Abstract
Schizophrenia is a heterogeneous psychiatric disorder of unknown cause or characteristic pathology. Clinical neuroscientists increasingly postulate that schizophrenia is a disorder of brain network organization. In this article we discuss the conceptual framework of this dysconnection hypothesis, describe the predominant methodological paradigm for testing this hypothesis, and review recent evidence for disruption of central/hub brain regions, as a promising example of this hypothesis. We summarize studies of brain hubs in large-scale structural and functional brain networks and find strong evidence for network abnormalities of prefrontal hubs, and moderate evidence for network abnormalities of limbic, temporal, and parietal hubs. Future studies are needed to differentiate network dysfunction from previously observed gray- and white-matter abnormalities of these hubs, and to link endogenous network dysfunction phenotypes with perceptual, behavioral, and cognitive clinical phenotypes of schizophrenia.
Keywords: schizophrenia, neuroimaging, connectome, network, hub
Abstract
La esquizofrenia es un trastorno psiquiátrico heterogéneo de causa o patología específica desconocidas. Los neurocientíficos clínicos postulan cada vez más que la esquizofrenia es un trastorno de la organización de la red cerebral. En este artículo se presenta el marco conceptual de la hipótesis de la desconexión, se describe el paradigma metodológico predominante para probar esta hipótesis y se revisa la evidencia reciente de una alteración de las regiones cerebrales centrales/concentradoras, como un ejemplo prometedor de esta hipótesis. Se resumen los estudios de los concentradores cerebrales en las redes cerebrales estructurales y funcionales a gran escala y se encuentra una fuerte evidencia para las alteraciones de la red de concentradores prefrontales y evidencias menores para las alteraciones de la red de concentradores límbicos, temporales y parietales. Se requiere de futuros estudios para diferenciar la disfunción de la red de las alteraciones observadas previamente en la sustancia gris y blanca de estos concentradores y relacionar fenotipos endógenos de disfunción de la red con fenotipos clínicos de perception, comportamiento y cognición en la esquizofrenia.
Abstract
La schizophrénie est un trouble psychiatrique hétérogène sans cause connue ni anatomo-pathologie caractéristique. Les neurosciences cliniques suggèrent de plus en plus que la schizophrénie est un trouble de l'organisation des réseaux cérébraux. Nous analysons dans cet article le cadre conceptuel de I'hypothèse de cette déconnexion, nous décrivons le modèle méthodologique prédominant pour vérifier cette hypothèse et nous étudions les preuves récentes d'une perturbation des régions centrales du cerveau comme exemple prometteur de cette hypothèse. Nous résumons les études sur les centres cérébraux des réseaux cérébraux anatomiques et fonctionnels à grande échelle. Les preuves d'anomalies de réseaux des centres préfrontaux sont solides ; elles le sont modérément pour les anomalies des réseaux des centres limbiques, temporaux et pariétaux. D'autres études seront nécessaires pour différencier les troubles des réseaux des anomalies déjà connues des substances grise ou blanche de ces centres, et pour établir des correspondances entre les dysfonctions endogènes des réseaux et les phénotypes cliniques cognitifs, comportementaux et perceptuels de la schizophrénie.
Schizophrenia and integration
Schizophrenia is a psychiatric disorder with an onset in early adulthood, a chronic course, and serious morbidity only modestly controlled by currently available treatments.1,2 The cause and characteristic abnormalities of schizophrenia are unknown; the disorder is thought to be underpinned by neurodevelopmental abnormalities of brain structure and function, but is onlydiagnosed using subjective criteria of psychiatric diagnostic manuals.3,4 These definitions emphasise the co-occurrence of positive/psychotic symptoms (eg, hallucinations and delusions), negative/deficit symptoms (eg, poverty of thought and loss of motivation), and cognitive symptoms (eg, impairment of memory and attention); the definitions encompass a wide range of heterogeneous presentations such that two patients with schizophrenia may share no common symptoms.5 These definitions may plausibly represent several distinct diseases and/or a spectrum of disease6; we return to this important point in the discussion, but for now we assume — as do most current investigators — that schizophrenia is a single entity.
Our current understanding of the causes of schizophrenia emphasizes interactions between diverse genetic and environmental factors.2,7 Conceptually, these diverse causes should converge on a small set of brain abnormalities pathognomonic of the disorder. Modern neuroimaging methods reveal a wide range of brain abnormalities in schizophrenia, including reductions in whole brain volume, increases in ventricular volume, reductions in frontal, temporal, limbic, and thalamic grey matter, and abnormalities in frontal and temporal white matter.8-10
Despite these promising findings, the abnormalities are insufficiently sensitive or specific to be individually or collectively diagnostic or prognostic of the disease in the clinical setting. In addition, the abnormalities have yet to be integrated into a clinically validated model of schizophrenia; such a model would for instance allow a rational approach to the search for treatment and prevention of the disorder.
Clinical neuroscientists increasingly postulate that schizophrenia is a disorder of integration of information between specialized brain regions. The emergence of complex perceptual, behavioral, and cognitive functions — the functions predominantly affected in schizophrenia — is contingent on such integration11,12; the binding of visual and other sensory stimuli into a unified perceptual whole is a well-studied instance of this phenomenon.13 Abnormality of integration hence represents an intuitive unifying hypothesis of schizophrenia. An early version of this hypothesis was posited by psychiatrists in the 19th century; modern versions of this hypothesis — including the constructs of dysconnection and cognitive dysmetria — have emerged in the last 20 years,14-19 driven by advances in neuroimaging and the consequent possibility of the study of integration in living humans. As part of the same broader trend, investigators recently proposed a roadmap towards reclassification of schizophrenia and other psychiatric disorders from entities based on subjective clinical diagnoses towards entities based on objective abnormalities of integration or brain networks.20
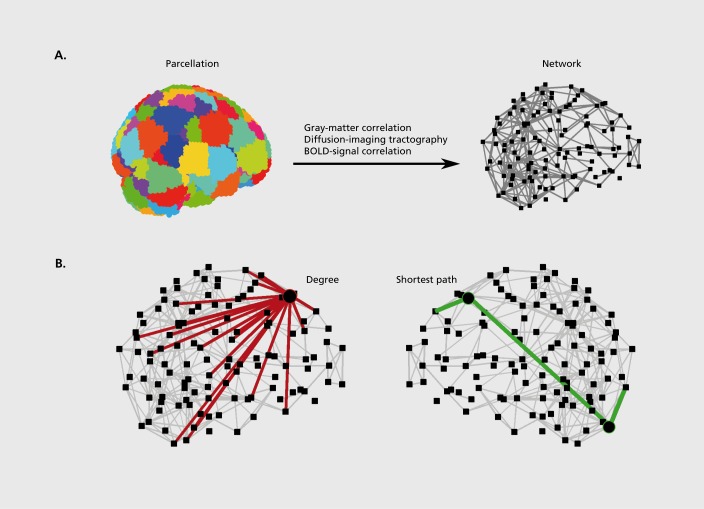
The study of integration is the study of brain networks.21 While the precise nature of integration remains an important question, neuroscientists increasingly emphasize the central role of reciprocal, distributed, and parallel interactions between brain regions, over serial and feedforward interactions in this process.22,23 Recent characterizations of large-scale structural and functional networks of the human brain broadly reveal several organizational principles supporting these properties; for instance, organization of brain networks is conducive to reciprocal interactions through a preponderance of symmetric connections and the presence of clusters; at the same time organization of brain networks is conducive to distributed and parallel interactions through the presence of high interconnectedness between most brain regions (Figure 1).24,25
Figure 1. Construction of brain networks from magnetic resonance imaging datasets and characterization of brain-network hubs. A) Brain networks are constructed by parcellation of the whole brain into nodes, and by definition of structural or functional links between these nodes. This procedure reduces the original data into a network model suitable for topological analysis (for clarity long-range connections are omitted from the present visualization). B) Measures of network centrality are most commonly based on the degree (the number of links between a node and other nodes in the network) and on the length of shortest paths (the smallest number of links connecting a pair of nodes). Hubs connect to many other nodes (in red) and have high degree. Hubs are also within close topological reach of other nodes (green path) and have a high closeness centrality or nodal efficiency. Finally, hubs lie on many short paths between other pairs of nodes (circles on green path) and have a high betweenness centrality.
An additional important property of large-scale brain network organization is the presence of central regions, or hubs. Hubs are brain regions which, by virtue of their many, diverse, strategic, or long-range connections are important in facilitating integration.26,27 Prominent hubs have been identified in prefrontal, temporal, and parietal multimodal association areas, and in limbic and subcortical areas.28-32 Abnormalities of brain hubs are increasingly implicated in brain disease32,33 and have potentially powerful explanatory capacity for a wide range of symptoms of schizophrenia. In this article we review methods used to describe hubs in large-scale brain networks and summarize recent studies which have begun to test abnormalities of these hubs in schizophrenia. Fornito et al34 comprehensively review more general properties of large-scale brain networks in schizophrenia.
Brain networks and hubs
Brain networks are maps of structural or functional interactions (termed links) between brain regions (termed nodes). The studied regions and interactions may span multiple spatial or temporal scales, although in practice the nature of these elements is limited by the spatiotemporal resolution of imaging methods. The present spatiotemporal resolution of magnetic resonance imaging (MRI) makes it the dominant method for imaging whole-brain networks; for instance, functional MRI is the only current method which allows noninvasive visualization of whole-brain networks of functional interactions, due to a reasonable trade-off between millimetre-scale spatial (node) resolution and second-scale temporal (link) resolution.35 However, it remains unclear if this resolution is sufficient for a fundamental understanding of integration, and alternative future approaches may define individual neurons as nodes,36 study structural and functional synaptic interactions in post-mortem brains37 or stem-cell-derived neuronal cultures,38 or improve spatial resolution in neurophysiological recordings39 to examine neuronal oscillations at the millisecond scale.40,41
The definition of brain networks involves the definition of nodes and links (Figure 1a). Nodes in brain networks represent structurally and functionally homogeneous brain regions. Parcellation of whole-brain MRI scans into a collection of such nodes is an active and important area of research.42 Links in brain networks represent anatomical or functional interactions. In MRI datasets, anatomical links are defined using two main methods. The method of structural correlation is based on the principle that anatomically connected regions share common trophic factors and correlate in size. This method defines links as correlations in interregional gray-matter volume or thickness inferred from a group of subjects, such that one network is constructed for the whole group.43 The method of diffusion-imaging tractography is based on the principle of anisotropic water diffusion along white-matter tracts and detects large-scale interregional anatomical links more directly such that one network is constructed for each subject.44 Functional links are defined as correlations of interregional low-frequency fluctuations in blood-oxygen-level-dependent (BOLD) signal, an indirect measure of neural activity based on concentration of oxygenated hemoglobin in brain tissue45; with this method one network is likewise constructed for each subject. These different types of connectivity are complementary and each offers distinct insights into interactions between brain regions.
The empirical study of brain networks has broadly and somewhat arbitrarily proceeded along two methodologically distinct lines of work. One line of work studies small networks of several brain regions46; the other line of work studies large networks of the whole brain.25 Investigators define small networks with methods such as seed correlation analysis47 (the detection of structurally or functionally similar neighbors for an a priori defined “seed” region) and independent component analysis48 (the delineation of the brain into a set of maximally independent small networks), and with modeling approaches.49,50 Small networks are usually associated with specific functional tasks: a classic example is of the language network associated with the comprehension and production of language51; a prominent recent example is the default network associated with internallyfocused cognition.52 Associations with functional tasks make small networks comparatively easy to interpret and the study of such networks has a long tradition in neurology.53 Nonetheless small networks may provide limited insight into characteristic abnormalities of schizophrenia if such abnormalities involve widespread disturbances of integration, as is likely to be the case. Calhoun et al54 review abnormalities of small functional brain networks in schizophrenia.
In this article we focus on large networks involving many nodes and describing the complete structural or functional maps of interregional interactions of the brain, the human connectome (Figure 1a).55,56 Large networks offer a powerful and general framework for the study of brain function in health and disease but — as a consequence of their size, nontrivial organization, and absence of associations with specific functional tasks — are more difficult to interpret. These networks are characterized with concepts from graph theory (the mathematical study of networks) and statistical physics.57 Early characterizations of these networks included computation of statistics for the propensity of networks to segregate into clusters (termed “the clustering coefficient”), the propensity of networks to be globally interconnected (termed “the characteristic path length”), and the simultaneous combination of these two properties (termed “small-worldness”), as recently reviewed.58,59
The analysis of whole-brain networks is however arguably at its most powerful when it localizes functionally distinct or functionally important brain regions solely on the basis of connection patterns associated with these regions.27 The concept of brain hubs is an example of this analysis, and is defined with measures of network centrality (Figure 1b). The archetypal measure of centrality is the degree which equals the total number of connections associated with a node. Other common measures are the closeness centrality and regional efficiency, both based on the average length of shortest paths from a node to all other nodes, the betweenness centrality, based on the fraction of all shortest paths traversing a node, and the eigenvector centrality, based on the extent with which a node is connected to important nodes in the network. Individual measures of centrality are often highly correlated, and hub nodes should score highly on several distinct measures.60
Brain hubs in schizophrenia
In this section we summarize all recent studies of abnormal hub organization in schizophrenia. These nine patient-control MRI studies have all been published in the last 5 years, and are evenly divided between structural correlation studies, diffusion-imaging tractography studies, and functional correlation studies. We summarize the main findings of these studies below and in Table I.
TABLE I. A summary of studies of hub abnormalities in schizophrenia. N, number of subjects; A, age of subjects; M, proportion of male subjects; PSS, positive-and-negative-symptom-scale positive symptoms; NSS, positive-and-negative-symptom-scale negative symptoms; AAL atlas, anatomical-automatic-labelling atias; BOLD signal, blood-oxygen-level-dependent signal.
| Study | Type of networks | Control subjects | Schizophrenia subjects | Clinical details | Definition of nodes | Definition of links | Definition of hubs | Hubs in control subjects | Hubs in schizophrenia subjects |
| Bassett et al, 200861 | Structural correlation | N: 259, A: 34±9.9, M: 0.46 | N: 203, A: 36±9.7, M: 0.75 | 81% schizophrenia, 12% schizoaffective disorder, 7% psychosis no otherwise specified PSS: 12.4±5.5, NSS: 18.4±9.2. All patients on antipsychotic medication | 104 regions: Brodmann areas, amygdala, hippocampus, striatum, and thalamus | Correlation between interregional grey-matter volume | Degree, closeness, betweenness and eigenvector centralities | Hubs in frontal association areas, insular and limbic areas | Fewer hubs in frontal areas, additional hubs in inferior temporal, primary motor, and subcortical areas |
| Zhang et al, 201262 | Structural correlation | H 101, A: 36±12, M 0.49 | N: 101, A,37±11, M: 0.5 | PSS: 13.3±4.9, NSS: 13.9±4.4. Most patients on antipsychotic medication | 78 regions based on the AAL atlas | Correlation between inter-regional grey-matter thickness | Betweenness centrality | Hubs in frontal, temporal and parietal association areas, limbic and paralimbic areas | Fewer hubs in association areas, more hubs in paralimbic areas, additional hubs in primary areas |
| Shi et al, 201263 | Structural correlation | N: 26, A: infants, M: 0.46 | N: 26, A infants, M: 0.46 | Infants of mothers with schizophrenia or schizoaffective disorder. Most mothers with schizophrenia on antipsychotic medication | 90 regions based on the AAL atlas adapted to neonatal space | Correlation between inter-regional grey-matter volume | Betweenness centrality | Hubs in frontal, temporal and parietal association areas, insular, limbic, paralimbic, primary and subcortical areas | Absence of hubs in parietal and subcortical areas, additional hubs in occipital area |
| van den Heuvel et al, 201064 | Structural white-matter | N: 40, A: 28±7.7, M: 0.73 | N: 40, A. 26.8±5.8, M: 0.75 | PSS: 15.7±5.6, NSS: 15.6±5.7 All on antipsychotic medication | 108 regions based on the AAL atlas | Diffusion-imaging tractography | Degree, closeness and betweenness centralities, clustering coefficient. | Hubs in frontal and parietal association areas, limbic and subcortical areas. | Less central hubs in frontal and limbic areas. |
| Wang et al, 201265 | Structural white-matter | N. 96, A: 37±13, M: 0.5 | N: 79, A: 37±11, M: 0.49 | PSS: 13±5, NSS: 14±4. Antipsychotic-medication status not specified | 90 regions based on the AAL atlas | Diffusion-imaging tractography | Regional efficiency | Hubs in frontal and temporal association areas, insular, limbic, paralimbic and subcortical areas | Less central hubs in frontal association areas, and in limbic, paralimbic and subcortical areas |
| van den Heuvel et al, 201366 | Structural white-matter | N: 45, A: 29±7.9, M: 0.64 | N: 4.8, A: 29±7.5, M:0.73 | 71% schizophrenia, 27% schizoaffective disorder, 21% schizophreniform disorder. PANSS total score: 63±11. Most patients on antipsychotic medication | 82 regions based on Freesurfer parcellation | Diffusion-imaging tractography | Degree centrality and rich-club coefficient | Hubs in frontal, and parietal association areas, insular area | Reduced interconnectivity between hubs in frontal and parietal association areas. |
| Bassett et al, 201267 | Functional correlation | N: 29, A: 41±11, M: 0.62 | N.29, A: 42±9.3, M: 0.62 | PSS: 6.9+3.2, NSS: 10.3±3.7. All patients on antipsychotic medication | 90 regions based on the AAL atlas, excluding cerebellum | Correlation between regional BOLD signal | Degree centrality | Hubs in temporal association areas, limbic areas, primary motor and sensory areas | Less central hubs in temporal association areas, limbic areas |
| Wang et al, 201068 | Functional correlation | N: 33, A: 27±7.6, M: 0.49 | N: 23, A: 30±9.2, M: 0.65 | 65% schizophrenia, 35% schizoaffective disorder. PANSS scores not directly specified. Most patients on antipsychotic medications | AAL atlas, number of regions not directly specified | Correlation between regional BOLD signal | Degree centrality | Hubs in frontal, parietal, temporal and occipital association areas, limbic areas | Increased number of frontal and occipital hubs, absence of temporal and limbic hubs |
| Alexander-BIoch et al, 201369 | Functional correlation | N: 20, A: 19±4.9, M: 0.5 | N: 19, A: 19±4.9, M: 0.47 | Childhood-onset schizophrenia. PANSS scores not directly specified All patients on clozapine | ~ 300 regions based on the Harvard-Oxford atlas and cerebellar probabilistic atlas | Correlation between regional BOLD signal | Degree and anatomical distance | Hubs in frontal, temporal and parietal association areas | More central hubs in frontal and parietal association areas |
Three studies examine hubs in structural correlation networks of patients with schizophrenia.61-63 Two of these studies61,62 construct networks from structural images of large cohorts of middle-aged subjects with schizophrenia and detect less central hubs in frontal and limbic association areas in schizophrenia; one study62 additionally detects hubs in paralimbic areas in healthy controls, and an increased number of these hubs in schizophrenia. The third study63 constructs correlation networks from structural images of a smaller cohort of neonates born to mothers with schizophrenia, and hence at high risk of developing the disorder. This study finds hubs in frontal, temporal, parietal, and subcortical areas in healthy control neonates, and a reduction in the total number of hubs with an absence of parietal and subcortical hubs in high-risk neonates.
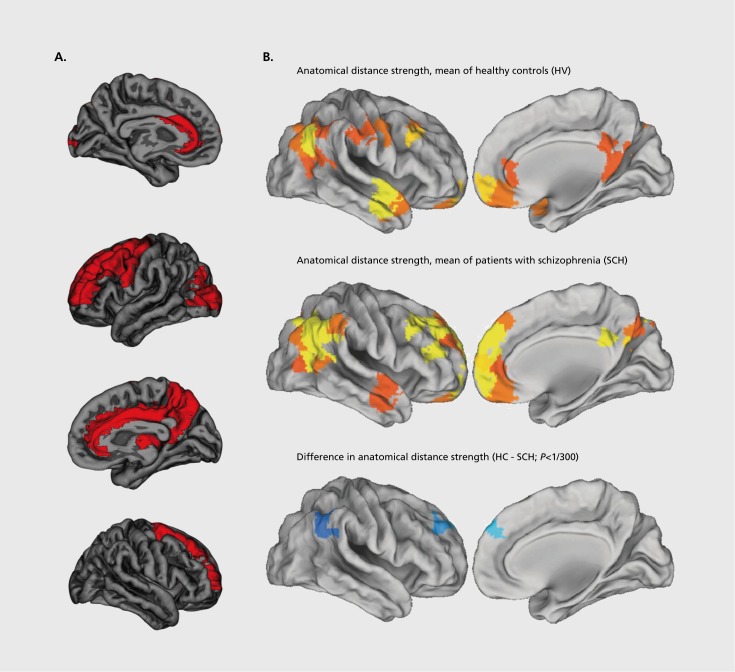
Three studies examine hubs in diffusion-imaging tractography networks of patients with schizophrenia.64-66 These studies find hubs in frontal and parietal association areas, as well as in limbic, paralimbic, and subcortical areas. All three studies find less central hubs in frontal association areas. Two studies64,65 additionally find less central hubs in limbic areas (Figure 2), while the third study66 extends the simple description of hubs and describes the pattern of interconnections between individual hubs as part of a so-called “rich club,” a small group of high-degree nodes which are additionally highly connected to each other. The study finds a weaker rich club in schizophrenia, reflecting a lower level of connectivity between hubs of schizophrenia subjects.
Figure 2. Abnormalities of brain hubs in schizophrenia. A) Less central hubs in the superior frontal gyrus and anterior cingulate cortex (in red) in structural white-matter networks of patients with schizophrenia. Reproduced from ref 64: van den Heuvei MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915-15926. Copyright © Society for Neuroscience 2010 B) Long-distance-connection hubs in functional networks (bright yellow) of healthy controls (top panel) and subjects with childhood-onset schizophrenia (middle panel). In both groups, hubs are present in anterior medial frontal lobe, dorsolateral prefrontal cortex, inferior lateral parietal cortex, and lateral temporal lobe. Hubs are more central (blue) in dorsolateral prefrontal and inferior lateral parietal cortices of subjects with childhood-onset schizophrenia. Reproduced from ref 69: Alexander-Bloch AF, Vertes PE, Stidd R, et al. The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb Cortex. 2013;23:127-138. Copyright © Oxford University Press 2013.
Three studies examine hubs in functional correlation networks of patients with schizophrenia.67-69 Two of these studies examine cohorts of middle-aged subjects and find less central hubs in temporal and limbic association areas67 or in frontal, temporal, limbic, and occipital association areas.68 The third study69 examines a group of adolescent subjects with childhood-onset schizophrenia, a rare and severe form of schizophrenia. This studyfocuses on the relationship between connection weight and anatomical distance, and finds hubs in frontal, temporal, and parietal association areas, and a greater proportion of long distance connections between hubs in the schizophrenia group (Figure 2b).
Discussion and conceptual issues
The above body of work broadly implicates abnormalities of association hubs and limbic hubs in schizophrenia. More specifically, the studies strongly implicate abnormalities of prefrontal hubs (9/9 studies) and moderately implicate abnormalities of limbic (6/9 studies), temporal (6/9 studies) and parietal (5/9 studies) hubs. The evidence points to a decreased centrality of these hubs in schizophrenia, at least in studies with adult populations.
The involvement of multimodal and limbic association areas has a relatively straightforward neurobiological interpretation in schizophrenia; these transmodal areas of the cerebral cortex are important in facilitating brain functions most visibly impaired in the disorder. Such functions include the integration or binding of distinct perceptual stimuli into complex unified representations, the cognitive and emotional contextualization of these representations, and higher cognitive functions such as executive control and working memory.11 In addition, the relatively late white-matter myelination and neuronal pruning of areas in multimodal cortex71 suggests that inherent neurodevelopmental abnormalities of these areas may only symptomatically manifest in adolescence or early adulthood, a common age of onset for schizophrenia70.
Despite this seemingly straightforward interpretation, there inevitably remain empirical and conceptual questions. Empirically, previous studies have already reported schizophrenia-associated reductions in gray matter in all presently implicated hubs, as well as abnormalities in white-matter tracts connecting most of these hubs8-10; indeed two of the present studies directly examine the relationship between regional gray-matter volumes and regional network centrality, and report substantial associations between these two properties.62,68 Hence, while the present body of work builds on previous studies to present a more global-network view of hub disorganization, the pathogenetic precedence of gray- or white-matter abnormalities and global network disorganization remains undetermined. On the one hand, it is simpler to consider the emergence of global network dysfunction following local abnormalities of gray and white matter. On the other hand, it is simpler to formulate a unitary model of schizophrenia based on the notion of abnormal disruption of integration and hubs, rather than on the notion of multiple focal lesions. For instance, one study69 reports that brain hubs have longer-distance and more metabolically costly functional connections; this arrangement implies that hubs are likely to be more susceptible to metabolic insult, and provides a conceptually straightforward potential pathogenetic mechanism.
An additional empirical question concerns the specificity of hub disruption as a characteristic phenotype of schizophrenia. It is possible that the same hubs are implicated in many other psychiatric and neurological disorders,32,33 making it difficult to associate endogenous phenotypes of hub dysfunction with perceptual, behavioural and cognitive clinical phenotypes of schizophrenia. Other empirical concerns include the absence of a standard methodological framework for the construction and characterization of brain networks, and low statistical power associated with some studies, resulting in potential for bias and inconsistent findings.72-74
Conceptually, the schizophrenia dysconnection hypothesis is hampered by the imprecision of both the notion of schizophrenia, and the notion of dysconnection. Neuroscientists commonly motivate the dysconnection hypothesis by invoking its long history dating back to psychiatrists in the 19th century. Yet there is no clear link between the work of early psychiatrists and present research75 nor would the presence of such a link imply conceptual validity. Schizophrenia remains a heterogeneous and poorly defined disorder, to the extent that a search for a single characteristic abnormality may be an a priori futile task. Brain networks are likewise heterogeneous, and the spatiotemporal resolution on which characteristic abnormalities of schizophrenia optimally manifest remains undetermined. The sceptically inclined may hence describe the dysconnection hypothesis of schizophrenia as an attempt at explanation of one imprecise concept with another.
In this context there is possibility for systematic progress if the constructs of schizophrenia and brain networks are both sufficiently close approximations to real and coherent entities. Progress may occur for instance through a series of iterative and mutual conceptual modifications of both constructs. In the case of schizophrenia, increased coherence may be achieved through a focus on more specific forms of the disorder, such as paranoid (primarily psychotic-symptom) and disorganized (primarily deficit-symptom) subtypes,3,4 or the focus on forms of the disorder with a seemingly higher genetic component, such as childhood-onset schizophrenia76 or 22qll.2 deletion syndrome, a genetic syndrome associated with a high occurrence of schizophrenia.77 In the case of brain networks, increased coherence is likely to follow from increasing spatial and temporal resolution associated with future methodological innovations. We hope that these developments will eventually lead to a substantial clarification in our understanding of schizophrenia.
Conclusion
There is now considerable conceptual and empirical evidence for the importance of network integration in healthy brain function, for the importance of topologically central nodes or hubs in brain network integration, and for abnormalities of both integration and hubs in schizophrenia. Despite this we will not be able to claim conclusively that schizophrenia is a disease of brain hubs, a hubopathy, until future studies have consolidated the preliminary findings based mainly on small- to medium-sized samples; resolved some of the discrepancies between functional and structural network phenotypes of schizophrenia; clarified how abnormal network hubs might emerge developmentally and in the context of growing awareness of the role of synaptic and postsynaptic risk alleles in the genetic predisposition to schizophrenia; and established the specificity of hub abnormalities in schizophrenia compared with other brain disorders. There is much still to do to substantiate and contextualize the new results arising from complex network analysis of the schizophrenia connectome. However, we suggest that the basic insight that brain network hubs may be central to the systems-level pathophysiology of schizophrenia is at least likely to prove heuristically valuable as we continue to make progress in understanding the neurobiological basis of psychotic disorders.
Acknowledgments
MR is supported by the NARSAD Young Investigator Grant and the Isaac Newton Trust. ETB is employed part-time by GlaxoSmithKline and part-time by the University of Cambridge. The Behavioural and Clinical Neuroscience Institute is supported by the Wellcome Trust and the Medical Research Council.
Contributor Information
Mikail Rubinov, Author affiliations: Brain Mapping Unit; Behavioural and Clinical Neuroscience Institute; Department of Psychiatry, University of Cambridge, UK; Churchill College, University of Cambridge, UK..
Ed. Bullmore, Brain Mapping Unit; Behavioural and Clinical Neuroscience Institute; Department of Psychiatry, University of Cambridge, UK; Clinical Unit, GlaxoSmithKline, Addenbrooke's Centre for Clinical Investigations, Cambridge, UK; Cambridgeshire & Peterborough NHS Foundation Trust, Cambridge, UK..
REFERENCES
- 1.van Os J., Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 2.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.4th ed. Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 4.World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders. Clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992 [Google Scholar]
- 5.Andreasen NC. A unitary model of schizophrenia: Bleuler's “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 6.Jablensky A. The diagnostic concept of schizophrenia: its history, evolution, and future prospects. Dialogues Clin Neurosci. 2010;12:271–287. doi: 10.31887/DCNS.2010.12.3/ajablensky. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Os J., Kenis G., Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 8.Ellison-Wright I., Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Shenton ME., Whitford TJ., Kubicki M. Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues Clin Neurosci. 2010;12:317–332. doi: 10.31887/DCNS.2010.12.3/mshenton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 12.Varela F., Lachaux JP., Rodriguez E., Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 13.Robertson LC. Binding, spatial attention and perceptual awareness. Wat. Rev Neurosci. 2003;4:93–102. doi: 10.1038/nrn1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friston KJ., Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 15.Bullmore ET., Frangou S., Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- 16.Andreasen NC., Paradiso S., O'Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcorticalcerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 17.Peled A. Multiple constraint organization in the brain: a theory for schizophrenia. Brain Res Bull. 1999;49:245–250. doi: 10.1016/s0361-9230(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 18.Tononi G., Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Res Brain Res Rev. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 19.Stephan KE., Baldeweg T., Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Insel T., Cuthbert B., Garvey M., et al Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 21.Sporns O. Networks of the Brain. Cambridge, Mass: MIT Press; 2011 [Google Scholar]
- 22.Calvert GA., Thesen T. Multisensory integration: methodological approaches and emerging principles in the human brain. J Physiol Paris. 2004;98:191–205. doi: 10.1016/j.jphysparis.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Ghazanfar AA., Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci. 2006;10:278–285. doi: 10.1016/j.tics.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Sporns O., Chialvo DR., Kaiser M., Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 26.Bullmore E., Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 27.Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol. 2013;23:162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Achard S., Salvador R., Whitcher B., Suckling J., Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagmann P., Cammoun L., Gigandet X., et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole MW., Pathak S., Schneider W. Identifying the brain's most globally connected regions. Neuroimage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Tomasi D., Volkow ND. Functional connectivity density mapping. Proc. Natl Acad Sci USA. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckner RL., Sepulcre J., Talukdar T., et al Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Achard S., Delon-Martin C., Vertes PE., et al. Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci USA. 2012;109:20608–20613. doi: 10.1073/pnas.1208933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fornito A., Zalesky A., Pantelis C., Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 35.Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Wature. 2010;468:194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- 36.Lewis DA., Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung K., Wallace J., Kim SY., et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennand KJ., Simone A., Jou J., et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassett DS., Bullmore ET., Meyer-Lindenberg A., Apud JA., Weinberger DR., Coppola R. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci USA. 2009;106:11747–11752. doi: 10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlhaas PJ., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 41.Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr Opin Neurobiol. 2013;23:283–290. doi: 10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Behrens TE., Sporns O. Human connectomics. Curr Opin Neurobiol. 2012;22:144–153. doi: 10.1016/j.conb.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander-Bloch A., Giedd JN., Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–336. doi: 10.1038/nrn3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 45.Fornito A., Bullmore ET. What can spontaneous fluctuations of the blood oxygenation-level-dependent signal tell us about psychiatric disorders? Curr Opin Psychiatry. 2010;23:239–249. doi: 10.1097/YCO.0b013e328337d78d. [DOI] [PubMed] [Google Scholar]
- 46.Fox MD., Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 47.Fox MD., Snyder AZ., Vincent JL., Corbetta M., Van Essen DC., Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Damoiseaux JS., Rombouts SA., Barkhof F., et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mcintosh AR., Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2:2–22. [Google Scholar]
- 50.Friston KJ., Harrison L., Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 51.Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- 52.Buckner RL., Andrews-Hanna JR., Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 53.Catani M., ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128(Pt 10):2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 54.Calhoun VD., Eichele T., Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sporns O., Tononi G., Kotter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biswal BB., Mennes M., Zuo XN., et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newman MEJ. xx. Networks: an Introduction. Oxford, UK: New York, NY: Oxford University Press; 2010 [Google Scholar]
- 58.Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Bullmore ET., Bassett DS. Brain graphs: graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- 60.Sporns O., Honey CJ., Kotter R. Identification and classification of hubs in brain networks. PLoS One. 2007;2:e1049. doi: 10.1371/journal.pone.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bassett DS., Bullmore E., Verchinski BA., Mattay VS., Weinberger DR., Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y., Lin L., Lin CP., et al. Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res. 2012;141:109–118. doi: 10.1016/j.schres.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Shi F., Yap PT., Gao W., Lin W., Gilmore JH., Shen D. Altered structural connectivity in neonates at genetic risk for schizophrenia: a combined study using morphological and white matter networks. Neuroimage. 2012;62:1622–1633. doi: 10.1016/j.neuroimage.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van den Heuvel MP., Mandl RC., Stam CJ., Kahn RS., Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–15926. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q., Su TP., Zhou Y., et al. Anatomical insights into disrupted smallworld networks in schizophrenia. Neuroimage. 2012;59:1085–1093. doi: 10.1016/j.neuroimage.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 66.vandenHeuvel MP., Sporns O., Collin G., et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- 67.Bassett DS., Nelson BG., Mueller BA., Camchong J., Lim KO. Altered resting state complexity in schizophrenia. Neuroimage. 2012;59:2196–2207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang L., Metzak PD., Honer WG., Woodward TS. Impaired efficiency of functional networks underlying episodic memory-for-context in schizophrenia. J Neurosci. 2010;30:13171–13179. doi: 10.1523/JNEUROSCI.3514-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexander-Bloch AF., Vertes PE., Stidd R., et al The anatomical distance of functional connections predicts brain network topology in health and schizophrenia. Cereb Cortex. 2013;23:127–138. doi: 10.1093/cercor/bhr388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lynall ME., Bassett DS., Kerwin R., et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ross CA., Pearlson GD. Schizophrenia, the heteromodal association neocortex and development: potential for a neurogenetic approach. Trends Neurosci. 1996;19:171–176. doi: 10.1016/s0166-2236(96)10022-9. [DOI] [PubMed] [Google Scholar]
- 72.loannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carp J. The secret lives of experiments: methods reporting in the fMRI literature. Neuroimage. 2012;63:289–300. doi: 10.1016/j.neuroimage.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Button KS., loannidis JP., Mokrysz C., et al. Power failure: why small sample size underminesthe reliability of neuroscience. Wat. Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 75.Berrios GE., Luque R., Villagran JM. Schizophrenia: a conceptual history. Int J Psychol Psychol Ther. 2003;3:111–140. [Google Scholar]
- 76.Asarnow JR. Annotation: childhood-onset schizophrenia. J Child Psychol Psychiatry. 1994;35:1345–1371. doi: 10.1111/j.1469-7610.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 77.Bassett AS., Chow EW. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry. 1999;46:882–891. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]