Abstract
The brain's default network is a set of regions that is spontaneously active during passive moments. The network is also active during directed tasks that require participants to remember past events or imagine upcoming events. One hypothesis is that the network facilitates construction of mental models (simulations) that can be used adaptively in many contexts. Extensive research has considered whether disruption of the default network may contribute to disease. While an intriguing possibility, a specific challenge to this notion is the fact that it is difficult to accurately measure the default network in patients where confounds of head motion and compliance are prominent. Nonetheless, some intriguing recent findings suggest that dysfunctional interactions between front-oparietal control systems and the default network contribute to psychosis. Psychosis may be a network disturbance that manifests as disordered thought partly because it disrupts the fragile balance between the default network and competing brain systems.
Keywords: functional MRI, default mode network, resting-state, prospection
Abstract
La red cerebral por defecto corresponde a un conjunto de regiones que se activa espontáneamente durante los momentos de reposo. Cuando los participantes requieren recordar hechos pasados o imaginar eventos futuros mediante tareas dirigidas la red también se activa. Una hipótesis es que la red facilita la construcción de modelos mentales (simulaciones) que pueden emplearse de manera adaptativa en muchos contextos. Existe abundante investigación que ha considerado que la alteración de la red por defecto puede contribuir a la patología. Si bien esta idea constituye una posibilidad atractiva, un desafío específico es la dificultad para medir con precisión la red por defecto en pacientes en que los movimientos de la cabeza y el acatamiento son importantes factores de confusión.
Sin embargo, algunos motivadores hallazgos recientes sugieren que las interacciones disfuncionales entre los sistemas de control frontoparietal y la red por defecto contribuyen a las psicosis. Las psicosis pueden ser una alteración de red que se manifieste como un trastorno del pensamiento, en parte porque perturba el frágil balance entre la red por defecto y los sistemas cerebrales que compiten.
Abstract
Le réseau cérébral par défaut est un ensemble de régions spontanément actives pendant les moments de repos. Le réseau est également actif lors de tâches dirigées qui demandent aux participants de se remémorer des événements anciens ou d'imaginer des événements à venir. Une des hypothèses est que le réseau facilite la construction de modèles mentaux (simulations) pouvant s'adapter à de nombreux contextes. La responsabilité éventuelle d'une interruption de ce réseau par défaut dans la maladie a été étudiée au cours de recherches poussées. Si cette hypothèse est intéressante, elle présente un problème spécifique car la mesure précise de l'activité du réseau par défaut est limitée par des facteurs d'erreur comme la compliance des patients ou leur difficulté à maintenir une position stable de la tête. Cependant, d'après des résultats récents passionnants, une dysfonction des interactions entre des systèmes de contrôle frontopariétaux et le réseau par défaut participe à la psychose. La psychose pourrait être une perturbation d'un réseau se manifestant par une désorganisation de la pensée, en partie parce que le fragile équilibre entre le réseau par défaut et des systèmes cérébraux rivaux est rompu.
Introduction
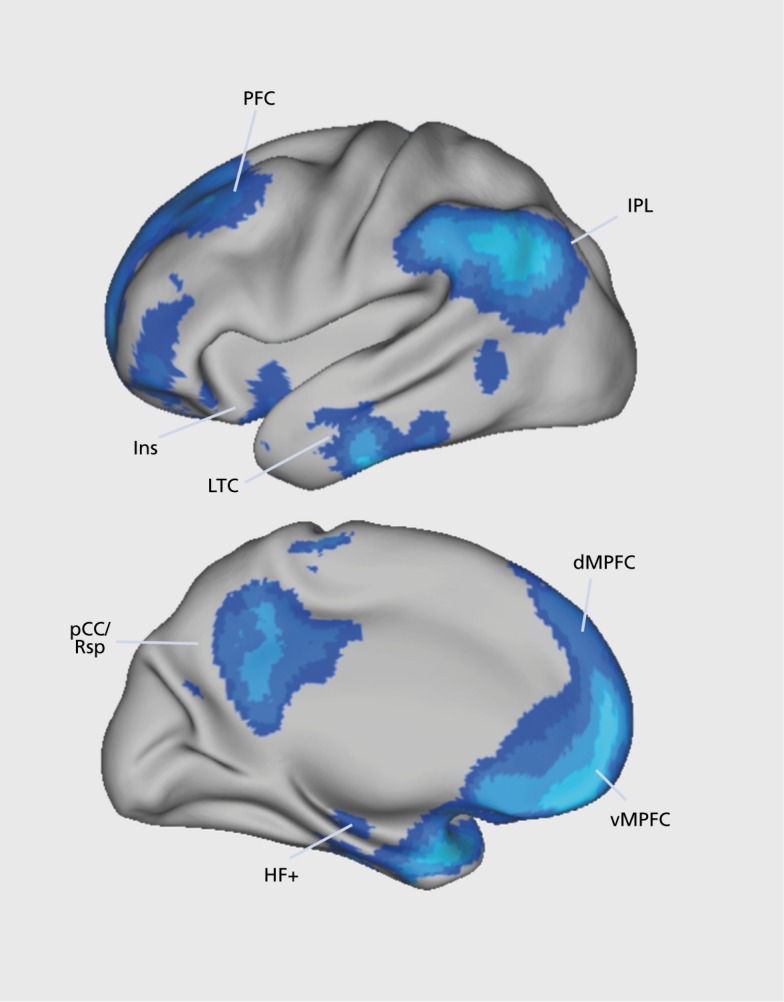
Considerable recent attention has been given to the brain's default network.1-5 The default network, illustrated in Figure 1, is a set of brain regions that are active in resting subjects compared with when they perform engaging externally oriented tasks.6,7 The term “default” arose from the discovery of the network's heightened activity during idle periods, implying that people's brains default to using the network when an externally directed task is not provided. The term, however, is a misnomer. The default network is also active during directed tasks, such as remembering one's past or thinking about what might happen in the future.8-10 By examining regions that are active in the passive individual, we may have stumbled upon the core network responsible for internal modes of cognition. One working hypothesis is that the default network's primary function is to support internal mental simulations that are used adaptively.3,5 From this perspective, the network can be engaged in a directed manner, such as recalling the location of a parked car, and also when the mind wanders from the immediate task at hand.
Figure 1. The brain's default network. The default network was discovered serendipitously when experimenters using neuroimaging began examining brain regions active in the passive control conditions of their experiments. The image shows brain regions more active in passive tasks as contrast to a wide range of simple, active task conditions.6 The network includes a distributed set of regions that involve association cortex and paralimbic regions but spare sensory and motor cortex. The challenge has been to understand the functional importance of the network. One possibility is that the network is suppressed in some manner by the active task conditions. Another possibility is that spontaneous cognitive processes that become dominant during passive moments rely on the default network. PFC, prefrontal cortex; Ins, insula, IPL, inferior parietal lobule, LTC, lateral temporal cortex; pCC/Rsp, posterior cingulate/retrosplenial cortex; HF+, the extended hippocampal formation; dMPFC, dorsal medial prefrontal cortex; vMPFC, ventral medial prefrontal cortex. Adapted from ref 6: Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II.: decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648-663. Copyright © MIT Press 1997.
The purpose of this article is to discuss how the concept of the default network has evolved since its discovery and how research on the default network might elucidate neuropsychiatry and neurological disease. A historical orientation is included, because a current snapshot of the literature on the topic reveals a complex collection of observations and loosely tied ideas. One would hope that, by starting from the beginning, the central issues will become clearer to the student or clinician interested in the topic. As such, this piece is not a comprehensive review, so it is recommended that readers explore several related reviews for a more thorough analysis.3-5 For those interested in a detailed historical account see refs 11 and 1211,12. The present piece will be especially useful to readers interested in a broad understanding of how the concept of the default network arose and how its discovery relates to contemporary research emphases.
Origins of discovery and implications
The default network was discovered serendipitously when investigators began noticing that specific, reproducible brain regions were more active during passive control tasks than during active tasks targeted by the experimenters.6,7 In many instances, responses in the passive (control) tasks were not reported, or were reported with minimal discussion. In one of our first studies of memory we noticed that a broad network of regions was active in the passive control task, during which participants simply fixated a crosshair. However the network was paradoxically less active in the targeted task, in which participants generated words.13 In an insightful anticipation of later work on the default network, Andreasen et al observed that passive tasks showed activation in regions that were also active when individuals recalled information from episodic memory.8 In an intentionally ironic twist, they labeled the passive “rest” condition “Random Episodic Silent Thinking” and suggested that “free-ranging mental activity (random episodic memory) produces large activations in association cortex and may reflect both active retrieval of past experiences and planning of future experiences.” They further argued that the regions involved were specifically regions of association cortex that “are more highly developed (ie, comprise a larger portion of the brain volume) in human beings than in nonhuman primates or other animals, have the most complex columnar cortical organization, and are the last to myelinate. Apparently, when the brain/mind thinks in a free and unencumbered fashion, it uses its most human and complex parts” (p1583).
The manner in which the default network was initially identified has had a lasting impact on how we think about its function and discuss the phenomena associated with the network. In typical task settings, the default network is most active in passive control tasks where the experimenter's demands required are minimized. The observation that the default network is active in passive tasks has led to a split in ideas about its functions. In one class of ideas, the network is seen as playing a role in the exploratory, unfocussed state that takes place during passive tasks. Passive task states differ from active task states in that they do not require attention to specific behaviorally relevant features of the external environment. This has led some to suggest that regions involved in the default network are actually suppressed by the active task. In other words, the appearance of increased activity in the passive task is really better conceived as a suppression of activity by the active task.
In a thoughtful description of one such form of hypothesized suppression, Corbetta and colleagues14 proposed that “deactivation” of certain regions overlapping the default network may be party caused by high tonic activity associated with the locus coeruleus/noradrenergic system. The locus coeruleus is a small midbrain nucleus that modulates cortical and subcortical brain activity through diffuse excitatory monoaminergic (norepinephrine) projections. During task-focused states, a decrease in tonic activity of the locus coeruleus to moderate levels, combined with an increase in task-locked transient activity, may promote optimal engagement in the immediate task.15 During passive task states, the system is characterized by a high tonic baseline. Deactivation of certain regions within the default network may be linked to activity modulation of the locus coeruleus as a mechanism of modulating the locus of attention.
It has been difficult to rule out the possibility that certain networks are actively suppressed by focused, attentionally demanding tasks and further that such suppression is the central cause of the observation of a “default network.” Adding a further complexity, studies of the monkey using intrinsic optical imaging of visual cortex suggest that anticipatory arousal can modulate blood flow (the basis of positron emission tomography, [PET], and functional MRI [fMRI] measures) via neuronal mechanisms that are distinct from the transient activity modulations, which are the target of task-based neuroimaging studies.16 While it is unclear how such a physiological observation relates to the default network observed in the human imaging studies, the observation of a sustained anticipatory signal raises the possibility of a class of attentional effects that are insufficiently understood and that may be the source of the default network's activity pattern during passive task states. Nonetheless, there is a favorably-alternative hypothesis that extends the ideas of Andreasen and colleagues.8
Internal mentation
During passive moments, when demands to engage the external environment are relaxed, the mind wanders.17,18 Self-report data from neuroimaging tasks that activate the default network reveal that mind wandering and spontaneous thoughts occur frequently.8,19,20 When probed, the participants report that they are often thinking about future plans or about recent personal events. Rarely do they report attending to stimuli in the environment. Imagined events tend to be practical and free of fantasy-like qualities. For example, during passive fixation one individual noted thinking about “events that happened during the weekend [and] what's for dinner,” whereas another reported, “Well, I am moving in two days, so I find myself writing mental “to do” lists and lists of things I had still to pack, and also just imagining life in a new apartment, new city, etc.”20 Killingsworth and Gilbert21 assessed the frequency of spontaneous mental thoughts in everyday life by using cell phones to probe participants at random times. They found that people's minds wander frequently, and do so during almost all activities. Spontaneous thoughts associated with mind wandering are pervasive in the laboratory and outside in the real world.
These observations lead to an interesting class of ideas: the brain's default network may be the collection of brain regions that, on average across people and over time, are most active during internal modes of cognition. The network of regions implicated in the default network are functionally22-24 and anatomically (see ref 4) linked to limbic structures including the parahippocampal cortex, suggesting a circuit that has access to mnemonic information. Within this possibility, the default network is proposed to support the construction of internal mental models based on mnemonic (limbic) systems. This simple idea may explain the common observation of increased activity in the default network during passive tasks when the mind is released to wander, as well as during active cognitive tasks when subjects are instructed to remember the past or mentally plan for a hypothetical future event (Figure 2).9,10,25 Thus, the serendipitous discovery of the default network during passive tasks and the origins of its name as the ”default“ network only partly captures its broad functions, which may extend to a range of internal modes of cognition.
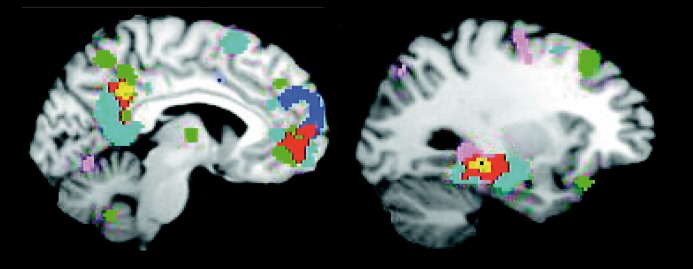
Figure 2. Remembering, thinking about the future, navigation, and theory of mind activate the default network. Images from a meta-analysis of tasks that require individuals to mentally project themselves into an alternative setting.10 Red and yellow represent overlap of at least two forms of task. The meta-analysis reveals that nodes of the default network are active during many forms of task where the participants must construct mental models of personally significant events. Differences do exist between task forms that are not emphasized by this display. One hypothesis is that the default network is important to many forms of active cognition but was serendipitously discovered during passive task states in the early years of human neuroimaging.11 Adapted from ref 10: Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489-510. Copyright © MIT Press 1997.
An interesting recent twist to the hypothesis that the default network supports certain forms of internally generated thought has proposed a relation to the locus coeruleus/noradrenergic system. As mentioned earlier, passive task states are associated with high tonic levels of locus ceoruleus activity. By contrast, focused tasks are associated with moderate tonic levels of locus ceoruleus activity with phasic responses time-locked to components of the task trials. Using measures of pupil diameter, which indirectly reflect locus ceoruleus activity when light responses are controlled, Smallwood and colleagues26 inferred that spontaneous thoughts arise most frequently during high tonic levels of locus ceoruleus activity. This is an interesting observation for two separate reasons. First, the observation suggests that spontaneous thoughts occur in an attentional state that is distinct from the modulatory pattern prominent during externally focused tasks (moderate tonic activity with task-locked transients). Second, the spontaneous thoughts occur during an aroused state. Low tonic locus ceoruleus activity characterizes drowsy, inattentive states.15 Smallwood et al's results suggest that spontaneous thoughts are linked to high tonic levels of activity.26 A speculative possibility is that default network activity could be an aroused state where cortical activity is not tuned to a specific set of temporally discrete task epochs but rather to internally generated cognitive operations that frequently occur, and are largely untethered to external perceptual events. While this state was discovered in passive task epochs, its role in internally directed modes of cognition is much broader.
Implications for study of disease
The default network is an interesting target for clinical exploration.3,27-29 Many psychiatric disorders are hallmarked by disturbances to internal modes of thought or impairment in remembering. Both sets of functions are associated with the default network. The link to these functions and the ease of making measurements of the default network have led to numerous reports of default network disruption across a wide range of conditions including schizophrenia, bipolar disorder, Alzheimer's disease, depression, autism, and others. At first glance disruption of the default network seems to be a nonspecific correlate of brain dysfunction. Alternatively, measurement of the default network may be confounded in ways that create an appearance of disturbance.
Many reported results about default network dysfunction in the literature may be due to confounding factors. For example, motion and respiratory artifacts have been demonstrated to alter functional connectivity measures of the default network.30-32 Patients often move their heads more than controls in brain imaging studies, and also may display differences in breathing patterns, eye movements, and swallowing that can affect data quality. A concern is that many of the patient findings reported in the literature are artifacts.33,34 We will need to undertake a process of sorting out what is artifact and what is insight. Nonetheless, studies paying careful attention to potential confounding influences have made observations that suggest a central role of the default network in mental illness.
The study of psychosis offers an intriguing clinical example of default network dysfunction. Among other symptoms, active psychosis is associated with disorganized thought patterns. A recent study from Whitfield-Gabrieli and colleagues35 found that patients with schizophrenia display a hyperactive default network and aberrant connectivity of the default network. Combined with other results,29,36 they suggested that default network dysfunction may be associated with the positive symptoms of schizophrenia. The idea stems from the default network's hypothesized contributions to internal modes of cognition. They noted that “constant overengagement of the default network could lead to an exaggerated focus on one's own thoughts and feelings as well as an ambiguous integration between one's own thoughts and feelings with events in the environment.”35
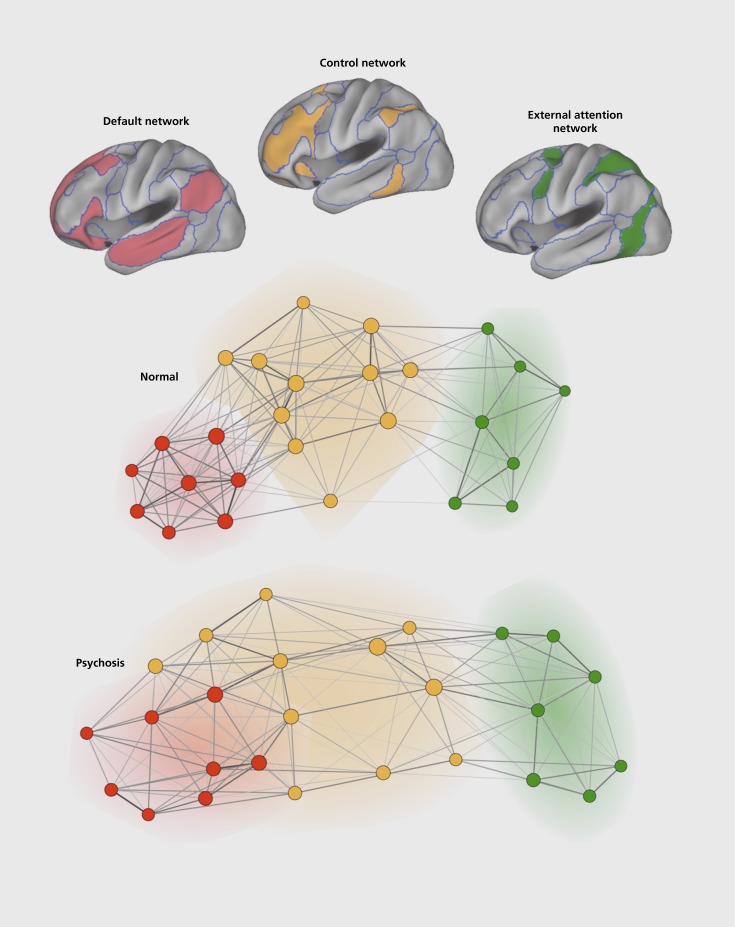
Schizophrenia has long been linked to disruption of frontally mediated control systems.37-40 How might the observation of control system disruption and disturbance of the default network be linked? One possibility is that control systems integrate the functions of the default network, which is primarily concerned with internal modes of cognition, with competing information supplied by networks linked to external attention. The frontoparietal control network,41,42 illustrated in Figure 3, is anatomically juxtaposed between the default network and networks that contribute to external attention. Dysfunction in psychosis may impact control networks and disrupt coordination between the default network and networks important to processing perceptual information from the external world.
Figure 3. (Opposite) Network dysfunction in psychosis. Brain function and dysfunction can be examined by exploring how different brain networks interact. The top panel illustrates three networks that include the default network (red), a frontoparietal control network thought to be important to executive control (orange), and an external attention network hypothesized to guide attention and actions toward external sensory stimuli, often called the dorsal attention network (green). In the middle row, the circles and lines represent a graphical representation of the relationship of the brain regions involved in all three networks. Each circle is a brain region and the line thickness between the circles represents the functional correlation strength between the regions. Regions that are strongly functionally coupled are plotted near to one another. What emerges in normal control subjects (n=100) is that regions within each network are tightly functionally coupled and distinct from the regions of the other networks. One hypothesis is that the frontoparietal control network regulates the interactions between the default network and external attention network. When the same analysis is applied to psychotic patients (n=100 including schizophrenia, schizoaffective disorder, and bipolar disorder with psychosis), the network interactions display an interesting difference: the frontoparietal control network shows a less modular structure and a less rigid boundary with the default network. Adapted from ref 43: Baker JT, Holmes AJ, Masters GA, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. In press. Copyright © American Medical Association.
In a recent study we directly explored the possibility that control system dysfunction may be linked to default network abnormalities by examining interactions between the frontoparietal control network and the default network43 (Figure 3). Using functional connectivity, 100 healthy control participants were compared with 100 psychotic patients (with schizophrenia, schizoaffective disorder, or bipolar disorder with psychosis). We discovered that functional connectivity between networks is different in the patients such that the networks possess less modular organization. The frontoparietal network, in particular a subnetwork of the frontoparietal network linked to the highest orders of executive control, showed altered coupling to other networks. Suggesting that the differences were a correlate of illness, the patterns linked to psychosis could not be mimicked in control participants, even when the data were degraded by head motion, although it is not possible to fully rule out more subtle confounds. Others have also recently observed network-wide differences in psychosis with some features shared between schizophrenia and bipolar disorder and other features unique to schizophrenia.44,45 In a particularly thorough study of a large sample of patients with schizophrenia, altered functional connectivity was found for both the frontoparietal control network and default network.44
It is difficult to decisively interpret these collective results, but it is intriguing that normal network interactions break down in psychotic patients in a manner that might blur the boundary between imagination and reality.3,35 Psychosis may be a network disturbance46 that manifests as disordered thought partly because it disrupts the fragile balance between processing systems that operate on external and internal channels of information.
Conclusions
The brain's default network is a set of regions more active during passive tasks than tasks demanding focused external attention. One hypothesis is that the default network contributes to internal modes of cognition used when remembering, thinking about the future, and mind wandering. An open question is whether dysfunction of the default network contributes to neurological and psychiatric illness. A specific challenge is that it is difficult to accurately measure the default network in patients where confounds of head motion and compliance are prominent. Several observations suggest that disruption in executive control processes may impact the function of the default network and contribute to disturbances of thought.
REFERENCES
- 1.Raichle ME., MacLeod AM., Snyder AZ., Powers WJ., Gusnard DA., Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gusnard DA., Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 3.Buckner RL., Andrews-Hanna JR., Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 4.Binder JR., Desai RH., Graves WW., Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shulman GL., Fiez JA., Corbetta M., et al Common blood flow changes across visual tasks: II.: decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 7.Mazoyer B., Zago L., Mellet E., et al Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 8.Andreasen NC., O'Leary DS., Cizadlo T., Arndt S., Rezai K. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL., Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Spreng RN., Mar RA., Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 11.Buckner RL. The serendipitous discovery of the brain's default network. NeuroImage. 2012;62:1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 12.Binder JR. Task-induced deactivation and the “resting” state. NeuroImage. 2012;62:1086–1091. doi: 10.1016/j.neuroimage.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckner RL., Petersen SE., Ojemann JG., Miezin FM., Squire LR., Raichle ME. Functional anatomical studies of explicit and implicit memory retrieval tasks. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbetta M., Patel G., Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aston-Jones G., Cohen JD. An integrative theory of locus coereleus-norpinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso MM., Sirotin YB., Lima B., Glushenkova E., Das A. The neuroimaging signal is a linear sum of neurally distinct stimulus- and task-related components. Nat Neurosci. 2012;15:1298–1306. doi: 10.1038/nn.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antrobus JS., Singer JL., Goldstein S., Fortgang M. Mindwandering and cognitive structure. Trans NY Acad Sci. 1970;32:242–52. doi: 10.1111/j.2164-0947.1970.tb02056.x. [DOI] [PubMed] [Google Scholar]
- 18.Smallwood J., Schooler JW. The restless mind. Psychol Bull. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- 19.Binder JR., Frost JA., Hammeke TA., Bellgowan PS., Rao SM., Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 20.Andrews-Hanna JR., Reidler JS., Huang C., Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104:322–335. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killingsworth MA., Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330:932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- 22.Greicius MD., Srivastava G., Reiss AL., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL., Snyder AZ., Fox MD., et al Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 24.Kahn I., Andrews-Hanna JR., Vincent JL., Snyder AZ., Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schacter DL., Addis DR., Buckner RL. Episodic simulation of future events: concepts, data, and applications. Ann NY Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 26.Smallwood J., Brown KS., Baird B., Mrazek MD., Franklin MS., Schooler JW. Insulation for daydreams: a role for tonic norepinephrine in the facilitation of internally guided thought. PLoS One. 2012;7:e33706. doi: 10.1371/journal.pone.0033706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broyd SJ., Demanuele C., Debener S., Helps SK., James CJ., Sonuga-Barke EJS. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D., Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 29.Whitfield-Gabrieli S., Ford JM. Default mode network activity and connectivity in psychopathology. Ann Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 30.Van Dijk KR., Sabuncu MR., Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Power JD., Barnes KA., Snyder AZ., Schlaggar BL., Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satterthwaite TD., Wolf DH., Loughead J. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. NeuroImage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buckner RL., Krienen FM., Yeo BTT. Opportunities and limitations of intrinsic connectivity MRI. Nat Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 34.Murphy K., Birn RM., Bandettini PA. Resting-state fMRI confounds and cleanup. NeuroImage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitfield-Gabrieli S., Thermenos HW., Milanovic S., et al Hyperactivity and hyperconnectivity of the default network in schizophrenia and in firstdegree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrity AG., Pearlson GD., McKiernan K., Lloyd D., Kiehl KA., Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 37.Weinberger DR., Berman KF., lllowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- 38.Carter CS., Perlstein W., Ganguli R., Brar J., Mintun M., Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 39.Barch DM., Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volk DW., Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508. doi: 10.1007/7854_2010_44. [DOI] [PubMed] [Google Scholar]
- 41.Vincent JL., Kahn I., Snyder AZ., Raichle ME., Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spreng RN., Sepulcre J., Turner GR., Stevens WD., Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 2013;25:74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker JT., Holmes AJ., Masters GA., et al Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. In press. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unschuld PG., Buchholz AS., Varvaris M., et al Prefrontal brain network connectivity indicates degree of both schizophrenia risk and cognitive dysfunction. Schizophr Bull. In press. doi: 10.1093/schbul/sbt077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khadka S., Meda S., Stevens MC., et al Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearlson GD., Petty RG., Ross CA., Tien AY. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharrnacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. [DOI] [PubMed] [Google Scholar]