Abstract
The current anti-cancer therapeutic armamentarium consists of surgery, chemotherapy, radiation, hormonal therapy, immunotherapy, and combinations thereof. Initial treatments usually result in objective clinical responses with prolongation of overall survival (OS) and progression-free survival (PFS) in a large subset of the treated patients. However, at the onset, there is a subset of patients who does not respond and another subset that initially responded but experiences relapses and recurrences. These latter subsets of patients develop a state of cross-resistance to a variety of unrelated therapies. Therefore, there is an urgent need to first unravel the underlying mechanisms of resistance and associated gene products that regulate the cross-resistance. Such gene products are potential therapeutic targets as well as potential prognostic/diagnostic biomarkers. In this context, we have identified three interrelated gene products involved in resistance, namely, Snail, YY1, and RKIP that are components of the dysregulated NF-κB/Snail/YY1/RKIP loop in many cancers. In this review, we will discuss the roles each of Snail, YY1 and RKIP in the regulation of tumor cell resistance to chemo and immunotherapies. Since these same gene products have also been shown to be involved in the regulation of the EMT phenotype and metastasis, we suggest that targeting any of these three gene products can simultaneously inhibit tumor cell resistance and metastasis.
Keywords: Snail, KRIP, YY1, resistance, cancer
I. Introduction
Clinically, most tumors initially respond to conventional therapies such as chemotherapy, radiation, hormonal therapy, immunotherapy and combinations. Such therapies invariably induce cell death in the sensitive tumor cell population. However, there preexists a cell subpopulation that is highly resistant to various cytotoxic therapies. In addition, several responding patients eventually experience relapses and recurrences and no longer respond to further treatments with either the original or different therapeutic regimens. Noteworthy, the cytotoxic therapies mediate their effects, largely, through the programmed cell death pathways or apoptosis. Hence, the resistant tumor cells are equipped with molecular mechanisms that evade the drug-induced activation of the apoptotic pathways.
Tumor cell resistance to apoptosis results, in large part, from the constitutive hyperactivation of several survival/anti-apoptotic pathways such as the NF-κB, the Raf1/MEK/ERK and the PI3K-Akt pathways. These pathways regulate the transcription, expression and activation of both pro- and anti-apoptotic gene products that regulate the apoptotic pathways.1,2 Studies reported by us and others delineated the establishment in tumor cells of the presence of a dysregulated NF-κB/Snail/YY1/RKIP loop3-7 and that this loop was found to be central in the regulation of tumor cell sensitivity or resistance to cytotoxic drug-induced apoptosis.
In this mini-review, we will briefly summarize the findings demonstrating the direct role mediated each by Snail, YY1, and RKIP in the regulation of tumor cell resistance/sensitivity to chemo-immunotherapeutic drugs-induced activation of apoptosis.
II. Role of Snail in the Regulation of Tumor Cell Resistance to Chemo-Immuno Cytotoxic Agents
A. General Properties
The Snail transcription factor is a member of the Snail subfamily of zinc-finger transcription factors. Snail has a conserved C-terminal region containing 4-6 C2H2-type zinc-finger repeats and a more divergent Snail/Gfi (SNAG) domain that is important for repression and which downregulates the expression of ectodermal genes within the mesoderm.8,9 Snail has highly conserved nuclear localization signal (NLS) motifs that are responsible for its nuclear transport. Snail has been shown to play a role in embryonic development, neural differentiation, cell division, and cell survival.10 Snail also plays an important role in the invasion and migratory properties of cancer cells during tumor progression by triggering the epithelial to mesenchymal transition (EMT).9 Snail is transcriptionally regulated by itself,11 by NF-κB12 and by YY1.13 Snail acts directly as a repressor for E-cadherin14 and for RKIP.15 In addition, the post-transcriptional regulation of Snail involves phosphorylation in the serine-rich domain of amino acids 104-107 by GSK3-β before Snail localization to the nucleus. Phosphorylation on Ser 96 and 100 by the same protein kinase induces Snail binding to β-TrCP1 and mediates ubiquitination and proteasomal degradation in the cytoplasm, preventing it for its transcriptional regulation in the nucleus.16
B. Regulation of chemo-immunoresistance by Snail
Snail plays an important role in the regulation of cell cycle and apoptosis through the activation of the MAPK and PI3K survival pathways. Snail was able to confer resistance to the lethal effect of serum depletion and TNF-α.17 In another report, human melanoma cells expressing Snail were protected from apoptosis induced by the chemotherapeutic drug adriamycin and significant induction of apoptosis in these cells was observed following treatment of these cells with shRNA Snail.18 In addition, knockdown of Snail via RNAi sensitized human lung adenocarcinoma cells to cisplastin-induced apoptosis.19 Vannini et al20 reported that treatment of highly resistant melanoma cells with siRNA Slug sensitized the cells to both cisplatin and fetomustin cell death. Since Snail and Slug belong to the same family, it is expected that siRNA Snail would also result in similar findings. Kurrey et al21 reported the involvement of Snail and Slug in the resistance to radiation and the chemotherapeutic drug paclitaxel. They showed that this phenomenon is a result of a novel subset of gene targets that is repressed under the conditions of stress by inactivating p53-mediated apoptosis.
Hoshino et al22 reported that transfection of colorectal cancer cells with Snail resulted in their being chemoresistant. Hsu et al23 investigated the mechanism of EMT-induced chemoresistance in head and neck squamous cancer cells (HNSCC). They examined a chemoresistant gene product, the excision repair cross complementation group 1 (ERCC1), and Snail in cancer cell lines from the NCI-60 database and from human HNSCC cell lines. They have found a correlation between the expression of Snail and ERCC1. In the HNSCC cell lines, the overexpression of Snail in the low endogenous Snail (ERCC1) lines increased the expression of ERCC1. Knockdown of Snail in the highly endogenous Snail/ERCC1 lines downregulated the expression of ERCC1 and attenuated resistance to cisplatin. Further, suppression of ERCC1 in Snail overexpressing cancer cells enhanced their sensitivity to cisplatin. Thus, the activation of ERCC1 by Snail regulates cisplatin resistance. Li et al24 reported that overexpression of Snail in the breast cancer MCF-7 cells resulted in slight enhancement of the MDR gene product, p-glycoprotein (p-gp). They suggested that Snail inhibition may be useful in modulating the MDR phenotype. Haslehurst et al25 reported in ovarian cancer cells that reducing the expression of Snail resulted in the reversal of the EMT phenotype and sensitization to cisplatin-induced cell death.
We have reported that treatment of human prostate cancer cell lines with the proteasome inhibitor NPI-0052 resulted in sensitizing the tumor cells to both chemo and immuno cytotoxic drugs.6 Treatment with this inhibitor resulted in the inhibition of NF-κB and its target gene product Snail. The direct role of NPI-0052-mediated inhibition of Snail in chemo-immuno-sensitization was shown by transfection of the cells (with high level of Snail) with siRNA Snail. Snail silencing resulted in the sensitization of the resistant cells to both CDDP and TRAIL-induced apoptosis, concomitantly with the induction of RKIP. Interestingly, the sensitization was due, in large part, to the induction of RKIP as transfection with RKIP siRNA abolished the apoptotic effects of CDDP on the tumor cells. Depletion of endogenous Snail by RNAi led to increased sensitization to DNA damage accompanied by increased expression of pro-apoptotic factors identified as targets of Snail. In addition to drugs, nitric oxide (NO) and proteasome inhibitors, antibodies also sensitized resistant tumors to apoptosis. Treatment of B-Non-Hodgkin's Lymphoma (B-NHL) cell lines with rituximab (chimeric anti-CD20 mAb) or galiximab (anti-CD80 mAb) resulted in the reversal of resistance and chemo-immunosensitization to apoptosis.17,26,27 These antibodies inhibited NF-κB and downstream Snail. Treatment with siRNA Snail sensitized the B-NHL cells to CDDP and TRAIL and, thus, mimicking the antibody-mediated sensitization.28
III. Role of YY1 in the Regulation of Tumor Cell Resistance to Chemo-Immuno Cytotoxic Agents
A. General properties
YY1 is a multi-functional DNA-binding protein that can activate, repress, or initiate transcription depending on the context in which it binds (directly or indirectly through complexing with DNA-binding proteins).29 As a repressor, YY1 has been reported to repress several genes such as IFN-α, GM-CSF, and IL-3.30-32 We have also reported that YY1 represses both the Fas and DR5 receptors.33,34
B. Regulation of chemo-immuno resistance by YY1
The transcription factor YY1 has been reported to activate or repress certain genes in different tissues. The first demonstration of the direct role of YY1 in the regulation of tumor cell resistance was reported in 2001 in which YY1 was shown to regulate tumor cell resistance to Fas-ligand (Fas L)-mediated apoptosis.33 The role of YY1 in Fas-L-apoptosis was the result of studies that demonstrated that treatment of ovarian carcinoma cell lines, resistant to the Fas-L agonist CH-11, were sensitized by treatment with IFN-γ and resulted in CH-11-induced apoptosis. Treatment with IFN-γ was shown to upregulate Fas expression.33 One mechanism by which IFN-γ sensitized the cells was through the induction of inducible nitric oxide synthase (iNOS) and the subsequent generation of NO in the tumor cells. The generation of NO by IFN-γ correlated with the increased expression of Fas transcription and protein expression.35 The relationship between NO and Fas gene expression was investigated by hypothesizing that NO may, directly or indirectly, modify the transcriptional machinery that is regulated for the initial expression of the Fas gene. Using a Fas promoter, driven by a luciferase reporter system, we demonstrated that NO-induced increase in the transactivation of the Fas promoter was inhibited by NOS inhibitors. The Fas promoter consists of a silencer, enhancer, and core-regions and the deletion of the silencing region upregulated the NO-mediated effect and, thus, suggesting that NO may be disrupting a repressor mechanism. We identified a relevant putative repressor cluster at the silencer region that matched the consensus sequence that binds the transcription factor, YY1. Three binding sites for YY1 are located in a very narrow sequence at -1619, -1590, -1543 base pairs from the translation initiation site of the Fas gene.
NO interferes with the DNA-binding activity of many zinc-fingers via S-nitrosylation of cysteine thiol groups and the formation of S-nitroso-thiols.36,37 Hence, treatment of tumor cells with NO inhibited YY1 DNA-binding activity and was restored by the addition of the reducing agent, DTT.33 We later reported directly the nitrosylation of YY1 by NO.38 These findings established, for the first time, a new role of YY1 in the regulation of tumor cell resistance to apoptosis.
In another report, we have investigated the role of the human chimeric anti-CD20 mAb (rituximab) in the sensitization of chemo-immune resistant B-NHL tumor cells to chemo immune-induced apoptosis. Initially, we reported that rituximab sensitized B-NHL lines to drug-induced apoptosis when used in combination with chemotherapeutic drugs in a synergistic fashion.39 Investigation of the molecular mechanisms underlying the sensitization revealed an association with the downregulation of the anti-apoptotic gene products, Bcl2 and BclxL, as a consequence of rituximab-mediated inhibition upstream of the constitutively activated survival/anti-apoptotic pathways, such as p38 MAPK, NF-κB and ERK1/2.40 Since NF-κB regulates YY1 transcription and expression41 and rituximab inhibits NF-κB, we hypothesized that rituximab-mediated sensitization to FasL apoptosis may be, due in part, to the inhibition of YY1 downstream of NF-κB. Indeed, treatment with rituximab inhibited YY1 expression and DNA-binding activity and correlated with the upregulation of Fas expression. In addition, treatment with siRNA YY1 also resulted in upregulation of Fas and sensitized the tumor cells to FasL apoptosis, mimicking rituximab. These findings established a mechanism of rituximab-mediated upregulation of Fas in the sensitization to FasL apoptosis via inhibition of the transcription repressor YY1,5 corroborating a previous report demonstrating YY1 repressing the Fas promoter.33
We investigated one mechanism by which tumor cells regulate the resistance to FasL apoptosis. Previous findings demonstrated that the activation of NF-κB regulates the resistance to FasL apoptosis and that this regulation is, in part, mediated by NF-κB induction of the transcription repressor YY1 that negatively regulates Fas expression and resistance to FasL. Thus, we have investigated a model with the prostate cancer cell line PC3. PC3 synthesizes and secretes TNF-α and expresses constitutively activated NF-κB. We first demonstrated that treatment of PC3 cells with recombinant TNF-α induced the activation of both NF-κB and YY1 concomitantly with the downregulation of Fas expression and augmented the resistance of PC3 cells to FasL apoptosis. In contrast, blocking TNF-α synthesis and secretion and inhibition of its interaction with the TNF-α receptors on PC3 cells, using soluble TNF receptor, reversed resistance and sensitized the cells to FasL apoptosis. This finding established the autocrine/paracrine loop by TNFα-TNF receptor interactions in PC3 cells in the regulation of FasL apoptosis via induction of YY1 expression and activity.42
The TNF-related apoptosis ligand, TRAIL, is expressed on cytotoxic lymphocytes (CTL, NK) and participates in cell killing via its interaction with its receptors DR4 and DR5 expressed on sensitive target cells. Unlike TNF-α and FasL, TRAIL is safe and minimally cytotoxic on normal tissues and, therefore, has been considered as a potential anti-cancer therapeutic agent. TRAIL has been reported to exhibit potent cytotoxicity against a variety of tumor cell lines and in vitro and in vivo murine models with minimal toxicity on normal issues.43 However, the majority of human tumors are resistant to TRAIL apoptosis and, therefore, must be sensitized to reverse the resistance. Several factors have been reported to contribute to the resistance of tumor cells to TRAIL apoptosis.44,45 Conventional chemotherapeutic drugs and radiation were reported to sensitize tumor cells to TRAIL apoptosis and several drugs resulted in the upregulation of DR5.46-48 However, the mechanisms by which certain chemotherapeutic drugs sensitize tumor cells to TRAIL apoptosis were not known and were subjected to our investigation. The transcriptional regulation of DR5 by drugs was reported as due to a functional SP1-binding site on the DR5 promoter,49 responsible for its regulation. We have also identified another binding site for the transcription factor YY1 on the DR5 promoter region. Thus, we hypothesized that drugs may inhibit NF-κB activity and downstream YY1 expression and resulting in the upregulation of DR5 and sensitization to TRAIL apoptosis. Several lines of evidence supported this hypothesis. Treatment of several human prostate cancer cell lines with various chemotherapeutic drugs (examples: CDDP, VP16, adriamycin, vincristine) inhibited both NF-κB and YY1 activities and sensitized the cells to TRAIL apoptosis concomitantly with the upregulation of DR5. Transfection of cells with the wild type DR5 reporter was significantly augmented with a DR5 construct carrying either a deletion or a mutation in the YY1 binding site. In addition, cells transfected with siRNA YY1 resulted in the upregulation of DR5 and sensitization to TRAIL apoptosis.34 In addition, we have demonstrated that treatment of TRAIL-resistant B-NHL cell lines with rituximab sensitized the cells to TRAIL apoptosis. Treatment of B-NHL cells with rituximab inhibited both YY1 DNA-binding activity and expression. Rituximab-mediated sensitization to TRAIL apoptosis was due, in large part, to rituximab-mediated inhibition of the transcription factor Yin Yang 1 (YY1). The direct role of YY1 in TRAIL sensitization by rituximab was also demonstrated by transfection with YY1 siRNA, and such cells mimicked rituximab and became sensitive to TRAIL-induced apoptosis.50
We have reported that treatment of tumor cells with nitric oxide (NO) sensitized tumor cells to FasL apoptosis via inhibition of NF-κB and YY1 expressions and activities through, in part, the S-nitrosylation of p50 and YY1. Therefore, the findings showing that YY1 also represses DR5 expression and that inhibition of YY1 by drugs upregulated DR5 and sensitized the cells to TRAIL apoptosis51 suggested that NO can also sensitize tumor cells to TRAIL apoptosis via inhibition by YY1 and its S-nitrosylation. Indeed, the findings corroborated this hypothesis and demonstrated that treatment of tumor cells with the NO donor DETANONOate sensitized tumor cells to TRAIL apoptosis. Treatment with DETANONOate inhibited NF-κB and YY1 and upregulated DR5 expressions.7 Another mechanism by which YY1 is involved in the regulation of DR5 and sensitization to TRAIL apoptosis was reported following treatment of tumor cells with proteasome inhibitors. Treatment with the inhibitors NP1-0052 or Bortezomib resulted in the inhibition of NF-κB and YY1, upregulation of DR5 and sensitization to TRAIL apoptosis.52 The above findings demonstrated the direct role of YY1 in the regulation of tumor cell resistance to both FasL and TRAIL apoptosis via its transcriptional repressor activity.
The role of YY1 in the resistance to chemotherapeutic drugs was previously not known. A reported study by Wink et al53 demonstrated that NO can sensitize tumor cells to various chemotherapeutic agents although the mechanism was not examined. We have shown that NO treatment resulted in the inhibition of NF-κB and YY1 expressions and activities as well as S-nitrosylation. These effects resulted in the upregulation of Fas and DR5 and sensitization to FasL and TRAIL apoptosis, respectively. Since NF-κB has been reported to regulate survival and drug resistance, we investigated whether YY1 was also involved in the regulation of drug resistance. Treatment of tumor cells with the NO donor DETANONOate resulted in the sensitization to drug-induced apoptosis along with inhibition of NF-κB, YY1 and BclxL. The direct role of YY1 in the regulation of drug resistance was corroborated by the use of tumor cells transfected with siRNA YY1 which were sensitized to drug apoptosis and concomitantly with the inhibition of both the YY1 and BclxL. Likewise, tumors cells transfected with siRNA BclxL inhibited both BclxL and YY1 and sensitization to drug apoptosis.54
IV. Role of RKIP in the Regulation of Tumor Cell Sensitivity to Chemotherapeutic Drugs
A. General properties
The Raf-1 kinase inhibitor protein (RKIP) is a member of the phosphatidylethanolamine-binding protein family (PEBP). It is a cytosolic protein initially shown to play a role in lipid metabolism and phospholipid membrane biogenesis.55 When initially cloned, it was shown to inhibit both the Raf-1/MEK/ERK and NF-κB pathways.56-58 Phosphorylation of RKIP at Serine-153 by protein kinase C abolishes RKIP's inhibition of Raf-1 and converts it to an inhibitor of the G-protein coupled receptor kinase, GRK-2, and in addition, Lorentz et al59 reported that this process facilitated the cross-talk between the EGF and GPCR signaling pathways. In a study by Fu et al,60 they demonstrated that the ectopic expression of RKIP suppressed invasion and metastasis of human prostate cancer cells implanted in mice. In another study, the ectopic expression of RKIP suppressed the metastatic ability of ovarian cancer cells in vivo and, in contrast, the down-regulation of RKIP led to invasiveness in vitro.61 Hence, RKIP has been named a “metastatic suppressor gene product.”
B. Role of RKIP in chemo-immunosensitization
Defining the genetic mechanisms underlying tumor resistance to drug-induced apoptosis is a prerequisite towards the development of new and successful approaches to reverse resistance, including improvement of host anti-tumor immunity. Tumors exhibit constitutively activated-survival/anti-apoptotic pathways, particularly NF-κB62,63 which regulate gene products involved in the apoptotic pathways. TRAIL and agonist monoclonal antibodies against DR4 and DR5 are being evaluated clinically in cancer therapy. However, most tumors are resistant to the direct effect of TRAIL44 and the reversal of TRAIL-resistance necessitates that the tumor cells become sensitized by other agents such as proteasome inhibitors64 and chemotherapeutic drugs.34,48 In addition, another gene product, namely, RKIP, a metastatic suppressor gene product, has also been shown to be involved in the regulation of resistance. Chatterjee et al.65 reported that drug-resistant cancer cells can be sensitized to drug apoptosis by expression of RKIP. RKIP has been characterized as a modulator of apoptosis and metastasis through the regulation of survival signaling cascades, such as the Raf/MEK/ERK kinase cascades, GPCR, and the NF-κB pathway.57,66-71 Due to the inhibitory role of RKIP in the regulation of above survival pathways, it invariably will also play a role in the regulation of apoptotic stimuli. Hence, we hypothesized for a new role of RKIP as a regulator of tumor sensitivity to TRAIL mediated apoptosis. Hence, induction or ectopic expression of RKIP in TRAIL-resistant tumor cells reversed resistance and sensitized the cells to TRAIL-apoptosis.52 The levels of RKIP in tumor cells are usually low and, therefore, are considered as critical determinants of tumor fate to apoptosis.72
RKIP overexpression reverses resistance to TRAIL apoptosis while knocking down RKIP by siRNA induced resistance to TRAIL apoptosis. RKIP overexpression modulated both type I and type II apoptotic pathways and in combination with TRAIL triggered apoptosis. To unravel the mechanism by which RKIP sensitizes tumor cells to TRAIL, we first examined its effect on the expression of DR5. Increased RKIP expression correlated with increased DR5 mRNA and protein as well as increased DR5 promoter activity. Since we reported previously that upregulation of DR5 is the results of inhibition of its transcription repressor YY1,34 we have found that overexpression of RKIP correlated with inhibition of the YY1 transcript and protein, due to RKIP-induced effects in inhibiting upstream NF-κB that in turn regulates YY1. The direct involvement of YY1 in DR5 upreguation by RKIP was consistent with the findings showing that either deletion or mutation of the putative YY1-binding site in the DR5 promoter upregulated the enhancing effect that RKIP overexpression had on the wild type DR5 promoter. There was no effect of RKIP overexpression on DR4 or decoy receptors. The involvement of NF-κB inhibition by overexpression of RKIP in the inhibition of YY1, up-regulation of DR5 and sensitization to TRAIL was shown in cells with overexpressed RKIP and with significant inhibition of NF-κB promoter activity and NIK phosphorylation. In addition, RKIP overexpression caused the depolarization of mitochondria markers and activation of pro-caspase 9 and contributed on the downregualtion of anti-apoptotic gene products, such as XIAP and BclxL, targets of NF-κB. In combination with TRAIL, overexpression of RKIP also resulted in the activation of caspase 8. Taking into account the notion that RKIP regulates immune surveillance and tumor metastasis, the levels of RKIP expression in tumors may be critical determinants in cancer progression. Therefore, the increased RKIP in tumors may be a therapeutic target for enhancing drug immune sensitivity of tumors.52
We have reported that resistance of tumor cells to chemo-and-immunocytotoxic agents may be reversed by sensitization with proteasome inhibitors such as Bortezomib and NPI-0052.54 We investigated the role of RKIP in proteasome inhibitors-induced sensitization to chemo and immuno-therapeutic drugs.6 This investigation was based on a report by Beach et al.15 who demonstrated that RKIP was under the transcriptional regulation of the transcription repressor Snail in prostate tumor cells. Snail is a member of the Snail superfamily of zinc-finger transcription factors with a pivotal role in embryonic development and cell survival.9 Snail is transcriptionally regulated, in part, by NF-κB12,73 and by itself via binding to its own promoter and repressor activity.11 Based on these findings, we hypothesized that inhibition of NF-κB by proteasome inhibitors will result downstream in the inhibition of Snail and consequently resulting in the derepression of Snail repressor activity and induction of RKIP expression. Hence, the induction of RKIP will then result in tumor cell sensitization to both drugs and immune cytotoxics as reported previously. We showed that treatment of tumor cells with the proteasome inhibitor NPI-0052 sensitized the tumor cells to both CDDP and TRAIL apoptosis. The sensitization was the result of NPI-0052-mediated inhibition of Snail and induction of RKIP and resulting in the modulation of the NF-κB/Snail/RKIP loop and, hence, it resulted in the continuous reinforcement of the inhibition of NF-κB by NIP-0052 directly and underwent inhibition of Snail and induction of RKIP. These findings suggested the role of the NF-κB/Snail/RKIP feedback loop established in cancer cells with a dominant effect on cell survival, resistance and metastasis; each component of the loop is independently involved and implicated in these phenomena.74-77
In addition to chemotherapeutic drugs and proteasome inhibitors effects on RKIP induction, we have reported that treatment of tumors cells with the NO donor DETANONOate also sensitized cells to apoptotic stimuli.54 We have also reported that NO inhibits NF-κB activity and since NF-κB regulates downstream Snail, therefore, we hypothesized that treatment of tumor cells with NO will result in the inhibition of both NF-κB and Snail and, consequently, derepressing Snail repressor activity on RKIP and resulting in the induction of RKIP. The induction of RKIP will, then, result in both chemo and immuno sensitization of tumor cells as reported above.6,3,4,52,62
V. Conclusions
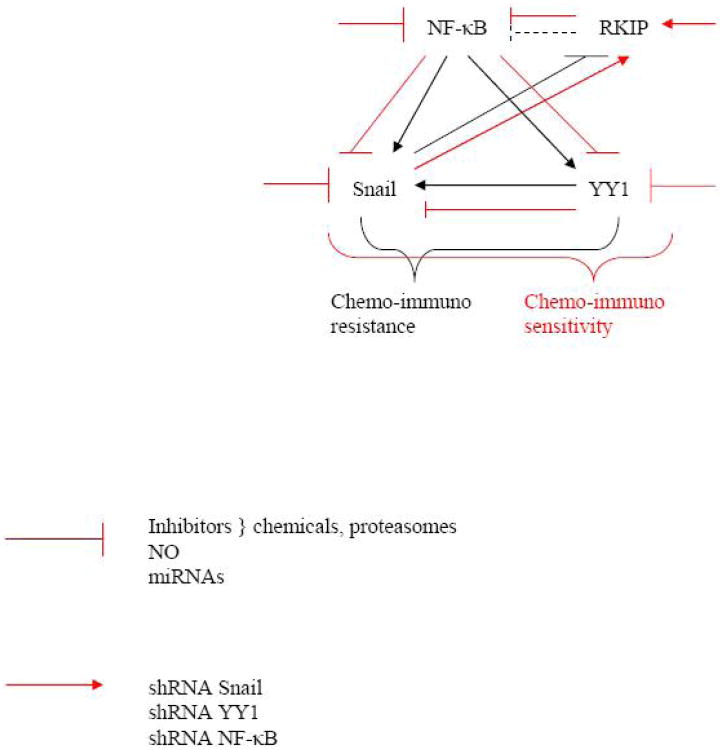
Conventional clinical treatment options for cancer patients include surgery, chemotherapy, radiotherapy, hormone- and immune-based therapies. Combinations of two or more of these approaches have also been clinically evaluated. These modalities induce apoptosis in sensitive tumor cell population. Owing to the heterogenous nature of tumors, a subpopulation of cells is inherently resistant to treatment. Additionally, patients whom initially respond to treatment experience tumor recurrences. Relapsed tumors are no longer responsive to either the initial treatment or alternative treatments. They basically develop cross-resistance to the apoptotic effects of various treatment modalities. Thus, tumor cells have developed various mechanisms to evade apoptosis, metastasize, with eventual demise of the patient. Recent technological advances such as array-based high-throughput gene expression analysis in understanding the specific genes involved and the signal transduction pathways and the comparative gene expression patterns of primary and metastatic tumors have provided unique opportunities to examine cancer in greater depth. In particular, these advances have presented opportunities to an improved understanding of the gene expression patterns involved with metastatic progression. This has led to the novel discovery of the NF-κB/Snail/YY1/RKIP circuitry as a key regulator of the EMT phenotype and tumor metastasis. In various tumor models, the activation of cell survival signal transduction pathways such as the NF-κB, MAPK, and AKT/PI3K pathways, which act as upstream regulators/activators of the Snail/YY1/RKIP circuitry, equip the cells with the drug-resistance and invasive phenotypes. (See scheme in Fig. 1). These tumors no longer respond to further therapy and metastasize. Thus, understanding the intricacies of these signaling pathways, their mode of activation, regulation, and suppression is urgently needed. Furthermore, small molecule chemical inhibitors or biological response modifiers (e.g., cytokines) that negatively regulate the activity of the NF-κB/YY1/RKIP loop will undoubtedly hamper the EMT phenotype and prevent tumor metastasis.
Fig. 1.
This schematic diagram represents the constitutively activated and deregulated NF-κB/Snail/YY1/RKIP loop in cancer cells. The black solid lines demonstrate that activated NF-κB regulates the transcription and expression of Snail. Snail is a transcription repressor of RKIP and, therefore, there is minimal suppression of NF-κB by RKIP. In addition, NF-κB activates the transcription and expression of YY1. YY1 has been shown to positively regulate the transcription and expression of Snail. Therefore, the activation of NF-κB, Snail and YY1 along with the inactivation of RKIP results in the regulation of tumor cells chemo-immuno-resistance to cytotoxic agents. However, inhibitors of the above loop (red solid lines), such as inhibitors of NF-κB (by chemicals, proteasome inhibitors, NO, etc.) result downstream in the inhibition of Snail and YY1 along with the induction of RKIP. Like the inhibition of NF-κB, the direct inhibition of Snail or YY1 (by shRNA, inhibitors, etc.) will also result in the induction of RKIP and further inhibiting the NF-κB/YY1/Snail circuit. Altogether, the various inhibitors will result in the reversal of tumor cells chemo-immuno-resistance to chemo-immuno-sensitivity to cytotoxic agents. Overall scheme of the roles each of Snail. YY1 and RKIP m the regulation of tumor cell chemo-immuno resistance.
Acknowledgments
This work was supported in part by the National Center for Research Resources and The National Cancer Institute (NCI) of the National Institutes of Health (NIH through Grant number R21149938 (AJ). The support of the Jonsson Comprehensive Cancer Center is acknowledged. The authors thank Daphne Liang and Melissa Cao for their assistance in the preparation of this manuscript.
Abbreviations
- EMT
epithelial to mesenchymal transition
- ERCC1
excision repair cross complementation group 1
- Fas L
Fas-ligand
- GSK3-β
Glycogen synthase kinase 3 beta
- HNSCC
head and neck squamous cancer cells
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- RKIP
Raf kinase inhibitor protein
- YY1
Yin Yang 1
References
- 1.Inoue J, Gohda J, Akiyama T, Semba K. NF-kappaB activation in development and progression of cancer. Cancer Sci. 2007;98:268–274. doi: 10.1111/j.1349-7006.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCubrey JA, Steelman LS, Chappel WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huerta-Yepez S, Vega M, Jazirehi A, Garban H, Hongo F, Cheng G, Bonavida B. Nitric oxide sensitizes prostate carcinoma cell lines to TRAIL-mediated apoptosis via inactivation of NF-kappa B and inhibition of Bcl-xl expression. Oncogene. 2004;23:4993–5003. doi: 10.1038/sj.onc.1207655. [DOI] [PubMed] [Google Scholar]
- 4.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway Bcl-xL down-regulation, and chemosensitization of non-Hodgkin's lymphoma B cells by Rituximab. Cancer Res. 2004;64:7117–7126. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
- 5.Vega MI, Jazirehi AR, Huerta-Yepez S, Bonavida B. Rituximab-induced inhibition of YY1 and Bcl-xL expression in Ramos non-Hodgkin's lymphoma cell line via inhibition of NF-kappa B activity: role of YY1 and Bcl-xL in Fas resistance and chemoresistance, respectively. J Immunol. 2005;175:2174–2183. doi: 10.4049/jimmunol.175.4.2174. [DOI] [PubMed] [Google Scholar]
- 6.Baritaki S, Yeung K, Palladino M, Berenson J, Bonavida B. Pivotal roles of snail inhibition and RKIP induction by the proteasome inhibitor NPI-0052 in tumor cell chemoimmunosensitization. Cancer Res. 2009;69:8376–8385. doi: 10.1158/0008-5472.CAN-09-1069. [DOI] [PubMed] [Google Scholar]
- 7.Simpson P. Maternal-zygotic gene interactions during formation of the dorsoventral pattern in Drosophila embryos. Genetics. 1983;105:615–632. doi: 10.1093/genetics/105.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huerta-Yepez S, Vega M, Escoto-Chavez SE, Murdock B, Sakai T, Baritaki S, Bonavida B. Nitric oxide sensitizes tumor cells to TRAIL-induced apoptosis via inhibition of the DR5 transcription repressor Yin Yang 1. Nitric Oxide. 2009;20:39–52. doi: 10.1016/j.niox.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Zhou BP. Snail: More than EMT. Cell Adh Migr. 2010;4:199–203. doi: 10.4161/cam.4.2.10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peiro S, Escriva M, Puig I, Barberà MJ, Dave N, Herranz N, Larriba MJ, Takkunen M, Francí C, Muñoz A, Virtanen I, Baulida J, García de Herreros A. Snail1 transcriptional repressor binds to its own promoter and controls its expression. Nucleic Acids Res. 2006;34:2077–84. doi: 10.1093/nar/gkl141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 13.Palmer MB, Majumder P, Cooper JC, Yoon H, Wade PA, Boss JM. Yin yang 1 regulates the expression of snail through a distal enhancer. Mol Cancer Res. 2009;7:221–229. doi: 10.1158/1541-7786.MCR-08-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, Del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 15.Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, Yeung KC. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008;27:2243–2248. doi: 10.1038/sj.onc.1210860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 17.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Neito MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kajita M, McClinic KN, Wafe PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–7566. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuo W, Wang Y, Zhuo X, Zhang Y, Ao X, Chen Z. Knockdown of Snail, a novel zinc finger transcription factor, via RNA interference increases A549 cell sensitivity to cisplatin via JNK/mitochondrial pathway. Lung Cancer. 2008;62:8–14. doi: 10.1016/j.lungcan.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Vannini I, Bonafe M, Tesei A, Rosetti M, Fabbri F, Storci G, Ulivi P, Brigliadori G, Amadori D, Zoli W. Short interfering RNA directed against the SLUG gene increases cell death induction in human melanoma cell lines exposed to cisplatin and fotemustine. Cell Oncol. 2007;29:279–87. doi: 10.1155/2007/540821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediated radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–68. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 22.Hoshino H, Miyoshi N, Nagai K, Tomimaru Y, Nagano H, Sekimoto M, Doki Y, Mori M, Ishii H. Epithelial-mesenchymal transition wit expression of SNAI1-induced chemoresistance in colorectal cancer. Biochem Biophys Res Commun. 2009;390:1061–5. doi: 10.1016/j.bbrc.2009.10.117. [DOI] [PubMed] [Google Scholar]
- 23.Hsu DS, Lan HY, Huang CH, Tai SK, Chang SY, Tsai TL, Chang CC, Tzeng CH, Wu KJ, Kao JY, Yang MH. Regulation of excision repair cross-complementation group 1 by Snail contribures to cisplatin resistance in head and neck cancer. Clin Cancer Res. 2010;16:4561–71. doi: 10.1158/1078-0432.CCR-10-0593. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Liu C, Tang Y, Li H, Zhou F, Lv S. Overexpression of Snail accelerates adriamycin induction of multidrug resistance in breast cancer cells. Asian Pac J Cancer Prev. 2011;12:2575–80. [PubMed] [Google Scholar]
- 25.Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, Davey S, Squire J, Park PC, Feilotter H. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer. 2012;12:91. doi: 10.1186/1471-2407-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jazirehi AR, Huerta-Yepez S, Cheng G, Bonavida B. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-{kappa}B signaling pathway in non-Hodgkin's lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosis. Cancer Res. 2005;65:264–76. [PubMed] [Google Scholar]
- 27.Vega MI, Baritaki S, Huerta-Yepez S, Martinez-Paniagua MA, Bonavida B. A potential mechanism of rituximab-induced inhibition of tumorgrowth through its sensitization to tumor necrosis factor-related apoptosis-inducing ligand-expressing host cytotoxic cells. Leuk Lymphoma. 2011;52:108–21. doi: 10.3109/10428194.2010.531408. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Paniagua MA, Baritaki S, Huerta-Yepez S, Ortiz-Navarrete VF, González-Bonilla C, Bonavida B, Vega MI. Mcl-1 and YY1 inhibition and induction of DR5 by the BH3-mimetic Obatoclax (GX15-070) contribute in the sensitization of B-NHL cells to TRAIL apoptosis. Cell Cycle. 2011;10:2792–805. doi: 10.4161/cc.10.16.16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 30.Ye J, Zhang X, Dong Z. Characterization of the human granulocyte–macrophage colony-stimulating factor gene promoter: an AP1 complex and an Sp1-related complex transactivate the promoter activity that is suppressed by a YY1 complex. Mol Cell Biol. 1996;16:157–167. doi: 10.1128/mcb.16.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye J, Young HA, Zhang X, Castranova V, Vallyathan V, Shi X. Regulation of a cell type-specific silencer in the human interleukin-3 gene promoter by the transcription factor YY1 and an AP2 sequence-recognizing factor. J Biol Chem. 1999;274:26661–26667. doi: 10.1074/jbc.274.38.26661. [DOI] [PubMed] [Google Scholar]
- 32.Guo J, Lin X, Williams MA, Hamid Q, Georas SN. Yin-Yang 1 regulates effector cytokine gene expression and T(H)2 immune responses. J Allergy Clin Immunol. 2008;122:195–201. doi: 10.1016/j.jaci.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garban HJ, Bonavida B. Nitric oxide inhibits the transcription repressor YinYang 1 binding activity at the silencer region of the Fas promoter: a pivotal role for nitric oxide in the up-regulation of Fas gene expression in human tumor cells. J Immunol. 2001;167:75–81. doi: 10.4049/jimmunol.167.1.75. [DOI] [PubMed] [Google Scholar]
- 34.Baritaki S, Huerta-Yepez S, Sakai T, Spandidos DA, Bonavida B. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and inhibition of Yin Yang 1. Mol Cancer Ther. 2007;6:1387–1399. doi: 10.1158/1535-7163.MCT-06-0521. [DOI] [PubMed] [Google Scholar]
- 35.Garban HJ, Bonavida B. Nitric oxide sensitizes ovarian tumor cells to Fas-induced apoptosis. Gynecol Oncol. 1999;73:257. doi: 10.1006/gyno.1999.5374. [DOI] [PubMed] [Google Scholar]
- 36.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 37.Kroncke KD, Carlberg C. Inactivation of zinc finger transcription factors provides a mechanism for a gene regulatory role of nitric oxide. FASEB J. 2000;14:166. doi: 10.1096/fasebj.14.1.166. [DOI] [PubMed] [Google Scholar]
- 38.Hongo F, Garban H, Huerta-Yepez S, Vega M, Jazirehi AR, Mizutani Y, Miki T, Bonavida B. Inhibition of the transcription factor Yin Yang 1 activity by S-nitrosation. Biochem Biophys Res Commun. 2005;336:692–701. doi: 10.1016/j.bbrc.2005.08.150. [DOI] [PubMed] [Google Scholar]
- 39.Demidem A, Lam T, Alas S, Hariharan K, Hanna N, Bonavida B. Chimeric anti-CD20 (IDEC-C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs. Cancer Biother Radiopharm. 1997;12:177–186. doi: 10.1089/cbr.1997.12.177. [DOI] [PubMed] [Google Scholar]
- 40.Jazirehi AR, Vega MI, Chatterjee D, Goodglick L, Bonavida B. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin's lymphoma B cells by rituximab. Cancer Res. 2004;64:7117–7126. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
- 41.Siednienko J, Maratha A, Yang S, Mitkiewicz M, Miggin SM, Moynagh PN. Nuclear factor κB subunits RelB and cRel negatively regulate Toll-like receptor 3-mediated β-interferon production via induction of transcriptional repressor protein YY1. J Biol Chem. 2011;286:44750–44763. doi: 10.1074/jbc.M111.250894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huerta-Yepez S, Vega M, Garban H, Bonavida B. Involvement of the TNF-α autocrine-paracrine loop, via NF-κB and YY1, in the regulation of tumor cell resistance to Fas-induced apoptosis. Clin Immunol. 2006;120:297–309. doi: 10.1016/j.clim.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 43.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Updat. 2004;2:139–56. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 46.Sheikh MS, Burns TF, Huang Y, Wu GS, Amundson S, Brooks KS, Fornace AJ, Jr, el-Deiry WS. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor α. Cancer Res. 1998;58:1593–8. [PubMed] [Google Scholar]
- 47.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis inducingligand in vitro and in vivo. Cancer Res. 2000;60:847–53. [PubMed] [Google Scholar]
- 48.Shankar S, Chen X, Srivastava RK. Effect of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate. 2005;62:165–186. doi: 10.1002/pros.20126. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida T, Maeda A, Tani N, Sakai T. Promoter structure and transcription initiation sites of the human death receptor 5/TRAIL-R2 gene. FEBS Lett. 2001;507:381–5. doi: 10.1016/s0014-5793(01)02947-7. [DOI] [PubMed] [Google Scholar]
- 50.Vega MI, Baritaki S, Huerta-Yepez S, Martinez-Paniagua MA, Bonavida B. A potential mechanism of rituximab-induced inhibition of tumor growth through its sensitization to tumor necrosis factor-related apoptosis-inducing ligand-expressing host cytotoxic cells. Leuk Lymphoma. 2011;52:108–21. doi: 10.3109/10428194.2010.531408. [DOI] [PubMed] [Google Scholar]
- 51.Kim YH, Park JW, Lee JY, Kwon TK. Sodium butyrate sensitizes TRAIL-mediated apoptosis by induction of transcription from the DR5 gene promoter through Sp1 sites in colon cancer cells. Carcinogenesis. 2004;25:1813–20. doi: 10.1093/carcin/bgh188. [DOI] [PubMed] [Google Scholar]
- 52.Baritaki S, Suzuki E, Umezawa K, Spandidos DA, Berenson J, Daniels TR, Penichet ML, Jazirehi AR, Palladino M, Bonavida B. Inhibition of Yin Yang 1-dependent repressor activity of DR5 transcription and expression by the novel proteasome inhibitor NPI-0052 contributes to its TRAIL-enhanced apoptosis in cancer cells. J Immunol. 2008;180:6199–210. doi: 10.4049/jimmunol.180.9.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wink DA, Cook JA, Christodoulou D, Krishna MC, Pacelli R, Kim S, DeGraff W, Gamson J, Vodovotz Y, Russo A, Mitchell JB. Nitric oxide and some nitric oxide donor compounds enhance the cytotoxicity of cisplatin. Nitric Oxide. 1997;1:88–94. doi: 10.1006/niox.1996.0108. [DOI] [PubMed] [Google Scholar]
- 54.Huerta-Yepez S, Baritaki S, Baay-Guzman G, Hernandez-Luna MA, Hernandez-Cueto A, Vega MI, Bonavida B. Contribution of either YY1 or BclXL-induced inhibition by the NO-donor DETANONOate in the reversal of drug resistance, both in vitro and in vivo. YY1 and BclXL are overexpressed in prostate cancer. Nitric Oxide. 2013;29:17–24. doi: 10.1016/j.niox.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Granovsky AE, Rosner MR. Raf kinase inhibitory protein: a signal transduction modulator and metastasis suppressor. Cell Res. 2008;18:452–457. doi: 10.1038/cr.2008.43. [DOI] [PubMed] [Google Scholar]
- 56.Yeung K, Seitz T, Li S, Janosch P, McFerrah B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. ppression of Raf-1 kinase activity and MAP kinase signaling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 57.Yeung K, Seitz T, Li S, Janosch P, McFerran B, Kaiser C, Fee F, Katsanakis KD, Rose DW, Mischak H, Sedivy JM, Kolch W. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 58.Tang H, Park S, Sun SC, Trumbly R, Ren G, Tsung E, Yeung KC. RKIP inhibits NF-kappaB in cancer cells by regulating upstream signaling components of the IkappaB kinase complex. FEBS Lett. 2010;584:662–668. doi: 10.1016/j.febslet.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574–579. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
- 60.Fu Z, Smith PC, Zhang L, Rubin MA, Dunn RL, Yao Z, Keller ET. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J Natl Cancer Inst. 2003;95:878–889. doi: 10.1093/jnci/95.12.878. [DOI] [PubMed] [Google Scholar]
- 61.Li HZ, Wang Y, Gao Y, Shao J, Zhao XL, Deng WM, Liu YX, Yang J, Yao Z. Effects of raf kinase inhibitor protein expression on metastasis and progression of human epithelial ovarian cancer. Mol Cancer Res. 2008;6:917–928. doi: 10.1158/1541-7786.MCR-08-0093. [DOI] [PubMed] [Google Scholar]
- 62.Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-κB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survuval signaling in B lymphocytes. Pro Natl Acad Sci USA. 1999;96:9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV. The role of IKK in constitutive activation of NF-κB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115:141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 64.Baritaki S, Chapman A, Yeung K, Spandidos DA, Palladino M, Bonavida B. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of Snail repression and RKIP induction. Oncogene. 2009;28:3573–3585. doi: 10.1038/onc.2009.214. [DOI] [PubMed] [Google Scholar]
- 65.Chatterjee D, Bai Y, Wang Z, Beach S, Mott S, Roy R, Braadstad C, Sun Y, Mukhopadhyay A, Aggarwal BB, Darnowski J, Pantazis P, Wyche J, Fu Z, Kitagwa Y, Keller ET, Sedivy JM, Yeung KC. RKIP sensitizes prostate and breast cancer cells to drug-induced apoptosis. J Biol Chem. 2004;279:17515–17523. doi: 10.1074/jbc.M313816200. [DOI] [PubMed] [Google Scholar]
- 66.Odabaei G, Chatterjee D, Jazirehi AR, Goodglick L, Yeung K, Bonavida B. Raf-1 kinase inhibitor protein: structure, function, regulation of cell signaling, and pivotal role in apoptosis. Adv Cancer Res. 2004;91:169–200. doi: 10.1016/S0065-230X(04)91005-6. [DOI] [PubMed] [Google Scholar]
- 67.Yeung KC, Rose DW, Dhillon AS, Yaros D, Gastafsson M, Chatterjee D, McFerran B, Wyche J, Kolch W, Sedivy JM. Raf kinase inhibitor protein interacts with NF-κB-inducing kinase and TAK1 and inhibits NF-κB activation. Mol Cell Biol. 2001;21:7207–7217. doi: 10.1128/MCB.21.21.7207-7217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Activation of Raf-1 signaling by protein kinase C through a mechanism involving Raf kinase inhibitory protein. J Biol Chem. 2003;78:13061–13068. doi: 10.1074/jbc.M210015200. [DOI] [PubMed] [Google Scholar]
- 69.Kroslak T, Koch T, Kahl E, Hollt V. Human phosphatidylethanolamine-binding protein facilitates heterotrimetric G protein-dependent signaling. J Biol Chem. 2001;276:39772–39778. doi: 10.1074/jbc.M106991200. [DOI] [PubMed] [Google Scholar]
- 70.Park S, Yeung ML, Beach S, Shields JM, Yeung KC. RKIP down-regulates B-Raf kinase activity in melanoma cancer cells. Oncogene. 2005;24:3535–3540. doi: 10.1038/sj.onc.1208435. [DOI] [PubMed] [Google Scholar]
- 71.Trakul N, Rosner MR. Modulation of the MAP kinase signaling cascade by Raf kinase inhibitory protein. Cell Res. 2005;15:19–23. doi: 10.1038/sj.cr.7290258. [DOI] [PubMed] [Google Scholar]
- 72.Jazirehi AR. Regulation of chemo- and immuno-induced apoptosis by Raf-1 kinase inhibitor protein (RKIP) In: Bonavida B, editor. Chemo-immunosensitization of Resistant Tumor Cells to Cell Death by Apoptosis. Transworld Research Network; Keraka, India: pp. 63–80. [Google Scholar]
- 73.Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiró S, Baulida J, Francí C, Dedhar S, Larue L, García de Herreros A. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–54. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 74.Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12:488–96. doi: 10.1245/ASO.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Fu Z, Kitagawa Y, Shen R, Shah R, Mehra R, Rhodes D, Keller PJ, Mizokami A, Dunn R, Chinnaiyan AM, Yao Z, Keller ET. Metastasis suppressor gene Raf kinase inhibitor protein (RKIP) is a novel prognostic marker in prostate cancer. Prostate. 2006;66:248–56. doi: 10.1002/pros.20319. [DOI] [PubMed] [Google Scholar]
- 76.Toyama T, Zhang Z, Iwase H, Yamashita H, Ando Y, Hamaguchi M, Mizutani M, Kondo N, Fujita T, Fujii Y, Iwata H. Low expression of the snail gene is a good prognostic factor in nodenegative invasive ductal carcinomas. Jpn J Clin Oncol. 2006;36:357–63. doi: 10.1093/jjco/hyl038. [DOI] [PubMed] [Google Scholar]
- 77.Scartozzi M, Bearzi I, Pierantoni C, Mandolesi A, Loupakis F, Zaniboni A, Catalano V, Quadri A, Zorzi F, Berardi R, Biscotti T, Labianca R, Falcone A, Cascinu S. Nuclear factor-κB tumor expression predicts response and survival in irinotecan-refractory metastatic colorectal cancer treated with cetuximab-irinotecan therapy. J Clin Oncol. 2007;25:3930–5. doi: 10.1200/JCO.2007.11.5022. [DOI] [PubMed] [Google Scholar]