the patient's dialysis vintage was >90 days. The presence or absence of diabetes at baseline and history of tobacco smoking were obtained by linking the DaVita database to the data from the Medical Evidence Form 2728 from the USRDS. The presence of preexisting comorbid conditions was similarly ascertained and grouped into 11 categories: diabetes mellitus, ischemic heart disease, congestive heart failure, history of HIV infection, presence of AIDS, history of hypertension, history of other cardiac diseases, cerebrovascular events, peripheral vascular disease, chronic obstructive pulmonary disease and cancer [28]. Patients were followed for outcomes through 30 June 2007.
Laboratory measures
Blood samples were drawn using uniform techniques in all dialysis clinics and were transported to the DaVita Laboratory in Deland, Florida within 24 h. All laboratory values, including hemoglobin, were measured by automated and standardized methods. We divided baseline TSAT into five categories (<20%, 20 to <30%, 30 to <40%, 40 to <50% and ≥50%) and baseline ferritin into five categories (<100, 100 to <500, 500 to <800, 800 to <1200 and ≥1200 ng/mL).
Epidemiologic and statistical methods
Data were summarized using proportions and means (± standard deviation). The significance of difference between categorical variables was determined using the χ2-test and continuous variables using Student's t-test or ANOVA as appropriate. Survival analysis was performed using baseline, time-dependent and time-averaged values to examine whether TSAT and/or ferritin levels predicted survival in PKD patients.
For each analysis, including subgroup analyses, four models were examined:
Unadjusted or minimally adjusted models included ferritin categories, change of ferritin categories and entry calendar quarter (q1 through q20).
Case-mix adjusted models that included unadjusted models' variables plus age, gender, race/ethnicity (African-Americans and other self-categorized Blacks, Non-Hispanic Caucasians, Asians, Hispanics and others), categories of dialysis vintage (<6 months, 6–12 months, 12–24 months and ≥2 years), primary insurance (Medicare, Medicaid, and others), marital status (married, single, divorced, widowed), type of vascular access (catheter, AV fistula, graft), dialysis dose as indicated by Kt/V (single pool), the 11 abovementioned pre-existing comorbid conditions and history of tobacco smoking.
Case-mix plus malnutrition inflammation complex syndrome (MICS) adjusted models included all of the covariates in the case-mix model as well as body mass index, and seven laboratory surrogates with known association with clinical outcomes in hemodialysis patients including serum levels of albumin, creatinine, calcium, bicarbonate, normalized protein catabolic rate (nPCR) as an indicator of daily protein intake, also known as the normalized protein nitrogen appearance, white blood cell (WBC) count and lymphocyte percentage.
The fully adjusted model included all of the covariates in the case-mix + MICS model in addition to intravenous (IV) iron administration and ESA administration in the form of recombinant human erythropoietin as two continuous variables.
We repeated the same analyses for TSAT categories instead of ferritin categories.
For all analyses, two-sided P-values were reported and results considered statistically significant if P < 0.05. All statistical analyses were carried out by the SAS, version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
The baseline demographic, clinical and laboratory characteristics of the 2247 PKD and 105 117 non-PKD patients, divided into five subgroups based on TSAT categories, are summarized in Table 1. PKD patients were 58 ± 13 years old and included 46% women and 15% African-Americans, whereas non-PKD patients were 62 ± 15 years old, 45% women and 32% African-Americans. Diabetes mellitus, atherosclerotic heart disease, heart failure and peripheral vascular disease were more prevalent in non-PKD patients and the mortality rate was higher in this group as compared with PKD patients. In PKD patients, lower TSAT was associated with higher prevalence of diabetes, lower creatinine and albumin and higher serum phosphorus. Lower TSAT in PKD patients was also associated with lower hemoglobin, higher total WBC but lower percentage of lymphocytes, higher BMI, lower Kt/v and lower nPCR. Table 1 of the suggested electronic appendix demonstrates baseline characteristics of the patients, divided into five ferritin categories.
Table 1.
Baseline characteristics of patients across baseline TSAT categories in 2247 MHD patients with and 105 117 patients without PKD
| Total population | All non-PKD patients | All PKD patients | Non-PKD (N = 105 117) |
PKD (N = 2247) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSAT <20% | TSAT 20 to <30% | TSAT 30 to <40% | TSAT 40 to <50% | TSAT ≥50% | P-value | TSAT <20% | TSAT 20 to <30% | TSAT 30 to <40% | TSAT 40 to <50% | TSAT ≥50% | P-value | ||||
| N = 107 364 | N = 105 117 | N = 2247 | N = 24 049 | N = 4 3656 | N = 2 3152 | N = 9008 | N = 5252 | N = 444 | N = 931 | N = 561 | N = 206 | N = 105 | |||
| Death rate [n (%)] | 53% | 53% | 31% | 57% | 52% | 50% | 54% | 53% | <0.0001 | 35% | 30% | 31% | 34% | 31% | 0.31 |
| Age (years) mean ± SD | 62 ± 15 | 62 ± 15 | 58 ± 13 | 63 ± 15 | 63 ± 15 | 61 ± 16 | 60 ± 16 | 59 ± 17 | <0.0001 | 57 ± 13 | 58 ± 13 | 58 ± 13 | 60 ± 13 | 56 ± 13 | 0.13 |
| Gender (% females) | 45% | 45% | 46% | 46% | 47% | 44% | 42% | 43% | <0.0001 | 44% | 46% | 47% | 47% | 49% | 0.87 |
| Race (%) | |||||||||||||||
| White | 43% | 43% | 65% | 50% | 43% | 39% | 39% | 37% | <0.0001 | 68% | 65% | 65% | 67% | 60% | 0.48 |
| Black | 32% | 32% | 15% | 28% | 33% | 34% | 33% | 33% | <0.0001 | 17% | 16% | 13% | 13% | 16% | 0.43 |
| Hispanic | 15% | 15% | 11% | 14% | 15% | 17% | 18% | 17% | <0.0001 | 8% | 11% | 14% | 10% | 12% | 0.03 |
| Asian | 3% | 3% | 2% | 3% | 3% | #### | 4% | 5% | <0.0001 | 2% | 2% | 3% | 1% | 5% | 0.26 |
| Other | 6% | 7% | 6% | 6% | 6% | 7% | 7% | 7% | 0.01 | 5% | 6% | 5% | 8% | 8% | 0.43 |
| Dialysis vintage (%) | |||||||||||||||
| <6 months | 16% | 16% | 11% | 23% | 16% | 12% | 11% | 15% | <0.0001 | 19% | 11% | 7% | 6% | 15% | <0.0001 |
| 6–24 months | 32% | 32% | 30% | 36% | 33% | 28% | 27% | 25% | <0.0001 | 32% | 31% | 28% | 27% | 23% | 0.13 |
| 2–5 years | 35% | 35% | 36% | 31% | 35% | 37% | 37% | 35% | <0.0001 | 34% | 37% | 37% | 39% | 30% | 0.42 |
| >5 years | 18% | 17% | 23% | 10% | 16% | 23% | 25% | 26% | <0.0001 | 15% | 21% | 29% | 27% | 31% | <0.0001 |
| Primary insurance (%) | |||||||||||||||
| Medicare | 68% | 68% | 56% | 65% | 68% | 69% | 69% | 68% | <0.0001 | 53% | 55% | 61% | 59% | 60% | 0.06 |
| Medicaid | 6% | 15% | 4% | 6% | 6% | 6% | 7% | 8% | <0.0001 | 5% | 5% | 3% | 4% | 3% | 0.53 |
| Other | 15% | 6% | 22% | 17% | 15% | 13% | 12% | 13% | <0.0001 | 29% | 23% | 18% | 17% | 17% | 0 |
| Marital status (%) | |||||||||||||||
| Married | 48% | 47% | 57% | 47% | 47% | 48% | 47% | 47% | 0.57 | 54% | 53% | 58% | 59% | 71% | 0.06 |
| Divorced | 8% | 8% | 9% | 8% | 9% | 8% | 8% | 8% | 0.26 | 11% | 9% | 9% | 10% | 4% | 0.31 |
| Single | 28% | 28% | 24% | 27% | 27% | 28% | 30% | 31% | <0.0001 | 26% | 25% | 23% | 21% | 20% | 0.69 |
| Widowed | 16% | 16% | 10% | 17% | 17% | 16% | 14% | 14% | <0.0001 | 9% | 11% | 10% | 10% | 5% | 0.46 |
| Vascular access (%) | |||||||||||||||
| Catheter | 46% | 46% | 34% | 58% | 46% | 39% | 38% | 39% | <0.0001 | 45% | 37% | 28% | 19% | 22% | <0.0001 |
| AVF | 25% | 25% | 39% | 20% | 24% | 28% | 31% | 30% | <0.0001 | 34% | 37% | 43% | 43% | 43% | 0.04 |
| Graft | 29% | 29% | 28% | 23% | 30% | 33% | 31% | 31% | <0.0001 | 21% | 27% | 29% | 39% | 35% | 0 |
| Comorbid conditions (%) | |||||||||||||||
| Diabetes mellitus | 59% | 60% | 14% | 64% | 62% | 58% | 53% | 49% | <0.0001 | 20% | 14% | 11% | 11% | 6% | <0.0001 |
| Cancer | 5% | 5% | 3% | 5% | 4% | 4% | 5% | 5% | 0 | 3% | 3% | 3% | 4% | 3% | 0.95 |
| Atherosclerotic heart disease | 21% | 22% | 12% | 25% | 22% | 20% | 17% | 16% | <0.0001 | 13% | 11% | 10% | 14% | 14% | 0.55 |
| Heart failure | 28% | 29% | 10% | 33% | 30% | 26% | 23% | 22% | <0.0001 | 11% | 10% | 9% | 9% | 5% | 0.35 |
| Other heart diseases | 6% | 6% | 5% | 7% | 6% | 5% | 4% | 4% | <0.0001 | 5% | 5% | 4% | 5% | 5% | 0.99 |
| Peripheral vascular disease | 12% | 12% | 3% | 14% | 12% | 10% | 10% | 9% | <0.0001 | 5% | 3% | 3% | 2% | 4% | 0.52 |
| Cerebrovascular disease | 5% | 8% | 5% | 9% | 8% | 7% | 7% | 6% | <0.0001 | 5% | 5% | 4% | 5% | 6% | 0.89 |
| History of hypertension | 79% | 79% | 79% | 79% | 80% | 79% | 77% | 75% | <0.0001 | 77% | 80% | 79% | 75% | 79% | 0.52 |
| COPD | 6% | 6% | 4% | 8% | 6% | 5% | 5% | 4% | <0.0001 | 3% | 4% | 3% | 5% | 7% | 0.14 |
| Non-ambulatory | 3% | 3% | 1% | 4% | 3% | 3% | 3% | 3% | <0.0001 | 1% | 1% | 1% | 1% | 1% | 0.82 |
| Smoker | 5% | 5% | 5% | 5% | 5% | 5% | 5% | 5% | 0.01 | 6% | 6% | 5% | 4% | 6% | 0.73 |
| Serum levels (at baseline) (mean ± SD) | |||||||||||||||
| Albumin (g/dL) | 3.65 ± 0.5 | 3.65 ± 0.5 | 3.89 ± 0.4 | 3.54 ± 0.5 | 3.65 ± 0.5 | 3.72 ± 0.5 | 3.72 ± 0.5 | 3.63 ± 0.6 | <0.0001 | 3.80 ± 0.4 | 3.88 ± 0.4 | 3.96 ± 0.3 | 3.92 ± 0.4 | 3.89 ± 0.4 | <0.0001 |
| Creatinine (mg/dL) | 7.82 ± 3.3 | 7.8 ± 33 | 9.06 ± 2.9 | 7.01 ± 3.1 | 7.71 ± 3.2 | 8.29 ± 3.4 | 8.56 ± 3.4 | 8.59 ± 3.5 | <0.0001 | 8.58 ± 2.8 | 9.01 ± 2.9 | 9.04 ± 3.0 | 9.01 ± 2.9 | 9.25 ± 3.0 | 0 |
| Bicarbonate (mmol/L) | 22.4 ± 3.1 | 22.4 ± 3.1 | 21.9 ± 2.9 | 22.5 ± 3.1 | 22.5 ± 3.0 | 22.4 ± 3.0 | 22.2 ± 3.1 | 22.1 ± 3.3 | <0.0001 | 22.8 ± 2.9 | 22.0 ± 2.8 | 21.9 ± 3.1 | 21.9 ± 3.0 | 22.3 ± 3.4 | 0.65 |
| Calcium (mg/dL) | 9.17 ± 0.71 | 9.16 ± 0.71 | 9.40 ± 0.7 | 9.10 ± 0.7 | 9.19 ± 0.7 | 9.20 ± 0.7 | 9.17 ± 0.7 | 9.08 ± 0.8 | <0.0001 | 9.36 ± 0.7 | 9.39 ± 0.7 | 9.43 ± 0.7 | 9.39 ± 0.7 | 9.40 ± 0.7 | 0.61 |
| Phosphorous (mg/dL) | 5.54 ± 1.5 | 5.53 ± 1.5 | 5.80 ± 1.5 | 5.50 ± 1.5 | 5.54 ± 1.4 | 5.54 ± 1.5 | 5.55 ± 1.5 | 5.46 ± 1.6 | <0.0001 | 5.93 ± 1.6 | 5.82 ± 1.5 | 5.78 ± 1.3 | 5.69 ± 1.4 | 5.46 ± 1.4 | 0.03 |
| Iron metabolism tests | |||||||||||||||
| Serum iron (µg/dL) | 57.8 ± 26 | 57.7 ± 25 | 61.0 ± 24 | 35 ± 10 | 51 ± 13 | 69 ± 16 | 87 ± 21 | 115 ± 38 | <0.0001 | 37 ± 9 | 54 ± 12 | 72 ± 17 | 89 ± 17 | 116 ± 30 | <0.0001 |
| Ferritin (ng/mL) | 500 ± 485 | 502 ± 487 | 432 ± 381 | 340 ± 361 | 467 ± 413 | 576 ± 487 | 670 ± 552 | 923 ± 814 | <0.0001 | 296 ± 307 | 403 ± 338 | 477 ± 386 | 559 ± 426 | 811 ± 550 | <0.0001 |
| TIBC (mg/dL) | 209 ± 47 | 209 ± 47 | 216 ± 44 | 225 ± 52 | 209 ± 44 | 202 ± 42 | 198 ± 44 | 188 ± 49 | <0.0001 | 234 ± 52 | 217 ± 41 | 211 ± 40 | 202 ± 36 | 192 ± 38 | <0.0001 |
| TSAT (%) | 28.1 ± 12 | 28.1 ± 12 | 28.7 ± 11 | 15.8 ± 3.0 | 24.5 ± 2.8 | 34.0 ± 2.8 | 43.9 ± 2.8 | 60.9 ± 11.5 | 15.9 ± 2.8 | 24.7 ± 2.8 | 34.1 ± 2.8 | 43.7 ± 2.8 | 59.8 ± 10.0 | ||
| Hematologic tests (mean ± SD) | |||||||||||||||
| Hemoglobin (g/dL) | 12.0 ± 1.4 | 12.0 ± 1.4 | 12.4 ± 1.4 | 12.2 ± 1.4 | 12.1 ± 1.3 | 12.2 ± 1.4 | 12.2 ± 1.4 | 11.9 ± 1.6 | <0.0001 | 12.1 ± 1.5 | 12.4 ± 1.3 | 12.6 ± 1.4 | 12.7 ± 1.2 | 12.5 ± 1.3 | <0.0001 |
| WBC (×103/µL) | 7.54 ± 2.6 | 7.56 ± 2.6 | 6.74 ± 2.1 | 8.32 ± 3.0 | 7.54 ± 2.4 | 7.16 ± 2.4 | 6.99 ± 2.5 | 6.98 ± 3.0 | <0.0001 | 7.16 ± 2.3 | 6.68 ± 1.9 | 6.39 ± 1.9 | 6.36 ± 2.0 | 6.11 ± 1.9 | <0.0001 |
| Lymphocyte (% of total WBC) | 20.4 ± 7.9 | 20.3 ± 7.9 | 20.7 ± 7.0 | 17.8 ± 7.3 | 20.2 ± 7.6 | 21.8 ± 7.9 | 22.6 ± 8.2 | 23.2 ± 8.9 | <0.0001 | 18.6 ± 6.7 | 20.5 ± 7.1 | 20.5 ± 7.1 | 22.7 ± 6.3 | 23.0 ± 7.7 | <0.0001 |
| Other variables (mean ± SD) | |||||||||||||||
| BMI (kg/m2) | 26.8 ± 6.9 | 26.8 ± 7.0 | 26.2 ± 6.0 | 26.9 ± 7.1 | 27.2 ± 7.1 | 26.6 ± 6.8 | 25.9 ± 6.6 | 25.1 ± 6.1 | <0.0001 | 26.5 ± 6.6 | 26.7 ± 6.0 | 26.0 ± 6.0 | 25.4 ± 5.3 | 23.9 ± 5.4 | <0.0001 |
| Kt/V (dialysis dose) | 1.52 ± 0.4 | 1.52 ± 0.4 | 1.56 ± 0.35 | 1.48 ± 0.4 | 1.52 ± 0.4 | 1.55 ± 0.3 | 1.57 ± 0.4 | 1.57 ± 0.4 | <0.0001 | 1.51 ± 0.4 | 1.53 ± 0.3 | 1.60 ± 0.3 | 1.62 ± 0.4 | 1.61 ± 0.3 | <0.0001 |
| Protein catabolic rate (g/kg/day) | 0.94 ± 0.3 | 0.94 ± 0.3 | 0.97 ± 0.24 | 0.90 ± 0.2 | 0.92 ± 0.3 | 0.97 ± 0.3 | 0.99 ± 0.3 | 0.98 ± 0.3 | <0.0001 | 0.93 ± 0.2 | 0.97 ± 0.2 | 1.00 ± 0.2 | 1.01 ± 0.3 | 0.99 ± 0.2 | <0.0001 |
TSAT, transferrin saturation.
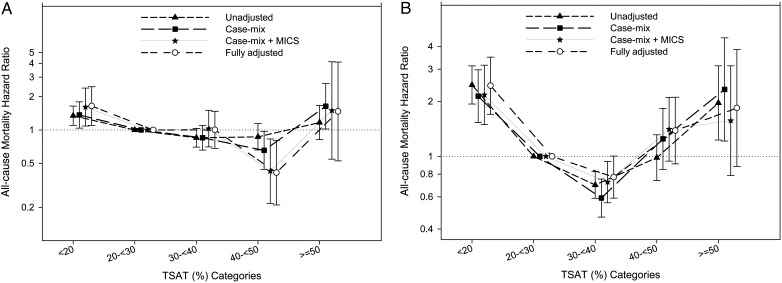
In terms of survival, in the overall study population with PKD and non-PKD patients combined, time-averaged TSAT between 30 and 40% was associated with the lowest mortality [hazard ratio (HR) = 0.86 (95% CI: 0.84–0.88); reference group: TSAT 20 to <30%]. Table 2 shows case-mix plus MICS adjusted and fully adjusted mortality HRs of time-dependent and time-averaged TSAT levels in PKD patients. As shown in Figure 1, in all models, a time-averaged TSAT between 30 and 40% was associated with the lowest mortality in PKD patients. In a fully adjusted model in PKD patients, a baseline TSAT of 40 to <50% was associated with the lowest mortality [HR = 0.41 (95% CI: 0.21–0.81); reference group: TSAT 20 to <30%].
Table 2.
Comparing the hazard ratios of death associated with time-dependent and time-averaged TSAT categories in 2247 MHD patients with PKD, by case-mix + MICS adjusted and fully adjusted models
| Case-mix + MICS adjusted |
Fully adjusted |
|||||
|---|---|---|---|---|---|---|
| Category | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Time-dependent TSAT (%) | ||||||
| <20% | 1.27 | 0.99–1.63 | 0.06 | 1.12 | 1.08–1.14 | <0.0001 |
| 20 to <30%a | 1 | 1 | 1 | 1 | ||
| 30 to <40% | 0.74 | 0.58–0.94 | 0.01 | 0.93 | 0.91–0.96 | <0.0001 |
| 40 to <50% | 0.99 | 0.73–1.35 | 0.96 | 0.93 | 0.89–0.96 | 0.0001 |
| ≥50% | 1.28 | 0.89–1.83 | 0.19 | 1.05 | 1.00–1.09 | 0.03 |
| Time-averaged TSAT (%) | ||||||
| <20% | 2.19 | 1.51–3.19 | <0.0001 | 2.44 | 1.7–3.49 | <0.0001 |
| 20 to <30%a | 1 | 1 | 1 | 1 | ||
| 30 to <40% | 0.74 | 0.57–0.95 | 0.02 | 0.77 | 0.59–1.0 | 0.0534 |
| 40 to <50% | 1.34 | 0.9–2.01 | 0.15 | 1.38 | 0.91–2.11 | 0.1291 |
| ≥50% | 1.59 | 0.80–3.16 | 0.19 | 1.84 | 0.88–3.85 | 0.1048 |
MHD, maintenance hemodialysis; PKD, polycystic kidney disease; TSAT, transferrin saturation; CI, confidence interval.
aReference group.
FIGURE 1:
Hazard ratio (95% CI) of mortality across the TSAT categories at baseline (A) and using time-averaged (B) cox regression analyses in MHD patients with polycystic kidney disease.
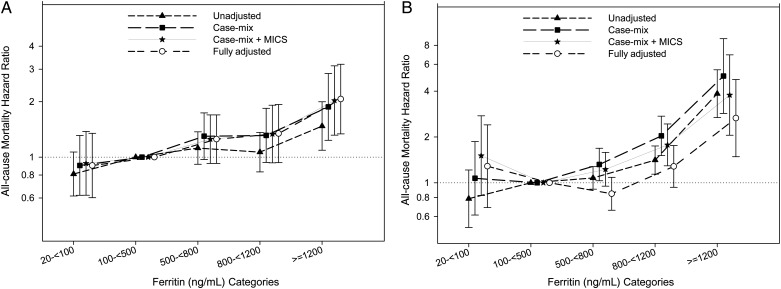
In the overall study population, time-averaged ferritin levels between 500 and 800 were associated with the lowest mortality [HR = 0.94 (95% CI: 0.91–0.96); reference group: ferritin 100 to <500 ng/mL]. The risk of mortality in PKD patients, increased incrementally with increasing baseline ferritin levels (Figure 2). This held true for time-averaged ferritin in an unadjusted model. However, after full adjustment, a ferritin level of 100 to <800 ng/mL appeared to be associated with the lowest mortality risk and ferritin levels <100 ng/mL displayed a trend toward increased mortality. Of note, in the fully adjusted model, for both baseline and time-averaged ferritin levels in PKD patients, only the association with increased mortality of ferretin levels ≥1200 reached statistical significance (reference group: ferritin 100 to <500 ng/mL) (Figure 2).
FIGURE 2:
Hazard ratio (95% CI) of mortality across the ferritin categories at baseline (A) and using time-averaged (B) cox regression analyses in MHD patients with polycystic kidney disease.
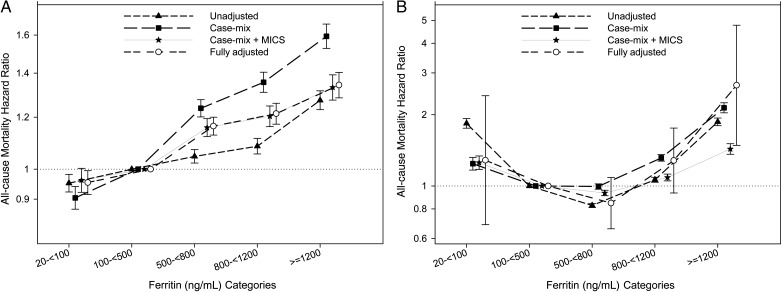
In non-PKD patients, the mortality hazard ratio increased incrementally with increasing baseline ferritin levels, and it reached statistical significance at all levels. However, in the fully adjusted model, time-averaged ferritin between 500 and <800 ng/mL was associated with the lowest mortality, with a J-shaped curve, which again reached statistical significance for increased mortality at ferritin levels ≥1200 (Figure 3).
FIGURE 3:
Hazard ratio (95% CI) of mortality across the ferritin categories at baseline (A) and using time-averaged (B) cox regression analyses in MHD patients without polycystic kidney disease.
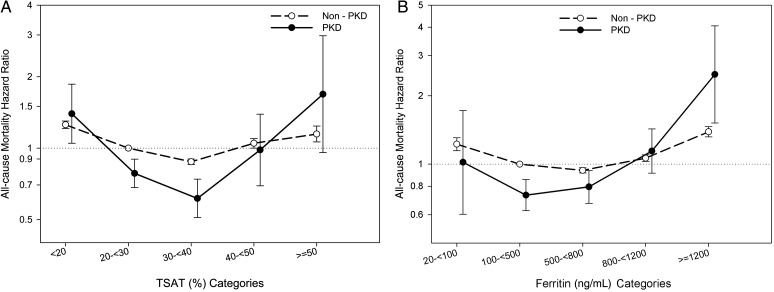
In a fully adjusted model for non-PKD patients, the association between time-averaged TSAT and mortality followed a similar pattern to that of PKD patients, with a TSAT of 30 to <40% being associated with the lowest mortality [HR = 0.88 (95% CI: 0.85–0.90); reference group: TSAT 20 to <30%] (Figure 4A). In the fully adjusted model, mortality hazard ratio of time-dependent TSAT for non-PKD patients was the lowest in the range of 40 to <50% [HR = 0.92 (95% CI: 0.88–0.97); reference group: TSAT 20 to <30%] with a small difference with the 30 to <40% group [HR = 0.95 (95% CI: 0.91–0.98); reference group: TSAT 20 to <30%]. Figure 4B compares the hazard ratios of mortality between MHD patients with and without PKD across the ferritin categories using a time-averaged cox regression analysis in a fully-adjusted model.
FIGURE 4:
Comparing the hazard ratio of mortality between MHD patients with and without polycystic kidney disease across the TSAT categories (A) and ferritin categories (B) using time-averaged cox regression analyses in a fully adjusted model.
Mean weekly dose of IV iron in PKD patients was 31.7 ± 41.5 mg/week and that of ESA was 23 431 ± 27 732 units/week, while in non-PKD patients, these numbers were 34.3 ± 44.7 mg/week and 27 116 ± 27 143 units/week, respectively.
DISCUSSION
This study showed that in MHD patients with PKD: (i) a baseline TSAT of 40–50% was associated with the lowest mortality rate. (ii) Time-averaged TSAT of 30–40% was associated with the best survival. (iii) There was a trend toward increase mortality with increments of ferritin at baseline and the baseline serum levels between 20 and 100 ng/mL were associated with the lowest mortality. (iv) Time-averaged ferritin levels between 100 and 800 ng/mL were associated with the best survival. Average ferritin levels <100 ng/mL and those of >800 ng/mL had a trend toward increased mortality and those ≥1200 ng/mL were significantly associated with increased mortality. In non-PKD patients on MHD, (i) time-averaged TSAT had the same pattern as that of PKD patients and TSAT levels of 30–40% were associated with the lowest mortality and (ii) time-averaged ferritin levels of 500–800 ng/mL were associated with the lowest mortality.
Our findings regarding levels of TSAT and ferritin associated with the lowest mortality in overall patient population, is consistent with the previous study of a subgroup of the same study population [29]. The 2012 KDIGO guidelines recommend administration of supplemental iron for patients on hemodialysis when TSAT ≤ 30% and serum ferritin level ≤500 ng/mL [7]. The 2006 KDOQI guidelines recommended administration of sufficient iron for patients on hemodialysis to maintain serum ferritin level >200 ng/mL and TSAT >20%. The upper limit for TSAT has not been specified and there had been insufficient evidence to assess harm and benefit of maintaining ferritin >500 ng/mL [8]. These recommendations are based on studies that compare the association of different levels of ferritin and TSAT with the outcomes such as required dose of ESAs or the need to initiate ESA to maintain target range hemoglobin levels [30–33]. However, the impact of different levels of these indices on outcomes such as mortality and morbidity is more conclusive and studies with these end points are necessary. Additionally, those guidelines do not differentiate between patients with and without PKD, even though in PKD patients plasma levels of erythropoietin, hemoglobin and hematocrit are different from those of CKD patients with etiologies other than PKD [14–16]; and therefore, optimal levels of TSAT and ferritin may vary between PKD and non-PKD patients on MHD. Our results show that further studies are necessary for this differentiation between the PKD and non-PKD patients.
In this study, we evaluated the association of serum ferritin level and TSAT with mortality. Time-averaged TSAT levels between 30 and 40% were associated with the best survival outcomes in both PKD and non-PKD patients on MHD. This is consistent with the previous studies [30, 31, 34], KDOQI guidelines that recommend maintenance of TSAT levels >20% [8] and more so with the more recent 2012 KDIGO guidelines which sets the TSAT level for administration of supplemental iron at 30% [7]. However, results of our study delineate the favorable TSAT level more precisely with both upper and lower limits, show its association with mortality and demonstrate its applicability in PKD patients as well as non-PKD patients.
Ferritin is both an index of iron storage and an acute-phase reactant. A higher ferritin at baseline may therefore represent a state of inflammation, hence a poorer prognosis. However, the increasing mortality trend with increments of baseline ferritin exists even after adjustment for markers of MICS both in PKD and non-PKD patients. A very low time-averaged ferritin, however, was associated with a higher mortality after the adjustments, which could be due to the fact that it is representative of lower iron storage and/or worse nutritional status. Maintaining ferritin between 100 and 800 ng/mL in PKD and 500 and 800 ng/mL in non-PKD patients were associated with the best survival and levels >800 ng/mL were associated with increases in mortality. The reason that in PKD patients, lower levels of ferritin, compared with non-PKD patients, might be associated with a better survival is unclear. It may be due to the fact that non-PKD patients in general tend to have lower hemoglobin levels compared with PKD patients and therefore, higher ferritin levels might be required to reach the same hemoglobin level. An alternative explanation would be the higher doses of IV iron and ESA administered in non-PKD patients. In line with this explanation is the fact that after adjustment for ESA and IV iron, the difference between ferritin level associated with the lowest mortality in PKD and non-PKD patients attenuated. The 2006 KDOQI guidelines for MHD patients recommended maintaining a ferritin level >200 ng/mL, whereas levels >500 ng/mL had not been recommended due to lack of evidence [8]. The 2012 KDIGO guidelines, however, recommends administration of iron supplement if TSAT ≤ 30% and ferritin is ≤500 ng/mL [7]. In our study, in the case-mix and MICS-adjusted model, ferritin levels >500 ng/mL show a trend of association with a worse outcome in PKD patients. Notwithstanding, in non-PKD patients, time-averaged ferritin levels of 500–800 ng/mL were associated with a better outcome, which is consistent with the newer 2012 KDIGO guidelines. As for TSAT, the KDOQI recommendations for ferritin level are mainly based on the studies designed to evaluate response to iron or ESA therapy measured by hemoglobin or its surrogate indices [31, 33–36]. Similarly, KDIGO 2012 guidelines in regard to the level of TSAT and ferritin are based on the fact that a substantial fraction of CKD patients with anemia and TSAT > 20% respond to iron supplementation as indicated by increase in hemoglobin concentration and/or reduction in ESA dose [7].
In our study, however, the association between mean ferritin and mortality was studied. Ferritin lacks accuracy in evaluation of functional iron status and anemia in hemodialysis patients [36–38]. On the other hand, increased serum levels of ferritin in MHD patients has been shown to be associated with increased morbidity and mortality [39]. In fact, levels of ferritin may also be affected by many other factors than the body's iron storage. Prospective randomized studies with hard primary outcomes need to be performed to achieve more accurate conclusions.
To the best of our knowledge, this is the first study assessing indices of iron status in PKD patients on MHD and their association with mortality. The large number of patients and their diverse nationwide geographical and racial distribution is in favor of the generalizability of the results. Furthermore, multiplicity of important covariates included in multivariate analysis boosts the validity of the results. However, as in any other observational study, potential confounding variables may affect the results and associations cannot prove causality. Another limitation of this study was the fact that we did not have C-reactive protein measurements as a marker of inflammation. Nevertheless, serum albumin, WBC count and lymphocyte percentage, which have significant associations with inflammation in dialysis patients [19, 40], were included in the analyses. Potential longer term effects of the studied parameters may have not been shown in this study. For instance, activation of inflammatory pathways may result in atherogenesis and subsequent related morbidity and mortality after several years. However, given the relatively short life expectancy of the patients on MHD, the follow-up time in this study appears to have a meaningful applicability to the target population.
Given the fact that these data were collected a few years ago and in the past few years some practice modalities might have changed, one may argue that the results of this study may not be completely applicable to the current practice. However, the time difference is minute and the practice guidelines and available modalities have not changed significantly throughout this time period. Moreover, as an observational study, this study does not recommend any clinical practice; rather, it creates new insight for further studies.
It was notable in our study that after incorporation of IV iron and ESA administration to the analyses, some confidence intervals widened. This can be due to the large variability of the doses of these medications used in the study population. However, even so, the direction and magnitude of the associations remained essentially the same.
CONCLUSION
In hemodialysis patients with PKD, as well as non-PKD patients, there was a U-shaped relationship between the average TSAT and mortality, in which a TSAT of 30–40% was associated with the best survival. A time-averaged ferritin of 100–800 ng/mL was associated with the best survival in MHD patients with PKD after full adjustment, and that of 500–800 ng/mL applied to non-PKD patients. Further studies are required to assess optimal levels for TSAT and ferritin in PKD and non-PKD patients. Prospective randomized controlled trials are necessary to determine the best management strategy based on these findings.
CONFLICT OF INTEREST STATEMENT
Dr K.K.-Z. was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA.
ACKNOWLEDGEMENTS
The authors thank DaVita Clinical Research for providing the clinical data. The study was supported by KKZ's research grant from the National Kidney Foundation of Southern California. Other funding sources include the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (K24-DK091419, R01-DK078106 and R21-DK078012) and a philanthropic grant from Mr Harold Simmons.
REFERENCES
- 1.Collins AJ, Li S, St Peter W. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39% J Am Soc Nephrol. 2001;12:2465–2473. doi: 10.1681/ASN.V12112465. Epub 2001/10/25. [DOI] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, et al. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int. 2006;69:560–564. doi: 10.1038/sj.ki.5000105. [DOI] [PubMed] [Google Scholar]
- 3.Besarab A, Bolton WK, Browne JK. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. Epub 1998/08/27. [DOI] [PubMed] [Google Scholar]
- 4.Besarab A, Goodkin DA, Nissenson AR. The normal hematocrit study – follow-up. N Engl J Med. 2008;358:433–434. doi: 10.1056/NEJMc076523. Epub 2008/01/25. [DOI] [PubMed] [Google Scholar]
- 5.Drueke TB, Locatelli F, Clyne N. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. Epub 2006/11/17. [DOI] [PubMed] [Google Scholar]
- 6.Singh AK, Szczech L, Tang KL. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. Epub 2006/11/17. [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcome (KDIGO) Anemia Work Group. KDIGO Clinical practice guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 8.National Kidney Foundation Disease Outcomes Quality Initiative (KDOQI) Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(Suppl. 3):S58–S70. doi: 10.1053/j.ajkd.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. Epub 1993/07/29. [DOI] [PubMed] [Google Scholar]
- 10.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens. 2009;18:99–106. doi: 10.1097/MNH.0b013e3283262ab0. Epub 2009/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins AJ, Foley RN, Herzog C. US Renal data system 2010 annual data report. Am J Kidney Dis. 2011;57(Suppl. 1) doi: 10.1053/j.ajkd.2010.10.007. A8, e1–526. Epub 2010/12/28. [DOI] [PubMed] [Google Scholar]
- 12.Abbott KC, Agodoa LY. Polycystic kidney disease in patients on the renal transplant waiting list: trends in hematocrit and survival. BMC Nephrol. 2002;3:7. doi: 10.1186/1471-2369-3-7. Epub 2002/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra M, Miller ME, Garcia JF, et al. Serum immunoreactive erythropoietin levels in patients with polycystic kidney disease as compared with other hemodialysis patients. Nephron. 1985;39:26–29. doi: 10.1159/000183332. Epub 1985/01/01. [DOI] [PubMed] [Google Scholar]
- 14.Gabow PA, Ikle DW, Holmes JH. Polycystic kidney disease: prospective analysis of nonazotemic patients and family members. Ann Intern Med. 1984;101:238–247. doi: 10.7326/0003-4819-101-2-238. Epub 1984/08/01. [DOI] [PubMed] [Google Scholar]
- 15.Milutinovic J, Fialkow PJ, Agodoa LY, et al. Autosomal dominant polycystic kidney disease: symptoms and clinical findings. Q J Med. 1984;53:511–522. Epub 1984/01/01. [PubMed] [Google Scholar]
- 16.Eckardt KU, Mollmann M, Neumann R. Erythropoietin in polycystic kidneys. J Clin Invest. 1989;84:1160–1166. doi: 10.1172/JCI114280. Epub 1989/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38:777–784. doi: 10.1053/ajkd.2001.27720. [DOI] [PubMed] [Google Scholar]
- 18.Fourtounas C, Panteris V, Valis D. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2002;39:660. doi: 10.1053/ajkd.2002.32161. [DOI] [PubMed] [Google Scholar]
- 19.Shah A, Molnar MZ, Lukowsky LR, et al. Hemoglobin level and survival in hemodialysis patients with polycystic kidney disease and the role of administered erythropoietin. Am J Hematol. 2012;87:833–836. doi: 10.1002/ajh.23255. Epub 2012/05/30. [DOI] [PubMed] [Google Scholar]
- 20.Streja E, Kovesdy CP, Molnar MZ. Role of nutritional status and inflammation in higher survival of African American and Hispanic hemodialysis patients. Am J Kidney Dis. 2011;57:883–893. doi: 10.1053/j.ajkd.2010.10.050. Epub 2011/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45:811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 22.Kilpatrick RD, McAllister CJ, Kovesdy CP, et al. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293–303. doi: 10.1681/ASN.2006070795. [DOI] [PubMed] [Google Scholar]
- 23.Molnar MZ, Lukowsky LR, Streja E. Blood pressure and survival in long-term hemodialysis patients with and without polycystic kidney disease. J Hypertens. 2010;28:2475–2484. doi: 10.1097/HJH.0b013e32833e4fd8. Epub 2010/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JE, Kovesdy CP, Nissenson AR. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55:100–112. doi: 10.1053/j.ajkd.2009.08.007. Epub 2009/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JE, Kovesdy CP, Norris KC. Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. Am J Nephrol. 2010;32:403–413. doi: 10.1159/000319861. Epub 2010/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalantar-Zadeh K, Miller JE, Kovesdy CP. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25:2448–2458. doi: 10.1002/jbmr.177. Epub 2010/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Streja E, Kovesdy CP. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85:991–1001. doi: 10.4065/mcp.2010.0336. Epub 2010/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longenecker JC, Coresh J, Klag MJ. Validation of comorbid conditions on the end-stage renal disease medical evidence report: the CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. J Am Soc Nephrol. 2000;11:520–529. doi: 10.1681/ASN.V113520. [DOI] [PubMed] [Google Scholar]
- 29.Kalantar-Zadeh K, Regidor DL, McAllister CJ, et al. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3070–3080. doi: 10.1681/ASN.2005040423. Epub 2005/07/22. [DOI] [PubMed] [Google Scholar]
- 30.Besarab A, Amin N, Ahsan M. Optimization of epoetin therapy with intravenous iron therapy in hemodialysis patients. J Am Soc Nephrol. 2000;11:530–538. doi: 10.1681/ASN.V113530. Epub 2000/03/07. [DOI] [PubMed] [Google Scholar]
- 31.Chang CH, Chang CC, Chiang SS. Reduction in erythropoietin doses by the use of chronic intravenous iron supplementation in iron-replete hemodialysis patients. Clin Nephrol. 2002;57:136–141. doi: 10.5414/cnp57136. Epub 2002/02/28. [DOI] [PubMed] [Google Scholar]
- 32.DeVita MV, Frumkin D, Mittal S, et al. Targeting higher ferritin concentrations with intravenous iron dextran lowers erythropoietin requirement in hemodialysis patients. Clin Nephrol. 2003;60:335–340. doi: 10.5414/cnp60335. Epub 2003/12/03. [DOI] [PubMed] [Google Scholar]
- 33.Fishbane S, Frei GL, Maesaka J. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis. 1995;26:41–46. doi: 10.1016/0272-6386(95)90151-5. Epub 1995/07/01. [DOI] [PubMed] [Google Scholar]
- 34.Fishbane S, Kowalski EA, Imbriano LJ, et al. The evaluation of iron status in hemodialysis patients. J Am Soc Nephrol. 1996;7:2654–2657. doi: 10.1681/ASN.V7122654. Epub 1996/12/01. [DOI] [PubMed] [Google Scholar]
- 35.Chuang CL, Liu RS, Wei YH, et al. Early prediction of response to intravenous iron supplementation by reticulocyte haemoglobin content and high-fluorescence reticulocyte count in haemodialysis patients. Nephrol Dial Transplant. 2003;18:370–377. doi: 10.1093/ndt/18.2.370. Epub 2003/01/25. [DOI] [PubMed] [Google Scholar]
- 36.Mittman N, Sreedhara R, Mushnick R. Reticulocyte hemoglobin content predicts functional iron deficiency in hemodialysis patients receiving rHuEPO. Am J Kidney Dis. 1997;30:912–922. doi: 10.1016/s0272-6386(97)90104-9. Epub 1997/12/16. [DOI] [PubMed] [Google Scholar]
- 37.Fishbane S, Galgano C, Langley RC, Jr., et al. Reticulocyte hemoglobin content in the evaluation of iron status of hemodialysis patients. Kidney Int. 1997;52:217–222. doi: 10.1038/ki.1997.323. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 38.Fishbane S, Shapiro W, Dutka P, et al. A randomized trial of iron deficiency testing strategies in hemodialysis patients. Kidney Int. 2001;60:2406–2411. doi: 10.1046/j.1523-1755.2001.00077.x. Epub 2001/12/12. [DOI] [PubMed] [Google Scholar]
- 39.Kalantar-Zadeh K, Don BR, Rodriguez RA, et al. Serum ferritin is a marker of morbidity and mortality in hemodialysis patients. Am J Kidney Dis. 2001;37:564–572. Epub 2001/03/03. [PubMed] [Google Scholar]
- 40.Kovesdy CP, George SM, Anderson JE, et al. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009;90:407–414. doi: 10.3945/ajcn.2008.27390. Epub 2009/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]