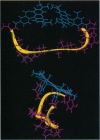
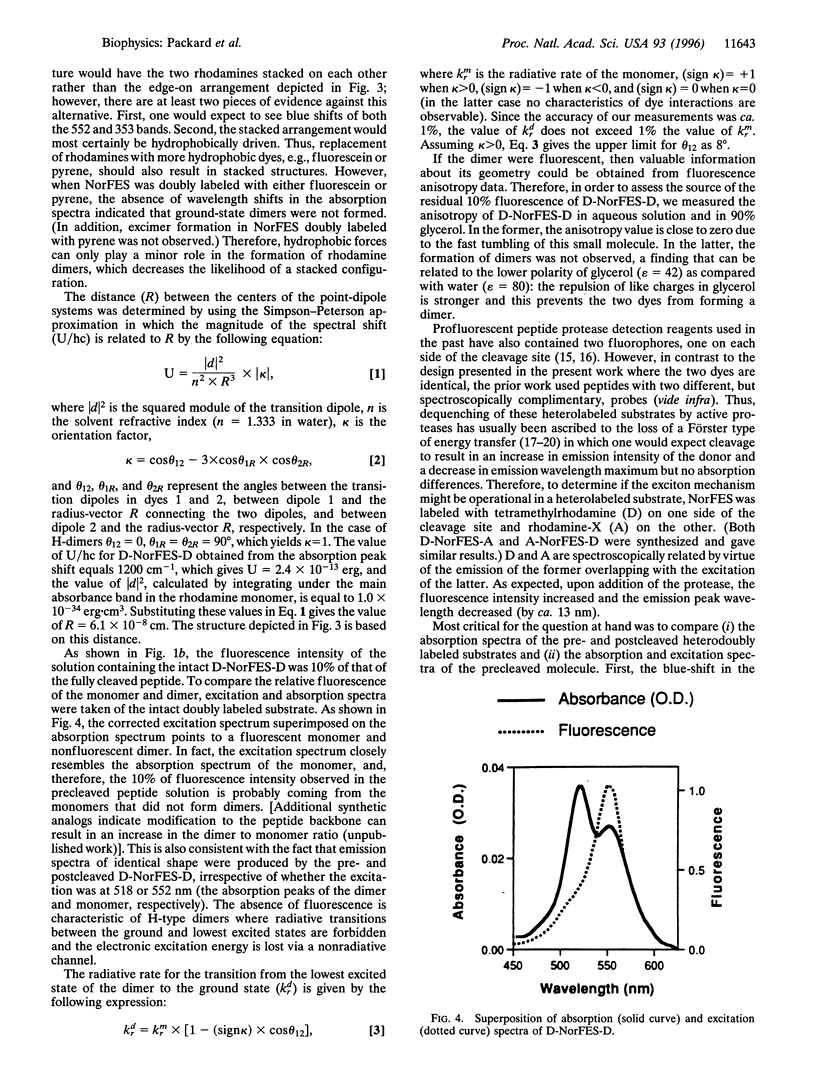
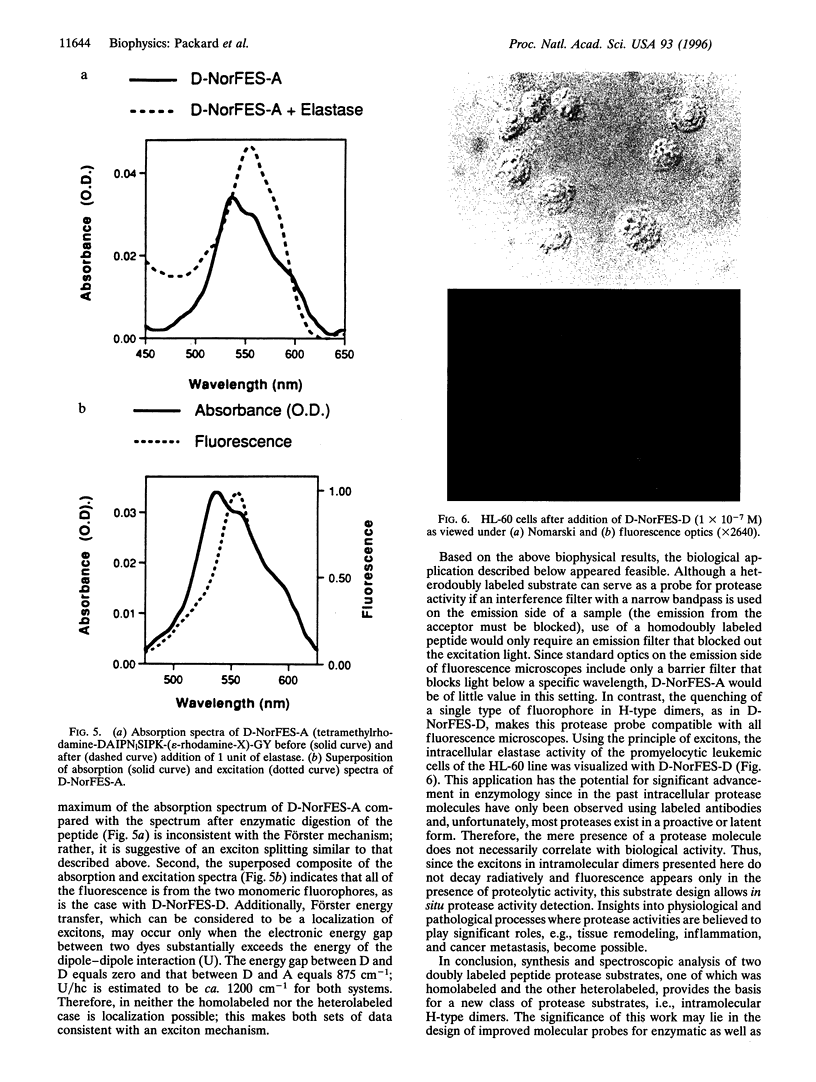
Abstract
Xanthene dyes are known to form dimers with spectral characteristics that have been interpreted in terms of exciton theory. A unique aspect of H-type dimers is the fluorescence quenching that accompanies their formation. Using the principles of exciton theory as a guide, a series of protease substrates was synthesized with a xanthene dye on each side of the cleavage site. To bring the attached dyes into spatial proximity to form a dimer, the molecular design included structure determinant regions in the amino acid sequence. In addition, chromophores were chosen such that changes in absorption spectra indicative of exciton splitting were anticipated. Cleavage of the peptides by a protease resulted in disruption of the dimers and indeed significant absorption spectral changes were observed. Furthermore, substrate cleavage was accompanied by at least an order of magnitude increase in fluorescence intensity. This has allowed determination of intracellular elastase activity using a fluorescence microscope equipped with standard optics.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajtai K., Ilich P. J., Ringler A., Sedarous S. S., Toft D. J., Burghardt T. P. Stereospecific reaction of muscle fiber proteins with the 5' or 6' isomer of (iodoacetamido)tetramethylrhodamine. Biochemistry. 1992 Dec 15;31(49):12431–12440. doi: 10.1021/bi00164a019. [DOI] [PubMed] [Google Scholar]
- Carmel Amos, Zur Margalit, Yaron Arieh, Katchalski Ephraim. Use of substrates with fluorescent donor and acceptor chromophores for the kinetic assay of hydrolases. FEBS Lett. 1973 Feb 15;30(1):11–14. doi: 10.1016/0014-5793(73)80607-6. [DOI] [PubMed] [Google Scholar]
- Clegg R. M. Fluorescence resonance energy transfer. Curr Opin Biotechnol. 1995 Feb;6(1):103–110. doi: 10.1016/0958-1669(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Hamman B. D., Oleinikov A. V., Jokhadze G. G., Bochkariov D. E., Traut R. R., Jameson D. M. Tetramethylrhodamine dimer formation as a spectroscopic probe of the conformation of Escherichia coli ribosomal protein L7/L12 dimers. J Biol Chem. 1996 Mar 29;271(13):7568–7573. doi: 10.1074/jbc.271.13.7568. [DOI] [PubMed] [Google Scholar]
- KASHA M. ENERGY TRANSFER MECHANISMS AND THE MOLECULAR EXCITON MODEL FOR MOLECULAR AGGREGATES. Radiat Res. 1963 Sep;20:55–70. [PubMed] [Google Scholar]
- Latt S. A., Auld D. S., Vallee B. L. Fluorescende determination of carboxypeptidase A activity based on electronic energy transfer. Anal Biochem. 1972 Nov;50(1):56–62. doi: 10.1016/0003-2697(72)90485-x. [DOI] [PubMed] [Google Scholar]
- Luttrull D. K., Valdes-Aguilera O., Linden S. M., Paczkowski J., Neckers D. C. Rose Bengal aggregation in rationally synthesized dimeric systems. Photochem Photobiol. 1988 Apr;47(4):551–557. doi: 10.1111/j.1751-1097.1988.tb08843.x. [DOI] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Selvin P. R. Fluorescence resonance energy transfer. Methods Enzymol. 1995;246:300–334. doi: 10.1016/0076-6879(95)46015-2. [DOI] [PubMed] [Google Scholar]
- Wu P., Brand L. Resonance energy transfer: methods and applications. Anal Biochem. 1994 Apr;218(1):1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]