Abstract
Introduction
The objective of this study was to examine the feasibility and toxicity of adjuvant dose-dense chemotherapy in older women with breast cancer.
Methods
A search of the Memorial Sloan-Kettering Cancer Center (MSKCC) breast cancer database was performed to identify all patients age 60 and older who underwent an initial consultation with a breast medical oncologist between October 1, 2002 and June 28, 2005. Inclusion criteria were: (1) age ≥ 60, (2) follow-up care obtained at MSKCC, (3) intent to treat with adjuvant dose-dense AC-T (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles followed by paclitaxel 175 mg/m2 every 2 weeks for 4 cycles, with white blood cell growth factor support).
Results
One hundred sixty-two patients (mean age 66, range 60–76) with breast cancer, stages I (n = 5), II (n = 111), and III (n = 46) according to the sixth edition of the AJCC staging system, were included in this analysis. Forty-one percent (n = 67) experienced a grade 3 or 4 toxicity, 9% a grade 3 infection (n = 14), 6% grade 3 fatigue (n = 9), 5% neutropenic fever (n = 8), and 4% thromboembolic events (n = 7). Twenty-two percent (n = 36) did not complete the planned 8 cycles of treatment. There was no statistically significant association between age and either toxicity or treatment discontinuation. In multivariate analysis including age, pretreatment hemoglobin, and comorbidity, the presence of comorbidity (Charlson score ≥ 1) and a lower baseline hemoglobin score were associated with an increased risk of any grade 3 or 4 toxicity.
Conclusions
We found that the risk of toxicity depended more on comorbid medical conditions and baseline hemoglobin value than age in this cohort of older adults receiving dose-dense adjuvant chemotherapy.
Keywords: Breast Cancer, Dose-Dense Chemotherapy, Older Patient
Introduction
Breast cancer is the most commonly diagnosed cancer in American women and the second leading cause of cancer deaths [1]. The greatest risk factor for breast cancer is age: the median age at diagnosis is 61, and the median age of death is 69 [2]. Over the next 30 years, the US population age 65 and older is expected to double. Thus, with the growing older population, an increase in the number of older individuals with breast cancer is also expected. Despite the relationship of breast cancer to aging, older women have been under-represented in breast cancer clinical trials [3, 4]. For example, in 4 randomized adjuvant chemotherapy trials performed by the Cancer and Leukemia Group B (CALGB) spanning almost a quarter of a century, only 8% of the patients were 65 and older, and 2% were age 70 and older [4]. As a result, data are sparse regarding the risks and benefits of adjuvant chemotherapy and its feasibility in older patients with breast cancer.
Intergroup trial C9741 [5] was a randomized adjuvant trial in women with node-positive breast cancer. Its 2 × 2 factorial design compared single sequential agent doxorubicin (A), paclitaxel (T), and cyclophosphamide (C) to concurrent doxorubicin and cyclophosphamide (AC), followed by paclitaxel (T), delivered either every 2 weeks (dose-dense) with growth factor support or every 3 weeks without growth factor support. The study results demonstrated that patients receiving dose-dense treatment experienced an improvement in disease-free and overall survival; however, there was no significant difference if the drugs were given in sequential or concurrent fashion. Toxicity patterns were similar between the two arms. There was an increase in red blood cell transfusions on the concurrent dose-dense arm; however, grade 4 neutropenia was less common with the dose-dense treatment. Based on these results, dose-dense chemotherapy has become an approved regimen for the adjuvant treatment of node-positive breast cancer. However, the median age of patients in Intergroup trial C9741 was 50, and only 3% of the patients were age ≥ 70. Therefore, additional information is needed to determine the feasibility and toxicity of dose-dense chemotherapy in older adults.
The objective of our retrospective analysis was to describe the feasibility and patterns of toxicity of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel in older patients with breast cancer. Additionally, we wanted to assess the association of age, comorbid medical conditions, and pretreatment hemoglobin levels with the risk of toxicity.
Methods
Database search
A search of the Memorial Sloan-Kettering Cancer Center (MSKCC) breast cancer database was performed to identify all patients age ≥ 60 who underwent an initial consultation with a breast medical oncologist between October 1, 2002 and June 28, 2005. Inclusion criteria for this analysis were: (1) age ≥ 60 at the time of initial consultation with a breast medical oncologist, (2) follow-up care obtained at MSKCC, (3) intent to treat with adjuvant dose-dense AC-T (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles followed by paclitaxel 175 mg/m2 every 2 weeks for 4 cycles with white blood cell growth factor support). Patients were excluded if they had a prior history of breast cancer or had already received any chemotherapy.
Retrospective chart review was performed, and the following data were gathered: patient age, sex, tumor stage (as defined by the sixth edition of the AJCC staging system) and characteristics, occurrence and type of surgery, comorbid medical problems (as defined by the Charlson comorbidity index), pretreatment hemoglobin, creatinine and liver function levels, timing and dosing of chemotherapy regimen, complications of chemotherapy course (including the percent of patients experiencing grade 3 or 4 toxicity), treatment delay, dose reduction, discontinuation of treatment, utilization of erythropoietin, hospitalization, and receipt of blood transfusion. Hematologic toxicities were included for blood count results checked when patients were symptomatic and at routinely scheduled treatment sessions within 5 days of the planned cycle. The Charlson comorbidity index [6] was used to assess the impact of co-existing medical conditions on the risk of toxicity from chemotherapy. For analysis purposes, the presence of comorbidity was defined as a Charlson comorbidity index score of 1 or higher and the absence of comorbidity was defined as a Charlson comorbidity index score of 0.
Age was analyzed as a categorical variable with two levels: age less than 70 years and age 70 or older. The relationship between age and each measure of feasibility and toxicity was assessed using Fisher’s exact tests. Multivariate logistic regression models were fit to assess the relationship of three toxicity measures (any grade 3 or 4 toxicity, grade 3 or 4 hematologic toxicity, and grade 3 or 4 nonhematologic toxicity) with age at breast cancer diagnosis, the presence of comorbid conditions, and pre-treatment hemoglobin levels. This study was approved by the MSKCC Institutional Review Board who granted a waiver of authorization to perform this retrospective review.
Results
A total of 2,600 patients age 60 and older were seen in consultation by breast medical oncologists at MSKCC between October 1, 2002 and June 28, 2005. Of these, 162 patients met the criteria of the cohort as defined above. Most of those who were ineligible did not receive chemotherapy or did not pursue follow-up care at MSKCC.
The average age of patients included in this analysis was 66 (SD 4.7; range 60–76). The median age was 65. Of 162 patients, 123 (76%) were aged 60–69 and 39 (24%) were older than 69. Ninety-nine percent (n = 160) of the patients were female, and had the following breast cancer stages: 3% stage I (n = 5), 69% stage II (n = 111), and 28% stage III (n = 46). Seventy-three percent (n = 119) of tumors were positive for estrogen and/or progesterone receptors. Seventeen percent (n = 27) were HER2-neu amplified by FISH analysis.
The prevalence of comorbid medical conditions was assessed using the Charlson comorbidity index. The majority of patients had a low comorbidity score. Seventy-three percent of patients (n = 118) had a score of zero, 18% (n = 29) had a score of 1, 7% (n = 11) had a score of 2, and 2% (n = 4) had a score of 3 (Fig. 1). Therefore, most patients had few other medical problems that would significantly contribute to a 1-year mortality risk. Eighty-five percent (n = 138) of patients were receiving adjuvant chemotherapy, and 15% (n = 24) were receiving neoadjuvant chemotherapy.
Fig. 1.
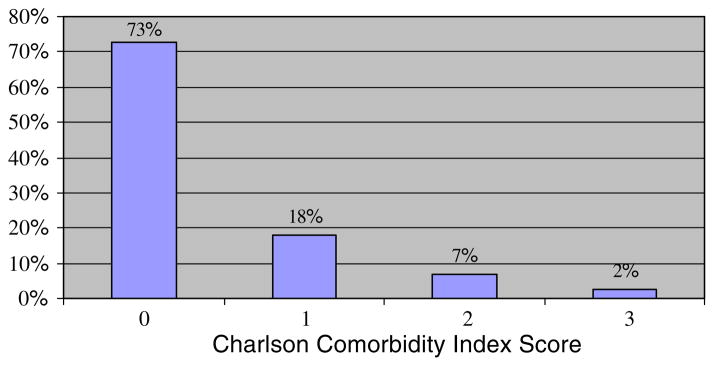
Percent of Older Breast Cancer Patients with Comorbid Medical Conditions as Assessed by Charlson Comorbidity Index* (N = 162). * The Charlson comorbidity index weights the following comorbid medical disease: myocardial infarction, congestive cardiac failure, peripheral vascular disease, chronic obstructive pulmonary disease, diabetes mellitus (without end-organ damage), cerebrovascular disease, dementia, ulcers, connective tissue disease, mild liver disease, hemiplegia, moderate to severe chronic renal failure, diabetes mellitus (with end-organ damage), other malignancy, leukemia, lymphoma, and moderate to severe liver disease. Please refer to the following article for the specific criteria for each comorbid medical condition and the scoring system: Charlson et al. [6]
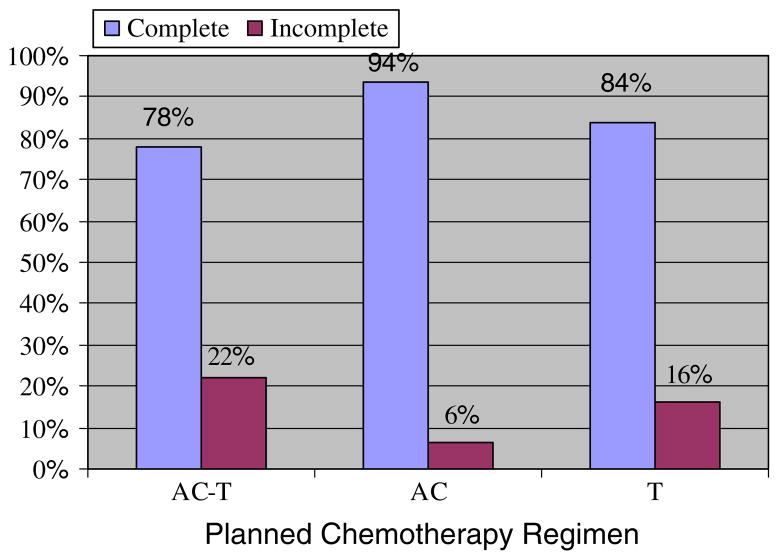
Figure 2 shows the percent of patients who did not complete the full course of chemotherapy. Twenty-two percent (n = 36) did not complete the planned 8 cycles of treatment, 6% (n = 10) during the AC portion and 16% (n = 26) during the taxane portion. The most common reasons for treatment discontinuation included patient preference (5%), allergic reaction to chemotherapy (3%), grade 3 neutropenic fever (2%), and grade 3 fatigue (2%). The less common reasons for discontinuation observed in <1% of patients included: grade 2 thrombocytopenia and sensory neuropathy; grade 3 mucositis, pneumonitis, depression, confusion, myopathy, infection, anemia, neutropenia; grade 4 neutropenia, hyponatremia, pulmonary embolism; and/or no change in tumor size. One patient experienced a treatment-related mortality secondary to grade 5 pneumonitis that occurred after the patient’s first cycle of paclitaxel.
Fig. 2.
Percent of Older Breast Cancer Patients Who Did Not Complete a Full Course of Dose-Dense Chemotherapy (N = 162). Abbreviations: AC, doxorubicin, cyclophosphamide; T, paclitaxel
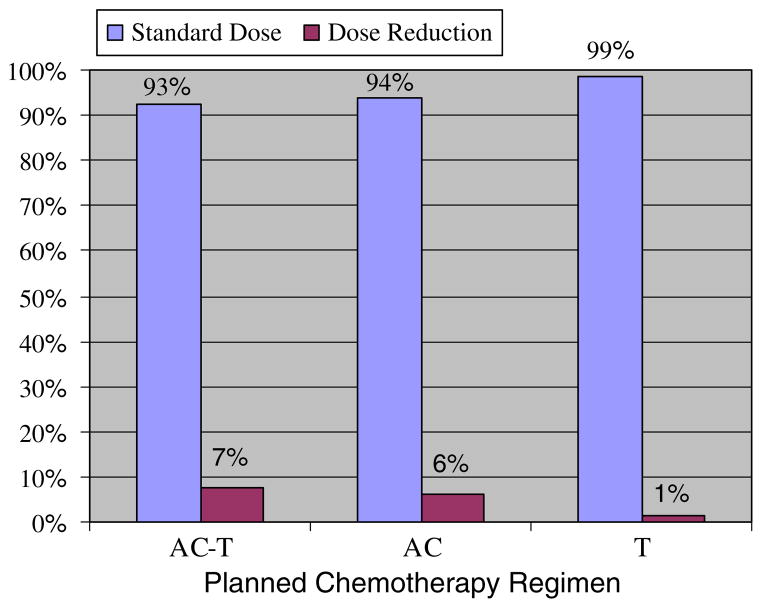
Figure 3 displays the percent of patients who received standard dosing and dose reductions. The standard dose of AC was defined as doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2; T was defined as paclitaxel 175 mg/m2. Twelve (7%) patients required dose reductions; 10 reductions occurred during the AC segment of treatment and 2 during the T portion. An additional 8 (5%) patients switched during the T segment of treatment from paclitaxel to docetaxel, most often for allergic reaction to the paclitaxel infusion.
Fig. 3.
Percent of Older Breast Cancer Patients Requiring Dose Reduction of Dose-Dense Chemotherapy (N = 162). Abbreviations: AC, doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2; T, paclitaxel 175 mg/m2
Table 1 summarizes the common toxicities documented during treatment. Forty-one percent of patients (n = 67) experienced at least one grade 3 or 4 toxicity. Seventeen percent (n = 27) experienced at least one grade 3 or 4 hematologic toxicity, while 36% (n = 59) experienced at least one grade 3 or 4 nonhematologic toxicity. The most common hematologic toxicities were neutropenia, leukopenia, and anemia; the most common nonhematologic toxicities were infection and fatigue. Five percent (n = 8) experienced neutropenic fever.
Table 1.
Common toxicities in older breast cancer patients treated with dose-dense AC-T chemotherapy
| Toxicitya | Number (%) |
|---|---|
| Any grade 3 or 4 toxicity | 67 (41) |
| Grade 3 or 4 hematologic toxicities | 27 (17) |
| Grade 3 or 4 nonhematologic toxicities | 59 (36) |
| Most common grade 3 and 4 hematologic toxicities | |
| Grade 3 neutropenia | 13 (8) |
| Grade 3 leukopenia | 6 (4) |
| Grade 3 anemia | 6 (4) |
| Grade 4 neutropenia | 6 (4) |
| Grade 4 leukopenia | 6 (4) |
| Grade 4 anemia | 0 (0) |
| Most common grade 3 nonhematologic toxicities | |
| Neutropenic fever | 8 (5) |
| Infection | 14 (9) |
| Fatigue | 9 (6) |
Specific toxicities are not mutually exclusive. A patient could have experienced more than one specific toxicity
Thromboembolic events affected 4% (n = 7) of patients. Toxicities observed in <6% of patients included grade 3 syncope, dyspnea, hyponatremia, pain, thrombosis, hypertension, chest pain, allergic reaction, nausea, rash, mucositis, creatinine increase, hyperglycemia, hypokalemia, cardiac ischemia, pneumonitis, embolism, depression, diarrhea, confusion, myopathy, sinus tachycardia, fracture, dizziness, dysphagia, central nervous system cardiovascular ischemia; and grade 4 central nervous system cardiovascular ischemia, dyspnea, hyponatremia, thrombosis; and grade 5 pneumonitis
Abbreviations: AC, doxorubicin, cyclophosphamide; T, paclitaxel
Age was not statistically associated with the risk of grade 3 or 4 toxicities (41% < age 70; 44% ≥ 70; P = 0.85); however, age 70 and older was associated with a statistically significant increase in the receipt of blood transfusion (5% < age 70; 15% ≥ 70; P = 0.04) (Table 2). Patients age 70 and older were more likely to have comorbid medical conditions (Charlson score 1, 2, or 3); however, this was not statistically significant (24% < age 70; 36% ≥ age 70; P = 0.21). A multivariate analysis was performed to determine the association of significant toxicity with age, presence of comorbidity, and baseline hemoglobin (Table 3). Even though age ≥ 70 was not significantly associated with risk of grade 3 or 4 toxicities in bivariate analyses, we still included it as a covariate in multivariate analysis in order to assess whether age became an important factor after adjusting for the presence of comorbidities. Age was not significantly associated with the probability of having any grade 3 or 4 toxicity, grade 3 or 4 hematologic toxicity, or grade 3 or 4 nonhematologic toxicity. However, a statistically significant association was found between comorbidity score and any grade 3 or 4 toxicity (P = 0.04) and grade 3 or 4 nonhematologic toxicity (P < 0.01). In addition, a statistically significant association was found between baseline hemoglobin score and any grade 3 or 4 toxicity (P = 0.04), grade 3 or 4 hematologic toxicity (P = 0.02), and grade 3 or 4 nonhematologic toxicity (P = 0.05). The odds of having a grade 3 or 4 hematologic toxicity is 1.57 times higher with every unit decrease in baseline hemoglobin. The odds of having a grade 3 or 4 nonhematologic toxicity among patients with a Charlson comorbidity index score of 1 or more is 2.97 times higher than the odds among patients with a Charlson comorbidity index score of 0.
Table 2.
Association of age category with outcomes in older patients with breast cancer given dose-dense chemotherapy
| Treatment outcomes | <70 (N = 123) % | ≥70 (N = 39) % | Overall (N = 162) % | P-value |
|---|---|---|---|---|
| Grade 3 or 4 toxicity | 41 | 44 | 41 | 0.85 |
| Grade 3 or 4 hematologic toxicity | 16 | 18 | 17 | 0.81 |
| AC grade 3 or 4 hematologic toxicity | 15 | 15 | 15 | 0.99 |
| T grade 3 or 4 hematologic toxicitya | 2 | 3 | 2 | 0.52 |
| Grade 3 or 4 nonhematologic toxicity | 36 | 39 | 36 | 0.85 |
| AC grade 3 or 4 nonhematologic toxicity | 28 | 28 | 28 | 0.99 |
| T grade 3 or 4 nonhematologic toxicitya | 12 | 17 | 13 | 0.54 |
| Hospitalizations | 27 | 31 | 28 | 0.68 |
| Febrile neutropenia | 6 | 3 | 5 | 0.68 |
| Dose reduction | 6 | 13 | 7 | 0.16 |
| Dose delay | 46 | 62 | 49 | 0.10 |
| Discontinuation of treatment | 19 | 33 | 22 | 0.08 |
| Receipt of transfusion | 5 | 15 | 7 | 0.04 |
| Receipt of erythropoietin | 46 | 64 | 50 | 0.07 |
| Presence of comorbidities | 24 | 36 | 27 | 0.21 |
Abbreviation: AC, doxorubicin, cyclophosphamide; T, paclitaxel
Based on N = 138 because some patients stopped after AC and did not receive T
Table 3.
Predictors of toxicity with dose-dense chemotherapy in older breast cancer patients
| Outcome | Parameter | Odds ratio | P-value |
|---|---|---|---|
| Grade 3 or 4 toxicity | Age ≥ 70 | 0.95 | 0.89 |
| Comorbiditya | 2.15 | 0.04 | |
| Baseline Hemoglobin | 0.73 | 0.04 | |
| Grade 3 or 4 hematologic toxicity | Age ≥ 70 | 0.97 | 0.95 |
| Comorbiditya | 1.21 | 0.69 | |
| Baseline Hemoglobin | 0.64 | 0.02 | |
| Grade 3 or 4 nonhematologic toxicity | Age ≥ 70 | 0.90 | 0.79 |
| Comorbiditya | 2.97 | <0.01 | |
| Baseline Hemoglobin | 0.73 | 0.05 |
Intercept term not shown. All models adjusted for whether patient completed therapy
Charlson comorbidity score of 1, 2, or 3 indicates the presence of a comorbidity
Discussion
The goal of this study was to examine the feasibility of and tolerance to dose-dense chemotherapy for the adjuvant treatment of breast cancer in a cohort of women age ≥ 60 at a large cancer center. A significant proportion (41%) of older adults in the study experienced grade 3 or 4 toxicity, and 22% did not complete the planned course of treatment. Higher Charlson comorbidity score and lower baseline hemoglobin value, but not age, were associated with an increased risk of grade 3 or 4 toxicity.
Many factors influence a clinician’s decision to recommend adjuvant chemotherapy and a patient’s decision to receive it. Typically, this decision process involves weighing the possibility of relapse, possible benefit from treatment, and the risks of therapy itself. For several reasons, this decision process is more complex in older adults than in younger patients. First, as an individual ages, the benefit from adjuvant chemotherapy decreases [7]. Data from the Early Breast Cancer Trialists’ collaborative group demonstrated that for patients less than age 50, receipt of polychemotherapy was associated with a 15-year decrease in mortality by 10%, compared with a 3% mortality decrease in patients age 50–69. The authors reported: “These trials of chemotherapy involved too few women older than 70 years of age to be reliably informative (even if ER status is ignored) as to whether it confers any net survival benefit among them [7].” Second, the risks of toxicity from chemotherapy increase with age. An analysis of toxicity patterns from 3 CALGB trials for node-positive disease (including data from Intergroup trial C9741) demonstrated that patients age < 50 compared to patients age ≥ 65 had a 0.2% (95% CI, 0.1–0.4) versus 1.5% (95% CI, 0.6–3.1) risk of treatment-related mortality, respectively. Third, the older adult is more likely to have comorbid medical conditions that may impact the ability to tolerate adjuvant chemotherapy. Such conditions can be competing causes of morbidity and mortality independent of the cancer [8–10]. These factors are among the main reasons why older women are less likely to receive adjuvant chemotherapy than younger women [11, 12].
However, as newer adjuvant treatments for breast cancer become available, applying the data to older adults becomes challenging. Since older adults have been under-represented on clinical trials, the factors that influence therapy tolerance are often not captured. Our study demonstrates the challenges of delivering adjuvant chemotherapy to an older adult. It also highlights the importance of specifically studying the feasibility and toxicity of new cancer therapies in older adults in order to understand what factors other than age contribute to toxicity.
In this study, there was no statistically significant association between age and grade 3 or 4 toxicity; however, an association was seen between lower pre-treatment hemoglobin and risk of grade 3 and 4 hematologic toxicity. This finding likely reflects the decreased bone marrow reserve that accompanies increased age [13], and a lower baseline hemoglobin may be a surrogate measure for lower hematologic reserve. The patients in this study received concurrent dose-dense doxorubicin and cyclophosphamide followed by paclitaxel. In Intergroup trial C9741, an alternate and equally effective means of delivering dose-dense chemotherapy was single sequential agent treatment with doxorubicin followed by paclitaxel and cyclophosphamide [5]. The feasibility and toxicity of the single sequential approach should be studied, as that approach may be less myelosuppressive.
This study also demonstrated a statistically significant association between comorbid medical conditions and the risk of any grade 3 or 4 toxicity and grade 3 or 4 nonhematologic toxicity. These data highlight the importance of assessing factors other than chronologic age when making adjuvant treatment decisions. A geriatric assessment evaluates functional status, comorbid medical conditions, cognition, nutritional status, social support, and psychological state. Each of these domains is an independent predictor of morbidity and mortality in older adults, independent of chronologic age [9, 14–19]. Assessing the correlation between these domains and the risk of toxicity from cancer therapy, along with a more detailed assessment of comorbid medical conditions, would be useful in determining the risk-to-benefit ratio of chemotherapy in an older patient. Ultimately, better assessment and stratification of the older population is needed in order to identify subsets that would derive the greatest benefit from chemotherapy.
The limitations of our study include its retrospective nature and our examination of only short-term toxicity. The patients included in this analysis were treated at a tertiary care cancer center and might not be representative of patients seen in a community-based practice. In addition, the oldest patient in this analysis was 76, so we have no information about tolerance to dose-dense therapy in patients beyond this age. Furthermore, we did not examine grade 1 and 2 toxicities which may play an important role in health outcomes for an elderly population.
On the other hand, this study has important strengths. In this group of patients, which has been under-represented in prospective clinical trials, we performed a detailed chart review to identify factors other than age that place older adults at increased risk for toxicity from adjuvant dose-dense chemotherapy. Our data highlight the need for further research to explore the risks and benefits of chemotherapy in older patients. Identifying comorbid conditions that can affect treatment outcomes, and understanding the impact of functional as opposed to chronologic age, would help clinicians and patients stratify the risks and benefits of adjuvant chemotherapy in older patients in order to make informed decisions about treatment. Prospective studies addressing these age-related questions are underway in this rapidly growing population, but more are needed.
Acknowledgments
The authors thank Carol Pearce, MFA, for her assistance in the preparation of this manuscript. This work was supported by Dr. Hurria’s K23 AG026749-01 (Paul Beeson Career Development Award in Aging Research) and American Society of Clinical Oncology-Association of Specialty Professors-Junior Development Award in Geriatric Oncology.
Contributor Information
Marjorie Zauderer, New York-Presbyterian Hospital, New York, USA.
Sujata Patil, Memorial Sloan-Kettering Cancer Center, New York, USA.
Arti Hurria, Email: ahurria@coh.org, City of Hope, 1500 E Duarte Road, Duarte, CA 91010, USA.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, MD: 2008. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 4.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 5.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 6.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 7.Early Beast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 8.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 9.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120(2):104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Frasci G, Lorusso V, Panza N, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18(13):2529–2536. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- 11.Du X, Goodwin JS. Patterns of use of chemotherapy for breast cancer in older women: findings from Medicare claims data. J Clin Oncol. 2001;19(5):1455–1461. doi: 10.1200/JCO.2001.19.5.1455. [DOI] [PubMed] [Google Scholar]
- 12.Elkin EB, Hurria A, Mitra N, Schrag D, Panageas KS. Adjuvant chemotherapy and survival in older women with hormone receptor-negative breast cancer: assessing outcome in a population-based, observational cohort. J Clin Oncol. 2006;24(18):2757–2764. doi: 10.1200/JCO.2005.03.6053. [DOI] [PubMed] [Google Scholar]
- 13.Dees EC, O’Reilly S, Goodman SN, et al. A prospective pharmacologic evaluation of age-related toxicity of adjuvant chemotherapy in women with breast cancer. Cancer Invest. 2000;18(6):521–529. doi: 10.3109/07357900009012191. [DOI] [PubMed] [Google Scholar]
- 14.Reuben DB, Rubenstein LV, Hirsch SH, Hays RD. Value of functional status as a predictor of mortality: results of a prospective study. Am J Med. 1992;93(6):663–669. doi: 10.1016/0002-9343(92)90200-U. [DOI] [PubMed] [Google Scholar]
- 15.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 16.Eagles JM, Beattie JA, Restall DB, Rawlinson F, Hagen S, Ashcroft GW. Relation between cognitive impairment and early death in the elderly. BMJ. 1990;300(6719):239–240. doi: 10.1136/bmj.300.6719.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolfson C, Wolfson DB, Asgharian M, et al. A reevaluation of the duration of survival after the onset of dementia. N Engl J Med. 2001;344(15):1111–1116. doi: 10.1056/NEJM200104123441501. [DOI] [PubMed] [Google Scholar]
- 18.Seeman TE, Kaplan GA, Knudsen L, Cohen R, Guralnik J. Social network ties and mortality among the elderly in the Alameda County Study. Am J Epidemiol. 1987;126(4):714–723. doi: 10.1093/oxfordjournals.aje.a114711. [DOI] [PubMed] [Google Scholar]
- 19.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279(21):1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]