Abstract
Because of the wide availability of hardware as well as of standardized analytic quantification tools, proton magnetic resonance spectroscopy (1H-MRS) has become widely used to study psychiatric disorders. 1H-MRS allows measurement of brain concentrations of more traditional singlet neurometabolites like N-acetylaspartate, choline, and creatine. More recently, quantification of the more complex multiplet spectra for glutamate, glutamine, inositol, and γ-aminobutyric acid have also been implemented. Here we review applications of 1H-MRS in terms of informing treatment options in schizophrenia, bipolar disorder, and major depressive disorders. We first discuss recent meta-analytic studies reporting the most reliable findings. Then we evaluate the more sparse literature focused on 1H-MRS-detected neurometabolic effects of various treatment approaches in psychiatric populations. Finally we speculate on future developments that may result in translation of these tools to improve the treatment of psychiatric disorders.
Keywords: schizophrenia, spectroscopy, NAA, glutamate, glutamine
Abstract
La espectroscopia de resonancía magnética de protón (1H-MRS) ha llegado a emplearse ampliamente en el estudio de los trastornos psiquiátricos. Hay una amplia disponibilidad de equipos como de herramientas estandarizadas de cuantificación analítica. La 1H-MRS permite la medición de concentraciones cerebrales de neurometabolitos con los singletes más tradicionales como el N-acetilaspartato, la colina y la creatina. Hace poco se ha implementado la cuantificación de espectros múltiples más complejos para el glutamato, la glutamina, el inositol y el ácido gama-amíno-butírico. En este artículo se revisan las aplicaciones de la 1H-MRS en relación con la información que pueda aportar para las alternativas terapéuticas en esquizofrenia, trastorno bipolar y trastornos depresivos mayores. Primero se discuten los estudios de meta-análisis recientes que dan cuenta de los hallazgos más confiables. Luego se evalúa la más amplia y diversa literatura orientada a los efectos neurometabólicos detectados con la 1H-MRS en varios esquemas terapéuticos en pacientes psiquiátricos. Finalmente se especula acerca de los futuros desarrollos que pueden surgir en la traslación de estas herramientas para mejorar el tratamiento de los trastornos psiquiátricos.
Abstract
Grâce au grand choix de matériels et d'outils de quantification analytique standardisés, la spectroscopie par résonance magnétique nucléaire protonique (RMN 1H) est largement utilisée dans I'étude des troubles psychiatriques. Elle permet de mesurer les concentrations cérébrales de métabolites singulets classiques comme le N-acétyl-aspartate, la choline, la créatine auxquels se sont rajoutés plus récemment la quantification des spectres plus complexes de multiplets comme le glutamate, la glutamine, l'inositol et I'acide gamma-aminobutyrique. Nous analysons dans cet article les applications de la RMN 1H en termes d'information sur les choix thérapeutiques dans la schizophrénie, les troubles bipolaires et la dépression majeure. Nous examinons tout d'abord les récentes métaanalyses rapportant les résultats les plus fiables, puis nous évaluons la littérature plus limitée sur les effets neurométaboliques détectés par RMN 1H des différents traitements chez des patients psychiatriques. Enfin, nous imaginons le développement à venir de ces outils dans le cadre du traitement des troubles psychiatriques.
Introduction
Technique overview
Proton magnetic resonance spectroscopy (1H-MRS) uses the same hardware as magnetic resonance imaging (MRI) to measure various metabolites in the brain. With suppression of the large proton signal from water molecules, the intensity of the relaxation signal from protons attached to other brain metabolites of interest can be quantified in the time domain, as free induction decays (FID). These FIDs are usually Fourier transformed to the frequency domain and depicted as spectra. The area under the curve at a particular frequency (usually in parts per million [ppm] to standardize across magnetic field strengths), can then be quantified and usually referenced to an internal standard (like water spectra or another neurometabolite, eg, total creatine).
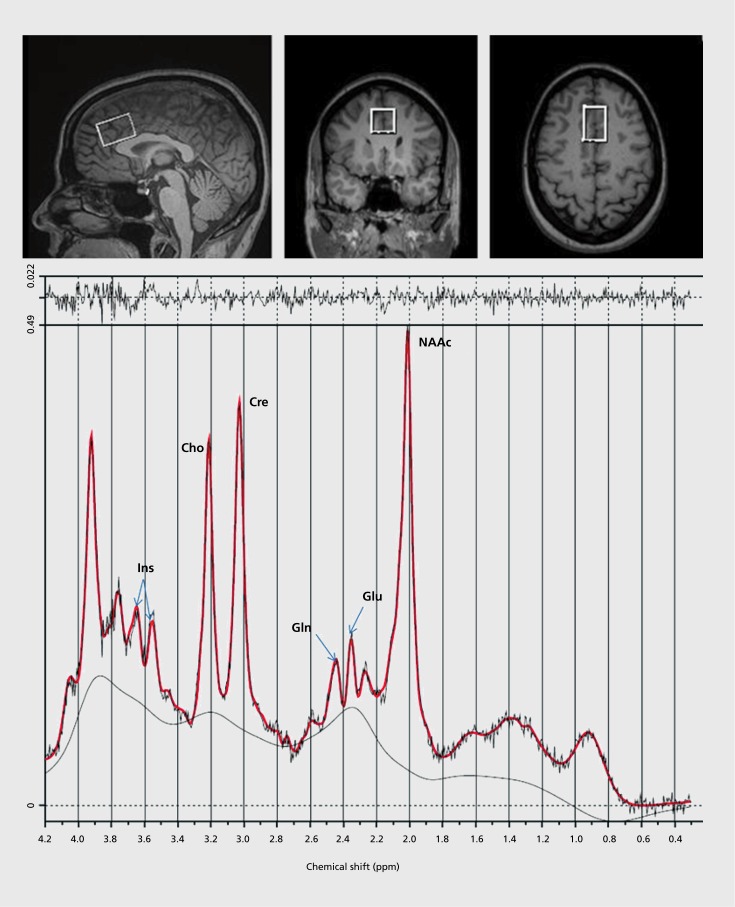
With traditional clinical 1.5-Tesla (T) scanners the main metabolites of interest include N-acetylaspartate compounds (NAA plus N-acetylaspartate-glutamate, NAAG), choline (Cho) and creatine (Cre). At higher field strengths (3,4, and 7 T), the more complex overlapping, multiplets corresponding to glutamate (Glu), glutamine (Gin) and myoinositol (Ins), can also be measured. γ-Aminobutyric acid (GABA) as well as gluthatione (GSII) can be quantified at the higher filed strengths with special editing techniques. Most studies have used single voxel acquisition (anywhere between 1 to 40 cc per voxel) (Figure 1). This is practical for populations where support for regional pathology is strong. Chemical shift Imaging (CSI), simultaneously acquires many voxels (20 to 100, usually 1 to 2 cc each) across a slab of tissue, providing better spatial coverage. However, spectral resolution can be compromised due to magnetic field inhomogeneity.
Figure 1. Single voxel location (2 x 2 x 3 cm) in the anterior dorsal cingulate cortex, shown in sagittal, coronal, and axial planes from MRI (top panel). A spectra, averaged from 196 acquisitions, collected from the above location at 3 Tesla, with a standard point resolved spectroscopic sequence (time to echo = 40 msec, time to repetition = 1500 msec), in about 5 mins. The fitted spectra (red line) has peak areas for glutamine (Gln), glutamate (Glu), N-acetyl-aspartate compounds (NAAc), total-creatine (Cre), myo-inositol (Ins) and choline (Cho) are labeled. Top irregular line represents the residual signal after fitting. Lower continuous line represents the baseline used for fitting with linear combination (LC) model (bottom panel).
Interpretation of the meaning of differences in levels measured depends on what is known about the physiology of the various neurometabolites. NAA is the second most concentrated aminoacid in the brain (after glutamate). NAA is synthesized in neuronal mitochondria and is located almost exclusively in neurons and their processes. Hence, in neurodegenerative and vascular disease NAA is reduced and it is absent from brain tumor tissue. Consequently, NAA is viewed as a marker of neuronal viability. However, NAA clearly is not a neurotransmitter or neuromodulator, and its function remains unclear. It has been hypothesized to serve as an osmolyte and an acetate donor involved in myelinization.
Choline compounds are trimethylamines but are chemically heterogeneous and have a twofold greater concentration in glial compared with neuronal cells. Consistently, the choline signal tends to be increased in neurodegenerative disorders with gliosis and/or increased membrane turnover.
Myoinositol is the most abundant biologically active stereoisomer of inositol in the brain. Myoinositol is a precursor in the phosphatidylinositol second messenger system, and is also a glial marker. In dementia, elevated Ins, in conjunction with reduced NAA, has been consistently found.
Glutamate is an aminoacid highly concentrated in all cells and involved in multiple metabolic functions. It is the principal excitatory neurotransmitter in the central nervous system (CNS). However, only a minimal proportion of 1H-MRS-measured glutamate signal comes from the synapses. Conversely, glutamine is mainly synthesized in the glia from synaptic glutamate and has been used as an index of glutamatergic neurotransmission.
GABA is the principal inhibitory neurotransmitter in the CNS. Although technically challenging, its 1H-MRS measurement can be more easily interpreted than the levels or glutamate or even glutamine. Finally, creatine and phosphocreatine are easily measured and are involved in energy metabolism. Although often used as a reference for other metabolites, interpretation of the meaning of creatine group differences is poorly understood.
Schizophrenia
Disease-related findings
Over the last three decades there have been many 1H-MRS studies comparing schizophrenic (Sz) and healthy control groups, and these have been summarized in three meta-analyses. Steen et al1 reported reduced NAA in frontal and medial temporal regions. Kraguljac et al2 confirmed NAA reductions in frontal and basal ganglia regions with no evidence of changes in Cho or Cre. A smaller number of studies have examined Glu, Gin, and Glx. Marsman et al3 reported increased Gin and Gln/Glu ratio in medial frontal cortex, more apparent earlier than later in the illness. In the last 4 years a few studies have examined GABA in Sz and the results have been inconsistent: two studies found reductions,4,5 two elevations,6,7 and one no differences.8 Finally, one study detected gluthathione reductions in frontal cortex as well as in cerebrospinal fluid of drug-free Sz.9
This literature confirms that in schizophrenia (mostly chronically treated patients) there are reductions in NAA in frontal and basal ganglia regions, consistent with neuronal dysfunction (postmortem literature has consistently failed to find neuronal loss). A more limited literature supports elevations of Gin in medial frontal regions, suggestive of increased glutamatergic neurotransmission.
Treatment-related studies
Treatment studies using 1H-MRS not surprisingly lag in number compared with cross-sectional investigations. Most of them have been naturalistic observations before and after treatment with antipsychotic agents. Theberge et al10 examined antipsychotic-naïve Sz subjects at baseline, 10, and 30 months following antipsychotic treatment. Baseline thalamic Gin elevations were decreased at 30 months, and correlated with widespread temporal and parietal gray matter reductions. This was interpreted as evidence of glutamate-related disease progression, not as a medication effect (at 10 months, Gin did not change).
Although a few studies have reported increases in NAA with treatment, the majority have failed to do so. Bertolino et al,11 in a retrospective study, reported higher NAA/Cre in the dorsolateral prefrontal cortex in patients while treated with antipsychotic medication compared with when they were medication-free. Fannon et al12 reported reduced medial temporal NAA/Cre at baseline, which was no longer statistically different from healthy subjects after 3 months of atypical antipsychotic treatment. However, Choe et al13 found low frontal NAA/Cre at baseline with no changes after treatment with typical and atypical agents (follow-up 1-6 months). We found frontal and striatal NAA reductions in minimally treated patients that did not change following 9-month randomized treatment with quetiapine or haloperidol.14 Szulc et al15 reported no NAA changes following treatment with various antipsychotics. Finally, Theberge et al10 found no NAA changes following a 30-month treatment.
These clinical studies are generally consistent with largely negative findings in the animal TI-MRS literature examining antipsychotic exposure in rats. Lindquist et al16 found no reductions of frontal NAA after 1 week of haloperidol but a reduction with olanzapine. We found no changes in NAA after 6 weeks of clozapine or haloperidol.17 Additionally, 6 months exposure to haloperidol produced no changes in NAA, Glu, Gin, or GABA.18 However, Ilarte et al19 did find increased NAA in the rat striatum with long-term haloperidol exposure, consistent with dendritic sprouting. Antipsychotic drugs are known to induce structural volume increases in the human striatum20 and cortical volume reductions,21 but no neuronal loss. Hence, the ability to detect changes in neuronal tissue concentration would depend on the spatial resolution of the 1H-MRS technique (currently limited to 1 cc, clearly suboptimal for the 2- to 3-mm thick human cortex) as well as the timing of acquisition.
A recent longitudinal study of antipsychotic-naïve schizophrenia examined Glu and NAA in the caudate before and 4 weeks following treatment with risperidone.22 Baseline Glu elevations normalized with treatment but there were no changes in NAA. However, the Glu normalization did not correlate with symptom improvement. These results are consistent with a dopamine D2 modulation of striatal hypeglutamalergia. Consistently, Goto et al23 reported similar Glx prefrontal reductions following 6 months of antipsychotic treatment.
Experimental agent studies
Few studies have used 1H-MRS to examine neurochemical effects of non-dopamine D2 blockers for potential applications in schizophrenia. Jarkog et al24 recently examined the effect of adjunct davunetide (a neurotrophic peptide) for 12 weeks in schizophrenia. Higher-dose davunetide (30 mg/day) resulted in marginal prefrontal NAA and Cho increases, suggesting a neuroprotective effect. However, there were no correlations with symptoms or cognitive measures.
Glycine has been examined for the treatment of negative symptoms, and an 1H-MRS sequence is sensitive enough to detect brain elevations in the context of oral administration in healthy volunteers.25 Future studies may examine the glycine spectra in clinically treated populations. Finally, in a knockout mouse model with reduced glutathione, N-acetyl cysteine administration during gestation was found to revert abnormally elevated prepuberal brain Gin and Gln/Glu.26 This is somewhat consistent with a studydocumenting effects of N-acetyi cysteine (NAC) on negative symptoms.27 However, there have been no reports examining the effects of NAC on 1H-MRS measures in schizophrenia.
Bipolar disorder
Disease-related findings
The Kraguljac et al2 meta-analysis reported NAA reductions only in basal ganglia and elevations in dorso-lateral prefrontal cortex (however, heterogeneity of studies was high for the latter). There were no differences in Cre or Cho. In another meta-analysis28 Glx was found to be elevated, mainly in the frontal region, regardless of medication or clinical state.29 One study reported reduced occipital GABA.30 These results suggest increased glutamatergic indices in bipolar disorder, without the more widespread neuronal dysfunction (reduced NAA) found in schizophrenia.
Treatment-related studies
Some studies have used 1H-MRS in bipolar mania to evaluate correlates of treatment with lithium, other mood stabilizers, and antipsychotic agents.
Lithium
Cross-sectional studies suggested that lithium therapyincreases NAA31,32 in bipolar patients. However, longitudinal human studies33,34 as well as a rodent study35 failed to detect NAA changes. Conversely, reductions in Glx have been reported in longitudinal human33 and rat35 studies with lithium, consistent with an improvement of Glx in bipolar disorder. However, documentation of a relationship between Glx reductions and symptom improvement is lacking. Additionally, lithium induced myoinositol reductions which did not relate to changes in mood,36 but another longitudinal study found increases in this metabolite.33 In general, no clear pattern of lithium related TI-MRS changes has been documented in clinical populations. However, spectroscopic measurement of brain lithium in treated bipolar subjects does correlate with serum lithium levels.37 This approach may result in a clinical application for lithium MRS in the future.
Other agents
Strawn et al38 studied adolescents in a manic episode before open-label treatment with divalproex and 1 and 4 weeks later. Remitters had lower Glx in the left ventro-lateral prefrontal cortex compared with nonremitters. However, in adult bipolar patients treated with valproic acid, Glx failed to predict response.33 In rats, sodium valproate did induce reductions in glutamate.35
Depression
Disease-related findings
Two meta-analyses have examined 1H-MRS neurometabolite findings in major depressive disorder. Yildiz-Yesiloglu and Ankerst39 reported an increase in Cho/Cre ratio in basal ganglia in major depression with no changes in NAA. This was interpreted as increased membrane turnover without evidence of neuronal damage. More recently, Glx was found reduced in the anterior cingulate cortex during a depressive episode.40 This suggests that contrary to bipolar mania, glutamatergic metabolism is reduced in a state-dependent relationship in depression. Finally, reduced occipital GABA has been found in MDD.41,42
Treatment-related studies
Since Cho levels tend to be increased in MDD, some studies have examined their relationship to treatment. Charles et al43 reported Cho/Cre reductions with nefazodone treatment. However, Cho increases have been reported in other drug44 and transcranial magnetic stimulation (TMS)45 studies in treatment responders.
Baseline reductions of Glx would be expected to increase with therapy. Indeed, electroconvulsive therapy (ECT) normalized reduced prefrontal Glx46 and successful TMS increased Glu44 in depression. Regarding GABA, baseline occipital reductions increased with serotonin reuptake inhibitors.41 Similarly, ECT increased GABA42 but cognitive behavioral therapy (CBT), failed to do so.47
Some studies have examined a neurotrophic effect of various treatments by examining NAA. The results for ECT have been mixed in terms of NAA changes.48,49 However, there is a modest but consistent literature suggesting increases in NAA with antidepressant medication.50-52 Finally, new strategies for depression with NMDA blockers have been studied recently and examinations of glutamate metabolism undertaken. Salvatore et al53 found that the pretreatment prefrontal Glx/Glu ratio was negatively correlated with improvement in depressive symptoms. However neither GABA nor Glu levels predicted treatment response. Still, the development of NMDA modulators for the treatment of depressive disorders is likely to benefit from the in- vivo examination of glutamate and GABA with 1H-MRS.
Summary of findings
1H-MRS has been widely used to study major psychiatric illness, and several findings have been consistently reported. In schizophrenia, prefrontal NAA is reduced and glutamine increased, suggestive of glutamate-related excitotoxicity. In bipolar disorder, there is increased prefrontal glutamatergic metabolism (elevated Glx) perhaps as a trait measure. In major depression, basal ganglia choline is increased, while prefrontal Glx and occipital GABA are reduced and these may represent state abnormalities. Presently, none of these effects are sufficiently sensitive or specific to have any diagnostic implication.
The literature regarding applicability of 1H-MRS to evaluate effects of treatment is, not surprisingly, more limited. In schizophrenia, NAA reductions are not caused, but also not restored, by antipsychotic agents. However, there is evidence that antipsychotics may reduce elevated glutamatergic indices, especially in the striatum, their primary site of action. In bipolar disorder, the 1H-MRS correlates of response to lithium and other mood stabilizers have not been elucidated. However, lithium quantification in brain is possible and may have future clinical applications. Regarding depression, it is encouraging that restoration of reduced glutamate and GABA have been documented with ECT, TMS, and antidepressant medication. Additionally, a small but reliable increase of NAA with medication is consistent with the neurotrophic effects of antidepressant drugs. However, the correlations with symptom improvement for these 1H-MRS /treatment relationships have been modest at best and no clinical applications are available. Table I summarizes the strengths and weaknesses of MRS.
TABLE I. Strengths and weaknesses of magnetic resonance spectroscopy.
| Strengths |
| • Accessible: available in most magnetic resonance (MR) centers with clinical scanners |
| • Safe: no radiation, allows for repeated studies in same individuals |
| • Transferable: easily transfers to animal models of disease to test mechanistic explanations of descriptive clinical findings |
| • Specific measurements: of neuroactive metabolites not currently available with radio-labelled PET studies (like γ-aminobutyric acid, glutamate, glutamine, glycine, glutathione) |
| • Integration with other modalities: easy to acquire in the same session with others (eg, MRI, diffusion tensor imaging, functional MR) |
| Weaknesses |
| • Spectral resolution: restricted to a few metabolites, some of doubtful significance |
| • Partial volume: because cortex macrostructure is in single millimeters and MRS voxels are in tens of millimeters, partial volume effects are significant and must be addressed. Tissue relaxation parameters: these can affect perceived metabolite concentrations and for several metabolites are not well known |
| • Spatial coverage: limited to single voxel acquisitions for the most potentially meaningful metabolites. Even multivoxel MRS typically includes a only few hundred voxels Because of spatial coverage and partial volume limitations, actual integration with other imaging modalities is difficult |
| • Signal to noise: low compared with water-based MR techniques. Effect sizes likely to be very small in psychiatric illnesses, so large samples generally necessary |
Future directions
In terms of technique development there is a need for sequences with broader spatial coverage so that true imaging of multiple metabolites is possible, with enough spatial resolution to allow full integration with other modalities. This would allow, for example, to test whether NAA reductions in white matter in schizophrenia, correspond or not to the well-described reductions in fractional anisotropy (FA), acquired with DTI. Additionally, techniques that reliably block lipid signal contamination, will permit more specific examination of peripheral cortical regions. Improved hardware and shimming techniques may allow measurements in deeper structures, like the amygdala or hippocampus, which are currently accessible mainly for the singlet peaks easier to measure. Editing techniques at higher field strength with improved spectral resolution may allow measurement of neuroactive metabolites in smaller, more physiologically plausible regions. Experiments in animals using microscopic and functional tools in addition to descriptive MRS measurements, would greatly advance the interpretation of clinical studies. Finally, in terms of clinical design, large samples (in the hundreds, like other modalities) of different clinical populations early in the illness, with long-term longitudinal follow-up, will be necessary. With renewed interest in excitatory/inhibitory neurotransmitter systems beyond traditional bioaminergic functions, more sophisticated TI-MRS studies of glutamate, glutamine, GABA, and glycine, in the context of various treatment modalities, may translate into clinical applications in psychiatry.
Selected abbreviations and acronyms
- 1H-MRS
proton magnetic resonance spectroscopy
- Cho
choline
- Cre
creatine
- GABA
γ-aminobutyric acid
- Gln
glutamine
- Glu
glutamate
- NAA
N-acetylaspartate
- TMS
transcranial magnetic stimillation
REFERENCES
- 1.Steen RG., Hamer RM., Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 2.Kraguljac NV., Reid M., White D., et al Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsman A., van den Heuvel MP., Klomp DW., Kahn RS., Luijten PR., Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and metaanalysis of H-MRS studies. Schizophr Bull. 2013;39:120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goto N., Yoshimura R., Moriya J., et al Reduction of brain gammaam inobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophr Res. 2009;112:192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Yoon JH., Maddock RJ., Rokem A., et al GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 2010;30:3777–3781. doi: 10.1523/JNEUROSCI.6158-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ongur D., Prescot AP., McCarthy J., Cohen BM., Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kegeles LS., Mao, Stanford AD., et al Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 8.Rowland LM., Kontson K., West J., et al In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophr Bull. In press. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do KQ., Trabesinger AH., Kirsten-Kruger M., et al Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 10.Theberge J., Williamson KE., Aoyama N., et al Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- 11.Bertolino A., Callicott JH., Mattay VS., et al The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry. 2001;49:39–46. doi: 10.1016/s0006-3223(00)00997-5. [DOI] [PubMed] [Google Scholar]
- 12.Fannon D., Simmons A., Tennakoon L., et al Selective deficit of hippocampal N-acetylaspartate in antipsychotic-naive patients with schizophrenia. Biol Psychiatry. 2003;54:587–598. doi: 10.1016/s0006-3223(03)00185-9. [DOI] [PubMed] [Google Scholar]
- 13.Choe BY., Suh TS., Shinn KS., Lee CW., Lee C., Paik IH. Observation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopy. Invest Radiol. 1996;31:345–352. doi: 10.1097/00004424-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Bustillo JR., Rowland LM., Jung R., et al Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33:2456–2466. doi: 10.1038/sj.npp.1301631. [DOI] [PubMed] [Google Scholar]
- 15.Szulc A., Galinska B., Tarasow E., et al Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry. 2011;44:148–157. doi: 10.1055/s-0031-1279739. [DOI] [PubMed] [Google Scholar]
- 16.Lindquist DM., Hawk RM., Karson CN., Komoroski RA. Effects of antipsychotic drugs on metabolite ratios in rat brain in vivo. Magn Reson Med. 2000;43:355–358. doi: 10.1002/(sici)1522-2594(200003)43:3<355::aid-mrm6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Bustillo J., Wolff C., Myers-y-Gutierrez A., et al Treatment of rats with antipsychotic drugs: lack of an effect on brain N-acetyl aspartate levels. Schizophr Res. 2004;66:31–39. doi: 10.1016/s0920-9964(02)00528-5. [DOI] [PubMed] [Google Scholar]
- 18.Bustillo J., Barrow R., Paz R., et al Long-term treatment of rats with haloperidol: lack of an effect on brain N-acetyl aspartate levels. Neuropsychopharmacology. 2006;31:751–756. doi: 10.1038/sj.npp.1300874. [DOI] [PubMed] [Google Scholar]
- 19.Harte MK., Bachus SB., Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophr Res. 2005;75:303–308. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Chakos MH., Lieberman JA., Bilder RM., et al Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- 21.Ho BC., Andreasen NC., Ziebell S., Pierson R., Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of firstepisode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Fuente-Sandoval C., Léon-Ortiz P., Azcárraga M., et al Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. In press. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goto N., Yoshimura R., Kakeda S., et al Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–122. doi: 10.2147/NDT.S25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarskog LF., Dong Z., Kangarlu A., et al Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology. 2013;38:1245–52. doi: 10.1038/npp.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufman MJ., Prescot AP., Ongur D., et al Oral glycine administration increases brain glycine/creatine ratios in men: a proton magnetic resonance spectroscopy study. Psychiatry Res. 2009;173:143–149. doi: 10.1016/j.pscychresns.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.das Neves Duarte JM., Kulak A., Gholam-Razaee MM., Cuenod M., Gruetter R., Do KQ. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry. 2012;71:1006–1014. doi: 10.1016/j.biopsych.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Berk M., Copolov D., Dean O., et al N-acetyl cysteine as a glutathione precursor for schizophrenia— a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Gigante AD., Bond DJ., Later B., Lam RW., Young LT., Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 2012;14:478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 29.Yuksel C., Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhagwagar Z., Wylezinska M., Jezzard P., et al Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 31.Hajek T., Bauer M., Pfennig A., et al Large positive effect of lithium on prefrontal cortex N-acetylaspartate in patients with bipolar disorder: 2-centre study. J Psychiatry Neurosci. 2012;37:185–192. doi: 10.1503/jpn.110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverstone PH., Wu RH., O'Donnell T., Ulrich M., Asghar SJ., Hanstock CC. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol. 2003;18:73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Friedman SD., Dager SR., Parow A., et al Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry. 2004;56:340–348. doi: 10.1016/j.biopsych.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Brambilla P., Stanley JA., Sassi RB., et al 1H MRS study of dorsolateral prefrontal cortex in healthy individuals before and after lithium administration. Neuropsychopharmacology. 2004;29:1918–1924. doi: 10.1038/sj.npp.1300520. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell T., Rotzinger S., Ulrich M., Hanstock CC., Nakashima TT., Silverstone PH. Effects of chronic lithium and sodium valproate on concentrations of brain amino acids. Eur Neuropsychopharmacol. 2003;13:220–227. doi: 10.1016/s0924-977x(03)00070-1. [DOI] [PubMed] [Google Scholar]
- 36.Moore GJ., Bebchuk JM., Parrish JK., et al Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am J Psychiatry. 1999;156:1902–1908. doi: 10.1176/ajp.156.12.1902. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH., Adler C., Norris M., et al 4-T 7Li 3D MR spectroscopy imaging in the brains of bipolar disorder subjects. Magn Reson Med. 2012;68:363–368. doi: 10.1002/mrm.24361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strawn JR., Patel NC., Chu WJ., et al Glutamatergic effects of divalproex in adolescents with mania: a proton magnetic resonance spectroscopy study. J Am Acad Child Adolesc Psychiatry. 201 2;51:642–651. doi: 10.1016/j.jaac.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yildiz-Yesiloglu A., Ankerst DP. Review of 1 H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Luykx JJ., Laban KG., van den Heuvel MP., et al Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Sanacora G., Mason GF., Rothman DL., Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002;159:663–665. doi: 10.1176/appi.ajp.159.4.663. [DOI] [PubMed] [Google Scholar]
- 42.Sanacora G., Mason GF., Rothman DL., et al Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry. 2003;160:577–579. doi: 10.1176/appi.ajp.160.3.577. [DOI] [PubMed] [Google Scholar]
- 43.Charles HC., Lazeyras F., Krishnan KR., Boyko OB., Payne M., Moore D. Brain choline in depression: in vivo detection of potential pharmacodynamic effects of antidepressant therapy using hydrogen localized spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1121–1127. doi: 10.1016/0278-5846(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 44.Sonawalla SB., Renshaw PF., Moore CM., et al Compounds containing cytosolic choline in the basal ganglia: a potential biological marker of true drug response to fluoxetine. Am J Psychiatry. 1999;156:1638–1640. doi: 10.1176/ajp.156.10.1638. [DOI] [PubMed] [Google Scholar]
- 45.Luborzewski A., Schubert F., Seifert F., et al Metabolic alterations in the dorsolateral prefrontal cortex after treatment with high-frequency repetitive transcranial magnetic stimulation in patients with unipolar major depression. J Psychiatr Res. 2007;41:606–615. doi: 10.1016/j.jpsychires.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Michael N., Erfurth A., Ohrmann P., Arolt V., Heindel W., Pfleiderer B. Metabolic changes within the left dorsolateral prefrontal cortex occurring with electroconvulsive therapy in patients with treatment resistant unipolar depression. Psychol Med. 2003;33:1277–1284. doi: 10.1017/s0033291703007931. [DOI] [PubMed] [Google Scholar]
- 47.Sanacora G., Fenton LR., Fasula MK., et al Cortical gamma-aminobutyric acid concentrations in depressed patients receiving cognitive behavioral therapy. Biol Psychiatry. 2006;59:284–286. doi: 10.1016/j.biopsych.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Michael N., Erfurth A., Ohrmann P., Arolt V., Heindel W., Pfleiderer B. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003;28:720–725. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- 49.Ende G., Braus DF., Walter S., Weber-Fahr W., Henn FA. The hippocampus in patients treated with electroconvulsive therapy: a proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry. 2000;57:937–943. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- 50.Gonul AS., Kitis O., Ozan E., et al The effect of antidepressant treatment on N-acetyl aspartate levels of medial frontal cortex in drug-free depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:120–125. doi: 10.1016/j.pnpbp.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 51.Block W., Traber F., von Widdern O., et al Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12:415–422. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- 52.Taylor MJ., Godlewska BR., Norbury R., Selvaraj S., Near J., Cowen PJ. Early increase in marker of neuronal integrity with antidepressant treatment of major depression: 1H-magnetic resonance spectroscopy of N-acetyl-aspartate. Int J Neuropsychopharmacol. 2012;15:1541–1546. doi: 10.1017/S1461145712000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salvadore G., van der Veen JW., Zhang Y., et al An investigation of aminoacid neurotransmitters as potential predictors of clinical improvement to ketamine in depression, Int J Neuropsychopharmacol. 2012;15:1063–1072. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]