Abstract
Background and Purpose
Previous research suggests greater risk of coronary heart disease with lower levels of the adrenal steroid dehydroepiandrosterone sulfate (DHEAS). No studies have examined the association between DHEAS and risk for ischemic stroke. DHEAS may influence ischemic stroke risk through atherosclerotic related mechanisms (endothelial function and smooth muscle cell proliferation) or insulin resistance.
Methods
Between 1989-1990, 32,826 women without prior stroke in the Nurses’ Health Study, an observational cohort, provided blood samples and were followed for cardiovascular events. Among this sample, using a nested-case control design, 461 ischemic strokes confirmed by medical records through 2006. Cases were matched to controls free of stroke at the time of the index case and by age, ancestry, menopausal status, postmenopausal hormone use, smoking status and date of sample collection. Multivariable conditional logistic regression was utilized.
Results
Median DHEAS levels did not differ between cases (median=58.7) and controls (median=66.0; p-value=0.10). Conditional on matching factors, the lowest DHEAS quartile exhibited a relative risk (RR) of 1.30 for ischemic stroke (95% confidence interval [CI]: 0.88-1.94), compared with the highest quartile and marginally unchanged when adjusted for confounders (RR=1.33; 95%CI: 0.87-2.02). When modeled as a binary variable dichotomized at the lowest quartile, women with low DHEAS (≤the lowest quartile) had a significantly increased multivariable adjusted risk of ischemic stroke compared to those with higher levels (RR=1.41; 95%CI: 1.03-1.92).
Conclusions
Lower DHEAS levels were associated with a greater risk of ischemic stroke, even after adjustment for potential confounders. These novel observations warrant confirmation in other populations.
Keywords: Ischemic stroke, dehydroepiandrosterone sulphate
Introduction
Dehydroepiandrosterone sulfate (DHEAS) the most abundant circulating human steroid hormone is secreted almost exclusively by the adrenal cortex.1 DHEAS serves as a precursor to approximately 50% of androgens in adult men, 75% of active estrogens in premenopausal and nearly 100% in postmenopausal women.1 DHEAS levels decline with age, peaking at age 20-30 years and declining by 20-30% by age 70-80.2 While no human studies have examined DHEAS and stroke, several studies have reported strong inverse associations between levels of DHEAS and risk of total mortality and cardiovascular disease (CVD).3-5 Recent results from the Women’s Ischemia Syndrome Evaluation (WISE) reported a greater than two-fold increased risk of CVD mortality among women in the lowest tertile of DHEAS compared to those in higher categories (HR=2.55; 95% CI:1.19-5.45).4 The associations between DHEAS and risk of CVD among women have been inconsistent in the few studies available, in contrast to observations among men.3-6 These data suggest divergent effects by gender, underscoring the need for gender-specific analyses with comprehensive evaluation of hormonal related factors.
The biologic plausibility for an association between DHEAS and risk of stroke is provided by inverse associations between DHEAS and several stroke risk factors including insulin resistance,7 carotid intima-media thickness8 and hypertension.9 In addition, low DHEAS levels have been associated with cognitive impairment.1 Higher DHEA levels have been shown to decrease atherogenic vascular remodeling post injury.10 Furthermore, higher DHEA/DHEAS levels have also been shown to improve endothelial cell function and promote insulin sensitivity11, 12 in human and animal models. Importantly, DHEAS may act directly or through conversion to sex steroid hormones; hence, the influence of DHEAS on risk may vary depending on the hormonal environment (e.g., gender, menopausal status and postmenopausal hormone use).13 Given its extensive use as an over-the-counter supplement, elucidating the role of DHEAS in the etiology of CVD could provide further support for randomized controlled trials evaluating its potential role in CVD prevention.
To further elucidate the relationship between DHEAS and risk for stroke, we evaluated its association with ischemic stroke among women. We hypothesized that lower levels of DHEAS were associated with an increased risk of ischemic stroke in women. Importantly, we explored potential variation of the association between DHEAS and ischemic stroke by: age, body mass index (BMI), smoking, history of diabetes, postmenopausal hormone use and ovarian status.
Materials and Methods
Nurses’ Health Study Cohort
The Nurses’ Health Study (NHS) enrolled 121,700 female registered nurses living in 11 US states aged 30-55 years who completed a mailed questionnaire in 1976. Follow-up questionnaires have been mailed biennially, with a semi-quantitative food frequency questionnaire (FFQ) mailed every 2-4 years since 1980; as previously published.14 Follow-up of the baseline population is >90% and mortality follow-up is >98% complete.15 Between 1989-1990, 32,826 women without prior evidence of stroke, 43-69 years, provided blood samples. Approximately 10 years later (2000-2001), 18,743 of these participants provided a second blood sample. Blood was drawn and shipped to our laboratory via overnight courier for processing. Samples were processed, archived and have been stored in continuously-monitored liquid nitrogen freezers as previously described.16
A nested case-control study of ischemic stroke was conducted among women with available blood samples (1989-1990). Stroke cases were participants free of known prior stroke or cancer at the time of the blood collection, but who experienced an ischemic stroke during the follow-up interval. For each stroke case, one NHS control participant who was free of known prior stroke or cancer at the time of the blood collection and had not had a stroke event at the time of the index case event was selected; hence cases and controls possessed the same amount of person-time at risk. Controls were s individually matched to the index case at blood collection by: age, ancestry, smoking, menopausal status, hormone therapy and date of sample collection (http://stroke.ahajournals.org).
Blood Sample Assay
Blood samples were collected from a sub-group of NHS participants (n=32,826) between 1989-1990 and a second sample was requested and received (n=18,743) approximately 10 years later (2000-2001). Estimation of the association between DHEAS and risk of ischemic stroke utilized DHEAS collected at one time point (1989-1990), replicate samples were only utilized to estimate measures of reliability. Case-control pair samples were handled identically and together, shipped to the laboratory in the same batch, and assayed in the same run. Each batch included replicate, blinded plasma samples to assess laboratory precision and ‘drift samples’ standardized to a particular level for the biomarker to track and correct for laboratory assay drift.
DHEAS was measured by competitive radioimmunoassay at Quest Diagnostics/Nichols Institute (San Juan Capistrano, California) for all cases and controls, with a mean intra-assay coefficient of variation (CV) of 4%. Previously, DHEAS levels have shown stability over 3 years in this population of postmenopausal women (intraclass correlation coefficient [ICC]=0.88 95% CI: 0.84-0.93),16 and up to 15 years stability in frozen serum in other populations.2 We estimated the ICC among 102 women with 2 measurements approximately 10 years apart and observed good reproducibility, ICC=0.73 (95% CI: 0.66-0.79).
Total, low density lipoprotein (LDL-C), high density lipoprotein (HDL-C) cholesterol, C-reactive protein (CRP) and sex-hormone binding globulin (SHBG) were measured at the Clinical and Epidemiological Research Laboratory at Children’s Hospital (Boston, MA) and estradiol and testosterone were measured at the Mayo Clinic (Rochester, MN) for all cases and controls using standard lab assays (http://stroke.ahajournals.org). All CVs for LDL-C, HDL-C, CRP and SHBG were <10% and <16% for estradiol and testosterone.
Cerebrovascular Disease Assessment
We included ischemic strokes that occurred from the return of the blood sample through 2006. Nonfatal stroke was reported on biennial questionnaires and confirmed by medical records. Deaths were detected through information provided by the next of kin, postal authorities or by systematic searches of the National Death Index. Classification of fatal stroke was confirmed by review of hospital records, autopsy, or death certificate. Women (or next-of-kin for decedents) reporting stroke on follow-up questionnaires were asked for permission to review medical records, which were reviewed by a physician blinded to exposure status. Stroke was classified according to the National Survey of Stroke17 criteria requiring evidence of a neurologic deficit with sudden or rapid onset that persisted for >24 hours or until death. Strokes were classified as ischemic stroke due to thrombotic or embolic occlusion of a cerebral artery with imaging data from CT or MRI or data on autopsy available for >92% of events, with high reproducibility.18
Statistical Analysis
Descriptive analyses for baseline characteristics were conducted by case-control status and across quartiles of DHEAS. Spearman’s correlation coefficient was calculated between DHEAS levels and age. Multivariable conditional logistic regression models estimated the association between DHEAS and risk of ischemic stroke. A priori DHEAS was modeled in quartiles based on the distribution among the controls and as a binary variable using the lowest quartile as the cut point to increase statistical power and facilitate interaction analyses. The odds ratio and 95% confidence intervals (CI) were used to approximate the relative risk (RR).
Lifestyle and diet covariates were utilized from the 1990 questionnaire or the closest year prior, with the exception of height (1976). We estimated 3 multivariable models: Model 1 adjusted for matching factors, Model 2 additionally included BMI, physical activity, aspirin use, alcohol consumption, and Alternative Healthy Eating Index (aHEI 2010), while in exploratory analysis Model 3 further adjusted for possible intermediates on the causal pathway: history of diabetes, hypertension, CHD or revascularization, HbA1c, and total/HDL-C (http://stroke.ahajournals.org). A missing indicator variable was assigned for missing values of alcohol and aspirin intake. In 17 case-control pairs with missing HbA1c, the median was imputed by case-control status.
In subgroup analyses, we evaluated effect modification of DHEAS, (the lowest quartile compared to all others) by selected risk factors (age, BMI, smoking, diabetes, postmenopausal hormone use, ovarian status) and time period (events occurring <8/≥8 years) based on a priori hypotheses. Significance of the interaction was assessed using the likelihood ratio test.. We examined the impact of estradiol, testosterone and SHBG on the association between DHEAS and risk of ischemic stroke. All p-values were two-sided. Analyses were conducted with SAS for UNIX statistical software (version 9.2; SAS Institute, Cary, NC).
Statement of Ethics
This study was approved by the Institutional Review Board of Brigham and Women’s Hospital and all procedures followed were in accordance with institutional guidelines. Participants provided informed consent to participate.
Results
DHEAS levels were available for 461 complete case-control pairs. The mean age was 61 years. As expected, women who developed stroke during follow-up were more likely to be diabetic, report a history of hypertension and family history of heart disease compared to controls (Table 1) at baseline.
Table 1.
Baseline Characteristics of the Population by Case-Control Status in 1990
| Cases (n=461) | Controls (n=461) | p-value | |
|---|---|---|---|
| Age (years) | 61 ± 6.0 | 61 ± 6.0 | 0.93 |
| DHEAS (μg/dL) | 76.7 ± 60.7 (median=58.7) |
79.9 ± 57.2 (median=66.0) |
0.10 |
| White, % | 97 | 98 | 0.37 |
| BMI (kg/m2) | 26.0 ± 5.1 | 25.4 ± 4.8 | 0.10 |
| Smoking, % | 0.84 | ||
| Never | 42 | 42 | |
| Past | 40 | 42 | |
| Current | 18 | 16 | |
| Postmenopausal Hormone Therapy Use | 0.99 | ||
| Premenopausal/Dubious | 11 | 11 | |
| Postmenopausal, Non-hormone therapy users | 47 | 47 | |
| Postmenopausal, Hormone therapy users | 42 | 42 | |
| Alcohol (g/day) | 5.9 ± 10.8 | 5.3 ± 10.2 | 0.48 |
| Physical Activity (METs/week) | 15.2 ± 19.8 | 16.2 ± 18.5 | 0.15 |
| Hypertension, % | 48 | 34 | <0.0001 |
| High Cholesterol, % | 48 | 46 | 0.64 |
| History of Heart Disease, % | 5 | 6 | 0.55 |
| Family History of Heart Disease, % | 12 | 7 | 0.01 |
| Diabetes, % | 13 | 6 | 0.0007 |
| HbA1c≥6, % | 17 | 11 | 0.01 |
| CRP≥3 (mg/L) | 40 | 34 | 0.08 |
| Year of Blood Draw | 1990 ± 0.33 | 1990 ± 0.33 | 0.51 |
Values are means ± SD (except where noted) or percentages
t-test, Wilcoxon rank-sum test or Chi-square test used as appropriate
Age was inversely correlated with DHEAS levels (Spearman r=−0.32, p<0.0001). Women in the lowest DHEAS quartile were older and less educated but less likely to be current smokers compared to the highest quartile (Table 2). Furthermore, the lowest quartile of DHEAS was associated with a higher prevalence of hypertension, high cholesterol and history of heart disease, as well as a higher proportion of hormone therapy use. In the lowest DHEAS quartile, the mean estradiol levels were 5.21±3.08 pg/mL among postmenopausal women not on hormone therapy and 18.00±19.00 pg/mL among hormone therapy users. For premenopausal women, there was not a clear relation between estradiol levels and DHEAS quartiles.
Table 2.
Baseline Characteristics of the Population by DHEAS Quartiles in 1990
| Characteristics | Quartile 1 | Quartile 2 | Quartile 3 | Quartiles 4 |
|---|---|---|---|---|
| n=259 | n=226 | n=210 | n=227 | |
| Age (years) | 63 ± 4.4 | 62 ± 5.3 | 60 ± 6.1 | 58 ± 6.6 |
| DHEAS (μg/dL) | 26.0 ± 11.3 (median=28.2) | 53.2 ± 6.6 (median=52.8) | 82.2 ± 10.0 (median=82.3) | 159.4 ± 59.1 (median=138.4) |
| BMI (kg/m2) | 25.4 ± 4.4 | 25.6 ± 5.3 | 26.4 ± 5.4 | 25.4 ± 4.7 |
| Current Smoker, % | 14 | 20 | 12 | 23 |
| Alcohol (g/d) | 4.9 ± 10.4 (median=0.9) | 5.0 ± 10.1 (median=0.9) | 5.4 ± 9.6 (median=1.1) | 7.1 ± 11.6 (median=1.8) |
| Physical Activity, (Mets/wk) | 16.1 ± 20.6 (median=8.4) | 15.4 ± 15.1 (median=10.9) | 16.0 ± 20.4 (median=8.0) | 15.2 ± 20.0 (median=10.2) |
| Master’s or Doctoral Level Education, % | 5 | 7 | 12 | 10 |
| Hypertension, % | 48 | 39 | 41 | 33 |
| High Cholesterol, % | 53 | 44 | 48 | 42 |
| History of Heart Disease, % | 8 | 4 | 5 | 3 |
| Diabetes, % | 9 | 11 | 7 | 11 |
| HbA1c≥6, % | 13 | 16 | 14 | 14 |
| CRP≥3 (mg/L), % | 41 | 38 | 37 | 31 |
| Postmenopausal-Hormone Therapy use, %* | 54 | 50 | 43 | 40 |
| Estradiol (pg/mL) | 13.3 ± 23.1 (median=7.3) | 15.3 ± 25.2 (median=7.7) | 16.7 ± 34.6 (median=34.6) | 16.9 ± 25.1 (median=8.5) |
| Testosterone (ng/dL) | 20.6 ± 13.4 (median=18.0) | 24.3 ± 15.0 (median=21.0) | 26.0 ± 12. 9 (median=12.9) | 30.2 ± 15.4 (median=25.0) |
| Sex Hormone-Binding Globulin (nmol/L) | 109.7 ± 72.5 (median=91.5) | 95.0 ± 60.6 (median=81.1) | 86.9 ± 60.2 (median=60.2) | 78.7 ± 50.4 (median=67.6) |
Values are means ± SD (medians) or percentages
Reference is postmenopausal non-hormone therapy use; premenopausal and dubious excluded
When adjusted for matching factors, women in the lowest versus the highest quartile of DHEAS had a non-significant greater risk of ischemic stroke (Table 3); adjustment for potential confounders did not alter results. The association was strengthened after adjusting for cardiovascular risk factors and biomarkers (HbA1c and total/HDL-C). When modeling binary DHEAS, women with DHEAS levels in the lowest quartile exhibited a significantly greater risk of ischemic stroke compared to higher levels in multivariable analyses (Figure 1, Model 2); estimates were strengthened and remained statistically significant after adjustment for cardiovascular risk factors and biomarkers (Figure 1, Model 3).
Table 3.
Multivariable adjusted relative risk (RR) and 95% CI of ischemic stroke by DHEAS* quartiles.
| Quartiles of DHEAS | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p-value for trend | |
| Range (μg/dL) | <42.0 | 42.0-65.2 | 65.6-101 | >101.5 | |
| Cases/Controls | 144/115 | 111/115 | 93/117 | 113/114 | |
| Model 1 | 1.30 (0.88-1.94) | 1.02 (0.68-1.53) | 0.80 (0.53-1.20) | 1.00 | 0.19 |
| Model 2 | 1.33 (0.87-2.02) | 1.03 (0.67-1.56) | 0.78 (0.51-1.20) | 1.00 | 0.18 |
| Model 3 | 1.44 (0.93-2.23) | 1.02 (0.66-1.58) | 0.82 (0.53-1.27) | 1.00 | 0.12 |
Quartiles of DHEAS based on distribution in controls
Model 1: Conditional on matching factors (age, menopausal status, hormone use, smoking)
Model 2: Model 1 ± BMI (kg/m2), aspirin use, alcohol intake, physical activity, aHEI 2010
Model 3: Model 2+ history of diabetes, hypertension, and CHD or revascularization, HbA1c and total/HDL-C
Figure 1.
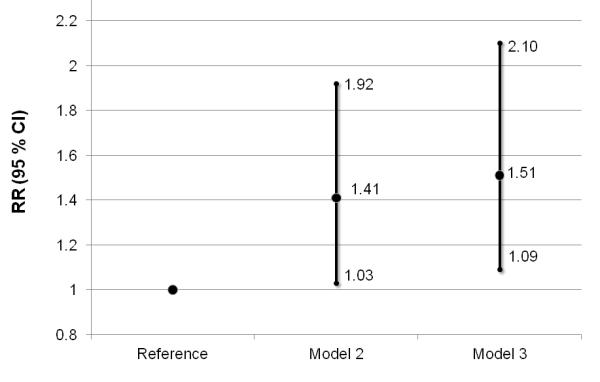
Multivariable association between DHEAS in the lowest quartile and ischemic stroke compared to quartiles 2-4. Model 2 and Model 3 adjusted for covariates in Table 3. RR= relative risk; 95% CI, 95% confidence interval
There was evidence to suggest the association between DHEAS and risk of ischemic stroke may vary by age, diabetes and smoking status, although Pinteraction>0.05 (Table 4). The risk of ischemic stroke for women in the lowest DHEAS quartile compared to higher quartiles was stronger among diabetics than for non-diabetics; however, confidence intervals were wide due to few events (Pinteraction=0.03). No significant interaction by time period was observed (results not shown). The association between DHEAS and risk of ischemic stroke was virtually unchanged after further adjustment for estradiol, testosterone and SHBG (results not shown).
Table 4.
Multivariable association between DHEAS* and ischemic stroke by key risk factors
| Stratification Factor | Cases/Controls | RR (95% CI) | Pinteraction |
|---|---|---|---|
| Age | |||
| <65 | 307/309 | 1.89 (1.26-2.85) | 0.06 |
| ≥65 | 154/152 | 1.09 (0.67-1.79) | |
| BMI | |||
| <25 | 226/254 | 1.39 (0.90-2.15) | 0.49 |
| ≥25 | 235/207 | 1.57 (0.98-2.52) | |
| Smoking | 0.11 | ||
| Never | 193/192 | 2.17 (1.32-3.56) | |
| Former | 186/193 | 1.30 (0.80-2.13) | |
| Current† | 82/76 | 0.85 (0.35-2.04) | |
| Diabetes ‡ | 0.03 | ||
| No | 403/433 | 1.36 (0.99-1.87) | |
| Yes | 58/28 | 2.00 (0.51-7.86) | |
| Postmenopausal Hormone Therapy Use § | 0.56 | ||
| No | 215/217 | 1.45 (1.07-1.98) | |
| Yes | 195/194 | 1.44 (1.05-1.96) | |
| Ovarian Status | 0.54 | ||
| Intact | 334/328 | 1.36 (0.93-1.98) | |
| >1 ovary removed | 127/133 | 1.83 (1.01-3.32) | |
Lowest quartile vs. quartiles 2-4
Models adjusted for covariates in Model 3 of Table 3, except stratification factor
Current smokers not adjusted for
Adjusted for matching factors only due to sample size constraints
Reference is postmenopausal non-hormone therapy use; premenopausal and dubious excluded
Discussion
In this nested case control study, we observed evidence to suggest that women with DHEAS levels in the lowest quartile had a greater risk of ischemic stroke during follow-up. The association was strengthened by adjustment for cardiovascular risk factors and biomarkers, and may be stronger among women with diabetes, those <65 years of age or never smokers. To our knowledge, this is the first report to evaluate DHEAS levels and risk of ischemic stroke.
Although no prior studies have examined DHEAS and stroke, several lines of evidence support a potential association. DHEAS levels have been significantly inversely associated with subclinical markers of vascular dysfunction among women: common carotid artery blood flow volume (β=0.21, p<0.05)19 and arterial stiffness (β=−0.27, p<0.03).8 Inverse association between DHEAS and CVD mortality has been suggested among men.5 Results from the Women’s Health and Aging Study, a population of older disabled women,3 reported low DHEAS levels were associated with an increased risk of CVD mortality (quartile 1 vs. 3(ref): HR=2.05, 95% CI: 1.27-3.32). Similarly, postmenopausal women in the Women’s Ischemia Syndrome Evaluation study4 with low DHEAS levels had a more than two-fold increased risk of CVD mortality (extreme tertiles; HR=2.43; 95% CI: 1.06-5.56). Despite suggestive data, the association between DHEAS and risk of total CVD has been inconsistent among women; 5 studies repored null associations,20-24 2 reported higher levels were associated with lower CVD mortality3, 4 and one reported higher levels of DHEAS were associated with increased risk of CHD.6 Inconsistencies between studies may be due to variations in age, cardiovascular risk profiles, race/ethnicity or other demographic factors, yet to be explored.3, 4, 6
DHEAS may influence the pathogenesis of CVD and ischemic stroke through several potential physiologic mechanisms. DHEAS may act through atherosclerotic-related mechanisms, such as inhibiting the migration and proliferation of cells within the vascular wall, and increasing vascular smooth muscle cell apoptosis, thereby reducing vascular remodeling after injury.10 DHEA administration has also been shown to improve endothelial cell function indicated by flow mediated dilation in vivo and increase endothelial cell proliferation independent of estrogen and androgen receptors in vitro.25 Furthermore, DHEAS may influence insulin resistance by affecting hepatocyte glucose production11 and improve peripheral tissue glucose clearance,26 shown in animal models in vivo. While unclear if DHEAS acts alone or predominately through conversion to estrogens/androgens, DHEAS can act independently in many cell types including vascular endothelium.27 However, the androgenic or estrogenic activity of DHEAS may vary given the underlying hormonal milieu, dependent on gender, hormonal status and hormone therapy use.13
It is unclear whether DHEAS plays a direct role in the pathogenesis of ischemic stroke or serves as a risk marker of subclinical vascular disease. DHEAS is almost exclusively synthesized in the zona reticluaris of the adrenal cortex, which is vulnerable to vascular damage; thus DHEAS levels may be a marker of subclinical vascular disease.28 The underlying biologic pathway is unclear; further research is needed to elucidate specific mechanisms.
Strengths of the current study include the nested case-control design with DHEAS levels collected prior to ischemic stroke events. DHEAS is a more stable marker than DHEA given a longer half-life, slower turnover rate and it does not exhibit diurnal variation, in contrast to DHEA. However, DHEAS levels vary substantially by gender; variation by race/ethnicity or socio-demographic factors is unclear, thus generalizability may be limited. Although we were unable to evaluate change in DHEAS over time with stroke risk due to a limited number of replicate plasma measurements, a high correlation between replicate measures approximately 10 years apart was indicated. This suggests the long term reproducibility of a single measure of plasma DHEAS levels in postmenopausal women, supporting previous work in this cohort.16 Moreover, the association between DHEAS and risk of stroke did not differ by time to event. We were unable to evaluate ischemic stroke classification by the TOAST criteria. Although, cases-control pairs were not matched on hypertension status, as it may be on the causal pathway, we did adjust for hypertension in sensitivity analyses (model 3). Furthermore, the sample size was moderate and while we examined variation by key cardiovascular risk factors which have not been previously explored, analyses were underpowered and warrant further study.
Summary
In this cohort of older women, these results suggest evidence for an inverse association between DHEAS and risk of ischemic stroke, where lower levels of DHEAS were associated with an increased risk of ischemic stroke. There was an indication that an elevated risk of ischemic stroke associated with low DHEAS levels in of this population may be strongest among diabetics, younger women (<65 yrs), and never smokers. Additional research is warranted to confirm these associations in other populations.
Supplementary Material
Acknowledgments
NHS Participants
Funding: HL088521-S1, HL34594, K99HL098459(Dr. Sun) from the National Heart, Lung and Blood Institute and CA87969 and CA49449 from the National Cancer Institute of the National Institutes of Health.
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. Dhea and dhea-s: A review. J Clin Pharmacol. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- 2.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 3.Cappola AR, Xue QL, Walston JD, Leng SX, Ferrucci L, Guralnik J, et al. Dheas levels and mortality in disabled older women: The women’s health and aging study i. J Gerontol A Biol Sci Med Sci. 2006;61:957–962. doi: 10.1093/gerona/61.9.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, et al. Dhea-s levels and cardiovascular disease mortality in postmenopausal women: Results from the national institutes of health--national heart, lung, and blood institute (nhlbi)-sponsored women’s ischemia syndrome evaluation (wise) J Clin Endocrinol Metab. 2010;95:4985–4992. doi: 10.1210/jc.2010-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thijs L, Fagard R, Forette F, Nawrot T, Staessen JA. Are low dehydroepiandrosterone sulphate levels predictive for cardiovascular diseases? A review of prospective and retrospective studies. Acta Cardiol. 2003;58:403–410. doi: 10.2143/AC.58.5.2005304. [DOI] [PubMed] [Google Scholar]
- 6.Page JH, Ma J, Rexrode KM, Rifai N, Manson JE, Hankinson SE. Plasma dehydroepiandrosterone and risk of myocardial infarction in women. Clin Chem. 2008;54:1190–1196. doi: 10.1373/clinchem.2007.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki M, Kanazawa A, Hasegawa M, Hattori Y, Harano Y. A close association between insulin resistance and dehydroepiandrosterone sulfate in subjects with essential hypertension. Endocr J. 1999;46:521–528. doi: 10.1507/endocrj.46.521. [DOI] [PubMed] [Google Scholar]
- 8.Creatsa M, Armeni E, Stamatelopoulos K, Rizos D, Georgiopoulos G, Kazani M, et al. Circulating androgen levels are associated with subclinical atherosclerosis and arterial stiffness in healthy recently menopausal women. Metabolism. 2012;61(2):193–201. doi: 10.1016/j.metabol.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Schunkert H, Hense HW, Andus T, Riegger GA, Straub RH. Relation between dehydroepiandrosterone sulfate and blood pressure levels in a population-based sample. Am J Hypertens. 1999;12:1140–1143. doi: 10.1016/s0895-7061(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 10.Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, et al. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the akt/gsk3-{beta}/nfat axis. Circulation. 2009;120:1231–1240. doi: 10.1161/CIRCULATIONAHA.109.848911. [DOI] [PubMed] [Google Scholar]
- 11.Aoki K, Taniguchi H, Ito Y, Satoh S, Nakamura S, Muramatsu K, et al. Dehydroepiandrosterone decreases elevated hepatic glucose production in c57bl/ksj323 db/db mice. Life Sci. 2004;74:3075–3084. doi: 10.1016/j.lfs.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita R, Saito T, Satoh S, Aoki K, Kaburagi Y, Sekihara H. Effects of dehydroepiandrosterone on gluconeogenic enzymes and glucose uptake in human hepatoma cell line, hepg2. Endocr J. 2005;52:727–733. doi: 10.1507/endocrj.52.727. [DOI] [PubMed] [Google Scholar]
- 13.Ebeling P, Koivisto VA. Physiological importance of dehydroepiandrosterone. Lancet. 1994;343:1479–1481. doi: 10.1016/s0140-6736(94)92587-9. [DOI] [PubMed] [Google Scholar]
- 14.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 15.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, et al. Test of the national death index. Am J Epidemiol. 1984;119:837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–654. [PubMed] [Google Scholar]
- 17.Walker AE, Robins M, Weinfeld FD. The national survey of stroke. Clinical findings. Stroke. 1981;12:I13–44. [PubMed] [Google Scholar]
- 18.Iso H, Rexrode K, Hennekens CH, Manson JE. Application of computer tomography-oriented criteria for stroke subtype classification in a prospective study. Ann Epidemiol. 2000;10:81–87. doi: 10.1016/s1047-2797(99)00040-x. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida S, Aihara K, Azuma H, Uemoto R, Sumitomo-Ueda Y, Yagi S, et al. Dehydroepiandrosterone sulfate is inversely associated with sex-dependent diverse carotid atherosclerosis regardless of endothelial function. Atherosclerosis. 2010;212:310–315. doi: 10.1016/j.atherosclerosis.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Barrett-Connor E, Goodman-Gruen D. Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women. The rancho bernardo study. Circulation. 1995;91:1757–1760. doi: 10.1161/01.cir.91.6.1757. [DOI] [PubMed] [Google Scholar]
- 21.Legrain S, Berr C, Frenoy N, Gourlet V, Debuire B, Baulieu EE. Dehydroepiandrosterone sulfate in a long-term care aged population. Gerontology. 1995;41:343–351. doi: 10.1159/000213706. [DOI] [PubMed] [Google Scholar]
- 22.Mazat L, Lafont S, Berr C, Debuire B, Tessier JF, Dartigues JF, et al. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: Relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc Natl Acad Sci U S A. 2001;98:8145–8150. doi: 10.1073/pnas.121177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilvis RS, Kahonen M, Harkonen M. Dehydroepiandrosterone sulfate, diseases and mortality in a general aged population. Aging (Milano) 1999;11:30–34. [PubMed] [Google Scholar]
- 24.Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab. 2001;86:4171–4177. doi: 10.1210/jcem.86.9.7838. [DOI] [PubMed] [Google Scholar]
- 25.Williams MR, Dawood T, Ling S, Dai A, Lew R, Myles K, et al. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J Clin Endocrinol Metab. 2004;89:4708–4715. doi: 10.1210/jc.2003-031560. [DOI] [PubMed] [Google Scholar]
- 26.Hansen PA, Han DH, Nolte LA, Chen M, Holloszy JO. Dhea protects against visceral obesity and muscle insulin resistance in rats fed a high-fat diet. Am J Physiol. 1997;273:R1704–1708. doi: 10.1152/ajpregu.1997.273.5.R1704. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in us women. Am J Clin Nutr. 2000;71:1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 28.Angeli A, Masera RG, Magri F, Ferrari E. The adrenal cortex in physiological and pathological aging: Issues of clinical relevance. J Endocrinol Invest. 1999;22:13–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


