Synopsis
Although it is widely appreciated that cats respond differently to certain drugs when compared with other companion animal species, the causes of these differences are poorly understood. This review critically evaluates published evidence for altered drug effects in cats, focusing on pharmacokinetic differences between cats, dogs and humans, and the molecular mechanisms underlying these differences. Pharmacokinetic studies indicate that acetaminophen, propofol, carprofen, and acetylsalicylic acid (aspirin) are cleared significantly more slowly in cats versus dogs and humans. All of these drugs are metabolized by conjugation. Cats lack the major phenol UDP-glucuronosyltransferase (UGT) enzymes, including UGT1A6 and UGT1A9, that glucuronidate acetaminophen and propofol. Deficient glucuronidation may also explain slower carprofen clearance, although there is no direct evidence for this. However, poor aspirin clearance in cats appears to be mainly a consequence of slower glycine conjugation. Cats are also deficient in several other conjugation enzymes, including N-acetyltransferase (NAT) 2 and thiopurine methyltransferase (TMPT). NAT2 deficiency may be the reason cats are more prone to developing methemoglobinemia rather than hepatotoxicity from acetaminophen. TMPT deficiency may predispose cats to azathioprine toxicity. No evidence was found for slower elimination of drugs cleared by oxidation or unchanged into urine or bile. Piroxicam, an oxidized drug, was cleared much more rapidly in cats than humans and dogs, although the mechanism for this difference is unclear. More work is needed to better understand drug metabolism and disposition differences in cats, thereby enabling more rational prescribing of existing medications, and the development of safer drugs for this species.
Keywords: Cat, Species differences, Glucuronidation, Pharmacokinetics
INTRODUCTION
Veterinarians are well aware that cats are not simply “small dogs” with regards to their physiology and pharmacology. However, there are relatively few papers that have critically evaluated the evidence for such species differences and their resultant impact on drug efficacy and toxicity in cats. In this paper, the primary literature is reviewed focusing on the available evidence for differences in drug metabolism and disposition between cats, dogs and humans, as well as the molecular and genetic mechanisms that may explain these differences.
DRUG PHARMACOKINETIC DIFFERENCES BETWEEN CATS, DOGS AND HUMANS
Fig. 1 shows the results of a preliminary survey of the current literature comparing elimination half-life values for 25 different drugs in cats, dogs, and humans. The particular drugs were chosen to represent a variety of drug elimination mechanisms, including conjugation (n = 8), oxidation (n = 9), and excretion of unchanged drug into the urine and/or bile (n = 8).
Figure 1.
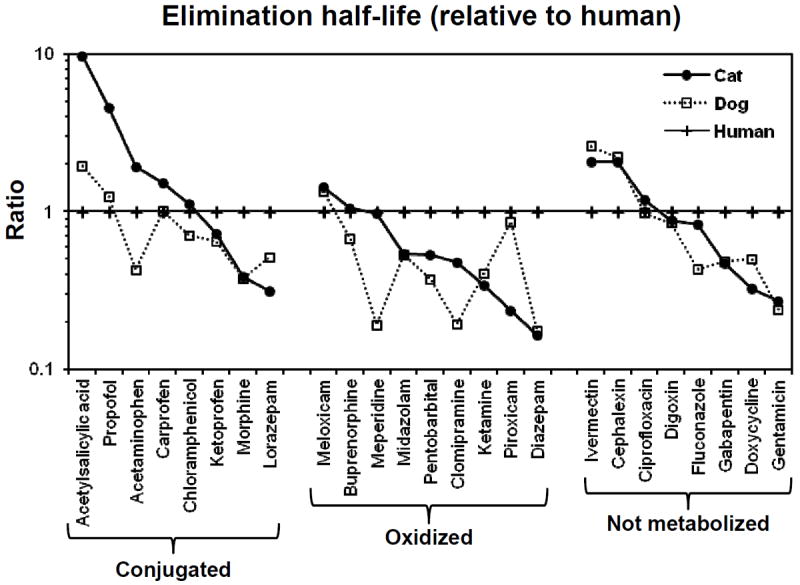
Pharmacokinetic evidence for differences in drug elimination rates between cats, dogs, and humans. Shown is a comparison of published elimination half-life values in cats (filled circle), dogs (open square) and humans (plus symbol) for representative drugs that are primarily eliminated by conjugation (glucuronidation, sulfation, and glycination), oxidation (CYP enzymes) or are excreted primarily unchanged into urine and/or bile. All values are expressed as a ratio of the human value. Complete pharmacokinetic data and literature references are given in Table 1 for acetylsalicylic acid, propofol, acetaminophen, carprofen and piroxicam. Because of space limitations, the references giving data for other drugs are available directly from the author.
Several trends are apparent in Figure 1 as follows.
All of the drugs that are eliminated more slowly in cats (i.e. aspirin, propofol, acetaminophen, and carprofen) are cleared by metabolic conjugation, including glucuronidation, sulfation and/or glycination.
Piroxicam, which is metabolized mainly by oxidation, is eliminated much more rapidly in cats as compared with dogs and humans (i.e. the opposite of the conjugated drugs).
Elimination half-life values were highly correlated between dogs and cats for the non-metabolized drugs, and poorly correlated for the metabolized (oxidized and/or conjugated) drugs.
Human elimination half-life data were poorly predictive of dog and cat elimination half-life data for most of the drugs evaluated.
Table 1 lists the elimination half-life, plasma clearance (CL) and volume of distribution (Vd) values of those drugs from Figure 1 that had longer (n = 4) or shorter (n = 1) elimination half-life values in cats compared with dogs and humans. The likely mechanisms for these species differences and their implications for drug use in the cat are discussed below.
Table 1.
Pharmacokinetic parameters determined for drugs that have longer (or shorter) elimination half-life values in cats versus dogs and humans. Results are the averages of published studies as referenced. Data from Obach et al, 2008 1 represent a compilation of all available human data up to 2008. Intravenous administration data were used if available to exclude bioavailability differences.
| Drug | Species | Half-life (h) | CL (mL/min/kg) | Vd (L/kg) |
|---|---|---|---|---|
| Acetylsalicylic acid* | Cat | 22 | 0.088 | 0.17 |
| Dog | 4.5 | 0.68 | 0.29 | |
| Human | 2.3 | 0.66 | 0.19 | |
| Acetaminophen | Cat** | 5.0 | 2.9 | 1.3 |
| Dog** | 1.1 | 13 | 1.3 | |
| Human** | 2.6 | 5 | 1.0 | |
| Propofol | Cat | 8.8 | 17 | 6.8 |
| Dog | 2.4 | 51 | 5.1 | |
| Human | 1.9 | 30 | 4.7 | |
| Carprofen | Cat | 18 | 0.10 | 0.15 |
| Dog | 12 | 0.28 | 0.17 | |
| Human** | 12 | 0.48 | 0.48 | |
| Piroxicam | Cat | 11 | 0.52 | 0.48 |
| Dog | 40 | 0.044 | 0.29 | |
| Human** | 47 | 0.050 | 0.16 |
Acetylsalicylic acid (aspirin) and salicylates
Aspirin is used in cats for acute pain and inflammation or more chronically as an antithrombotic. However, because of slow elimination of aspirin relative to dogs, the recommended doses are 2-4 times lower and the dose frequencies are 4 to 6 times longer in cats 16. Although slow elimination of aspirin in cats has been frequently attributed to deficient glucuronidation 16, a review of the available literature on aspirin metabolism suggests that other causes are more likely responsible.
After ingestion, aspirin is normally rapidly converted to the major circulating active metabolite, salicylic acid. Salicylic acid is then excreted in the urine unchanged, or following conjugation with glucuronic acid (forming phenolic and acyl glucuronides) or with glycine (forming salicylurate) 17. However, as shown in Figure 2, there is considerable variation in utilization of these pathways between species. After a therapeutic dose of aspirin (650 mg orally), humans excrete primarily salicylurate (69%), and salicylurate glucuronide (10%), with some salicyl glucuronides (12%) and unchanged salicylate (8%) 17. In comparison, dogs excreted approximately equal amounts of unchanged salicylate, salicylurate, and salicyl glucuronides into their urine after administration of a 44 mg/kg iv dose of sodium salicylate 18. In that same study, cats excreted mostly salicyl glucuronides (60-80%) with some unchanged salicylate (12-23%) but only a minor amount of salicylurate (~5%).
Figure 2.
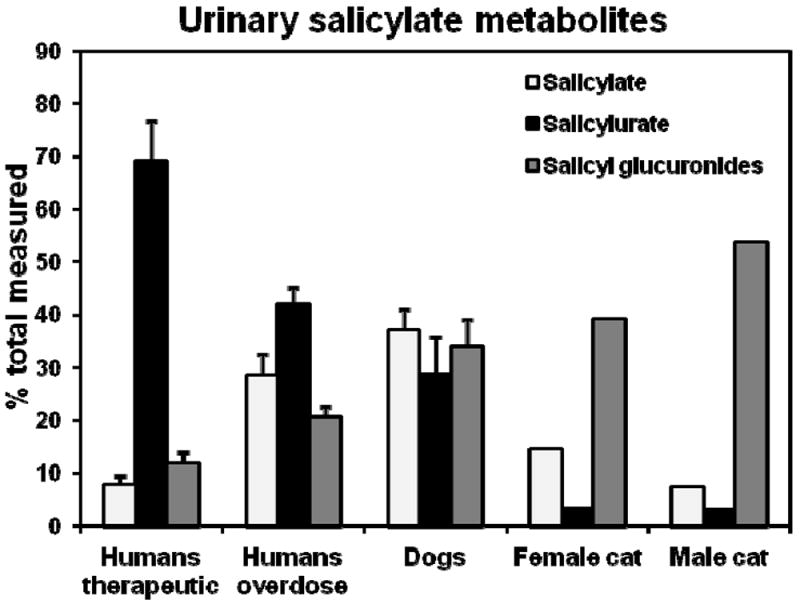
Cats can readily glucuronidate salicylate, but they poorly conjugate salicylate with glycine (forming salicylurate). Shown are data from several studies comparing the urinary metabolites of salicylate when administered as the sodium salt to 7 dogs and 2 cats (one male, one female) at 44 mg / kg intravenously, or orally as acetylsalicylic acid to 25 human volunteers at a therapeutic dose of 650 mg, or to 24 human patients that had intentionally taken a moderate aspirin overdose. Data are from Davis et al (1972) for cat and dog, Chen et al (2007) for human volunteers, and Patel et al (1990) for overdose patients.
These data suggest that cats can readily glucuronidate salicylic acid, although the type of glucuronide formed is unclear (phenolic and acyl glucuronides are possible). This is supported by in vitro studies that showed significant glucuronidation of salicylic acid by liver tissue slices from cats 19.
On the other hand the urinary metabolite data (Figure 2) indicates that cats are deficient in the conjugation of salicylate with glycine to form salicylurate 18. Although relatively little is known about the enzyme process that mediates salicylic acid glycination, available evidence suggests that it is a two-step process involving activation of salicylic acid with coenzyme A to form a salicyl-coA thioester, followed by conjugation with the amino group of glycine to form an amide linked glycine conjugate 20. Enzymes thought to be involved in this process in humans include acyl-CoA synthetase medium-chain 2B (ACSM2B, also called HXM-A) 21, and glycine N-acyltransferase (hGLYAT) 22, respectively. Both enzymes are localized to the mitochondrial matrix of liver and kidney tissues. A cDNA encoding a feline ortholog of GLYAT was recently identified (Genbank accession number JV729374) 23, while a feline ACSM2B ortholog has yet to be reported.
Slower elimination in cats might be expected for salicylate drugs other than sodium salicylate and aspirin, such as methylsalicylate (oil of wintergreen; Bengay®) and trolamine salicylate (Aspercreme®), although there is no direct evidence for this in the literature.
Acetaminophen
Although acetaminophen is one of the most widely used non-prescription treatments for mild pain and fever in humans, this drug is rarely used in dogs, and is contraindicated for use in cats. One of the reasons is that dogs and especially cats show significant methemoglobinemia and other signs of oxidative injury to erythrocytes (Heinz bodies and anemia) following acetaminophen doses that would be considered nontoxic to humans and other species5, 24.
In humans, acetaminophen toxicity with increasing doses is typically manifested as acute hepatocellular injury that can proceed to liver failure if not appropriately treated25. The mechanism of acetaminophen hepatotoxicity is a consequence of saturation of the major conjugative metabolic pathways (glucuronidation and sulfation) and increased metabolism of acetaminophen by CYP in liver to form a highly reactive metabolite N-acetyl-p-quinone-imine (NAPQI) (see Figure 3) 26. NAPQI is normally detoxified by glutathione conjugation, but once glutathione supplies are depleted (following overdose), NAPQI causes cellular damage. Acetaminophen hepatotoxicity is normally treated by administering the glutathione precursor N-acetylcysteine.
Figure 3.
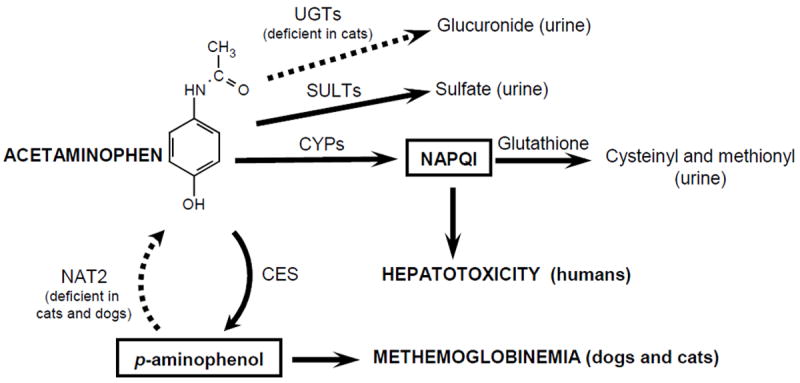
Proposed mechanisms for species differences in acetaminophen toxicity. Acetaminophen overdose in humans (and most other species) results in acute hepatotoxicity. The mechanism involves saturation of the detoxifying conjugation pathways (sulfation, glucuronidation, and glutathione conjugation), resulting in accumulation of the oxidative reactive metabolite N-acetyl p-quinoneimine (NAPQI) in the liver with resultant cellular damage. However in cats and dogs, acetaminophen toxicity primarily manifests as methemoglobinemia with Heinz body anemia. McConkey et al (2009) has proposed the existence of a futile cycle in erythrocytes that involves deacetylation of acetaminophen to p-aminophenol by carboxyesterases (CES) and then re-acetylation of p-aminophenol back to acetaminophen by N-acetyltransferase (NAT) isoform 2. p-aminophenol is a reactive compound that can co-oxidate with hemoglobin to form methemoglobin. Although methemoglobin can be reduced back to hemoglobin by NADH cytochrome b5 reductase, this capacity is limited. p-aminophenol is proposed to accumulate in cat and dog erythrocytes (and not in human erythrocytes) since both cat and dog (unlike human and most other species) lack NAT2. Cats may be more susceptible than dogs to this toxicity since they also lack several UGTs, including UGT1A6 and UGT1A9, which are essential for efficient elimination of acetaminophen by glucuronidation. Acetaminophen clearance is lower in cats resulting in increased levels of acetaminophen (and probably p-aminophenol).
Several explanations for acetaminophen sensitivity in cats have been proposed, including their hemoglobin, which may be more sensitive to oxidative injury, as well as a lower antioxidant capacity of their erythrocytes 27. However, these explanations do not account for the sensitivity of dogs to methemoglobinemia. Also, NAPQI is formed by CYPs primarily in the liver and not in the blood. Given the reactivity of this compound, it is unlikely that NAPQI could reach significant levels in erythrocytes. An alternate hypothesis has been explored by McConkey et al (2009) 27 as shown in Figure 3. They propose the existence of a futile cycle in erythrocytes that involves deacetylation of acetaminophen to p-aminophenol by carboxyesterases (CES) and then re-acetylation of p-aminophenol back to acetaminophen by N-acetyltransferase (NAT) isoform 2. p-aminophenol is known to be a reactive compound that can co-oxidate with hemoglobin to form methemoglobin. Although methemoglobin can be reduced back to hemoglobin by NADH cytochrome b5 reductase, this capacity is limited. p-aminophenol is proposed to accumulate in cat and dog erythrocytes (and not in human erythrocytes) since both cat and dog (unlike human and most other species) lack NAT2.
Cats may be more susceptible than dogs to acetaminophen toxicity since they also lack several UGTs, including UGT1A6 and UGT1A9, which are essential for efficient elimination of acetaminophen by glucuronidation (see further discussion of this below) 28. In support of this, acetaminophen glucuronidation by cat liver microsomes is very slow 29 and acetaminophen glucuronide is a relatively minor metabolite of acetaminophen in cat urine, while it is the main metabolite in dogs and humans (see Figure 4) 5, 30, 31. As a result acetaminophen clearance is lower, and half-life is longer, in cats (Table 1) resulting in increased circulating levels of acetaminophen, and probably higher p-aminophenol levels in blood.
Figure 4.
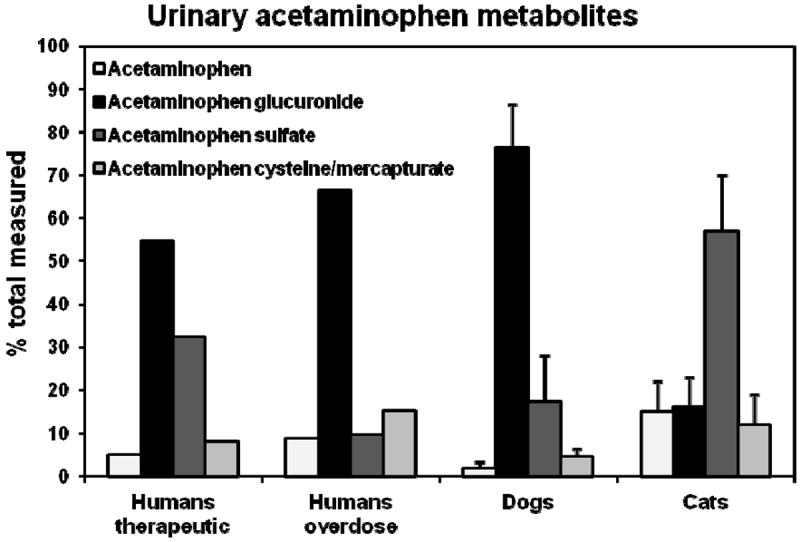
Cats are sensitive to the toxic effects of acetaminophen, in part because they glucuronidate acetaminophen less efficiently than humans or dogs. Shown are data from several studies comparing the urinary metabolite profiles of acetaminophen following oral administration of a nontoxic dose of 100 mg / kg to 4 dogs, a toxic dose of 120 mg / kg to 6 cats, a therapeutic dose of 20 mg / kg to healthy human volunteers, and an intentional overdose taken by human patients. Data are from Savides et al (1984) for cat and dog, and Prescott (1980) for human volunteers and overdose patients.
Propofol
Propofol administered intravenously is commonly used for induction and short duration anesthesia in dogs and humans. However, repeated dosing or the use of continuous infusions of propofol in cats has been associated with prolonged anesthetic recoveries 32, 33. Furthermore, repeated daily dosing of propofol results in oxidative injury to erythrocytes and increased Heinz body formation after 3 days, and more severe symptoms including malaise, anorexia, and diarrhea after 5 days 34. A recent study suggested that increasing the dosing interval to 48 hours between doses may ameliorate these latter symptoms, although there was still evidence for significant Heinz body formation following the first dose 32.
A toxicity syndrome (called propofol infusion syndrome, or PRIS) in human patients has been associated with administration of relatively high doses of propofol by infusion for up to 48 hours in the intensive care setting 35, 36. The most frequent symptoms include metabolic acidosis, bradyarrhythmias and progressive myocardial failure, with less frequent symptoms of rhabdomyolysis and renal failure. The mortality rate is about 80% in published cases, and 30% in cases reported directly to the FDA. A recent study suggests that the incidence of PRIS is about 1.1% in patients admitted to an ICU and receiving propofol for at least 24 hours, with an 18% mortality rate35. The molecular mechanism underlying PRIS is currently unknown, although various studies have implicated inhibition of several mitochondrial proteins including carnitine palmitoyl-transferase I and the mitochondrial respiratory chain at complex II and IV, either directly by propofol, or by one of its metabolites 36. It is unknown whether inter-individual differences in the metabolism of propofol can contribute to this syndrome.
Propofol is normally eliminated either by glucuronidation (directly) or by CYP mediated oxidation to form 4-hydroxypropofol that is glucuronidated or sulfated and then excreted into the urine and the bile 37. The relative utilization of these pathways differs between species. In humans, about 60% of the dose is eliminated by direct glucuronidation (primarily by UGT1A9), while 40% is eliminated by oxidation (primarily by CYP2B6) followed by conjugation 38, 39. However in dogs, propofol is eliminated almost entirely by oxidation (primarily by CYP2B11) with only 2% of the dose eliminated by direct glucuronidation 37, 40.
Unfortunately, the metabolism of propofol in cats is unknown. However, given that cats do not express an ortholog of UGT1A9 28, it might be speculated that propofol is mainly metabolized by alternate pathways including oxidation and sulfation. Consequently, the relatively slow clearance and prolonged elimination half-life of propofol in cats relative to humans and dogs (Table 1) might be explained by deficient glucuronidation in this species. The reason for oxidative injury to feline erythrocytes is less clear, although might involve the same adverse mitochondrial effects of propofol (or a metabolite) that were proposed as the cause of PRIS in humans. On the other hand, propofol is considered to have direct antioxidant effects and has been shown to protect against hemoglobin oxidation, although the antioxidant (or pro-oxidant) effects of its metabolites are unknown 41.
Carprofen
Carprofen is a nonsteroidal anti-inflammatory drug that is commonly used for the treatment of mild to moderate acute and chronic pain in dogs. It is currently approved for use in cats by regulatory agencies in a number of countries (not the USA) for postoperative analgesia given as a single dose of 4 mg / kg by injection 42, 43. Although longer term use is discouraged because of a lack of safety data, it is also being used orally in cats for treatment of chronic pain 42. Carprofen was marketed for about 10 years for use in humans, but was withdrawn in 1995 for commercial reasons. The most common adverse side effects are gastrointestinal irritation and ulceration, which are more likely with prolonged use of the oral preparation. Available pharmacokinetic data (Table 1) indicate that carprofen is cleared significantly slower in cats than in dogs and humans (by 2.8 to 4.8-fold) and has a 50% longer half-life. Consequently lower doses and/or a longer dose interval would likely need to be used in cats for chronic administration to achieve the same plasma drug levels as dogs and humans.
Carprofen is cleared primarily by glucuronidation in the liver 44. No studies have been published that identify which human UGT glucuronidates carprofen. Slower elimination of carprofen in cats could be a consequence deficient glucuronidation, although there is no direct evidence to support this. For example, it is not known whether cat liver microsomes glucuronidate carprofen more slowly than human or dog liver. Arguing against this contention is that several structurally related compounds, including pirprofen, flurbiprofen, and ibuprofen, are readily glucuronidated by cat liver microsomes 45. Carprofen is also highly bound to plasma proteins (<1% unbound in humans and dogs) and so pharmacokinetic differences might also be a consequence of species differences in protein binding although such an analysis has not been reported.
Piroxicam
Piroxicam is a nonsteroidal anti-inflammatory drug that was initially approved for use in humans as an anti-inflammatory and analgesic, but has garnered a novel off-label use as a cancer chemotherapeutic in both human and veterinary medicine 13. Piroxicam pharmacokinetics are distinctly different in cats compared with dogs and humans (Table 1). Relative to dogs and humans, cats show over 10-fold higher clearance of piroxicam and a 3- to 4-fold faster elimination half-life. The mechanism for this difference is unclear.
In humans, about 50% of the dose is oxidized by CYP2C9 to 5’-hydroxypiroxicam 46 and most of the remainder is hydrolysed at the amide bond, presumably by an esterase. Resultant metabolites and some unchanged drug are excreted into urine and bile. 5’-hydroxypiroxicam is also glucuronidated and excreted in the urine 47. There is no evidence for direct glucuronidation of piroxicam. Piroxicam (or possibly a metabolite) undergoes significant enterohepatic recirculation in people since pharmacokinetic studies have consistently demonstrated secondary elimination peaks after administration, and a decrease in elimination half-life and faster clearance was observed in people coadministered the anionic sequestrant, cholestyramine 15, 48. Piroxicam is also highly bound (>99%) to human plasma proteins 49.
The excretory pathways in dogs is similar to humans except that an additional cyclodehydrated metabolite was identified representing as much as 12% of metabolites. Pharmacokinetic studies showing strong secondary elimination peaks also suggest that piroxicam undergoes significant enterohepatic recirculation in dogs 14. The metabolism and excretion of piroxicam in cats is unknown.
A number of possibilities exist that might explain faster clearance of piroxicam in cats. Interestingly pharmacokinetic elimination profiles of piroxicam in cats do not show any evidence for enterohepatic recirculation (no secondary peaks) 13. Consequently, it is possible that the mechanism enabling recirculation in dogs and humans is deficient in cats leading to faster clearance. Alternatively, cats might have a higher capacity for clearance of piroxicam via hydroxylation or hydrolysis, or by elimination of unchanged drug. Finally, there may be lower plasma protein binding of piroxicam (and/or metabolites) in cats compared with dogs and humans that would tend to favor faster elimination.
MOLECULAR BASIS FOR DIFFERENCES IN CATS VERSUS OTHER SPECIES
Although a relatively understudied area of research, over the last 20 years there has been considerable progress in understanding the molecular and genetic basis for differences in drug metabolism and disposition in cats compared with other species. Deficiencies in four different drug elimination pathways have been explored in some detail, including glucuronidation (UGTs), acetylation (NATs), methylation (TPMT), and active transport (ABCG2).
Glucuronidation deficiency
Glucuronidation catalyzed by the UGT enzymes is an important metabolic process that transfers glucuronic acid to many different drugs, toxins, and endogenous compounds (such as steroids and bilirubin) thereby promoting efficient elimination into urine and/or bile. Humans express 19 different UGT isoforms that are classified based on genetic similarity into 2 families and 3 subfamilies (UGT1A, UGT2A, and UGT2B). UGT1A isoforms are encoded by a single gene that produces 9 different enzymes in humans by differential mRNA splicing (see Figure 5) 50. Human UGT2A1 and UGT2A2 are also generated by splicing from a single gene, while UGT2A3 and all human UGT2B isoforms are products of separate genes 50. UGTs are primarily expressed in liver, kidney and intestinal mucosa, the primary sites of drug metabolism 50.
Figure 5.
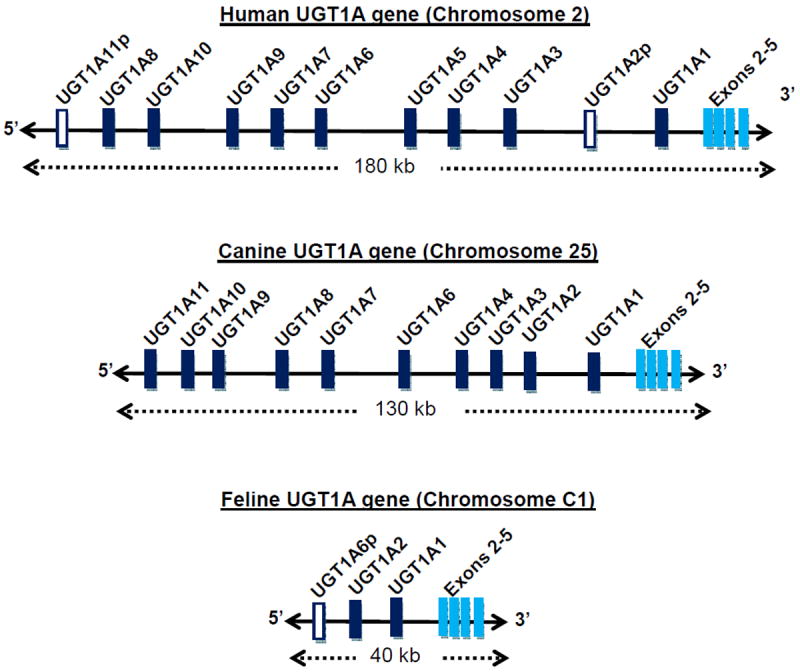
Comparison of the size, structure, and exon content of the human, canine and feline UGT1A genes. Each gene consists of multiple exons 1 (designated UGT1A1 up to UGT1A11) each with their own promoter that are differentially spliced with the conserved exons 2 to 5. Exon 1 encodes for the UGT enzyme protein domain that binds to and determines substrate specificity, while the conserved exons code for the UDPGA binding domain shared by all UGT1A enzymes. The human gene spans 180 kilobases and contains 9 functional exons 1 that encode 9 different UGT enzymes. The canine gene is somewhat smaller (130 kilobases) but includes 10 functional exons 1, encoding 10 different UGT enzymes. However, the feline UGT1A gene is much smaller (only 40 kilobases) and has only two functional exons 1 that encode 2 different UGT enzymes. Also shown are 2 exons 1 in human and one exon 1 in cat that are considered pseudogenes (p) since they contain multiple mutations that prevent protein coding. UGT1A1 is conserved across all 3 species probably because it encodes the only known enzyme capable of high efficiency glucuronidation of bilirubin. Data are from Li and Wu (2007) for human and dog genes, or directly from the University of California at Santa Cruz genome browser for the feline gene.
Deficient glucuronidation is one of the oldest and most widely appreciated pharmacologic idiosyncrasies of cats (perhaps only second to “morphine mania”). Reports regarding the inability of cats to glucuronidate drugs and toxins originated in the scientific literature nearly 60 years ago 51. Since then various studies have determined that this deficiency in cats is not generalized to all glucuronidated drugs, but is dependent on drug structure. Specifically the defect appears to mainly affect compounds with a simple planar phenolic structure 52, 53.
Studies of UGT isoform substrate specificity in humans and other species indicate that simple planar phenolic compounds are mainly metabolized by several UGT1A isoforms expressed in liver, particularly UGT1A6 and UGT1A9 29. Furthermore, it has been shown that feline liver only expresses two different UGT1A isoforms, including UGT1A1 and UGT1A2, while humans express 5 different UGT1As in liver 28. UGT1A1 is likely to be conserved amongst species since it is essential for glucuronidating and clearing bilirubin. Although little is known about UGT1A2, it is most related to human UGT1A3 and UGT1A4, which glucuronidate carboxylic acid and amine containing drugs. Importantly, no UGT1A isoform related to UGT1A6 or UGT1A9 was expressed in cat liver. The same study went on to identify the UGT1A6 gene by DNA sequencing but found that it contained multiple mutations in all cats 28. This finding suggests that a functional UGT1A6 gene had been present at one point in cats (or a cat species ancestor), but it had been permanently disabled in this species to form what is commonly called a pseudogene 28. As shown in Figure 5, this finding was confirmed by the recent sequencing of the feline genome, which found only 2 isoforms (UGT1A1 and UGT1A2) and the UGT1A6 pseudogene. In comparison, based on available genome sequences, dogs express up to 10 different UGT1As, while humans express 9 different UGT1As (Figure 5).
Deficient glucuronidation of phenolic compounds has also been demonstrated for other nondomestic Felid species, including the African lion and caracal 54, 55. This was confirmed by a recent molecular genetic study of a large number of carnivore species that showed UGT1A6 mutations in 17 different nondomestic Felid species, representing all major Felid lineages, including African lion, tiger, leopard, snow leopard, jaguar, Asiatic golden cat, African golden cat, serval, margay, Geoffrey’s cat, tigrina, Canada lynx, bobcat, puma (cougar), Florida panther, cheetah, and leopard cat 56. However, these mutations in UGT1A6 did not extend beyond the Felidae family to other Carnivora species, such as wolves, ferrets, bears, racoon indicating that that the first UGT1A6 mutation had evolved during the separation of the Felidae from other Carnivora species between 11 and 35 million years ago 56.
Finally, although it is clear that cats are deficient in a number of UGT1A enzymes, it is unknown whether other UGT isoforms are also different. In particular, it would be important to know whether cats express feline orthologs of human UGT2B7 and UGT2B15 which glucuronidate many different drugs. UGT2B7 selectively glucuronidates morphine in humans, and there is evidence for reduced morphine glucuronidation by cat liver 52. This contrasts with the pharmacokinetic data shown in Figure 1 indicating that morphine is eliminated at a similar rate in dogs and even faster than in humans. This may be because morphine is sulphated extensively in cats which might compensate for slow glucuronidation 57. UGT2B15 selectively glucuronidates lorazepam in humans, but this benzodiazepine appears to be glucuronidated readily in cats suggesting that cats express a feline ortholog of human UGT2B15 58.
Drugs with evidence for poor glucuronidation in cats
Pharmacology texts and other sources frequently cite deficient glucuronidation as the cause of toxicity or need for dose reduction of glucuronidated drugs given to cats without adequate justification. For example, current evidence points to poor glycine conjugation (see above) rather than poor glucuronidation as the cause of slow aspirin clearance in cats 16. Table 2 lists drugs with clear evidence that they are glucuronidated either more slowly or with similar efficiency as compared with other mammalian species. Evidence includes either comparative in vitro glucuronidation studies or in vivo metabolic studies.
Table 2.
Drugs with direct evidence that they are glucuronidated more slowly (left column) or with similar efficiency (right column) in cats compared with other mammalian species
| Drugs glucuronidated slowly in cats | Drugs glucuronidated efficiently in cats |
|---|---|
| Acetaminophen | Flurbiprofen |
| Chloramphenicol | Ibuprofen |
| Clofibrate | Lorazepam |
| Morphine | Phenolphthalein |
| Orbifloxacin | Pirprofen |
| Valproate | Salicylate |
| Telmisartan |
It is also worthwhile mentioning here that benzyl alcohol and benzoic acid are compounds that are frequently added to drugs as preservatives. Benzyl alcohol is metabolized to benzoic acid and then excreted as the glucuronide or glycine conjugate in most species. Cats are unable to glucuronidate benzoic acid, but can glycinate it although relatively slowly 63. Benzoic acid poisoning has been reported in cats and it has been recommended that the amounts of benzyl alcohol and benzoic acid used in pharmaceutical preparations for cats be minimized 64, 65.
NAT2 deficiency
N-acetylation catalysed by the N-acetyltransferase enzymes NAT1 and NAT2 is an important metabolic pathway in humans and most other species for a number of arylamine drugs including isoniazid, various sulphonamide antibiotics, dapsone, hydralazine, and procainamide 66. However, dogs (and all other Canid species) completely lack this metabolic pathway since they lack both genes encoding these enzymes 67. Cats also lack NAT2, but express NAT1, albeit with somewhat lower enzyme activity compared with other species 66. NAT2 deficiency has been associated with very low acetylation of sulfamethazine, sulphanilamide, sulfadimethoxine, and isoniazid by cat liver 52, 66. It is not known whether dapsone and hydralazine are acetylated more slowly in cats. As mentioned above, the deficiency of NAT2 in cats, and of both NATs in dogs, are proposed to contribute to the mechanism of toxicity of acetaminophen that are specific to these species.
TPMT deficiency
Cats are highly susceptible to the adverse effects of azathioprine 68. This difference is most likely associated with low thiopurine methyltransferase (TPMT) activity that has been found in cat erythrocytes compared with several other species including humans 69-71. S-Methylation by TPMT is an important detoxification mechanism for several drugs used for treatment of cancer (6-mercaptopurine) and for immunosuppression (azathioprine). The reason for the lower TPMT activity in cats is not known but could involve gene sequence differences (from other species) that affect enzyme level and/or enzyme affinity for substrate. A number of polymorphisms in the coding sequence of the feline TPMT gene have been identified that affect enzyme protein levels and activity 69.
ABCG2 deficiency
Fluroquinolone antibiotic use has been associated with the development of temporary and permanent blindness in cats 72. A recent study suggests that this may be the result of inefficient efflux of fluorquinolones by the ATP-binding cassette (ABC) G2 transporter from the feline eye 72. A more complete discussion of this is provided elsewhere in this issue (Mealey Chapter??).
CONCLUSIONS
Cats are deficient in a number of drug conjugation pathways that can lead to relatively slow elimination of certain drugs, and the need for dose adjustment or alternative therapies to avoid serious adverse effects. The most well understood conjugation defect in cats causes reduced glucuronidation of phenolic drugs, such as acetaminophen and propofol. Cats lack UGT1A6 and UGT1A9, which glucuronidate these drugs in other species. Slower clearance of carprofen might also result from deficient glucuronidation, although direct evidence for this is lacking. On the other hand, slower aspirin clearance does not appear to result from deficient glucuronidation, and is more likely a consequence of poor glycine conjugation. Cats are also deficient in several other conjugation pathways, including N-acetylation by NAT2 and S-methylation by TMPT. NAT deficiency may be the reason cats (and dogs) are more prone to acetaminophen-induced methemoglobinemia rather than hepatotoxicity. TMPT deficiency likely results in sensitivity to azathioprine toxicity. No evidence was found for slower clearance of drugs that are eliminated by oxidation or unchanged into urine or bile. Piroxicam, an oxidized drug, was cleared much more rapidly in cats than humans and dogs, although the mechanism for this difference is unclear. Species differences in plasma protein binding might also explain observed differences in pharmacokinetics especially for drugs that are highly bound. Much work is still needed to better understand the molecular causes of drug metabolism and disposition differences in cats, thereby enabling more rational prescribing of existing medications, and the development of more effective and safer drugs for this species.
Key points.
Acetaminophen, propofol, carprofen, and aspirin are eliminated much more slowly in cats, and are all metabolized by conjugation.
Cats lack UGT1A6 and UGT1A9, which glucuronidate acetaminophen and propofol, respectively.
Slower aspirin clearance results mainly from deficient glycine conjugation and not deficient glucuronidation.
Cats lack NAT2, which may be the reason they are prone to developing methemoglobinemia rather than hepatotoxicity from acetaminophen.
Cats have low thiopurine methyltransferase (TPMT) activity, which would cause sensitivity to azathioprine toxicity.
Piroxicam is eliminated more quickly in cats versus humans and dogs, but the reason for this is unknown.
Acknowledgments
This work was supported by funds provided by the William R. Jones Endowed Chair in Veterinary Medicine at Washington State University.
Footnotes
Disclosures: There are no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Obach RS, Lombardo F, Waters NJ. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos. 2008;36(7):1385–1405. doi: 10.1124/dmd.108.020479. [DOI] [PubMed] [Google Scholar]
- 2.Parton K, Balmer TV, Boyle J, et al. The pharmacokinetics and effects of intravenously administered carprofen and salicylate on gastrointestinal mucosa and selected biochemical measurements in healthy cats. J Vet Pharmacol Ther. 2000;23(2):73–79. doi: 10.1046/j.1365-2885.2000.00253.x. [DOI] [PubMed] [Google Scholar]
- 3.Waters DJ, Bowers LD, Cipolle RJ, et al. Plasma salicylate concentrations in immature dogs following aspirin administration: comparison with adult dogs. J Vet Pharmacol Ther. 1993;16(3):275–282. doi: 10.1111/j.1365-2885.1993.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 4.Greenblatt DJ, Abernethy DR, Boxenbaum HG, et al. Influence of age, gender, and obesity on salicylate kinetics following single doses of aspirin. Arthritis and rheumatism. 1986;29(8):971–980. doi: 10.1002/art.1780290805. [DOI] [PubMed] [Google Scholar]
- 5.Savides MC, Oehme FW, Nash SL, et al. The toxicity and biotransformation of single doses of acetaminophen in dogs and cats. Toxicol Appl Pharmacol. 1984;74(1):26–34. doi: 10.1016/0041-008x(84)90266-7. [DOI] [PubMed] [Google Scholar]
- 6.Volak LP, Hanley MJ, Masse G, et al. Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2013;75(2):450–462. doi: 10.1111/j.1365-2125.2012.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleale RM, Muir WW, Waselau AC, et al. Pharmacokinetic and pharmacodynamic evaluation of propofol administered to cats in a novel, aqueous, nano-droplet formulation or as an oil-in-water macroemulsion. J Vet Pharmacol Ther. 2009;32(5):436–445. doi: 10.1111/j.1365-2885.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Ghim JL, Song MH, et al. Pharmacokinetics and pharmacodynamics of a new reformulated microemulsion and the long-chain triglyceride emulsion of propofol in beagle dogs. Br J Pharmacol. 2009;158(8):1982–1995. doi: 10.1111/j.1476-5381.2009.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor PM, Delatour P, Landoni FM, et al. Pharmacodynamics and enantioselective pharmacokinetics of carprofen in the cat. Res Vet Sci. 1996;60(2):144–151. doi: 10.1016/s0034-5288(96)90009-0. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt M, Guentert TW. Biopharmaceutical evaluation of carprofen following single intravenous, oral, and rectal doses in dogs. Biopharm Drug Dispos. 1990;11(7):585–594. doi: 10.1002/bdd.2510110704. [DOI] [PubMed] [Google Scholar]
- 11.Holazo AA, Chen SS, McMahon FG, et al. The influence of liver dysfunction on the pharmacokinetics of carprofen. J Clin Pharmacol. 1985;25(2):109–114. doi: 10.1002/j.1552-4604.1985.tb02810.x. [DOI] [PubMed] [Google Scholar]
- 12.Iwakawa S, Suganuma T, Lee SF, et al. Direct determination of diastereomeric carprofen glucuronides in human plasma and urine and preliminary measurements of stereoselective metabolic and renal elimination after oral administration of carprofen in man. Drug Metab Dispos. 1989;17(4):414–419. [PubMed] [Google Scholar]
- 13.Heeb HL, Chun R, Koch DE, et al. Single dose pharmacokinetics of piroxicam in cats. J Vet Pharmacol Ther. 2003;26(4):259–263. doi: 10.1046/j.1365-2885.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- 14.Galbraith EA, McKellar QA. Pharmacokinetics and pharmacodynamics of piroxicam in dogs. The Veterinary record. 1991;128(24):561–565. doi: 10.1136/vr.128.24.561. [DOI] [PubMed] [Google Scholar]
- 15.Guentert TW, Defoin R, Mosberg H. The influence of cholestyramine on the elimination of tenoxicam and piroxicam. Eur J Clin Pharmacol. 1988;34(3):283–289. doi: 10.1007/BF00540957. [DOI] [PubMed] [Google Scholar]
- 16. [25 January, 2013];Merck Veterinary manual. http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/214009.htm.
- 17.Chen Y, Kuehl GE, Bigler J, et al. UGT1A6 polymorphism and salicylic acid glucuronidation following aspirin. Pharmacogenet Genomics. 2007;17(8):571–579. doi: 10.1097/01.fpc.0000236339.79916.07. [DOI] [PubMed] [Google Scholar]
- 18.Davis LE, Westfall BA. Species differences in biotransformation and excretion of salicylate. Am J Vet Res. 1972;33(6):1253–1262. [PubMed] [Google Scholar]
- 19.Schachter D, Kass DJ, Lannon TJ. The biosynthesis of salicyl glucuronides by tissue slices of various organs. J Biol Chem. 1959;234(1):201–205. [PubMed] [Google Scholar]
- 20.Forman WB, Davidson ED, Webster LT., Jr Enzymatic conversion of salicylate to salicylurate. Mol Pharmacol. 1971;7(3):247–259. [PubMed] [Google Scholar]
- 21.Vessey DA, Lau E, Kelley M, et al. Isolation, sequencing, and expression of a cDNA for the HXM-A form of xenobiotic/medium-chain fatty acid:CoA ligase from human liver mitochondria. J Biochem Mol Toxicol. 2003;17(1):1–6. doi: 10.1002/jbt.10056. [DOI] [PubMed] [Google Scholar]
- 22.Kelley M, Vessey DA. Isolation and characterization of mitochondrial acyl-CoA: glycine N-acyltransferases from kidney. Journal of biochemical toxicology. 1993;8(2):63–69. doi: 10.1002/jbt.2570080203. [DOI] [PubMed] [Google Scholar]
- 23.Irizarry KJ, Malladi SB, Gao X, et al. Sequencing and comparative genomic analysis of 1227 Felis catus cDNA sequences enriched for developmental, clinical and nutritional phenotypes. BMC genomics. 2012;13:31. doi: 10.1186/1471-2164-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nash SL, Savides MC, Oehme FW, et al. The effect of acetaminophen on methemoglobin and blood glutathione parameters in the cat. Toxicology. 1984;31(3-4):329–334. doi: 10.1016/0300-483x(84)90114-8. [DOI] [PubMed] [Google Scholar]
- 25.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 26.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31(12):1499. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 27.McConkey SE, Grant DM, Cribb AE. The role of para-aminophenol in acetaminophen-induced methemoglobinemia in dogs and cats. J Vet Pharmacol Ther. 2009;32(6):585–595. doi: 10.1111/j.1365-2885.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 28.Court MH, Greenblatt DJ. Molecular genetic basis for deficient acetaminophen glucuronidation by cats: UGT1A6 is a pseudogene, and evidence for reduced diversity of expressed hepatic UGT1A isoforms. Pharmacogenetics. 2000;10(4):355. doi: 10.1097/00008571-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Court MH, Greenblatt DJ. Molecular basis for deficient acetaminophen glucuronidation in cats. An interspecies comparison of enzyme kinetics in liver microsomes. BiochemPharmacol. 1997;53(7):1041. doi: 10.1016/s0006-2952(97)00072-5. [DOI] [PubMed] [Google Scholar]
- 30.Gelotte CK, Auiler JF, Lynch JM, et al. Disposition of acetaminophen at 4, 6, and 8 g/day for 3 days in healthy young adults. Clin Pharmacol Ther. 2007;81(6):840–848. doi: 10.1038/sj.clpt.6100121. [DOI] [PubMed] [Google Scholar]
- 31.Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. 1980;10(Suppl 2):291S–298S. doi: 10.1111/j.1365-2125.1980.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PM, Chengelis CP, Miller WR, et al. Evaluation of propofol containing 2% benzyl alcohol preservative in cats. Journal of feline medicine and surgery. 2012;14(8):516–526. doi: 10.1177/1098612X12440354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascoe PJ, Ilkiw JE, Frischmeyer KJ. The effect of the duration of propofol administration on recovery from anesthesia in cats. Vet Anaesth Analg. 2006;33(1):2–7. doi: 10.1111/j.1467-2995.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 34.Andress JL, Day TK, Day D. The effects of consecutive day propofol anesthesia on feline red blood cells. Vet Surg. 1995;24(3):277–282. doi: 10.1111/j.1532-950x.1995.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 35.Roberts RJ, Barletta JF, Fong JJ, et al. Incidence of propofol-related infusion syndrome in critically ill adults: a prospective, multicenter study. Critical care. 2009;13(5):R169. doi: 10.1186/cc8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diedrich DA, Brown DR. Analytic reviews: propofol infusion syndrome in the ICU. Journal of intensive care medicine. 2011;26(2):59–72. doi: 10.1177/0885066610384195. [DOI] [PubMed] [Google Scholar]
- 37.Simons PJ, Cockshott ID, Douglas EJ, et al. Species differences in blood profiles, metabolism and excretion of 14C-propofol after intravenous dosing to rat, dog and rabbit. Xenobiotica. 1991;21(10):1243–1256. doi: 10.3109/00498259109043199. [DOI] [PubMed] [Google Scholar]
- 38.Favetta P, Degoute CS, Perdrix JP, et al. Propofol metabolites in man following propofol induction and maintenance. Br J Anaesth. 2002;88(5):653–658. doi: 10.1093/bja/88.5.653. [DOI] [PubMed] [Google Scholar]
- 39.Court MH, Duan SX, Hesse LM, et al. Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology. 2001;94(1):110. doi: 10.1097/00000542-200101000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Hay Kraus BL, Greenblatt DJ, Venkatakrishnan K, et al. Evidence for propofol hydroxylation by cytochrome P4502B11 in canine liver microsomes: breed and gender differences. Xenobiotica. 2000;30(6):575. doi: 10.1080/004982500406417. [DOI] [PubMed] [Google Scholar]
- 41.Stratford N, Murphy P. Effect of lipid and propofol on oxidation of haemoglobin by reactive oxygen species. Br J Anaesth. 1997;78(3):320–322. doi: 10.1093/bja/78.3.320. [DOI] [PubMed] [Google Scholar]
- 42.Lascelles BD, Court MH, Hardie EM, et al. Nonsteroidal anti-inflammatory drugs in cats: a review. VetAnaesthAnalg. 2007;34(4):228. doi: 10.1111/j.1467-2995.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 43.Sparkes AH, Heiene R, Lascelles BD, et al. ISFM and AAFP consensus guidelines: long-term use of NSAIDs in cats. Journal of feline medicine and surgery. 2010;12(7):521–538. doi: 10.1016/j.jfms.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray JE, Wade DN. The pharmacokinetics and metabolism of 14C-carprofen in man. Biopharm Drug Dispos. 1982;3(1):29–38. doi: 10.1002/bdd.2510030105. [DOI] [PubMed] [Google Scholar]
- 45.Magdalou J, Chajes V, Lafaurie C, et al. Glucuronidation of 2-arylpropionic acids pirprofen, flurbiprofen, and ibuprofen by liver microsomes. Drug Metab Dispos. 1990;18(5):692–697. [PubMed] [Google Scholar]
- 46.Tracy TS, Hutzler JM, Haining RL, et al. Polymorphic variants (CYP2C9*3 and CYP2C9*5) and the F114L active site mutation of CYP2C9: effect on atypical kinetic metabolism profiles. Drug Metab Dispos. 2002;30(4):385–390. doi: 10.1124/dmd.30.4.385. [DOI] [PubMed] [Google Scholar]
- 47.Richardson CJ, Blocka KL, Ross SG, et al. Piroxicam and 5’-hydroxypiroxicam kinetics following multiple dose administration of piroxicam. Eur J Clin Pharmacol. 1987;32(1):89–91. doi: 10.1007/BF00609964. [DOI] [PubMed] [Google Scholar]
- 48.Hobbs DC, Twomey TM. Piroxicam pharmacokinetics in man: aspirin and antacid interaction studies. J Clin Pharmacol. 1979;19(5-6):270–281. doi: 10.1002/j.1552-4604.1979.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 49.Richardson CJ, Blocka KL, Ross SG, et al. Effects of age and sex on piroxicam disposition. Clin Pharmacol Ther. 1985;37(1):13–18. doi: 10.1038/clpt.1985.4. [DOI] [PubMed] [Google Scholar]
- 50.Court MH, Zhang X, Ding X, et al. Quantitative distribution of mRNAs encoding the 19 human UDP-glucuronosyltransferase enzymes in 26 adult and 3 fetal tissues. Xenobiotica. 2012;42(3):266. doi: 10.3109/00498254.2011.618954. [DOI] [PubMed] [Google Scholar]
- 51.Hartiala KJ. Studies on detoxication mechanisms. III. Glucuronide synthesis of various organs with special reference to the detoxifying capacity of the mucous membrane of the alimentary canal. Annales medicinae experimentalis et biologiae Fenniae. 1955;33(3):239–245. [PubMed] [Google Scholar]
- 52.Gregus Z, Watkins JB, Thompson TN, et al. Hepatic phase I and phase II biotransformations in quail and trout: comparison to other species commonly used in toxicity testing. Toxicol Appl Pharmacol. 1983;67(3):430–441. doi: 10.1016/0041-008x(83)90327-7. [DOI] [PubMed] [Google Scholar]
- 53.Watkins JB, 3rd, Klaassen CD. Xenobiotic biotransformation in livestock: comparison to other species commonly used in toxicity testing. J Anim Sci. 1986;63(3):933–942. doi: 10.2527/jas1986.633933x. [DOI] [PubMed] [Google Scholar]
- 54.French MR, Bababunmi EA, Golding RR, et al. The conjugation of phenol, benzoic acid, 1-naphthylacetic acid and sulphadimethoxine in the lion, civet and genet. FEBS Lett. 1974;46(1):134–137. doi: 10.1016/0014-5793(74)80352-2. [DOI] [PubMed] [Google Scholar]
- 55.Capel ID, French MR, Millburn P, et al. The fate of (14C)phenol in various species. Xenobiotica. 1972;2(1):25–34. doi: 10.3109/00498257209036231. [DOI] [PubMed] [Google Scholar]
- 56.Shrestha B, Reed JM, Starks PT, et al. Evolution of a major drug metabolizing enzyme defect in the domestic cat and other felidae: phylogenetic timing and the role of hypercarnivory. PLoS One. 2011;6(3):e18046. doi: 10.1371/journal.pone.0018046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh SY, Chernov HI, Woods LA. Metabolism of morphine by cats. J Pharm Sci. 1971;60(3):469–471. doi: 10.1002/jps.2600600330. [DOI] [PubMed] [Google Scholar]
- 58.Schillings RT, Sisenwine SF, Schwartz MH, et al. Lorazepam: glucuronide formation in the cat. Drug Metab Dispos. 1975;3(2):85–88. [PubMed] [Google Scholar]
- 59.Emudianughe TS, Caldwell J, Sinclair KA, et al. Species differences in the metabolic conjugation of clofibric acid and clofibrate in laboratory animals and man. Drug Metab Dispos. 1983;11(2):97–102. [PubMed] [Google Scholar]
- 60.Matsumoto S, Takahashi M, Kitadai N, et al. A study of metabolites isolated from the urine samples of cats and dogs administered orbifloxacin. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 1998;60(11):1259–1261. doi: 10.1292/jvms.60.1259. [DOI] [PubMed] [Google Scholar]
- 61.Zoran DL, Boeckh A, Boothe DM. Hyperactivity and alopecia associated with ingestion of valproic acid in a cat. J Am Vet Med Assoc. 2001;218(10):1587–1589. 1580. doi: 10.2460/javma.2001.218.1587. [DOI] [PubMed] [Google Scholar]
- 62.Ebner T, Schanzle G, Weber W, et al. In vitro glucuronidation of the angiotensin II receptor antagonist telmisartan in the cat: a comparison with other species. J Vet Pharmacol Ther. 2012 doi: 10.1111/j.1365-2885.2012.01398.x. [DOI] [PubMed] [Google Scholar]
- 63.Bridges JW, French MR, Smith RL, et al. The fate of benzoic acid in various species. Biochem J. 1970;118(1):47–51. doi: 10.1042/bj1180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janz R. Benzoic acid hazard in cat preparations. The Veterinary record. 1989;124(22):595. doi: 10.1136/vr.124.22.595-b. [DOI] [PubMed] [Google Scholar]
- 65.Bedford PG, Clarke EG. Experimental benzoic acid poisoning in the cat. The Veterinary record. 1972;90(3):53–58. doi: 10.1136/vr.90.3.53. [DOI] [PubMed] [Google Scholar]
- 66.Trepanier LA, Cribb AE, Spielberg SP, et al. Deficiency of cytosolic arylamine N-acetylation in the domestic cat and wild felids caused by the presence of a single NAT1-like gene. Pharmacogenetics. 1998;8(2):169–179. doi: 10.1097/00008571-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Trepanier LA, Ray K, Winand NJ, et al. Cytosolic arylamine N-acetyltransferase (NAT) deficiency in the dog and other canids due to an absence of NAT genes. Biochem Pharmacol. 1997;54(1):73–80. doi: 10.1016/s0006-2952(97)00140-8. [DOI] [PubMed] [Google Scholar]
- 68.Beale KM, Altman D, Clemmons RR, et al. Systemic toxicosis associated with azathioprine administration in domestic cats. Am J Vet Res. 1992;53(7):1236–1240. [PubMed] [Google Scholar]
- 69.Salavaggione OE, Yang C, Kidd LB, et al. Cat red blood cell thiopurine S-methyltransferase: companion animal pharmacogenetics. J Pharmacol Exp Ther. 2004;308(2):617–626. doi: 10.1124/jpet.103.059055. [DOI] [PubMed] [Google Scholar]
- 70.White SD, Rosychuk RA, Outerbridge CA, et al. Thiopurine methyltransferase in red blood cells of dogs, cats, and horses. J Vet Intern Med. 2000;14(5):499–502. doi: 10.1892/0891-6640(2000)014<0499:tmirbc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 71.Foster AP, Shaw SE, Duley JA, et al. Demonstration of thiopurine methyltransferase activity in the erythrocytes of cats. J Vet Intern Med. 2000;14(5):552–554. doi: 10.1892/0891-6640(2000)014<0552:dotmai>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 72.Ramirez CJ, Minch JD, Gay JM, et al. Molecular genetic basis for fluoroquinolone-induced retinal degeneration in cats. Pharmacogenet Genomics. 2011;21(2):66–75. doi: 10.1097/FPC.0b013e3283425f44. [DOI] [PubMed] [Google Scholar]


