Abstract
In the course of development, the brain undergoes a remarkable process of restructuring as it adapts to the environment and becomes more efficient in processing information. A variety of brain imaging methods can be used to probe how anatomy, connectivity, and function change in the developing brain. Here we review recent discoveries regarding these brain changes in both typically developing individuals and individuals with neurodevelopmental disorders. We begin with typical development, summarizing research on changes in regional brain volume and tissue density, cortical thickness, white matter integrity, and functional connectivity. Space limits preclude the coverage of all neurodevelopmental disorders; instead, we cover a representative selection of studies examining neural correlates of autism, attention deficit/hyperactivity disorder, Fragile X, 22q11.2 deletion syndrome, Williams syndrome, Down syndrome, and Turner syndrome. Where possible, we focus on studies that identify an age by diagnosis interaction, suggesting an altered developmental trajectory. The studies we review generally cover the developmental period from infancy to early adulthood. Great progress has been made over the last 20 years in mapping how the brain matures with MR technology. With ever-improving technology, we expect this progress to accelerate, offering a deeper understanding of brain development, and more effective interventions for neurodevelopmental disorders.
Keywords: development, MRI, DTI, rsfMRI, brain structure, brain connectivity, neurodevelopmental disorder, autism, ADHD, 22q, fragile X, Turner syndrome, Williams syndrome, Down syndrome
Abstract
Le cerveau subit au cours du développement une restructuration remarquable par son adaptation à l'environnement et son efficacité croissante dans le traitement de l'information. Plusieurs méthodes de neuro-imagerie peuvent être utilisées pour mettre en évidence les modifications anatomiques, fonctionnelles et de connectivité dans le cerveau en cours de développement. Nous analysons ici les découvertes récentes sur les modifications cérébrales à la fois chez les sujets en cours de développement classique et chez ceux souffrant de troubles neurodéveloppementaux. Débutant par le développement classique, un résumé de la recherche sur les modifications du volume cérébral régional et la densité tissulaire, l'épaisseur corticale, l'intégrité de la substance blanche et la connectivité fonctionnelle, est présenté. Par manque d'espace nous ne pouvons traiter tous les troubles neurodéveloppementaux et nous avons plutôt sélectionné des études représentatives des caractéristiques neurologiques de l'autisme, du trouble déficit de l'attention/hyperactivité, de l'X fragile, du syndrome de délétion 22q11.2, du syndrome de Williams, du syndrome de Down et du syndrome de Turner. Lorsque cela est possible, nous nous intéressons aux études qui identifient une interaction âge/diagnostic, en faveur d'un trouble de la trajectoire du développement. Les études examinées couvrent généralement la période de la petite enfance à l'adulte jeune. La cartographie de la maturation cérébrale par résonance magnétique a considérablement progressé ces 20 dernières années et, la technologie s'améliorant sans cesse, nous espérons aller plus vite afin de mieux comprendre le développement cérébral et d'être plus efficaces dans les troubles neurodéveloppementaux.
Abstract
Durante el curso del desarrollo el cerebro experimenta un notable proceso de reestructuración para adaptarse al ambiente y llegar a ser más eficiente en el procesamiento de la información. Se puede emplear una variedad de métodos de imágenes cerebrales para evaluar cómo cambia la anatomía, la conectividad y el funcionamiento durante el desarrollo. Se revisan los descubrimientos recientes en relación con estos cambios cerebrales en sujetos que tienen un desarrollo típico y en quienes tienen trastornos del neurodesarrollo. El artículo comienza con el desarrollo clásico, resumiendo la investigación acerca de los cambios en el volumen regional y la densidad del tejido cerebral, el espesor cortical, la integridad de la sustancia blanca y la conectividad funcional. La limitación de espacio impide cubrir todos los trastornos del desarrollo y se aborda una selección representativa de estudios que examinan los correlatos neurales en autismo, trastorno por déficit de atención/hiperactividad, Síndrome X frágil, Síndrome de deleción 22q11.2, Síndrome de Williams, Síndrome de Down y Síndrome de Turner. Cuando es posible se destacan los estudios que identifican una interacción entre la edad yel diagnóstico, lo que sugiere una alteración en el curso del desarrollo. Los estudios revisados en general cubren el período de desarrollo entre la infancia y la adultez inicial. En los últimos 20 años, con tecnología de resonancia magnética, se han realizado grandes progresos en el mapeo de cómo madura el cerebro. Se espera que con tecnologías cada vez mejores se acelere este progreso, se posibilite una comprensión más profunda del desarrollo cerebral y se puedan realizar intervenciones más efectivas para los trastornos del neurodesarrollo.
Introduction
Brain development is a dramatic process that unfolds throughout the first decades of life, gradually transforming the brain, and involving both microscopic and macroscopic changes. By far the greatest developmental changes occur by the early twenties, and frontal brain regions are among the last to fully mature1; even so, many developmental processes, such as myelination, continue throughout life, only to be overtaken by degenerative changes in old age.
Using postmortem examinations of tissue, the age at which synaptic density peaked for a range of cortical areas was investigated by tracking changes in synaptic density at different ages.2 Among the last regions to mature are those responsible for higher-level cognition, which is still developing in adolescents (reviewed in ref 3)3. Some neuropsychiatric disorders emerge in childhood or adolescence and distinctly alter the developmental trajectory for both brain structure and function. By studying characteristic patterns of abnormalities in these disorders, many clues emerge about biological mechanisms contributing to a range of psychiatric illnesses and neurodevelopmental disorders. A more mechanistic understanding of each disorder is crucial—for more effective diagnosis, to better design interventions, and better understand treatment effects. With constantly improving technology, we can now visualize neural structures, axonal pathways, and functional connections with ever-increasing precision.
Here we review recent neuroimaging research in the fields of typical and atypical development, focusing primarily on studies from age 4 to early adulthood. There are now many studies of infancy and even fetal development with magnetic resonance imaging (MRI),4 but the vast majority of pediatric MRI studies evaluate children old enough to keep still for the duration of a scan, making later ages somewhat easier to study. We cover structural imaging methods, such as voxel-based and tensor-based morphometry (VBM and TBM), structural connectivity analyses, using diffusion tensor imaging and high angular resolution diffusion imaging (DTI and HARDI), and functional connectivity analyses, using graph theory, independent components analysis (ICA), and seed-based methods. We describe these approaches in the Typical Development section. We will not cover functional development, as many studies are task-specific and would require much more space to review. In addition to covering development of healthy individuals (Table I), we review the neuroimaging literature on a number of neurodevelopmental disorders (Table II), including autism, attention deficit-hyperactivity disorder (ADHD), Fragile X, 22q11.2 deletion syndrome, Williams syndrome, Down syndrome, and Turner syndrome. Where possible, we selected studies that examined the interaction of age and diagnosis, but in some cases we discuss studies simply addressing the effects of a disorder on the brain, as fewer studies have mapped disease effects on the entire developmental trajectory. A few other recent reviews focus on the development of brain structure,5 functional connectivity,6,7 or structural connectivity8-11 either in typically developing or atypically developing individuals.7,12 In this review, we address each of these topics, but readers are encouraged to refer to these reviews, in addition to the articles we cite here.
Table I. Studies investigating typical development that are reviewed in this paper. Bold indicates study that examined age* diagnosis effect. *, no gender information; AD, autism; ADHD, attention deficit/hyperactivity disorder; PBD, pediatric bipolar disorder; FX, fragile X; DD, developmental delay; 22q, 22q11.2 deletion syndrome; WS, Williams syndrome; DS, Down syndrome; TS, Turner syndrome; TD, typically developing; (# F), number of female participants; yo, years old; mo, months old; MRI, magnetic resonance imaging; GMD, gray matter density; DTI, diffusion tensor imaging; ROI, region of interest; HARDI, high angular resolution diffusion imaging; ICA, independent components analysis; ‡ indicates study for which we have included a figure.
| Study | N | Ages | Modality |
| Structural MRI | |||
| Giedd et al, 1999 | 145 (56 F) | 4-22 overall | Brain wolume |
| Gogtay et al, 2004‡ | 13 (7 F) | 4-21 yo overall | Brain volume |
| Sowell et al, 2002 | 35 (15 F) | 7-16 yo | Brain volume |
| Sowell et al, 2003 | 1796(86 F) | 7-87 yo | Brain volume (GMD) |
| Sowell et al, 2004 | 45 (22 F) | 5-10 yo baseline | Cortical thickness |
| Hua et al, 2009‡ | 13 (6 F) | 6-15 yo baseline | Brain volume |
| Tamnes et al, 2010 | 168 (87 F) | 8-30 yo | Brain volume, cortical thickness, and DTI |
| Diffusion-weighted imaging | |||
| Klingberg et al, 1999 | 7 children, 5 adults, all male | 8-12 and 20-31 yo | DTI - ROI |
| Schmithorst et al, 2002 | 33 (17 F) | 5-18 yo | Voxel-wise DTI |
| Barnea-Goralyetal, 2005 | 34 (16 F) | 6-19 yo | Voxel-wise DTI |
| Bonekamp et al, 2007 | 40 (18 F) | 5-19 yo | DTI - ROI |
| Eluvathingal et al, 2007 | 31 (16 F) | 6-17 yo | DTI - ROI (tract-based) |
| Qiu et al, 2008 | 1: 24 (11 F), 2: 27 (11 F), 3: 24 (13 F) | 1:6-8, 2:9-12, 3:18-26 yo | Voxel-wise DTI |
| Lebel et al, 2008‡ | 202 (98 F) | 5-30 yo | DTI - ROI (tract based) |
| Kochunov et al, 2010 | 831 (485 F) | 11-90 yo | DTI - ROI (tract-based) |
| Hagmann et al, 2010 | 30 (17 F) | 18 mo-18 yo | DTI - graph theory |
| Dennis et al, 2013‡ | 439 (267 F) | 12,16, 20-30 yo | HARDI - graph theory |
| Dennis et al, 2013b | 438(266 F) | 12,18, 20-30 yo | HARDI - graph theory |
| Functional connectivity | |||
| Fair et al, 2007, 2008‡ | 210 (*) | 7-9,10-15,19-31 yo | Seed-based func. conn. |
| Kelly et al, 2009 | 1:14 (4 F), 2: 12 (5 F), 3: 14 (5 F) | 1:8-13, 2:14-17, 3:20-24 yo | Seed-based func. conn. |
| Dosenbach et al, 2010 | 238 (115 F) | 7-30 yo | Seed-based func. conn. |
| Supekar et al, 2010 | 1: 23 (13 F), 2: 22 (11 F) | 1: 7-9, 2: 19-22 yo | DTI, ICA func. conn. |
| Thomason et al, 2011 | 65 (32 F) | 9-15 yo baseline | Seed/ICA func. conn. |
| JoIIes et al, 2011 | 1:19(10 F), 2: 29 (16 F) | 1: 11-13, 2: 19-25 yo | ICA func. conn. |
Table II. Studies investigating neurodevelopmental disorders that are reviewed in this paper. Bold indicates study that examined age* diagnosis effect. AD, autism disorder; TD, typically developing; DTI, diffusion tensor imaging; FX, Fragile X; DD, developmental delay; ADHD, attention deficit-hyperactivity disorder; WS, Williams syndrome; ICA, independent components analysis; * = no gender information; (# F), number of female participants. ‡ indicates study for which we have included a figure; # indicates study listed in multiple places.
| Study | N | Ages | Modality |
| Autism | |||
| Langan et al, 2009 | 99 AD (8 F), 89 TD (7 F) | 6-25 yo | Brain volume |
| Hardan et al, 2009 | 18 AD, 16 TD, all male | 8-12 yo baseline | Brain volume, cortical thickness |
| Brun et al, 2009 | 24 AD, 26 TD, all male | 6-16 yo | Brain volume |
| Mengotti et al, 2011 | 20 AD (2 F), 22 TD (2 F) | 4-14 yo | Brain volume, DTI |
| Hua at al, 2013 | 13 AD,7 TD, all male | 6-13 yo baseline | Brain volume |
| Barnea-Goraly et al, 2004 | 7 AD, 9 TD, all male | AD mean=14.6, TD mean=13.4 | DTI |
| Bashat et al, 2007 | 7 AD, 41 TD (23 F) | 1.8-3.3 AD, 4 mo-23 yo TD | DTI |
| Keller et al, 2007 | 34 AD, 31 TD, all male | 10-35 yo | DTI |
| Barnea-Goraly et al, 2010 | 13 AD (2 F), 13 same-sex siblings, 11 TD (2 F) | 6-13 yo | DTI |
| Shukla et al, 2010‡ | 26 AD (1 F), 24 TD (1 F) | 9-20 yo | DTI |
| Cherkassky et al, 2006 | 57 AD (4 F), 57 TD (5 F) | AD mean=24, TD mean=24 | Seed-based func. conn. |
| Just et al, 2007 | 18 AD (1 F), 18 TD (5 F) | AD mean=27.1, TD mean=24.5 | Seed-based func. conn. |
| Kennedy et al, 2008 | 12 AD, 12 TD, all male | 15-52 yo | Seed-based func. conn. |
| Monk et al, 2009 | 12 AD (1 F), 12 TD (2 F) | AD mean=26, TD mean=27 | Seed-based func. conn. |
| Noonan et al, 2009 | 10 AD, 10 TD, all male | 14-43 yo | Seed-based func. conn. |
| Weng et al, 2010 | 16 AD (2 F), 15 TD (1 F) | 13-18 yo | Seed-based func. conn. |
| Assaf et al, 2010 | 15 AD (1 F), 15 TD (2 F) | 10-23 yo | ICA func. conn. |
| Rudie et al, 2012‡ | 23 AD (2 F), 25 TD (3 F) | 8-17 yo | Seed-based func. conn. |
| Rudie et al, 2013 | 42 AD (6 F), 37 TD (6 F) | 9-18 yo | Graph theory func. conn |
| Attention-deficit hyperactivity disorder | |||
| Castellanos et al, 2002 | 152 ADHD (63 F), 133 TD (56 F) | 4-19 yo | Brain volume |
| Sowell et al, 2003 | 27 ADHD (11 F), 46 TD (17 F) | 8-18 yo | Brain volume |
| Carmona et al, 2005 | 25 ADHD (4 F), 25 TD (4 F) | 6-16 yo | Brain volume |
| Shaw et al, 2006 | 163 ADHD (*), 168 TD (*) | Maan baseline=8 yo | Cortical thickness |
| Shaw et al, 2007 | 223 ADHD (82 F), 223 TD (82 F) | Maan baseline=10 yo | Cortical thickness |
| Brieber et al, 2007 | 15 AD, 15 ADHD, 15 TD | ADHD mean=13.1, TD mean=13.3 | Bra in volume |
| Luders et al, 2009 | 19 ADHD, 19 TD, all male | 7-16 yo | Callosal thickness |
| Kobel et al, 2010 | 14 ADHD, 12 TD, all male | 9-13 yo | Brain volume, DTI, ICA func. conn. |
| Ashtari et al, 2005 | 18 ADHD (6 F), 15 ADHD (6 F) | 7-11 yo | DTI |
| Hamilton et al, 2008 | 17 ADHD, 16 TD, all male | ADHD mean=12.0, TD mean=11.7 | DTI |
| Pavuluri et al, 2009 | 13 PBD (3 F), 13 ADHD (1 F), 13 TD (7 F) | ADHD mean=13.4, TD mean=13.7 | DTI |
| Silk et al, 2O09a,b | 15 ADHD, 15 TD, all male | 8-18 yo | DTI |
| Tamm et al, 2012‡ | 12 ADHD, 12 TD, all male | 14-18 yo | DTI |
| Lawrence et al, 2013 | 56 ADH D {17 F), 31 siblings (24 F),17 TD (11 F) | 6-18 yo | DTI |
| Cao et al, 2006 | 29 ADHD, 27 TD, all male | 11-16.5 yo | Regional homogeneity (func. conn.) |
| Wang et al, 2009 | 19 ADHD, 20 TD, all male | ADHD mean=13.6, TD mean=13.3 | graph theory func. conn. |
| Fair et al, 2010 | 23 ADHD (7 F), 23 TD (11 F) | 7-16 yo | Seed-based func. conn. |
| Qiu et al, 2011 | 15 ADHD, 15 TD, all male | 10-15 yo | Brain volume, cortical thickness, DTI, ICA func. conn. |
| Fragile X | |||
| Lee et al, 2007 | 36 FX (18 F), 33 TD (17 F) | FX mean=14.7, TD mean=14.7 | Brain volume |
| Hoeft et al, 2010 | 41 FX, 28 TD, all male | 1-3 yo baseline | Brain volume |
| Hoeft et al, 2011‡ | 52 FX, 31 TD, all male | 1-4 yo | Brain volume |
| Hazlett et al, 2012 | 53 FX, 50 TD, all male | 18-42 mo baseline | Brain volume |
| Peng et al, 2013 | 48 FX (*), 28 DD (*), 36 TD (*) | 15-27 yo | Brain volume |
| Barnea-Goraly et al, 2003a | 10 FX, 10 TD, all female | 11-23 yo | DTI |
| Haas et al, 2009 | 17 FX, 13 TD, 8 DD, all male | 1 5-4 yo | DTI |
| Villalon et al, 2013# | 18 FX, 25 22q, 17 TS, 41 TD, all female | 7-14 yo | DTI |
| 22q11.2 Deletion syndrome | |||
| Simon et al, 2005 | 18 22q (11 F), 18 TD (7 F) | 7-14 yo | Brain volume |
| Campbell et al, 2006 | 39 22q (19 F), 26 siblings (10 F) | 22q mean=11, TD mean=11 | Brain volume |
| Bearden et al, 2007 | 21 22q (11 F), 13 TD (6 F) | 8-17 yo | Cortical thickness |
| Bearden et al, 2009 | 21 22q (11 F), 13 TD (6 F) | 8-17 yo | Cortical thickness |
| Schaer et al, 2009‡ | 59 22q (35 F), 80 TD (44 F) | 6-40 yo baseline | Cortical thickness |
| Shashi et al, 2010 | 22 22q (7 F), 16 TD (6 F) | 8-17 yo | Brain volume |
| Gothelf et al, 2011 | 19 22q (8 F), 18 TD (8 F) | 22q mean=13, TD mean=13.4 baseline | Brain volume |
| Srivastava et al, 2012 | 49 22q, 37 TD* | 6-15 yo | CorticaI gyrification |
| Barnea-Goraly et al, 2003b | 19 22q (6 F), 19 TD (8 F) | 7-22 yo | DTI |
| Villalon et al, 2013# | 18 FX, 25 22q, 17 TS, 41 TD, all female | 7-14 yo | DTI |
| Debbane et al, 2012 | 27 22q (15 F), 33 TD (14 F) | 12-19 yo | ICA func. conn. |
| Williams syndrome | |||
| Thompson et al, 2005 | 42 WS (23 F), 40 TD (24 F) | 12-50 yo | Cortical thickness |
| Eckert et al, 2006 | 42 WS (23 F), 40 TD (24 F) | 12-50 yo | Cortical morphology |
| Gaser et al, 2006 | 42 WS (23 F), 40 TD (24 F) | 12-50 yo | Cortical morphology |
| Boddaert et al, 2006 | 9 WS (2 F), 11 TD (5 F) | 5-15 yo | Brain volume |
| Tosun et al, 2006 | 39 WS (22 F), 39 TD (23 F) | WS mean=29.9, TD mean=27.1 | Cortical morphology |
| Chiang et al, 2007 | 41 WS (23 F), 33 TD (23 F) | 12-50 yo | Brain volume |
| Luders et al, 2007 | 12 WS (5 F), 12 TO (5 F) | 13-30 yo | CallosaI thickness |
| Meda et al, 2012 | 31 WS (11 F), 50 TD (23 F) | WS mean=26.3, TD mean=28.0 | Brain volume, cortical thickness |
| Hoeft et al, 2007 | 10 WS (4 F), 10 DD (7 F), 10 TD (3 F) | WS mean=26.8, TD mean=27.8 | DTI |
| Arlinghaus et al, 2011‡ | 16 WS (6 F), 16 TD (8 F) | 16-33 yo | DTI |
| Jabbi et al, 2012 | 14 WS (7 F), 23 TD (10 F) | WS mean=27.8, TD mean=32.1 | Brain volume, DTI |
| Haas et al, 2012 | 20 WS (9 F), 10 DD (7 F), 10 TD (2 F) | WS mean=23.7,TD mean=27.8 | DTI |
| Haas et al, 2012 | 39 WS (24 F), 40 TD (23 F) | WS mean=26.1, TD mean=21.3 | Brain volume |
| Down syndrome | |||
| Pinter et al, 2001 | 16 DS (5 F), 15 TD (*) | DS mean=11.3, TD mean=11.9 | Brain volume |
| Smigielska-Kuzia et al, 2011 | 23 DS (9 F), 26 TD (11 F) | 3-15 yo | Brain volume |
| Turner syndrome | |||
| Murphy et al, 1993 | 18 TS, 19 TD, a 11 female | TS mean=30, TD mean=27 | Brain volume |
| Reiss et al,1995 | 30 TS, 30 TD, all female | 6-17 yo | Brain volume |
| Brown et al, 2002 | 26 TS, 26 TD, all female | TS mean=13.2, TD mean=13.4 | Brain volume |
| Kester et al, 2004 | 30 TS, 23 TD, all female | 6-33 yo | Brain volume |
| Molko et al, 2004 | 14 TS, 14TS, all female | 18-36 yo | DTI |
| Holzapfel et al, 2006 | 10 TS, 10 TD, all female | 7-24 yo | Brain volume, DTI |
| Yamagata et al, 2012‡ | 26 TS, 20 TD, all female | 3-12 yo | Brain volume, DTI |
| Villalon et al, 2013# | 18 FX, 25 22q, 17 TS, 41 TD, all female | 7-14 yo | DTI |
| Bray et al, 2011 | 37 TS,18 TD, all female | 7-13 yo | Seed-based func. conn. |
| Bray et al, 2012 | 15 TS,14 TD, all female | 7-14 yo | Seed-based func. conn. |
Typical development
An exhaustive review of all studies of typical development with various neuroimaging methods is beyond the scope of this paper, so we will highlight illustrative examples that reflect some general trends in the field (Table I).
Structural MRI
A vast number of methods have been used to investigate changes in brain structure. The most traditional way to measure anatomical changes in the brain is to identify the substructures of brain—often by manual tracing, or more recently by using automated computer programs to measure their volumes. By parcellating the brain into regions with different functions, such as the major lobes, the subcortical nuclei, and cortical regions, several early landmark studies generated “growth curves,” or norms, to show how the size of different brain regions increases—or decreases—with age. Around the year 2000, the first studies were published describing data from large cohorts of children scanned with MRI. Distinct and characteristic growth trajectories were found for each brain region,13 with some notable sex differences.14-16
A more detailed picture of the developmental trajectory emerged with the advent of voxel-based brain mapping methods.17,18 Voxel-based statistical approaches can create a color-coded 3D map of growth rates at each location in the brain, or changes in cortical thickness. Alternatively, they can simply test if the density or thickness of a tissue in a brain region is affected by age, or if it relates to a clinical variable of interest. One such statistical mapping method, VBM, begins by spatially normalizing all MRI scans from a group of subjects into the same space. The scans are then segmented into gray matter, white matter, and cerebrospinal fluid (CSF), maps of each tissue are smoothed, and statistical tests are performed at each voxel—or 3D location—in the brain, to determine where age related changes occur, and what other factors affect the brain measures. The values in VBM analyses then represent the average proportion of gray matter in a small region around any given voxel. To be clear, “density” here is not intended to measure axonal or cellular packing density,19 but it offers a gross anatomical measure of regional tissue volumes, offering spatial detail on the pattern of tissue in the brain.
TBM is a more computationally intensive method, in which a deformation field is obtained for each subject, warping their brain to match a common brain template, and encoding the relative positions of various brain landmarks. Tensor fields, or Jacobian matrices, are then calculated from the gradient of the deformation field, at each point, representing the volume of the unit-cube after the deformation.20 From these, it is possible to determine the degree of regional volume expansion or shrinkage across scans taken at different times,21 or to determine anatomical differences in a set of scans. These can then be analyzed statistically, to identify characteristic diagnostic group differences, age effects, or links with clinical or cognitive measures.
Cortical thickness assessments use semiautomated methods to reconstruct 3D representations of the gray matter–white matter boundary and the pial surface, and they calculate the distance between the two for every point across the cortex.22,23 Using cortical thickness maps, timelapse movies have been created to show the shifting pattern of cortical thinning in typically developing children between ages 4 and 21,1 and in disorders such as childhood-onset schizophrenia,24,25 or before and after the onset of bipolar disorder.26
Between age 5 and adulthood, important changes occur in higher cognitive functions that in part reflect changes in brain structure. Total brain volume increases with age, and many studies have found that the growth rate varies across the brain, and over time. Gray matter volume increases into adolescence, when it plateaus and begins to decline, but white matter volume usually increases into adulthood.13,27
Myelination continues throughout life—even well into old age—and white matter volume reductions only begin to be observed when the balance between myelin production and degeneration tilts in favor of white matter loss.28 If this is recognized, it avoids the misconception that some of the developmental processes “stop,” when in fact they continue but are often dominated by other more dramatic changes.
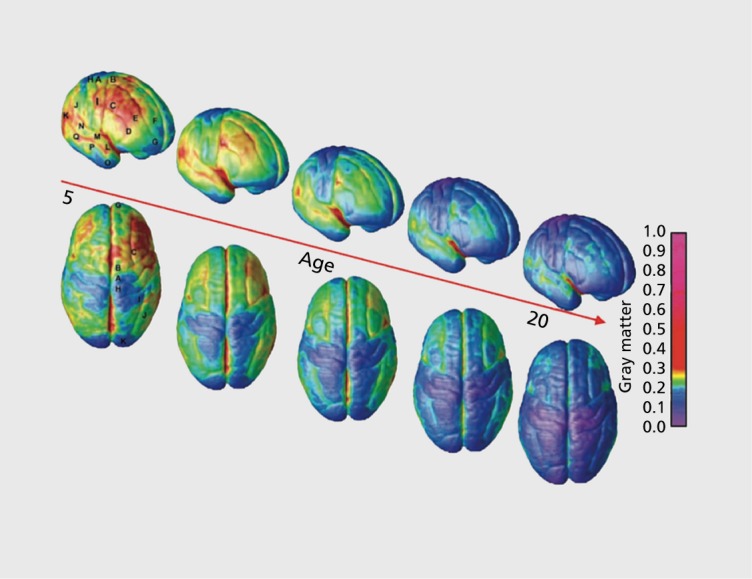
The age at which gray matter volume plateaus varies across the lobes, and temporal gray matter volume tends to reach a maximum last.13 Within the lobes too, there is a great deal of variation in time to mature. In a whole-brain study, it was found that the prefrontal cortex and the posterior part of the superior temporal gyrus were shown to be the last to mature (Figure 1).1 In general, phylogenetically earlier structures—those supporting vision, hearing, and sensorimotor function—develop the most rapidly in infancy. To some extent, ‘ontogeny recapitulates phylogeny.’ Brain areas that support speech, language comprehension, and finally executive function, tend to develop in roughly the same sequence as they emerged during human evolution. Sowell et al similarly found that the posterior temporal cortex had a more protracted development.29 For subcortical structures, they showed that as the brain grows in size, the proportion taken up by subcortical structures decreases, but at a different rate for males and females.15 Additionally, they proposed that the decrease in gray matter, while due in part to cortical pruning (ie, synapse elimination and dendritic pruning), was also due in large part to the ongoing increase in white matter. They also examined cortical thickness between ages 5 and 11.30 While large areas of cortex became thinner with age, cortical gray-matter in Broca's and Wernicke's areas thickened.
Figure 1. Gray matter maturation between ages 5 and 20. The side bar shows a color representation in units of gray matter volume. Images are stills from a movie available online from ref 1: Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174-8179. Copyright © National Academy of Sciences 2004.
Hua et al used TBM to show regional brain changes in a longitudinal dataset from children, finding expansion of cerebral white matter and shrinkage of parietal, temporal, and occipital gray matter (Supplementary Figure 1).31 Using TBM, one can create a picture of the mean growth rate, for each brain region, at any age. Tamnes et al examined age-related changes in a large cohort of subjects between ages 8 and 30 with both structural MRI (sMRI) and diffusion tensor imaging (DTI—described below)32 They found prominent cortical thinning across the parietal lobe, superior medial frontal lobe, cingulate gyrus, prefrontal cortex, and occipital cortex. The rate of thinning was greatest in the youngest subjects, after which the rate slowed down.
Supplementary Figure 1. Gray matter maturation between ages 7-15. Tissue growth maps modeled by linear regression, for all subjects and males and females separately. Reproduced from ref 31: Hua X, Leow AD, Levitt JG, Caplan R, Thompson PM, Toga AW. Detecting brain growth patterns in normal children using tensor-based morphometry. Hum Brain Mapp. 2009;30:209-219. Copyright © Wiley-Liss 2009.
Diffusion-weighted imaging
Diffusion-weighted imaging (DWI) is a variant of MRI scanning, which allows us to visualize, at a gross level, the rate of diffusion of water along axons. It can therefore be used to visualize axonal pathways. The MR signal is reduced when water is diffusing,33 and it is possible to design an MRI protocol whose signals are depleted by water diffusing in a particular direction (a diffusion gradient image). By measuring diffusion in a large set of different directions (at least 6, but often as many as 30 to 256 directions), we can identify the primary directions of water diffusion in each voxel in the brain.
Diffusion tensor imaging (DTI) models water diffusion at each voxel as an ellipsoid or “tensor,” after which tractography may be used to follow and reconstruct the major white matter fiber bundles. HARD I is similar to DTI, but can map crossing fibers better, as it does not rely on the assumption that there is only one dominant fiber present in each voxel.34 HARDI collects diffusion information from more angles and uses orientation distribution functions (ODFs), or other spherical functions—instead of tensors—to map the probability of water diffusion in every direction, leading to more accurate tractography.35-40 Fractional anisotropy (FA), the degree to which water diffuses in one direction (along the axon), is one of the most widely used measures of axonal integrity. As a rule of thumb—which has many exceptions—higher FA and lower mean diffusivity (MD) tend to reflect more highly developed, more strongly myelinated tracts, with a higher axonal conduction speed. These measures are reproducible in children, providing reliable developmental biomarkers.41
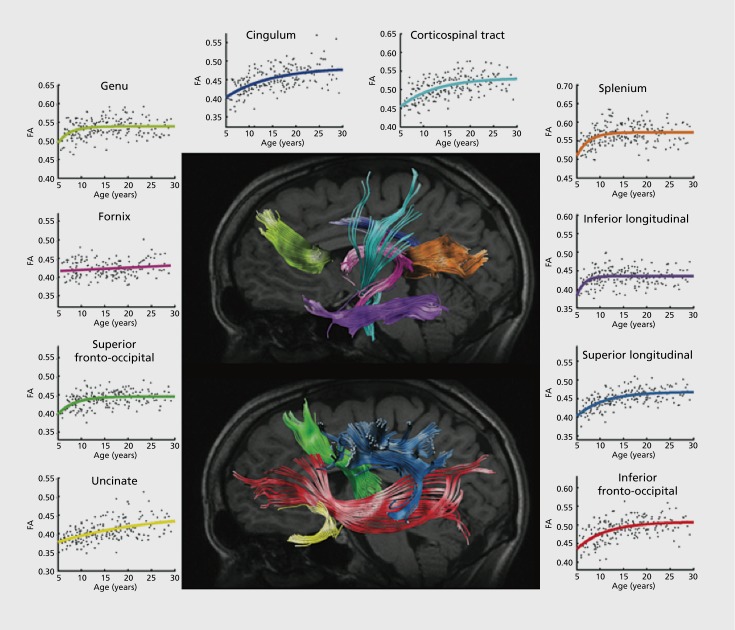
Specifically examining the frontal lobe white matter, Klingberg et al found significantly greater white matter fractional anisotropy in adults than in children.42 They attributed this to a lesser degree of myelination in children; this is also consistent with visual inspection of brain MRI scans from infants, which often show limited white-matter contrast in poorly myelinated regions. Schmithorst et al expanded on earlier work, examining a range of specific tracts in subjects between 5 and 18 years old.43 FA increased with age in the internal capsule, corticospinal tract, left arcuate fasciculus, and right inferior longitudinal fasciculus. Similar trajectories have been reported in DTI studies of the entire lifespan.44 In one study, FA increased with age in the internal capsule, the white matter of the prefrontal cortex, corpus callosum, basal ganglia and thalamic pathways, and visual pathways.45 Several of these regions underlie cognitive functions such as memory and attention, as well as motor skills. Eluvathingal et al examined 6 specific tracts and found three patterns in the results.46 Various parts of the arcuate fasciculus, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, uncinate fasciculus, and corticospinal tract showed either increased in FA with decreases in other measures of diffusivity, or no detectable effect on FA and decreases in diffusivity. Only the somatosensory pathway showed no detectable age effect at all, probably because it matures very early in infancy, prior to the age range examined in that study. In a voxel-wise analysis, Qiu et al found widespread age effects on FA across the cerebellum, temporal, frontal, and parietal lobes.47 Additionally, they found that reading scores (in Chinese and English) were associated with higher FA in a number of regions. Lebel et al found that the developmental trajectory of measures of anisotropy and diffusivity across most tracts were best fit with an exponential curve (Figure 2). 48 Echoing structural studies above, they found the last tracts to mature were frontotemporal connections. In one of the largest brain imaging studies to date, Kochunov et al detailed how 11 major tracts change over the lifespan (age 11 to 90) in 831 subjects.49 By charting the FA of these tracts across their subject pool, they reported the “age-at-peak” for each tract, as well as the rate of increase/decrease, along with sex differences, in some cases.
Figure 2. White matter maturation between ages 5 and 30. Age-related fractional anisotropy increases measured by tractography in 202 individuals across 10 tracts. Reproduced from ref 48: Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neurolmage. 2008;40:1044-1055. Copyright © Elsevier 2008.
Using DTI-based connectivity analysis, Hagmann et al used graph theory to show that the efficiency of the brain's anatomical network increased with age—as did the number of detectable connections for each brain region.50 Graph theory represents the brain as a set of nodes (brain regions) and edges (the connections between them). A number of standard parameters such as path length and modularity, to name a few, are used to describe network topology.51 Characteristic path length measures the average path length in a network. It does not refer to the physical length of the tracts, but the number of edges, or individual “jumps,” between nodes in the network. Modularity is the degree to which a system may be subdivided into smaller networks. Graph theory can quantify more global features in brain connectivity patterns. These include network efficiency, or the degree to which the network is differentiated into modules. Using cortical connectivity matrices calculated from HARDI data, Dennis et al examined the developmental trajectory of graph theoretical measures of structural connectivity (Figure 3).52 Path length and modularity, among other measures, decreased with age, suggesting an increase in network integration. Interestingly, the left and right intrahemispheric networks, when analyzed separately, showed opposing age trends; some parameters increased with age in the left hemisphere, but decreased in the right. If this is corroborated in the future, it could point to different developmental processes in each hemisphere, perhaps due to the known structural asymmetry of the brain, which also increases with age.15,53 The level of structural brain asymmetry is also known to relates to the morphometry of corpus callosum, the major interhemispheric commissure,54 so it may also relate to detectable differences in connectivity.
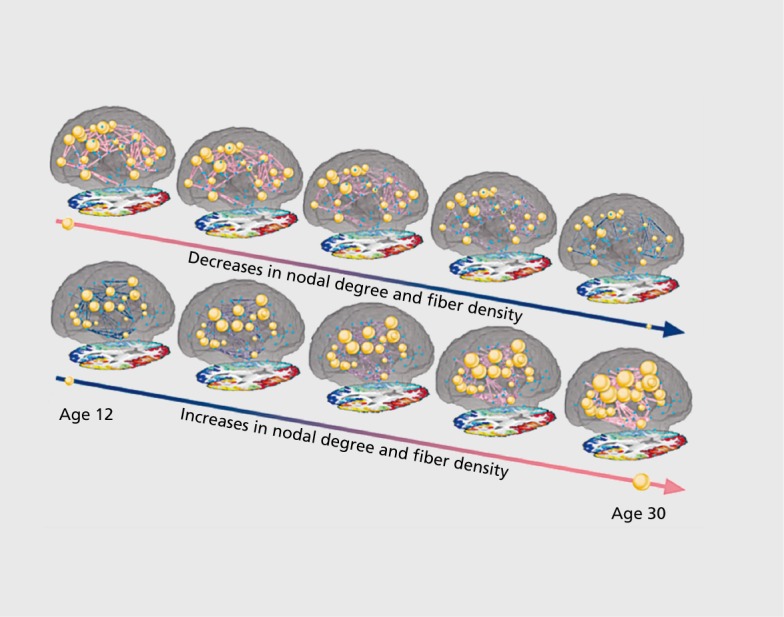
Figure 3. Development of structural connectivity between age 12 and age 30. Still images from videos available online from ref 55 displaying the increases and decreases in degree and fiber density between age 12 and age 30. For this image, node size is proportional to the degree (number of connections), and connection thickness is proportional to relative fiber density. The connection color is simulated to make the connections easier to see. The rate of increase or decrease for each node and connection was the regression coefficients from the age analyses for those nodes and connections. Small blue dots indicate nodes for which there was no significant age-related increase or decrease in degree. Only connections that had a significant age-related increase or decrease in fiber density are included in this image, other connections exist but are not drawn in for clarity. In this image are both weighted (fiber density) and binary (degree) measures. These images are created from the results when analyses were restricted to only connections existing in at least 95% of subjects. Reproduced from ref 52. Dennis EL, Jahanshad N, McMahon KL, et al. Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. Neurolmage. 2013;64:671-684. Copyright © Elsevier 2013.
Dennis et al also found differences in the structural core of the brain, as the “rich club” is restructured and strengthened.55 The “rich club” of the brain is the core of the network, made up of high-degree nodes that are highly interconnected and play an important role in network efficiency.56
Functional connectivity
Resting-state fMRI (rsfMRI) is a branch of research based on the theory that distributed brain regions are functionally coupled, even if they are not directly structurally connected. In fact, the coherence (temporal correlations) in brain activity across disparate brain regions may be used to identify systems or networks in the brain that interact. Resting-state functional connectivity can be assessed through blood oxygenation level dependent (BOLD) time-courses of these distant regions, resulting in a number of intrinsic connectivity networks (ICNs) that are reliably found across individuals, and across studies. The main methods to assess functional connectivity are independent components analysis (ICA), seed-based analysis, and graph theory. ICA is a model-free approach, in which the four-dimensional resting-state data (the time-series) is decomposed into time courses and associated spatial maps, describing the temporal and spatial characteristics of the components making up the data.57 Seed-based analysis is a model-based approach in which the researcher selects a seed region of interest, and extracts the time course of that seed. They then correlate that time course with the time-course of activations in the rest of the brain, searching for those that are most similar.58 Regions whose time course is highly correlated with the seed are considered to be functionally coupled. Lastly, graph the ory can also be applied to functional images, exactly as discussed in the previous section. Graph theory is applicable to functional, anatomical, or diffusionweighted MRI—any scans that measure the relationship between brain regions in terms of correlation, coherence, mutual information, or physical measures of connectivity such as fiber density.
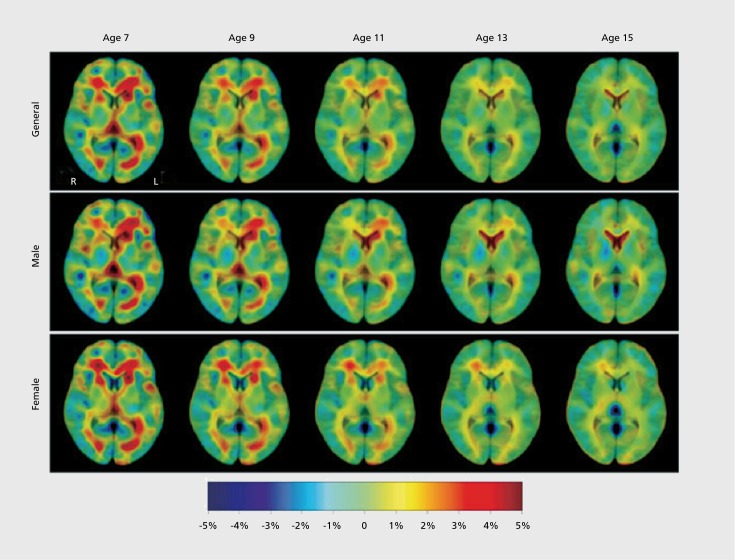
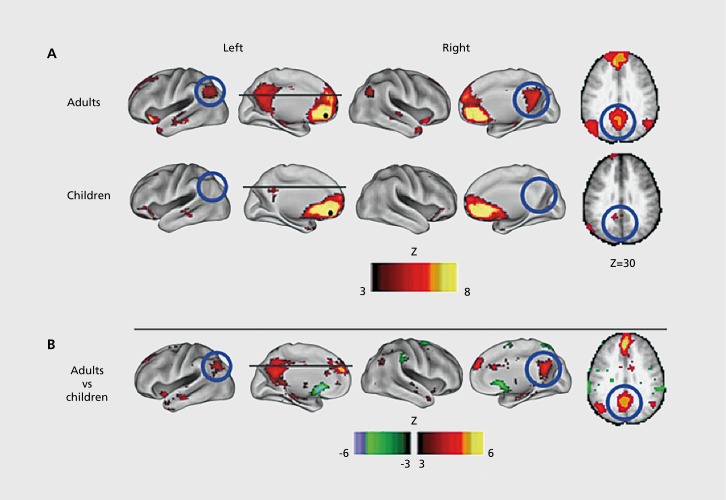
Focusing on regions involved in task control, Fair et al found that the period of development between 7 and 31 was marked by increases in segregation and integration, as distinct networks mature.59 In the same dataset, they examined the maturation of the default mode network, and it was found to be only sparsely connected in children (Figure 4).60 The default mode network (DMN) is a network usually including the precuneus/posterior cingulate (PCC), medial prefrontal cortex (mPFC), hippocampus, inferior parietal lobule, and lateral temporal cortex.61 These regions are more active during rest than during a task, hence the name “default mode” or “task negative” network.62
Figure 4. Development of functional connectivity. Voxelwise resting-state functional connectivity maps for a seed region (solid black circle) in medial prefrontal cortex—mPFC (ventral: -3, 39, -2). (A) Qualitatively, the resting-state functional connectivity MRI (rs-fcMRI) map for the mPFC (ventral) seed region reveals the commonly observed adult connectivity pattern of the default network. The connectivity map in children, however, significantly deviates from that of the adults. Functional connections with regions in the posterior cingulate and lateral parietal regions (highlighted with blue open circles) are present in the adults but absent in children. (B) These qualitative differences between children and adults are confirmed by the direct comparison (random effects) between adults and children. mPFC (ventral) functional connections with the posterior cingulate and lateral parietal regions are significantly stronger in adults than children. Reproduced from ref 60: Fair DA, Cohen AL, Dosenbach NUF, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028-4032. Copyright © National Academy of Sciences 2008.
Using five seeds in distinct regions of the anterior cingulate cortex it was found that over development, local patterns of connectivity evolved from diffuse to focused, and networks changed from exhibiting mostly local connectivity to include more distant brain regions.63 Subjects' resting state data were able to be used to predict their age—their maturational curve accounted for more than half of the variation in their data.64 Examining both structural and functional connectivity of DMN regions, it was found that the connectivity of the PCC-mPFC along the cingulum was the least mature in children.65 Some regions that were poorly connected structurally in children still had strong functional connectivity. This suggests that the saying “what fires together, wires together”66 may hold on a larger scale—the functional coupling of some brain regions may strengthen their structural connectivity over time.
In a cohort of subjects scanned multiple times—both within scan session and between sessions separated by a few years—it was demonstrated that rsfMRI can reliably map brain networks in children and adolescents.67 A study that focused less on the specific regions connected and more on the quality of the connections found that children's functional networks tended to include more voxels and than did those of adults.68 This supports earlier hypotheses that maturation is marked by a process of refining and “focusing” of brain networks.
Neurodevelopmental disorders
While we cannot cover all neurodevelopmental disorders, here we review some of the more common or more commonly studied neurodevelopmental disorders (Table II).
Autism
Autism is a neurodevelopmental disorder characterized by deficits in social interaction and communication, and by repetitive behaviors. The prevalence of autism is estimated to be around 2.5 %69 and is usually diagnosed by age 3.70 Autism has a partially genetic basis, although the specific mechanisms that contribute to the disorder are complex and are not expected to be the same in all children with autism.71
Structural MRI
A number of studies have compared individuals of a specific age group with autism with typically developing individuals; fewer have examined changes in the developmental trajectory associated with autism. In an impressively large study (N=188), Langen et al examined the development of the striatum in autistic and typicallydeveloping individuals.72 They found opposite developmental trends in individuals with autism in the caudate and the nucleus accumbens, and an altered trajectory, albeit in the same direction, in the putamen. Ilardan et al examined longitudinal changes in cortical thickness in autistic boys, finding a greater decrease with age in cortical thickness in the autistic individuals than the typically developing boys.73 Brun et al found that autistic boys had enlarged lobes compared with typically developing boys, but voxel-wise analyses also showed gray matter deficits in parietal, temporal, and occipital lobes.74 Mengotti et al examined changes in the developmental trajectory of both regional brain volume and structural connectivity in individuals with autism and found that the volume of the inferior temporal cortex, superior and inferior parietal lobule, and superior occipital lobe were larger in individuals with autism, while the volumes of the inferior frontal cortex and supplementary motor cortex were smaller.75 Hua et al examined longitudinal data, and the trajectory of white matter growth was slowed in autistic boys, especially in the parietal lobe.76 In gray matter, they found accelerated growth in the anterior cingulate cortex and putamen.
Diffusion-weighted imaging
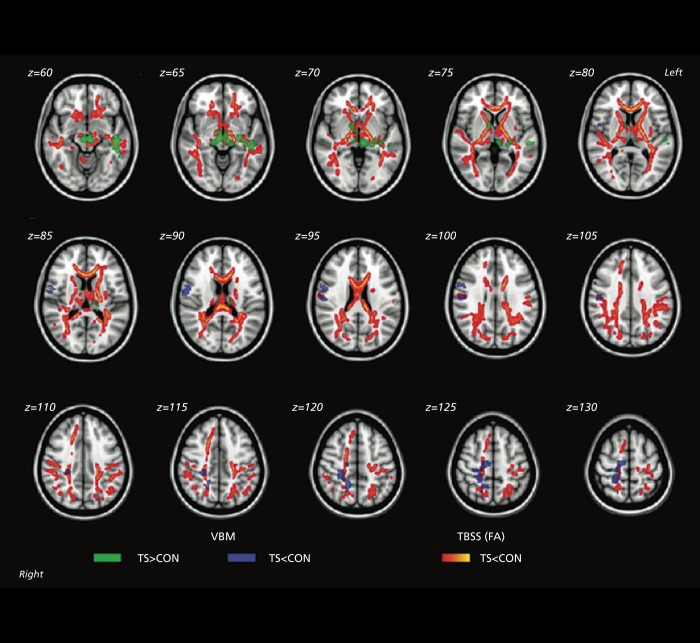
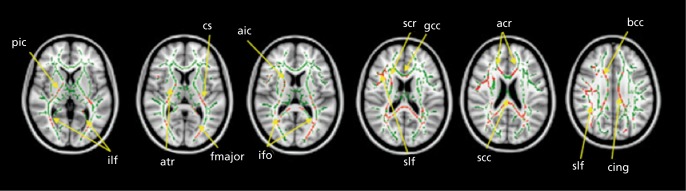
Diffusion imaging studies of autism show widespread disruption of white matter tracts, especially between regions implicated in social behavior (Figure 5).77-79
Figure 5. Differences in white matter integrity in autism. Tract-based spatial statistics revealed regions of reduced fractional anisotropy in children with autism spectrum disorder compared with the typically developing group. Red color symbolizes significant voxels at P<.05 (corrected for multiple comparisons at cluster level). Mean skeleton of detected fiber tracts is overlaid in green on standard T1-weighted anatomical image), ilf, inferior longitudinal fasciculus; ifo, inferior fronto-occipital fasciculus; slf, superior longitudinal fasciculus; cs, corticospinal tract; cing, cingulum; bcc, body of corpus callosum; gcc, genu of corpus callosum; sec, splenium of corpus callosum; aic, anterior internal capsule; pic, posterior internal capsule; fmajor, forceps major; acr, anterior corona radiata; scr, superior corona radiata; atr, anterior thalamic radiation Reproduced from ref 79: Shukla DK, Keehn B, Lincoln AJ, Muller RA. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:1269-1278.e2. Copyright © Elsevier 2010.
According to one theory of autism, at least a subset of children with autism experience an initial brain “overgrowth,” after which typically developing children catch up and surpass autistic children. This is a debated hypothesis in the field, however,80,81 and it may apply to some autistic children but not others. Various findings support this. Significantly accelerated maturation of the white matter has been found in autistic children.82 Following this overgrowth, the autistic brain may fail to effectively prune connections, leading to disorganization. One region has been found to show an interaction of age with diagnostic group: the right posterior limb of the internal capsule decreased in FA with age in typically developing individuals, but it increased with age in individuals with autism.83 It has also been found that the apparent diffusion coefficient (ADC) was negatively associated with age across most of the cortex and the splenium of the corpus callosum in autistic individuals, but no detectable associations with age in typically developing individuals were found.75
Functional connectivity
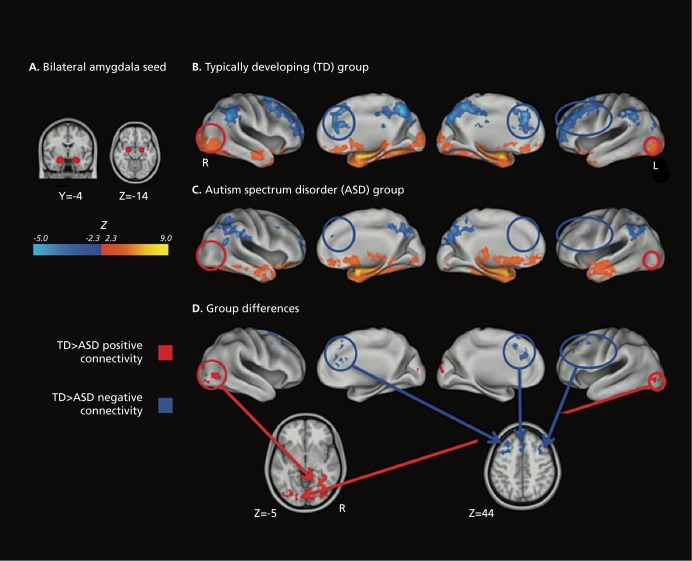
We were unable to find any reports of an age by diagnosis interaction effect on functional connectivity in autism. A number of studies have reported effects of autism diagnosis on intrinsic connectivity networks (ICNs). One set of studies supports the theory that autism results from widespread underconnectivity in the brain.84-88 Monk et al found a correlation between social functioning and the degree of connectivity between the posterior cingulate and the superior frontal gyrus; those with poorer skills exhibited weaker connectivity.89 They also found stronger functional connectivity in autistic subjects between the posterior cingulate and the temporal lobe and parahippocampal gyrus. One study reported generally more extensive functional connectivity in autistic individuals, challenging the underconnectivity hypothesis, but this study had fewer subjects than any of the others.90 Addressing the question in a different way, some have found both reduced integration within network and reduced segregation between networks in individuals with autism (Figure 6).91,92
Figure 6. Functional connectivity in autism. Bilateral amygdala connectivity. (A) The Harvard-Oxford bilateral amygdala (25% probability) used as seed region and displayed on the 1 mm MNI152 T1 standard brain. (B) Typically developing (TD) within-group connectivity maps, (C) Autism spectrum disorder within-group connectivity maps, and (D) direct between-group contrasts rendered on the Inflated PALS B12 brain using CARET (Computerized Anatomical Reconstruction and Editing Toolkit) and on the 1 mm Montreal Neurological Institute (MNI)152 T1 standard brain using Analysis of Functional Neurolmages. Maps are thresholded at Z > 2.3 (P< 0.01) with correction for multiple comparisons applied at the cluster level (P< 0.05). Red circles highlight areas of greater positive connectivity with the seed region for the TD group. Blue circles highlight areas of greater negative connectivity with the seed region for the TD group. The original paper also details the connectivity of the right inferior frontal gyrus, pars opercularis. Adapted from ref 91: Rudie JD, Shehzad Z, Hernandez LM, et al. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 2012;22:1025-1037. Copyright © Oxford University Press 2012.
Attention deficit/hyperactivity disorder
Attention deficit/hyperactivity disorder (ADHD) is a common neurodevelopmental disorder, disproportion-ately diagnosed in boys.93 It is a heterogeneous disorder with a strong familial factor.94
Structural MRI
There are many studies reporting brain structural differences in individuals with ADHD, but they do not appear to have arrived at a consensus. Widespread differences in gray matter volume are reported, while other reports discuss more specific regions of differences in volume, focusing on the parietal and temporal areas of the brain, respectively.95-98 Some studies have found a thinner corpus callosum,99 and widespread differences in cortical thickness.100 In a longitudinal study, Shaw et al reported thinner cortex in children with ADHD, across a number of areas important for attentional control.101 Additionally, while mean cortical thickness showed consistent differences across development, there was an age by diagnosis effect in the right parietal cortex. A similar longitudinal study by these authors found that the “age at peak” for cortical thickness was significantly delayed in children with ADHD, especially in the prefrontal cortex.102
Diffusion-weighted imaging
A number of studies have found disruption in white matter tracts implicated in the pathophysiology of ADHD.103-106 Silk et al examined subjects with ADHD between ages 8 and 18.107 The mean FA of the whole brain for both groups increased with age, with localized increases in FA in the ADHD subjects. Further analysis suggested that these increases were in fact due to decreased neural branching in subjects with ADHD. Looking specifically at the FA of the basal ganglia in the same subject pool, they also found differences in the developmental trajectory of the caudate.108 Similarly, Tamm et al also found greater FA and axial diffusivity in ADHD subjects specifically in frontostriatal pathways, perhaps due to decreased branching (Supplementary Figure 2).109 Lawrence et al found that white matter disruptions in individuals with ADHD were also found to some degree in their siblings, suggesting a strong familial factor.110
Supplementary Figure 2. Compromised white matter integrity in attention deficit-hyperactivity disorder (ADHD). Regions of significant differences between adolescents with ADHD and controls shown in coronal, axial and sagittal views from the tract-based spatial statistics analysis. The white matter skeleton used in this analysis is displayed in yellow. Regions in which children with ADHD had higher fractional anisotropy (FA) are shown in red. Regions in which children with ADHD had higher axial diffusivity (AD) values than controls are shown in light blue. Group differences were “thickened” for visualization purposes, shown in red and blue for FA and AD respectively (ie, lighter colors represent the actual skeleton and the darker colors are the areas that were “thickened”). The bottom right panel of the figure shows the frontostriatal mask used in the analysis. Reproduced from ref 109: Tamm L, Bamea-Goralv N, Reiss AL. Diffusion tensor imaging reveals white matter abnormalities in Attention-Deficit/Hyperactivity Disorder. Psychiatry Res: Neuroimage. 2012;202:150-154. Copyright © Elsevier 2012.
Functional connectivity
A few studies have found that the functional connectivity within the DMN (default mode network) is disrupted or decreased in ADHD.106,111 Along with increases in the regional homogeneity in the occipital cortex, decreases in the regional homogeneity of the frontostriatal-cerebellar circuits were found in boys with ADHD.112 This fits with some current hypotheses regarding the pathophysiology of ADHD. Using graph theory, decreased global efficiency and increased local efficiency in ADHD were found, pointing to a shift from the typical “small-world” networks towards less biological “regular” networks.113 Small-world networks have a balance of network integration and segregation and are most efficient, while a regular or lattice network is highly segregated, a topology that is rarely found in functioning biological networks.
Neurogenetic disorders
Fragile X syndrome
Fragile X (FX) is caused by an expansion of the CGG repeat in the 5’ untranslated region of the fragile X mental retardation 1 (FMR1) gene, leading to a loss or decrease in functionality of fragile X mental retardation protein (FMRP). It is a common genetic cause of intellectual disability,114 especially in boys.
Structural MRI
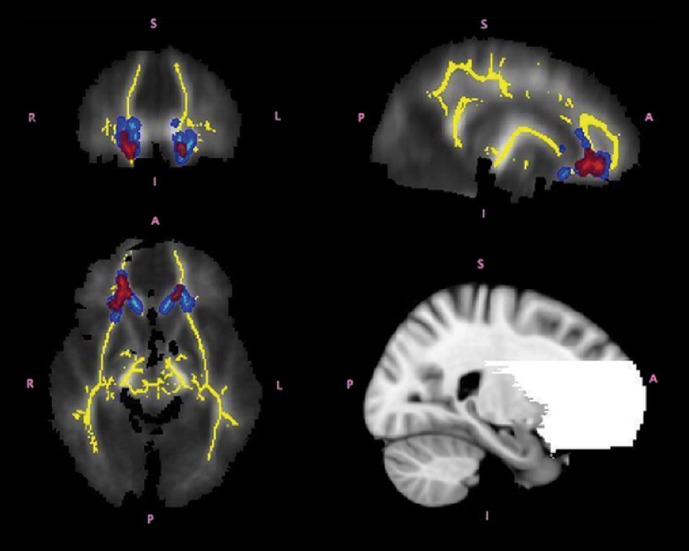
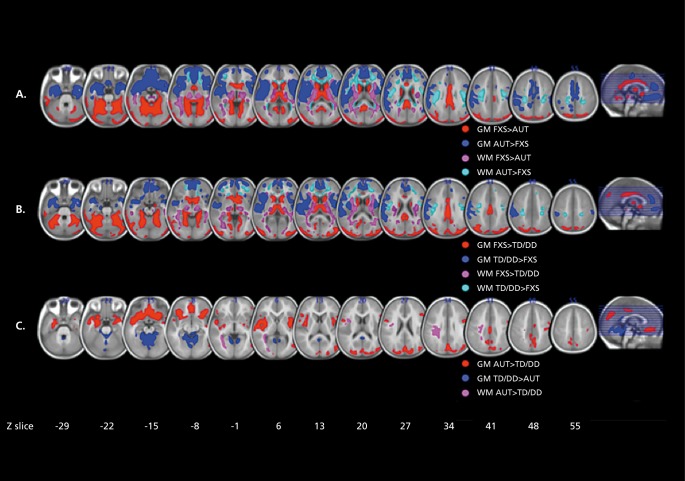
In a longitudinal study, Hoeft et al found altered developmental trajectories in the gray matter volume of the orbital gyri, basal forebrain, and thalamus in young boys with FX, along with a number of differences that persisted across development.115 Differences in the white matter volume of the frontostriatal regions became more pronounced with age. Also using a longitudinal design, Hazlett et al found generalized brain overgrowth in boys with FX, especially in the temporal lobe, cerebellum, and caudate.116 Looking at a main effect of diagnosis, Lee et al found volumetric increases in the caudate and ventricles—abnormalities that correlated with the degree of reduction in the FMRP protein in females.117 Comparing boys with FX with those with AD, idiopathic developmental delay, and typically developing boys, Hoeft et al found widespread reductions in frontal and temporal gray and white matter in young boys with FX (Figure 7).118
Figure 7. Differences in regional brain volume in fragile X. A: Regions showing significant differences in regional gray matter (GM) volume and white matter (WM) volume between fragile X syndrome (FXS) and idiopathic autism (iAUT) (panel A), FXS and typically developing (TD) and idiopathic developmentally delayed (DD) controls (panel B), and iAUT and TD/DD controls (panel C). The left side shows the right hemisphere. The statistical threshold is set at P=0.01 , familywise error cluster-level corrected. Montreal Neurological Institute coordinates. Adapted from ref 118: Hoeft F, Walter E, Lightbody A, et al. Neuroanatomies! differences in toddler boys with fragile X syndrome and idiopathic autism. Arch Gen Psychiatry. 2011;68:295-305. Copyright © American Medical Association 2011.
Diffusion-weighted imaging
Studies of white matter integrity in fragile X report abnormalities mainly in the frontostriatal pathways. Decreased FA in females with FX, and increased fiber density in males with FX, have both been found in frontostriatal regions.119,120
Functional connectivity
We were unable to find any studies of functional connectivity in Fragile X.
22q11.2 Deletion syndrome
22q11.2 Deletion syndrome (22q DS), also called velocardiofacial syndrome and DiGeorge syndrome (among other names), is caused by a deletion on chromosome 22 and results in a heterogeneous spectrum of physiological, neurological, and psychological symptoms.121 Several of the 30 genes encoded in the deleted segment are highly expressed in the developing brain and known to affect early neuronal migration. Several neuroimaging studies have pointed to abnormal patterns of cortical thinning and white matter impairments.
Structural MRI
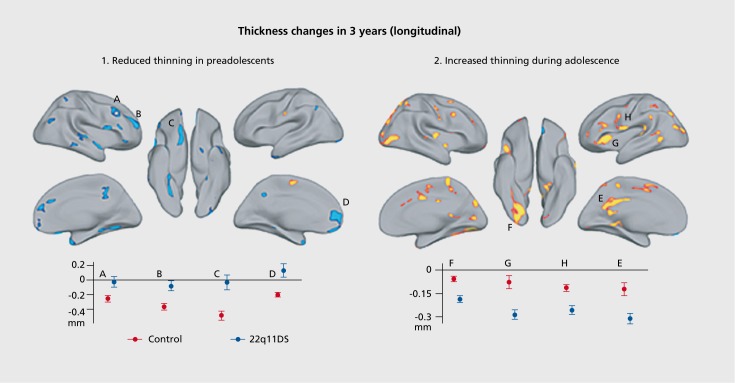
In a cross-sectional study, Schaer et al found altered developmental trajectories of cortical thickness in 22q DS, with a decreased rate of thinning in childhood followed by an increased rate of cortical thinning in late adolescence (Supplementary Figure 3).122
Supplementary Figure 3. Differences in cortical thickness in 22q11.2 DS. Using repeated-measures with the longitudinal subsample, they confirm the different trajectories of cortical thickness changes observed with cross-sectional design. 122 In preadolescents (before 9 of age at Time 1), they observe numerous clusters where no thickness changes occur in patients, whereas thinning is observed in controls. In clusters A to D, this pattern of delayed thinning reaches significance at a threshold of P< 0.007. Contrarily, they observed greater thickness loss in affected adolescents compared with controls (older than 9 at Time 1). This greater thinning with age in patients compared with controls is significant at P< 0.002. Reproduced from ref 122: Schaer M, Debbane M, Cuadra MB, et al. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): Across-sectional and longitudinal study. Schizophr Res. 2009;115:182-90. Copyright © Elsevier 2009.
This study built on earlier work by Bearden et al suggesting regionally specific cortical thinning in 22q DS, in superior parietal cortices and right parie to-occipital cortex, regions critical for visuospatial processing, and bilaterally in the most inferior portion of the inferior frontal gyrus (pars orbitalis), a key area for language development.123 A later study of the same cohort also used fractal dimension analysis to reveal altered complexity and gyrification in 22q DS,124 a further index of disturbed cortical development. Studies of volumetric changes in the gray matter in 22q DS have found reductions in the cerebellum and posterior areas of the posterior and occipital areas and expansions in the frontal lobes,125,126 although one group found reductions in the frontal lobe as well.127 Looking specifically at which individuals with 22q DS developed psychosis, Gothelf et al found that greater reduction of the left DLPFC predicted greater psychotic symptoms. 22q DS is a heterogeneous disorder, which predisposes individuals to a range of other psychiatric and neurological issues.128 This heterogeneity might explain some of the lack of agreement across studies. In the white matter, volume is reduced in individuals with 22q DS, across the cerebellum, internal capsule, and frontal cortex.125,126 Srivastava et al found abnormalities in the development of the cortical gyri in children with 22q DS, specifically in areas important for visuospatial, attentional, and numerical cognition tasks.129
Diffusion-weighted imaging
Widespread changes in FA in 22q DS, including decreased FA in the corpus callosum, and increased FA in the cingulate and parietal lobe,125 and widespread effects, with individuals with 22q DS (velocardiofacial syndrome in the paper) having decreased FA across the frontal, parietal, and temporal white matter130 have also been found. In a more recent study, Villalon et al found that girls with 22q11.2DS showed lower fractional anisotropy (FA) than controls in the association fibers of the superior and inferior longitudinal fasciculi, the splenium of the corpus callosum, and the corticospinal tract.131
Functional connectivity
Only one study, to our knowledge, has examined howindividuals with 22q DS differ in functional connectivity. Debbane et al found widespread changes in functional connectivity using ICA to compare networks between groups.132 Individuals with 22q DS had altered connectivity in the default mode network, with both increased connectivity between lateral frontal regions and the inferior parietal lobule and decreased connectivity between medial frontal regions and the precuneus. They also found altered connectivity in visual, sensorimotor, and visuospatial networks.
Williams syndrome
Williams syndrome (WS) is a disorder caused by a hemizygous deletion of chromosome 7qll.23 resulting in physiological, intellectual, and behavioral abnormalities.133
Structural MRI
Thompson et al found a pattern of excesses and deficits in cortical thickness in WS, along with alterations in the complexity of the gyral pattern.134 Using a fractal dimension analysis of the cortical surface, abnormalities of gyral folding were found, consistent with reports of altered sulcal patterns, especially in perisylvian regions, in people with WS.135 The analysis of cortical patterns using surface-based analyses of local curvature has also revealed gyral-sulcal anomalies in WS.136,137 The shape anomalies are also found subcortically at midline,138 and have been characterized using mesh-based shape analysis methods. Boddaert et al found reductions in the gray matter volume of the left parieto-occipital region in children with WS, which overlapped with prior findings in adults with WS.139 Chiang et al similarly found reductions in the parietal and occipital regions, along with subcortical structures including the basal ganglia and thalamus, and the volumes of these structures were positively correlated with IQ.140 Meda et al also found the basal ganglia to be significantly reduced in WS.141 Additionally, individuals with WS had increased cortical thickness and/or decreased surface area in a number of ROIs across parietal, occipital, and frontal regions. Even the smaller subcortical gray matter nuclei, such as the amygdala142 and caudate nucleus,143 show shape anomalies in WS, and their implications for cognition and behavior are only just beginning to be understood.
Diffusion-weighted imaging
DWI studies of Williams syndrome have shown increases in FA in the superior longitudinal fasciculus and inferior longitudinal fasciculus, association pathways important for language, memory, visuospatial processing, and object processing, to name a few (Figure 8).144-146 Reduced FA in the uncinate fasciculus—one of the fiber tracts connecting the limbic system—has also been found.145-147 Looking specifically at tracts related to the fusiform gyrus, Haas et al found both an increased volume of fibers and increased FA in individuals with WS. Face processing is altered in WS, and these results may explain these abnormalities.148
Figure 8. (opposite) Compromised white matter integrity in Williams syndrome (WS). Voxel-based comparison of fractional anisotropy (FA) in WS compared with norma! controls. Overlay of regions of significantly increased (warm colors) and reduced (cool colors) FA in WS compared with controls on coronal slices of the average FA template in Talairach space (images displayed according to radiological convention, ie, the left hemisphere is shown on the right). Reproduced from ref 145: Arlinghaus LR, Thornton-Wells TA, Dykens EM, Anderson AW. Alterations in diffusion properties of white matter in Williams syndrome. Magn Res Imaging. 2011;29:1165-1174. Copyright © Elsevier 2011.
Functional connectivity
We were unable to find any studies examining functional connectivity in WS.
Chromosomal disorders
Down syndrome
Down syndrome (DS), or trisomy 21, is a common chromosomal disorder and the most common cause of intellectual disability.149 There are surprisingly few brain imaging studies of DS in children. DS increases the risk of developing Alzheimer's-like dementia with age, so many more studies focus on adults with DS.150
Structural MRI
Total brain volume is decreased in DS,151,152 and certain structures are disproportionately affected. Consistent with adult imaging studies, the hippocampus is reduced in DS, but there is conflicting information as to whether the amygdala is as well.151,152 Children with DS were found to have reduced frontal and temporal lobe volumes.152 The differences in the hippocampus are particularly intriguing given the increased risk for dementia in DS individuals.
Diffusion-weighted imaging
We were unable to find any studies of white matter integrity in DS in children.
Functional connectivity
To our knowledge, no studies have examined functional connectivity in DS.
Turner syndrome
Another chromosomal disorder, Turner syndrome (TS) results from the absence of one X chromosome in girls, resulting in a number of changes physically, hormonally, and neurologically.153
Structural MRI
A number of studies have examined brain volume in TS, generally finding decreased brain volume in the parietal and occipital regions.154-156 The hippocampus and subcortical structures such as the thalamus and basal ganglia are also reduced in TS,154,157 but the amygdala is larger.157
Diffusion-weighted imaging
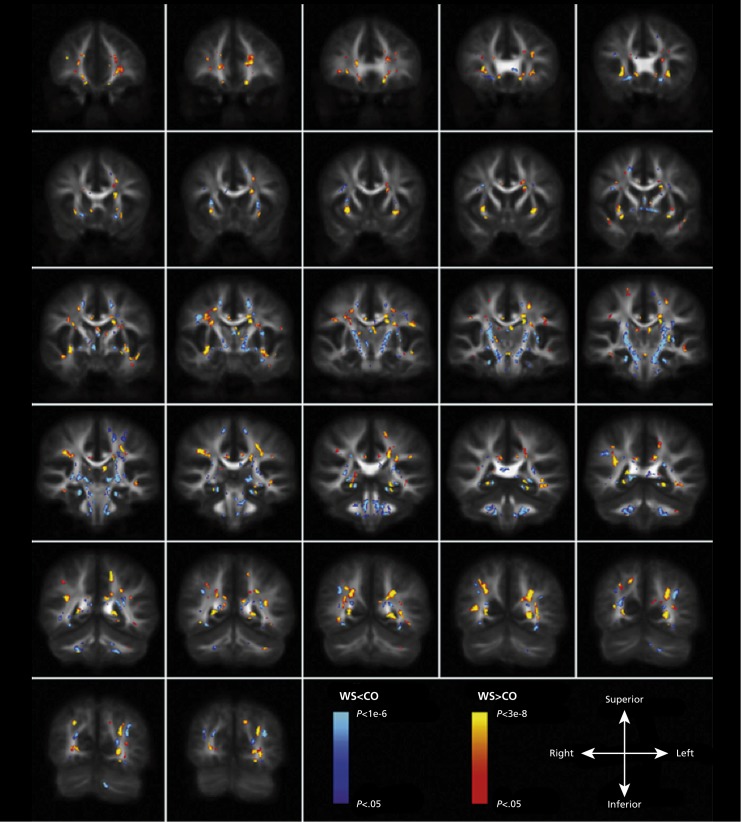
DWI studies in TS reveal abnormalities across a large area of the white matter. Molko et al found microstructural differences in the temporal lobe, especially tracts running anterior-posterior in the temporal lobe.158 Holzapfel et al found lower FA in the pallidum, internal capsule, and prefrontal cortex, as well as in the parieto-occipital region, extending into the superior longitudinal fasciculus.159 Yamagata et al found lower FA in a wide array of regions implicated in visuopatial processing, face processing, and sensorimotor and social abilities, including a number of association, commissural, and projection fibers (Supplementary Figure 4).160 In one DTI study comparing TS with Fragile X syndrome and 22q DS, Villalon et al, found that girls with TS had lower FA in the inferior longitudinal fasciculus, right internal capsule and left cerebellar peduncle.131 Even so, partially overlapping white matter anomalies were detected in all three neurogenetic disorders. They suggested that altered white matter integrity in the superior and inferior longitudinal fasciculi and thalamic to frontal tracts may contribute to the behavioral characteristics of all of these disorders.
Supplementary Figure 4. Differences in white matter and gray matter in Turner syndrome (TS). Superimposed results of voxels showing significant fractional anisotropy (FA) reduction in the tract-based spatial statistics (TBSS) and voxel-based morphometry (VBM) clusters showing significant white matter volume (WMV) differences between the groups (P< 0.05, familywise error rate corrected). Group differences in TBSS were “thickened” (for visualization purposes) by expanding the significant white matter skeleton cluster to the full extent of the local FA map. 1) FA reduction in TS relative to controls (CON) is shown in red-yellow. 2) Greater WMV in TS relative to CON is shown in green. 3) Reduced WMV in TS relative to CON is shown in blue. Results are mapped onto a standard T1 -weighted Montreal Neurological Institute 152 template. Reproduced from ref 160: Yamagata B, Barnea-Goraly N, Marzelli MJ, et al. White matter aberrations in prepubertal estrogen-naive girls with monosomic Turner syndrome. Cereb Cortex. 2012;22:2761-2768. Copyright © Oxford University Press 2012.
Functional connectivity
Based on the known deficits girls with TS experience in working memory tasks, one study examined functional connectivity during a working memory task.161 Reduced connectivity was found between parietal and dorsal frontal regions, which correlated with task performance. A second study examined the specific connectivity of the posterior parietal cortex, finding differential clustering in TS, which may underlie the visuospatial processing deficits in TS.162
Conclusion
In this paper, we have reviewed representative research over the last 20 years investigating brain development using neuroimaging techniques. We discussed both healthy development and neurodevelopmental disorders, including autism, ADHD, fragile X, 22q DS, Williams syndrome, Down syndrome, and Turner syndrome. Hie brain undergoes remarkable changes in structure and connectivity as it matures into adulthood. The developmental trajectory of these brain measures is important to identify for our fundamental understanding of the brain and of neurodevelopmental disorders. Disrupted brain structure or connectivity can lead to neurodevelopmental or neuropsychiatric disorders. Understanding these disorders and their developmental trajectory in greater detail should expedite the discovery and more efficient evaluation of effective interventions.
Selected abbreviations and acronyms
- DMN
default mode network
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FX
fragile X
- HARDI
high angular resolution diffusion imaging
- ICA
independent components analysis
- TBM
tensor-based morphometry
- VBM
voxel-based morphometry
Contributor Information
Emily L. Dennis, Imaging Genetics Center, Laboratory of Neuro Imaging, Dept of Neurology & Psychiatry, UCLA School of Medicine, Los Angeles, California, USA.
Paul M. Thompson, Imaging Genetics Center, Laboratory of Neuro Imaging, Dept of Neurology & Psychiatry, UCLA School of Medicine, Los Angeles, California, USA.
REFERENCES
- 1.Gogtay N., Giedd JN., Lusk L., et al Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huttenlocher PR., Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore S-J., Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 4.Thomason ME., Dassanayake MT., Shen S., et al Cross-hemispheric functional connectivity in the human fetal brain. Sci Transl Med. 2013;5:173ra24. doi: 10.1126/scitranslmed.3004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedman AM., Van Haren NEM., Schnack HG., Kahn RS., Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33:1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Power JD., Fair DA., Schlaggar BL., Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uddin LQ., Supekar K., Menon V. Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci. 2010;4:1–12. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paus T., Collins DL., Evans AC., Leonard G., Pike B., Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 9.Cascio CJ., Gerig G., Piven J. Diffusion tensor imaging: application to the study of the developing brain. J Am Acad Child Psychiatry. 2007;46:213–223. doi: 10.1097/01.chi.0000246064.93200.e8. [DOI] [PubMed] [Google Scholar]
- 10.Assaf Y., Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 11.Schmithorst VJ., Wilke M., Dardzinski BJ., Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222:212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walter E., Mazaika PK., Reiss AL. Insights into brain development from neurogenetic syndromes: evidence from fragile X syndrome, Williams syndrome. Turner syndrome and velocardiofacial syndrome. Neuroscience. 2009;164:257–271. doi: 10.1016/j.neuroscience.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giedd JN., Blumenthal J., Jeffries NO., et al Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 14.Lenroot RK., Gogtay N., Greenstein DK., et al Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neurolmage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowell ER., Trauner DA., Gamst A., Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 16.Sowell E., Peterson BS., Kan E., et al Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreasen NC., Arndt S., Swayze V., et al Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 18.Paus T., Zijdenbos A., Worsley K., et al Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 19.Ashburner J., Friston KJ. Voxel-based morphometry - the methods. Neurolmage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 20.Ashburner J., Friston KJ. Morphometry. In: Ashburner J, Friston KJ, Penny W, eds. Human Brain Function. 2nd ed. San Diego, CA: Academic; 2003 [Google Scholar]
- 21.Thompson PM., Giedd JN., Woods RP., MacDonald D., Evans A., Toga A. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nat Lett. 2000;404:1–4. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 22.Dale AM., Fischl B., Sereno Ml. Cortical surface-based analysis. Neurolmage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 23.Thompson PM., Hayashi KM., Sowell ER., et al Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. Neurolmage. 2004;23:S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PM., Vidal C., Giedd JN., et al Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very earlyonset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidal CN., Rapoport JL., Hayashi KM., et al Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63:25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Gogtay N., Ordonez A., Herman DH., et al Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PM., Sowell ER., Gogtay N., et al Structural MRI and brain development. Int Rev Neurobiol. 2005;67:285–323. doi: 10.1016/S0074-7742(05)67009-2. [DOI] [PubMed] [Google Scholar]
- 28.Bartzokis G., Lu PH., Tingus K., et al Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sowell ER., Peterson BS., Thompson PM., Welcome SE., Henkenius AL., Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 30.Sowell ER., Thompson PM., Leonard CM., Welcome SE., Kan E., Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua X., Leow AD., Levitt JG., Caplan R., Thompson PM., Toga AW. Detecting brain growth patterns in normal children using tensor-based morphometry. Hum Brain Mapp. 2009;30:209–219. doi: 10.1002/hbm.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamnes CK., Ostby Y., Fjell AM., Westlye LT., Due-Tonnessen P., Walhovd KB. Brain maturation in adolescence and young adulthood: regional agerelated changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 33.Stejskal EO., Tanner JE. Spin Diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- 34.Jahanshad N., Aganj I., Lenglet C., et al Sex differences in the human connectome: 4-Tesla high angular resolution diffusion imaging (HARDI) tractography in 234 young adult twins. In: Proc. 8th IEEE ISBI, Chicago. 2011:939–943. [Google Scholar]
- 35.Tuch DS., Reese TG., Wiegell MR., Makris N., Belliveau JW., Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- 36.Tournier JD., Calamante F., Gadian DG., Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neurolmage. 2004;23:1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 37.Zhan L., Leow AD., Barysheva M., et al Investigating the uncertainty in multi-fiber estimation in High Angular Resolution Diffusion Imaging. In: Pohl K, Joshi S, Wells S. Workshop on Probabilistic Modeling in Medical Image Analysis (PMMIA), Medical Image Computing and Computer Assisted Intervention (MiCCAl). London, UK; 2009 [Google Scholar]
- 38.Cetingul HE., Afsari B., Wright MJ., Thompson PM., Vidal R. Group action induced averaging for HARDI processing. In: Proc 9th IEEE ISBI, Barcelona. 2012:1389–1392. doi: 10.1109/ISBI.2012.6235827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cetingul HE., Nadar M., Thompson P., Sapiro G., Lenglet C. Simultaneous ODF Estimation and Tractography in HARDI. In: Proc. 24th IEEE EMBS. San Diego; 2012:86–89. doi: 10.1109/EMBC.2012.6345877. [DOI] [PubMed] [Google Scholar]
- 40.Jin Y., Shi Y., Zhan L., et al Automatic population hardi white matter tract clustering by label fusion of multiple tract atlases. Paper presented at: Workshop on Multimodal Brain Imaging Analysis, Medical Image Computing and Computer Assisted Intervention (MICCAI). 2012. Nice doi: 10.1007/978-3-642-33530-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonekamp D., Nagae LM., Degaonkar M., et al Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and agerelated differences. Neurolmage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klingberg T., Vaidya CJ., Gabrieli JD., Moseley ME., Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. NeuroReport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 43.Schmithorst VJ., Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Kochunov P., Thompson PM., Lancaster JL., et al Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neurolmage. 2007;35:478–187. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Barnea-Goraly N., Menon V., Eckert M., et al White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 46.Eluvathingal TJ., Hasan KM., Kramer L., Fletcher JM., Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu D., Tan L-H., Zhou K., Khong P-L. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: Voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neurolmage. 2008;41:223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neurolmage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 49.Kochunov P., Williamson DE., Lancaster J., et al Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2010:1–12. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagmann P., Sporns O., Madan N., et al White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci U S A. 2010;107:19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sporns O., Chialvo DR., Kaiser M., Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–25. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Dennis EL., Jahanshad N., McMahon KL., et al Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. Neurolmage. 2013;64:671–684. doi: 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toga AW., Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 54.Luders E., Rex DE., Narr KL., et al Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- 55.Dennis EL., Jahanshad N., Toga AW., et al Development of the “rich club” in brain connectivity networks from 438 adolescents & adults aged 12 to 30. In: Proc. 10th IEEE ISBI, San Francisco. 2013:620–623. doi: 10.1109/ISBI.2013.6556552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van den Heuvel MP., Sporns O. Rich-Club Organization of the Human Connectome. J Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beckmann CF., DeLuca M., Devlin JT., Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos T Roy Soc B. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fox MD., Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 59.Fair DA., Dosenbach NUF., Church JA., et al Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fair DA., Cohen AL., Dosenbach NUF., et al The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raichle ME., MacLeod AM., Snyder AZ., Powers WJ., Gusnard DA., Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox MD., Snyder AZ., Vincent JL., Corbetta M., Essen DCV., Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly AMC., Di Martino A., Uddin LQ., et al Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 64.Dosenbach NUF., Nardos B., Cohen AL., et al Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Supekar K., Uddin LQ., Prater K., Amin H., Greicius MD., Menon V. Development of functional and structural connectivity within the default mode network in young children. Neurolmage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hebb DO. The Organization of Behavior. New York, NY: Wiley & Sons; 1949 [Google Scholar]
- 67.Thomason ME., Dennis EL., Joshi AA., et al Resting-state fMRI can reliably map neural networks in children. Neurolmage. 2011;55:165–175. doi: 10.1016/j.neuroimage.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jolles DD., van Buchem MA., Crone EA., Rombouts SARB. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb Cortex. 2011;21:385–91. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- 69.Kim YS., Leventhal BL., Koh Y-J., et al Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168:904–912. doi: 10.1176/appi.ajp.2011.10101532. [DOI] [PubMed] [Google Scholar]
- 70.Lord C., Risi S., Lambrecht L., Cook EH Jr., et al The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Dis. 2000;30:205–223. [PubMed] [Google Scholar]
- 71.Szatmari P., Paterson AD., Zwaigenbaum L., et al Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langen M., Schnack HG., Nederveen H., et al Changes in the developmental trajectories of striatum in autism. Biol Psychiat. 2009;66:327–333. doi: 10.1016/j.biopsych.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 73.Hardan AY., Libove RA., Keshavan MS., Melhem NM., Minshew NJ. A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol Psychiatry. 2009;66:320–326. doi: 10.1016/j.biopsych.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brun CC., Nicolson R., Lepore N., et al Mapping brain abnormalities in boys with autism. Hum Brain Mapp. 2009;30:3887–900. doi: 10.1002/hbm.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mengotti P., D'Agostini S., Terlevic R., et al Altered white matter integrity and development in children with autism: a combined voxel-based morphometry and diffusion imaging study. Brain Res Bull. 2011;84:189–195. doi: 10.1016/j.brainresbull.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Hua X., Thompson PM., Leow AD., et al Brain growth rate abnormalities visualized in adolescents with autism. Hum Brain Mapp. 2013;34:425–436. doi: 10.1002/hbm.21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barnea-Goraly N., Kwon H., Menon V., Eliez S., Lotspeich L., Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 78.Barnea-Goraly N., Lotspeich L., Reiss A. Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Arch Gen Psych. 2010;67:1052–1060. doi: 10.1001/archgenpsychiatry.2010.123. [DOI] [PubMed] [Google Scholar]
- 79.Shukla DK., Keehn B., Lincoln AJ., Mtiller RA. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:1269–1278.e2. doi: 10.1016/j.jaac.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Courchesne E., Carper R., Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 81.Herbert MR., Ziegler DA., Makris N., et al Larger brain and white matter volumes in children with developmental language disorder. Dev Sci. 2003;6:F11–F22. [Google Scholar]
- 82.Bashat DB., Kronfeld-Duenias V., Zachor DA., et al Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neurolmage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 83.Keller TA., Kana RK., Just MA. A developmental study of the structural integrity of white matter in autism. NeuroReport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- 84.Cherkassky VL., Kana RK., Keller TA., Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17:1687–16890. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- 85.Just MA., Cherkassky VL., Keller TA., Kana RK., Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an fMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kennedy DP., Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neurolmage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 87.Weng S-J., Wiggins JL., Peltier SJ., et al Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Assaf M., Jagannathan K., Calhoun VD., et al Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neurolmage. 2010;53:247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monk CS., Peltier SJ., Wiggins JL., et al Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neurolmage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noonan SK., Haist F., Muller R-A. Aberrant functional connectivity in autism: Evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rudie JD., Shehzad Z., Hernandez LM., et al Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 2012;22:1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rudie JD., Brown JA., Beck-Pancer D., et al Altered functional and structural brain network organization in autism. Neurolmage. 2013;2:79–94. doi: 10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson JC., Williams S., McGee R., Silva PA. DSM-III disorders in preadolescent children. Prevalence in a large sample from the general population. Arch Gen Psych. 1987;44:69–76. doi: 10.1001/archpsyc.1987.01800130081010. [DOI] [PubMed] [Google Scholar]
- 94.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 95.Carmona S., Vilarroya O., Bielsa A., et al Global and regional gray matter reductions in ADHD: a voxel-based morphometry study. Neurosci Lett. 2005;389:88–93. doi: 10.1016/j.neulet.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 96.Castellanos FX., Lee PP., Sharp W., et al Developmental trajectories of brain volume abnormalities in children and adolescents with attentiondeficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 97.Brieber S., Neufang S., Bruning N., et al Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J Child Psychol Psyc. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 98.Kobel M., Bechtel N., Specht K., et al Structural and functional imaging approaches in attention deficit/hyperactivity disorder: does the temporal lobe play a key role? Psychiat Res: Neurolmage. 2010;183:230–236. doi: 10.1016/j.pscychresns.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 99.Luders E., Narr KL., Hamilton LS., et al Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry . 2009;65:84–88. doi: 10.1016/j.biopsych.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sowell ER., Thompson PM., Welcome SE., Henkenius AL., Toga AW., Peterson BS. Cortical abnormalities in children and adolescents with attention deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- 101.Shaw P., Lerch J., Greenstein D., et al Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attentiondeficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 102.Shaw P., Eckstrand K., Sharp W., et al Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ashtari M., Kumra S., Bhaskar SL., et al Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 104.Hamilton LS., Levitt JG., O Neill J., et al Reduced white matter integrity in attention-deficit hyperactivity disorder. NeuroReport. 2008;19:1705–1708. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pavuluri MN., Yang S., Kamineni K., et al Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attentiondeficit/hyperactivity disorder. Biol Psychiatry . 2009;65:586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qiu M-G., Ye Z., Li Q-Y., Liu G-J., Xie B., Wang J. Changes of brain structure and function in ADHD children. Brain Topogr. 2011;24:243–252. doi: 10.1007/s10548-010-0168-4. [DOI] [PubMed] [Google Scholar]
- 107.Silk TJ., Vance A., Rinehart N., Bradshaw JL., Cunnington R. Structural development of the basal ganglia in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Psychiatry Res: Neuroimage. 2009;172:220–225. doi: 10.1016/j.pscychresns.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 108.Silk TJ., Vance A., Rinehart N., Bradshaw JL., Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009;30:2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tamm L., Barnea-Goraly N., Reiss AL. Diffusion tensor imaging reveals white matter abnormalities in attention-deficit/hyperactivity disorder. Psychiatry Res: Neurolmage. 2012;202:150–154. doi: 10.1016/j.pscychresns.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lawrence KE., Levitt JG., Loo SK., et al white matter microstructure in attention-def icit/hyperactivity disorder subjects and their siblings. J Am Acad Child Psychiatry. 2013:1–15. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fair DA., Posner J., Nagel BJ., et al Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cao Q., Zang Y., Sun L., et al Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting- state functional magnetic resonance imaging study. NeuroReport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- 113.Wang L., Zhu C., He Y., et al Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:638–649. doi: 10.1002/hbm.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verkerk AJ., Pieretti M., Sutcliffe JS., et al Identification of a gene (FMR1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 115.Hoeft F., Carter JC., Lightbody AA., Hazlett HC., Piven J., Reiss AL. Regionspecific alterations in brain development in one- to three-year-old boys with fragile X syndrome. Proc Natl Acad Sci U S A. 2010;107:9335–9339. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hazlett HC., Poe MD., Lightbody AA., et al Trajectories of early brain volume development in fragile X syndrome and autism. J Am Acad Child Adolesc Psychiatry. 2012;51:921–933. doi: 10.1016/j.jaac.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee AD., Leow AD., Lu A., et al 3D Pattern of brain abnormalities in fragile X syndrome visualized using tensor-based morphometry. Neurolmage. 2007;34:924–938. doi: 10.1016/j.neuroimage.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoeft F., Walter E., Lightbody A., et al Neuroanatomical differences in toddler boys with fragile X syndrome and idiopathic autism. Arch Gen Psychiatry. 2011;68:295–305. doi: 10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barnea-Goraly N., Eliez S., Hedeus M., et al White matter tract alterations in fragile X syndrome: Preliminary evidence from diffusion tensor imaging. Am J Med Genet. 2003;1186:81–88. doi: 10.1002/ajmg.b.10035. [DOI] [PubMed] [Google Scholar]
- 120.Haas BW., Barnea-Goraly N., Lightbody A., et al Early white-matter abnormalities of the ventral frontostriatal pathway in fragile X syndrome. Dev Med Child Neurol. 2009;51:593–599. doi: 10.1111/j.1469-8749.2009.03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shprintzen RJ. Velo-cardio-facial syndrome: a distinctive behavioral phenotype. Ment Retard Dev D R. 2000;6:142–147. doi: 10.1002/1098-2779(2000)6:2<142::AID-MRDD9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 122.Schaer M., Debbane M., Cuadra MB., et al Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): Across-sectional and longitudinal study. Schizophr Res. 2009;115:182–90. doi: 10.1016/j.schres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 123.Bearden CE., Van Erp TGM., Dutton RA., et al Alterations in midline cortical thickness and gyrif ication patterns mapped in children with 22q11.2 deletions. Cereb Cortex. 2009;19:115–126. doi: 10.1093/cercor/bhn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bearden CE., Van Erp TGM., Dutton RA., et al Mapping cortical thickness in children with 22q11.2 deletions. Cereb Cortex. 2007;17:1889–1898. doi: 10.1093/cercor/bhl097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simon TJ., Ding L., Bish JP., McDonald-McGinn DM., Zackai EH., Gee J. Volumetric, connective, and morphologic changes in the brains of children with chromosome 22q11.2 deletion syndrome: an integrative study. Neurolmage. 2005;25:169–180. doi: 10.1016/j.neuroimage.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 126.Campbell LE., Campbell LE., Daly E., et al Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006;129:1218–1228. doi: 10.1093/brain/awl066. [DOI] [PubMed] [Google Scholar]
- 127.Shashi V., Kwapil TR., Kaczorowski J., et al Evidence of gray matter reduction and dysfunction in chromosome 22q11.2 deletion syndrome. Psychiatry Res: Neuroim. 2010;181:1–8. doi: 10.1016/j.pscychresns.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gothelf D., Hoeft F., Ueno T., et al Developmental changes in multivariate neuroanatomical patterns that predict risk for psychosis in 22q11.2 deletion syndrome. J Psychiatry Res. 2011;45:322–331. doi: 10.1016/j.jpsychires.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Srivastava S., Buonocore MH., Simon TJ. Atypical developmental trajectory of functionally significant cortical areas in children with chromosome 22q11.2 deletion syndrome. Hum Brain Mapp. 2012;33:213–223. doi: 10.1002/hbm.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barnea-Goraly N., Menon V., Krasnow B., Ko A., Reiss A., Eliez S. Investigation of white matter structure in velocardiofacial syndrome: a diffusion tensor imaging study. Am J Psychiatry. 2003;160:1863–1869. doi: 10.1176/appi.ajp.160.10.1863. [DOI] [PubMed] [Google Scholar]
- 131.Villalon J., Jahanshad N., Beaton E., Toga AW., Thompson PM., Simon TJ. White matter microstructural abnormalities in children with chromosome 22q11.2 deletion syndrome. Fragile X or Turner syndrome as evidenced by diffusion tensor imaging. Neurolmage. In press. doi: 10.1016/j.neuroimage.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Debbane M., Lazouret M., Lagioia A., Schneider M., Van De Ville D., Eliez S. Resting-state networks in adolescents with 22q11.2 deletion syndrome: Associations with prodromal symptoms and executive functions. Schizophr Res. 2012;139:33–39. doi: 10.1016/j.schres.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 133.Bellugi U., Lichtenberger L., Jones W., Lai Z., George MS. I. The neurocognitive profile of Williams syndrome: a complex pattern of strengths and weaknesses. J Cogn Neurosci. 2001;12:7–29. doi: 10.1162/089892900561959. [DOI] [PubMed] [Google Scholar]
- 134.Thompson PM., Lee AD., Dutton RA., et al Abnormal cortical complexity and thickness profiles mapped in Williams syndrome. J Neurosci. 2005;25:4146–4158. doi: 10.1523/JNEUROSCI.0165-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eckert MA., Galaburda AM., Karchemskiy A., et al Anomalous sylvian fissure morphology in Williams syndrome. Neurolmage. 2006;33:39–45. doi: 10.1016/j.neuroimage.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 136.Gaser C., Luders E., Thompson PM., et al Increased local gyrification mapped in Williams syndrome. Neurolmage. 2006;33:46–54. doi: 10.1016/j.neuroimage.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 137.Tosun D., Reiss AL., Lee AD., et al Use of 3-D cortical morphometry for mapping increased cortical gyrification and complexity in Williams syndrome. In: Proc. 3rd IEEE ISBI, Arlington. 2006:1172–1175. [Google Scholar]
- 138.Luders E., Di Paola M., Tomaiuolo F., et al Callosal morphology in Williams syndrome: a new evaluation of shape and thickness. NeuroReport. 2007;18:203–207. doi: 10.1097/WNR.0b013e3280115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Boddaert N., Mochel F., Meresse l., et al Parieto-occipital grey matter abnormalities in children with Williams syndrome. Neurolmage. 2006;30:721–725. doi: 10.1016/j.neuroimage.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 140.Chiang M-C., Reiss AL., Lee AD., et al 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. Neurolmage. 2007;36:1096–1109. doi: 10.1016/j.neuroimage.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meda SA., Pryweller JR., Thornton-Wells TA. Regional brain differences in cortical thickness, surface area and subcortical volume in individuals with Williams syndrome. PLoS ONE. 2012;7:e31913. doi: 10.1371/journal.pone.0031913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haas BW., Sheau K., Kelley RG., Thompson PM., Reiss AL. Regionally specific increased volume of the amygdala in Williams syndrome: evidence from surface-based modeling. Hum Brain Mapp. In press. doi: 10.1002/hbm.22219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peng D., Kelley R., Quintin E-M., Raman M., Thompson P., Reiss AL. Cognitive and behavioral correlates of caudate subregion shape variation in Fragile X syndrome. Hum Brain Mapp. In press. doi: 10.1002/hbm.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoeft F., Barnea-Goraly N., Haas BW., et al More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci. 2007;27:11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Arlinghaus LR., Thornton-Wells TA., Dykens EM., Anderson AW. Alterations in diffusion properties of white matter in Williams syndrome. Magn Res Imaging. 2011;29:1165–1174. doi: 10.1016/j.mri.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Catani M., de Schotten MT. Atlas of Human Brain Connections. Oxford, UK: Oxford University Press; 2012 [Google Scholar]
- 147.Jabbi M., Kippenhan JS., Kohn P., et al The Williams syndrome chromosome 7q11.23 hemideletion confers hypersocial, anxious personality coupled with altered insula structure and function. Proc Natl Acad Sci U S A. 2012:E860–E866. doi: 10.1073/pnas.1114774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Haas BW., Hoeft F., Barnea-Goraly N., Golarai G., Bellugi U., Reiss AL. Preliminary evidence of abnormal white matter related to the fusiform gyrus in Williams syndrome: a diffusion tensor imaging tractography study. Genes Brain Behav. 2012;11:62–68. doi: 10.1111/j.1601-183X.2011.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nadel L. Down syndrome in cognitive neuroscience perspective. In: Tager-Flusberg, H, ed. Neurodevelopmental Disorders. Boston, MA: The MIT Press; 1999 [Google Scholar]
- 150.Stanton LR., Coetzee RH. Down's syndrome and dementia. Advances Psych Treatment. 2004;10:50–58. [Google Scholar]
- 151.Pinter JD., Brown WE., Eliez S., Schmitt JE., Capone GT., Reiss AL. Amygdala and hippocampal volumes in children with Down syndrome: a high- resolution MRI study. Neurology. 2001;56:972–974. doi: 10.1212/wnl.56.7.972. [DOI] [PubMed] [Google Scholar]
- 152.Smigielska-Kuzia J., Bo kowski L., Sobaniec W., et al A volumetric magnetic resonance imaging study of brain structures in children with Down syndrome. Neurology. 2011;45:363–369. doi: 10.1016/s0028-3843(14)60107-9. [DOI] [PubMed] [Google Scholar]
- 153.Lippe B. Turner syndrome. Endocrin Metab Clin. 1991;20:121–152. [PubMed] [Google Scholar]
- 154.Murphy DGM., DeCarli C., Daly E., et al X-chromosome effects on female brain: a magnetic resonance imaging study of Turner's syndrome. Lancet. 1993;342:1197–1200. doi: 10.1016/0140-6736(93)92184-u. [DOI] [PubMed] [Google Scholar]
- 155.Reiss AL., Mazzocco MMM., Greenlaw R., Freund LS., Ross JL. Neurodevelopmental effects of X monosomy: a volumetric imaging study. Ann Neurol. 1995;38:731–738. doi: 10.1002/ana.410380507. [DOI] [PubMed] [Google Scholar]
- 156.Brown WE., Kesler SR., Eliez S., et al Brain development in Turner syndrome: a magnetic resonance imaging study. Psychiat Res: Neuroim. 2002;116:187–196. doi: 10.1016/s0925-4927(02)00086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kesler SR., Garrett A., Bender B., Yankowitz J., Zeng SM., Reiss AL. Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologic. 2004;42:1971–1978. doi: 10.1016/j.neuropsychologia.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Molko N., Cachia A., Riviere D., et al Brain anatomy in Turner syndrome: evidence for impaired social and spatial-numerical networks. Cereb Cortex. 2004;14:840–850. doi: 10.1093/cercor/bhh042. [DOI] [PubMed] [Google Scholar]
- 159.Holzapfel M., Barnea-Goraly N., Eckert MA., Kesler SR., Reiss AL. Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in Turner syndrome. J Neurosci. 2006;26:7007–7013. doi: 10.1523/JNEUROSCI.1764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yamagata B., Barnea-Goraly N., Marzelli MJ., et al White matter aberrations in prepubertal estrogen-naive girls with monosomic Turner syndrome. Cereb Cortex. 2012;22:2761–2768. doi: 10.1093/cercor/bhr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bray S., Dunkin B., Hong DS., Reiss AL. Reduced functional connectivity during working memory in Turner syndrome. Cereb Cortex. 2011;21:2471–2481. doi: 10.1093/cercor/bhr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bray S., Hoeft F., Hong DS., Reiss AL. Aberrant functional network recruitment of posterior parietal cortex in turner syndrome. Hum Brain Mapp. In press. doi: 10.1002/hbm.22131. [DOI] [PMC free article] [PubMed] [Google Scholar]