Abstract
Over decades, cancer treatment has been mainly focused on targeting cancer cells and not much attention to host tumor microenvironment. Recent advances suggest that the tumor microenvironment requires in-depth investigation for understanding the interactions between tumor cell biology and immunobiology in order to optimize therapeutic approaches. Tumor microenvironment consists of cancer cells and tumor associated reactive fibroblasts, infiltrating non-cancer cells, secreted soluble factors or molecules, and non-cellular support materials. Tumor associated host immune cells such as Th1, Th2, Th17, regulatory cells, dendritic cells, macrophages, and myeloid-derived suppressor cells are major components of the tumor microenvironment. Accumulating evidence suggests that these tumor associated immune cells may play important roles in cancer development and progression. However, the exact functions of these cells in the tumor microenvironment are poorly understood. In the tumor microenvironment, NF-κB plays an important role in cancer development and progression because this is a major transcription factor which regulates immune functions within the tumor microenvironment. In this review, we will focus our discussion on the immunological contribution of NF-κB in tumor associated host immune cells within the tumor microenvironment. We will also discuss the potential protective role of zinc, a well-known immune response mediator, in the regulation of these immune cells and cancer cells in the tumor microenvironment especially because zinc could be useful for conditioning the tumor microenvironment toward innovative cancer therapy.
Keywords: Tumor microenvironment, T helper cells, Tregs, MDSCs, DCs, TAMs
1. Introduction
Traditionally cancer therapy has been mainly focused on targeting the cancer cell itself. However, recent advances suggest that the microenvironment within or in the surrounding tissue area of the tumor, called tumor microenvironment, could play important role in cancer development and progression, and thus conditioning of the tumor microenvironment could be useful for designing optimal therapy to eradicate tumors. The tumor microenvironment consists of cancer cells, reactive fibroblasts and a wide range of surrounding non-cancer cells such as infiltrating immune cells, secreted soluble factors such as cytokines and cell growth signaling molecules, chemicals such as oxygen and nitrogen, and non-cellular support materials such as extracellular matrix (ECM) [1–6]. The relative contribution of each of these components varies between tumor types, between patients with the same type of tumor, and even between regions within of a tumor. The tumor microenvironment changes over the course of tumor development and progression, and changes in response to therapeutic interventions [7].
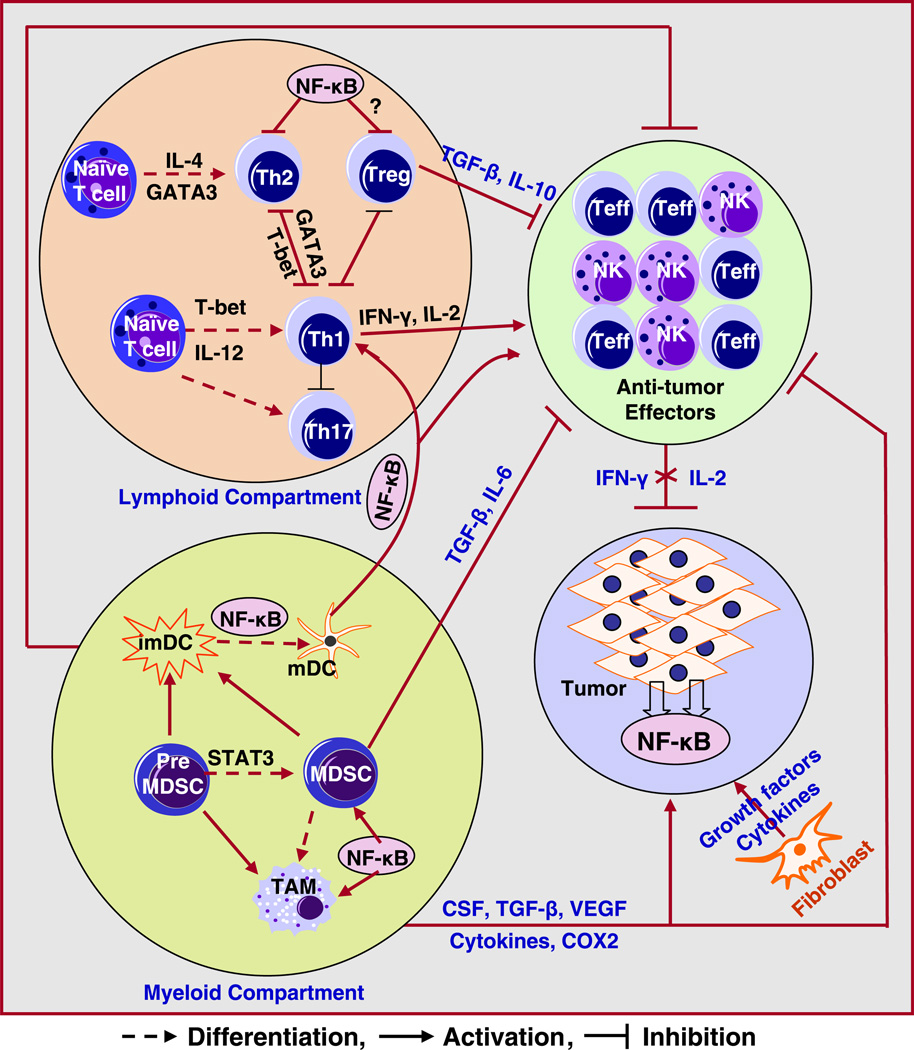
In the tumor microenvironment, the surrounding normal epithelial cells become benign tumor cells, which can be transformed to malignant cells. Furthermore, malignant cells develop into metastatic cells with invasive characteristics depending on the molecular signals between the cells and the surrounding area of tumor [8,9]. Therefore, the cancer cell behavior may depend on the microenvironment for its proliferation, progression, invasion and metastasis, suggesting that therapy targeted at the tumor microenvironment could be useful for designing innovative treatments to eradicate tumors. Such a strategy has recently been documented in an animal tumor model whereby the delivery and efficacy of gemcitabine was improved by conditioning the tumor microenvironment using hedgehog inhibitor [10]. Accumulating evidence clearly suggests that the tumor microenvironment plays a critical role in tumor initiation, progression, invasion, metastasis, as well as contributes to resistance to chemo-, radio-, and immuno-therapy. Both pro-and anti-tumor interactions between tumor cells and host surrounding cells may promote or inhibit tumor initiation and progression (Fig. 1). Therefore, targeting tumor microenvironment, in addition to tumor cells, would provide a novel therapeutic strategy for tumor treatment.
Fig. 1.
Schematic diagram of possible interactions between tumor cells, lymphoid, myeloid and stromal components involved in immune escape and tumor progression. Various cytokines and growth factors produced by tumors or stroma may recruit and activate several immune suppressive cells, resulting in tumor progression. (MDSC: myeloid derived suppressor; imDC: immature dendritic cells; mDC: mature dendritic cells; Treg: T regulatory cells; Teff: effector T cells; NK: natural killer cell; TAM: tumor associated macrophages).
In this review, we focus our discussion on the role of host immune cells in the tumor microenvironment. Furthermore, our focus is centered on the role of NF-κB in the host immune cells within the tumor microenvironment and the role of zinc, a well-known immune mediator, for conditioning the tumor microenvironment.
2. Th1 and Th2 cells and its cytokines
Differentiation of functionally distinct subsets Th1 and Th2 of T helper cells plays an important role in tumor microenvironment, which could impact tumor initiation and progression. Differentiation of naïve CD4+ T cells into Th1 or Th2 cells is an important and critical part of the immune response [11]. Th1 cells produce IFN-γ and IL-2, which activate CD8+ T cells and natural killer (NK) cells and promote cellular immunity whereas Th2 cells produce IL-4, IL-5, IL-10 and IL-13, and promote humoral immunity.
T helper cell differentiation is regulated at many levels. Interactions of peptide antigen with the T cell receptor (TCR), cytokine signaling, actions of co-stimulatory molecules and induction of key transcription factors are major players in determining the type of T helper differentiation. The classic cytokine used for in vitro differentiation of Th1 effector cells from naïve T cell precursors is IL-12 [12–14]. IL-12 also induces IFN-γ production in NK and T cells. IL-12 has been reported to enhance the activity of NK and T cells. IL-12 with IFN-γ antagonizes Th2 differentiation and inhibits the production of IL-4, IL-5, and IL-13 [15–17]. The action of IL-12 is mediated through its receptor and IL-12 receptor (IL-12R), which consists of two β-receptor subunits, β1 and β2. Resting T cells do not express β1 and β2. These receptors are induced upon T cell differentiation. Maintenance of the expression of IL-12Rβ2 is required for normal Th1 differentiation as well. Thus, the expression of IL-12Rβ2 can be considered as a marker for Th1 differentiation [18–20]. The major sources of IL-12 are antigen-presenting cells (APCs) such as dendritic cells and macrophages, thereby establishing an important link between innate and adaptive immune responses.
Another key cytokine that regulates Th1 differentiation is IFN-γ. It is the “signature” cytokine of Th1 effector cells and is important for the stabilization of the Th1 phenotype itself. Differentiated Th1 cells normally do not produce Th2 cytokines. However, Th1 cells from IFN-γ deficient mice retain the ability to produce IL-4 if re-stimulated under Th2 polarizing conditions [21]. IFN-γ induces the transcription factor T-box protein expressed by T cells (T-bet) [22,23]. T-bet, in turn, promotes the expression of IL-12Rβ2 and IFN-γ in CD4+ T cells, thereby establishing a potential feedback loop. Transcription factor STAT4 is also involved in Th1 polarization through regulation in the expression of IL-12Rβ2 and IFN-γ gene in CD4+ T cells. T-bet is now considered to be a major candidate as the Th1 master gene. CD4+ T cells from T-bet−/− mice have a severe defect in Th1-polarized responses [17,24], suggesting that T-bet is essential for Th1 polarization.
Evidences suggest that Th1 polarized response plays an important role in tumor surveillance. IFN-γ has been shown to have an antitumor response in tumor immuno-surveillance [25]. Our recent study demonstrates that both human breast cancer cells SK-BR-3 (Her2/neu with epitheloid morphology) and MDA-MB-231 (a triple negative and highly invasive cell line) cultured in the presence of IFN-γ and IL-2 cytokines exhibited smaller tumorspheres in 3D culture condition compared to larger tumorspheres in regular medium without IFN-γ and IL-2, suggesting that Th1 cytokines enriched mi-croenvironment may inhibit tumor growth [26].
On the other hand, Th2 differentiation is central to the promotion of humoral immunity, allergic reactions to environmental antigens, and resistance to parasite infections. Th2 cells produce IL-4, IL-5, IL-10, and IL-13, which act with toxic mediators of innate immune cells to establish environments that are inhospitable to parasites and other organisms. IL-4 is a known major determinant that regulates Th2 differentiation. Naive CD4+ T cells stimulated through the TCR in the presence of IL-4, develop into Th2 effector cells capable of producing IL-4, IL-5 and the related cytokine, IL-13, another important component of Th2 responses. IL-4 also suppresses the production of IFN-γ from Th1 cells, resulting in the switch of from Th1 to Th2 polarization [27].
The transcription factors STAT6 and GATA-3 play central roles in modulating Th2 differentiation. Upon binding, IL-4 recruits STAT6 to its receptor, where it is activated. GATA-3 expression is STAT6 activation dependent and is up-regulated during Th2 differentiation and down-regulated during Th1 differentiation. Activation of GATA-3 also strongly inhibits the production of IFN-γ and down regulates IL-12Rβ2 expression in an IL-4 independent manner [11]. The role of Th2 in tumor microenvironment has not been fully elucidated. Several studies showed that in Hodgkin's lymphoma, most abundant infiltrating CD4+ T lymphocytes display a phenotype of Th2 and regulatory T cells, a group of immunosuppressive cells [28–30]. The evidence from mouse tumor model studies suggests that Th2 cells and Th2 cytokines are involved in tumor development and progression through various mechanisms such as the activation of macro-phages and myeloid-derived suppressor cells (MDSCs) [31–33]. The balance between the Th1 and Th2 polarizations is important for immune response in tumor microenvironment. Therefore, further studies are required to explore the function of Th2 polarization in tumor microenvironment.
3. Regulatory T cells
Another type of tumor infiltrating immune cells is the regulatory T cells (Tregs). In general Tregs are CD4+CD25+FOXP3+ T cells defined as inhibitors of the T effector cell activity by suppressing the secretion of IFN-γ and IL-2 while increasing the production of IL-10 and TGF-β. In the presence of Tregs, the cytolytic functions of CD8+ and natural killer (NK) cell activity are suppressed [34,35]. The regulation mediated by Tregs is desirable in transplantation patients since they suppress immune responses to alloantigens. Excess accumulation of Tregs has been described as a cause of fatal course in infections and malignancies [34,36,37], which was related to the suppressive action of Tregs. The ranges of immune cells that can be suppressed by Tregs are quite wide and include CD4+CD25− T cells, natural killer (NK) cells, dendritic cells, monocytes and CD8+ T cells. An increased frequency and/or altered function of Tregs may contribute to the documented immuno-suppressed states such as aging and cancer. Non-antigen specific Tregs are anergic to mitogenic stimuli; they fail to proliferate after stimulation via its T-cell receptor (TCR) in a cytokine-independent but cell contact-dependent manner [37].
In prostate cancer tissues, tumor infiltrating T cells are skewed towards Treg (FOXP3+) and Th17 cells [38]. The number of Tregs in peripheral blood is increased in men with prostate cancer compared to normal healthy individuals [38]. Furthermore, studies revealed that tumor infiltrating Tregs, defined as CD8+FOXP3+ cells, suppressed naive T-cell proliferation mainly through a cell contact-dependent mechanism [39]. Interestingly, Tregs isolated from patients with prostate cancer exert a significantly greater suppressive activity than Tregs isolated from healthy donors [40]. These results suggest that Tregs may play potential pro-tumor role in tumor microenvironment; however, the exact molecular mechanism of Tregs action on the suppression of T cell activation in tumor immunology is unknown and requires further investigation.
4. Th17 cells and IL-17 cytokine
Th17 positive cells play an important role in tumor microenvironment and may impact cancer biology and therapeutics. Th17 cells are defined as CD4+ T helper effector cells that produce extensive amounts of IL-17, a pro-inflammatory cytokine that attracts and activates granulocytes and monocytes [41]. Development of Th17 cells is regulated by multiple cytokines [35,42,43] and the transcription factor retinoic acid receptor-related orphan receptor γ T (RORγT) [42,43].
Accumulating evidence suggests that Th17 cells exert an antitumor effect within the tumor microenvironment. In ovarian cancer patients, ascites level of IL-17, solely produced from Th17 cells was found to be positively associated with disease-free survival. Even after controlling for surgical therapy and other parameters, tumor-associated IL-17 had death hazard ratio that was negative. Average survival of cancer patients with greater than 220 pg/ml of IL-17 in ascitic fluid was 78 months, whereas death hazard ratio of patients with less IL-17 in their ascitic fluid was 27 months. In the tumor microenvironment, IL-17 synergized with IFN-γ to induce the production of CXCL9 and CXCL10, the Th1-type chemokines that recruit T cell effector populations within the tumor itself. Furthermore, ascites levels of CXCL9 and CXCL10 was correlated directly with the presence of tumor-infiltrating natural killer and CD8+ T cells [44]. Another recent study examined the role of tumor-infiltrating Th17 cells in 30 patients with lung adenocarcinoma or squamous cell carcinoma [45]. The results showed that malignant pleural effusion from these patients was chemotactic for Th17 cells, and this activity was partially abrogated by CCL20 and/or CCL22 blockade. Interestingly, higher accumulation of Th17 cells in malignant pleural effusion was positively associated with improved patient survival [45]. Several animal studies have also confirmed that Th17 cells has better anti-tumor efficacy, and especially synergized with IFN-γ or Th1 cells or activated CD8+ T cells [46–48].
However, other evidence suggests that Th17 cells may exert a pro-tumor effect within the tumor microenvironment. One clinical study demonstrated an inverse correlation between pretreatment circulating levels of Th17 cells and time to disease progression in prostate cancer patients [49]. Another study with 20 prostate cancer patients undergoing radical prostatectomy found an inverse correlation between the differentiation stage of Th17 cells in prostate glands of cancer patients and its association with tumor progression [38]. Furthermore, those prostate cancer patients responsive to immunotherapy with significant reductions in PSA level showed a Th17 profile similar to healthy male controls, but a significant difference from the patients who did not respond [49].
These controversial data regarding the role of Th17 cells in cancer may arise from the studies examining the function of cytokine IL-17 itself. IL-17 is a pro-inflammatory cytokine, which can induce the production of other pro-inflammatory cytokines, chemokines and prostaglandins. Currently, it has been identified that six family members of IL-17 (A–F) are expressed by a variety of innate and adaptive immune cell types, including mast cells, epithelial cells, smooth muscle cells, invariant natural killer T cells, natural killer cells, Paneth cells, lymphoid-tissue inducer -like cells, neutrophils, and γ6 and αβ T cells [43]. Although the role of IL-17 remains controversial, it is clear that IL-17 plays an important role in tumor microenvironment. For example, one clinical observation was that higher levels of IL-17 are positively associated with cancer patient survival [50]. However, other studies showed that high levels of IL-17 are associated with tumor microvessel density, poor prognosis, and short disease survival [51,52]. Further studies are needed to elucidate the function of Th17 cells and IL-17 in the tumor microenvironment.
5. Dendritic cells
Dendritic cells (DCs) are one group of antigen-presenting cells (APCs) that possess the ability to transport antigen from peripheral sites of infection into draining lymph nodes via activation of tolllike receptors (TLRs) [2]. Immature DCs effectively capture and trim exogenous antigen within the peripheral tissue where DCs initiate maturation [53]. After antigen uptake, the immature DCs migrate to the draining lymph nodes where immature DCs differentiate into phenotypically and functionally mature DCs [54]. Mature DCs express high levels of surface molecules such as CD80, CD86, MHC class I and MHC class II and activate naïve T cells, thereby inducing their differentiation toward Th1, Th2 or regulatory T cells [55]. Furthermore, mature DCs can produce various pro-inflammatory cytokines and activate innate immune cells [56], and act as a link between innate and adaptive immune responses. Mature DCs are the major source of pro-inflammatory cytokine IL-12 [2], which can be responsible for the activation of T cells to differentiate Th1 cells, suggesting a role of mature DCs in the activation of Th1 immune response.
DCs play a dual role in tumor biology, showing either anti-tumor activity or tumor promoting activity, depending on the cytokine milieu encountered within the tumor microenvironment, and the degree of maturation. A decreased population of mature DCs in tissue is shown to impair the ability of immune system to induce and maintain an effective anti-tumor immune response [57,58]. Accumulating evidence suggests that DCs play important roles in the tumor microenvironment; however, the exact mechanism(s) of DC function in tumor microenvironment is not fully understood.
6. Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are a group of myeloid cells comprising of immature macrophages, granulocytes, DCs, and other myeloid cells at earlier stages of differentiation. MDSCs can suppress the proliferation and function of both CD4+ and CD8+ T cells [59,60], and are responsible for significant immune dysfunctions. The murine cell-surface phenotype of MDSCs was identified as CD11b+CD11c+Gr-1+IL-4Rα+, indicating that these cells belong to one class of inflammatory monocytes [61]. In addition, a subset of the bone marrow-derived MDSC population that matures at the tumor site can differentiate into tumor-infiltrating macrophages [59,62]. Prior to exerting effector functions, MDSCs are first activated by cytokines such as IL-4, IL-13, or IFN-γ, which are generated by tumor-infiltrating lymphocytes or by MDSCs themselves in an autocrine feedback pathway [63].
MDSCs can promote tumor growth and development by secretion of various molecules and factors such as inflammatory cytokines essential for tumor growth and angiogenesis and also exert a profound inhibitory activity on both tumor specific and non-specific CD4+ and CD8+ T cells [63–65]. The functional plasticity of MDSC has been reported to be regulated by T helper cytokines. Particularly, the sup-pressive activity of MDSCs can be enhanced by the addition of Th2 cytokines such as IL-4 and IL-10 to the cultures. However, the co-culture of MDSCs with Th1 cytokines can enhance antigen specific T cell cyto-toxicity. Our recent study reports a reduced percentage of granulocytic CD33+/CD11b+/CD14−/HLA-DR− and monocytic CD33+/CD11b+/ CD14−/HLA-DR+ MDSC populations in the presence of Th1 cytokines for human breast cancer SK-BR-3 and MDA-MB-231 cells, compared to co-culture without Th1 cytokines. Low levels of IFN-γ, CXCL9 and CXCL10 were associated with increased numbers of MDSC. Increased levels of IFN-γ, CXCL9 and CXCL10 corroborate with reduced number of MDSC, suggesting that Th1 cytokine enriched microenvironment inhibits the development of MDSC [26].
7. Tumor-associated macrophages
Macrophages play critical roles in the regulation of inflammatory responses, wound healing, tissue repair and tissue remodeling. Due to its antigen-presenting activity, macrophages are critically involved in cellular immunity and can destroy tumor cells via cytotoxic mechanisms [66,67], which can then impact tumor progression. Usually, tumor cell damage and hypoxia attract macrophages within or in the surrounding tissue area of tumors, namely tumor-associated macrophages (TAM). TAMs are usually abundant in the tumor microenvironment and are known to play an essential role in tumor progression. TAMs have varying functions, depending on the microenvironment of tumor tissues. Although some studies have reported that an increased number of TAMs represses tumor growth and progression [68,69], most clinical and experimental data demonstrate that an increased population of TAMs is correlated with reduced disease-free and overall survival of cancer patients.
Many tumors secrete molecules or factors that prevent macrophages from signaling other immune cells in the presence of tumor cells, resulting in the loss of capacity of the immune system to recognize the tumor cells. TAMs themselves secrete factors such as inflammatory cytokines that enhance tumor cell proliferation and invasion, and promote angiogenesis. In addition, TAMs release reactive oxygen species and other mutagenic compounds that may cause mutations in surrounding host cells. Ablation of macrophages in mouse tumor models results in the inhibition of tumor angiogenesis and metastasis [70–72]. Increased numbers of TAMs have been found to be associated with increased tumor angiogenesis, invasion and metastasis, or poor prognosis in cancer [73,74]. Therefore, TAMs play a critical role in tumor growth, progression, invasion and metastasis, suggesting that the conditioning of tumor microenvironment by targeting TAMs could be a useful strategy for the treatment of human malignancies.
7.1. M1 and M2 subpopulations of TAMs
TAMs constitute the majority of tumor-infiltrating leukocytes in the tumor microenvironment, and are defined as two distinct and polarized TAM sub-populations [75]. Classical, or M1, macrophages are characterized by the expression of high levels of iNOS and tumor necrosis factor-α (TNF-α) and exhibit an anti-tumor response [76]. Whereas alternatively activated M2 macrophages are characterized by the high expression of arginase-1 (ARG1) and IL-10 [77,78].
Polarized TAMs differ in their expression of receptors, effector function and, cytokine and chemokine production. Differential cytokine production is a key feature of polarized macrophages. Cytokines produced by M1 macrophages such as IL-12 and TNF induce T-cell effector functions [79] while M2 macrophages largely produce immune suppressive cytokine such as IL-10 [80]. In addition to cytokine polarization in M1 and M2 macrophages, chemokine receptors and ligands are also differentially regulated. M1 macrophages produce IFN-γ-inducible chemokines IP-10 (CXCL10) and MIG (CXCL9) that attract Th1 cells and M2 macrophages produce CCL22 that attracts Th2 or Tregs [81]. At the tumor site, M2-like macrophages constitute the predominant population of TAMs, which can suppress T cell-mediated anti-tumor responses and promote tumor progression, metastasis, and angiogenesis [79,80,82].
8. Immunological role of NF-κB in the tumor microenvironment
NF-κB is one of the major transcription factors for the development of immune responses, and thus alterations of NF-κB activation are known to play critical roles in the development of chronic diseases including cancer. This transcription factor is activated by many stimuli including inflammatory cytokines, LPS, protein kinase C activators, reactive oxygen species (ROS), ultraviolet light and ionizing radiation, and other cellular stresses [83,84]. NF-κB activation regulates the expression of numerous genes such as inflammatory cytokines, chemokines, enzymes (iNOS and inducible cyclooxygenase), transforming growth factor 2, adhesion molecules, receptors, and other immune mediators [83–85]. NF-κB is a central player involved in critical innate and adaptive immune and stress responses as well as cell survival and proliferation responses (Table 1).
Table 1.
NF-κB regulated immune modulators.
| Activity | Mediators/receptors | References |
|---|---|---|
| NF-κB Regulation of Inflammation and adaptive immunity | ||
| NF-κB induction and activation | IL-1/IL-1R, TNF/TNF-R, TCR/BCR, TLRs, CD40/BAFF-R | Refs. [200–205] |
| NF-κB regulation of adaptive immunity (Defects in B- and T-cell proliferation, activation, cytokine production and B cells isotype switching) |
STAT3, STAT5a, B7-1 (CD80) and B7-2 (CD86), IL-2, IL-18 and IFN-γ, mutation or NF-κB1 and NF-κB2 deficiency |
Refs. [206–210] |
| NF-κB regulation of inflammation | IL-1, IL-8, TNF-α, MIP-2, MCP-1, COX-2, ICAM-1 | Refs. [211–215] |
| NF-κB mediated regulation of tumor promoting factors | ||
| Survival | Bcl-2, Bcl-XL, XIAP, TRAF1/2, cFLIP, cIAP, Survivin | Refs. [216–220] |
| Proliferation | Cyclin D, c-Myc | Refs. [221–224] |
| Angiogenesis | MCP-1, IL-8, IL-1, IL-6, VCAM-1 | Refs. [225,226] |
| Epithelial-to-mesenchymal transition (EMT) | Vimentin, Cathepsis B, MMPs, Twist | Refs. [227,228] |
| Invasion and metastasis | MMP-2, MMP-9, VCAM-1, ICAM-1, uPA | Ref [221] |
NF-κB is normally present in the cytoplasm in an inactive form bound to an inhibitory protein of NF-κB (IκB), which has several sub-units such as IκB-α, IκB-β, and IκB-γ [83–85]. Classically, the NF-κB is activated when it is dissociated from IκB. Cytosolic NF-κB becomes active and translocates to the nucleus only when IκB is dissociated from the NF-κB heterodimer p50/p65. Many stimuli activate NF-κB via phosphorylation of IκB proteins through the action of specific kinases such as IκB kinase (IKK). Phosphorylation of IκB results in the attachment of ubiquitin residues and subsequent degradation by the multifunctional proteolytic enzyme proteasome complex [83–85]. Dissociation of NF-κB from IκB results in the rapid translocation of NF-κB to the nucleus for activation of NF-κB target genes.
8.1. Role of NF-κB in tumor progression
Loss of regulation of the normally latent NF-κB contributes to the deregulated growth, resistance to apoptosis, and propensity to metastasize as observed in many human cancers, and thus targeted inactivation of NF-κB has been recognized as a promising strategy for the prevention and/or treatment of human cancers. High levels of NF-κB activity are associated with increased expression of NF-κB-regulated genes such as proinflammatory cytokines IL-1α, IL-6, and IL-8, which may contribute to tumor progression and metastasis [86].
The molecular mechanisms of NF-κB pathway in cancer cells have been thoroughly investigated. However, the immunological contribution of NF-κB to the tumor microenvironment remains unclear. The immunological role of NF-κB in tumor growth and progression within the context of tumor microenvironment is very complex, due to its different functions depending on different cell lineages. Limited studies have been conducted to examine the role of NF-κB activation in the regulation of tumor associated host immune cells, especially T helper cells, Treg cells, and DCs that comprises the tumor microenvironment.
8.2. Role of NF-κB in the development of innate immune responses
The activation and nuclear translocation of NF-κB have been associated with increased transcription of chemokines, cytokines and adhesion molecules, which constitute important components of the innate immune response to invading microorganisms. Induction of chemokines, cytokines and adhesion molecules can facilitate recruitment and activation of inflammatory cells at the site of NF-κB activation. In addition to direct NF-κB activation as a consequence of inflammation and infection, there are indirect pathways that lead to NF-κB activation. For example, the release of IL-1 can activate the NF-κB pathway which, in turn, leads to the production of the other cytokines and chemokines [87].
NF-κB can be activated by many bacterial products; however, the recognition of the Toll-like receptors (TLRs) which has been shown to be a specific pattern recognition molecules [88] leads to the activation of NF-κB, and this result enhanced our understanding of pathogens stimulated NF-κB activation. TLRs are widely distributed on macrophage, dendritic and B-cells [89]. Signaling through the TLR has been linked to increased expression of a number of genes known to be regulated by NF-κB, including cytokines, co-stimulatory molecules, nitric oxide, and further contributed to the susceptibility to apoptosis as well as an autocrine increase in TLR expression.
IL-12 is one of the most important cytokines involved in the activation of innate and adaptive responses; its production leads to the innate activation of NK cells and enhances their cytolytic activity and production of IFN-γ. IL-12p40 promoter was demonstrated to have NF-κB binding sites that are involved in the transcriptional production of IL-12 [90,91].
RelB, an NF-κB family member, has been reported to be responsible for DC differentiation [92]. In DCs, the activation of NF-κB induced by pro-inflammatory cytokines and toll-like receptor (TLR) signal results in the induction of transcription of genes involved in DC maturation, and antigen-processing and presentation [93]. Inhibition of NF-κB activation could maintain DCs in an immature state promoting immune tolerance [93]. Specifically, the loss of RelB in DCs resulted in a shift of a Th1/Th17 to a Th2 and FOXP3 + Treg cell profile in the co-culture system [92]. These studies suggest that NF-κB plays a key role in maturation and function of DC, which impact cancer development and progression within the context of tumor microenvironment [93].
8.3. Role of NF-κB in adaptive immune responses
T-cell responses can be broadly divided into two Th1 (characterized by IL-2 and IFN-γ production) or Th2 (characterized by IL-4 and IL-5 production) functional subsets. NF-κB can directly be involved in the polarization T-cell responses or via IL-12 production that is required for the generation of Th1 responses [94,95].
Maintenance of long-term memory cells is the hallmark of adaptive immunity. NF-κB signaling has recently been implicated in the signals that allow development of memory phenotype. NF-κB protein families have been identified to be involved in the regulation of IL-2 and IFN-γ gene expression in T lymphocytes with co-activation of MAPK pathway [20,24,96] and controlling the activation of T cells via TCR and CD28 ligation [97]. One study showed that the loss of NF-κB activation induced by its inhibitor resulted in decreased level of IFN-γ and IL-17 in activated CD4+ T cells [98]. NF-κB also promotes T-cell proliferation; transgenic mice that express the degradation-deficient ΔIκB transgene which acts as a global inhibitor of NF-κB activity showed impaired T cell proliferation [99]. Another study showed that signaling through the T-cell receptor (TCR) in mature T cells requires NF-κB activity [100] which may be important in TCR-mediated proliferative signals.
Involvement of NF-κB in the development of Th1-type responses is well known but its regulation of Th2-type responses is less clear. Studies using ΔIκB mutant mice indicated that it did not alter the ability of these mice to develop Th2-type responses in vivo [101]. In experimental allergic encephalomyelitis [102] and pulmonary inflammation [103], NF-κB1 is required for the development of Th2 responses through the regulation of transcription factor GATA3 signaling [104], which plays an important role in differentiation of Th2 cells and their production of IL-4 and IL-10 [105,106].
8.4. Role of NF-κB in B-cell activation and effector function
Presence of constitutive NF-κB activity in B cells and identifying that a nuclear factor was able to bind to the κB site in the immunoglobulin κ light chain enhancer underscore the importance of NF-κB in the control of B-cell functions. In fact multiple studies using mice deficient in NF-κB1, NF-κB2, RelA, RelB, c-Rel, or Bcl-3 showed compromised humoral immune responses and documented its role in immunoglobulin class switching [107,108]. These studies suggest that NF-κB is an important regulator of B cell survival, cell cycle progression, and their effector functions.
8.5. Role of NF-κB in regulatory T cells, TAM and MDSC
It has been found that c-Rel/RelB but not NF-κB1 (p50) is important for Treg cell development by up-regulation of FOXP3 expression, a major transcription factor for Tregs [109–112]. However, more studies are required to examine the role of NF-κB in the regulation of Tregs in the tumor microenvironment.
The role of NF-κB in the regulation of macrophage in cancer biology has been intensively investigated. Activation of NF-κB in macro-phages is required for the onset of tumor development in several inflammation-induced cancer models [113–119]. Inhibition of NF-κB signaling pathway induces TAMS to become cytotoxic to tumor cells [71,120]. NF-κB is also known to regulate the expression of many important genes including tumor-promoting genes such as such as VEGF, IL-6, TNF-α, and COX2, which support its crucial role in the activation of tumor-associated macrophages in the tumor microenvironment [121,122].
8.6. Potential target of NF-κB for cancer therapy
NF-κB transcription factors have a key role in many physiological processes such as innate and adaptive immune responses, cell proliferation, cell death, and inflammation. Aberrant regulation of NF-κB and the signaling pathways that control its activity promote cancer development, progression and resistance to therapy, which makes it a potential therapeutic target. There are many strategies that have been experimentally employed to inhibit NF-κB activation or function and one of the strategies to inhibit the NF-κB activation is by using proteasome inhibitors. Inhibitors of the 26S proteasome were shown to inhibit IκB degradation and NF-κB nuclear translocation. At least one proteasome inhibitor, bortezomib (Velcade; Millenium), has entered into clinical development for the treatment of myeloma; however, prolonged NF-κB inhibition can be detrimental due to its role in innate immunity. Therefore, NF-κB inhibition strategies that are transient and reversible would be more beneficial while avoiding long-term immuno-suppression.
9. The protective role of zinc in tumor microenvironment
Zinc, an essential trace mineral element in humans and animals, has been known to participate in the activation of approximately 300 enzymes and is involved in the regulation of over 2000 zinc-dependent transcription factors, which are involved in DNA synthesis, protein synthesis, cell division, and other metabolisms. Therefore, zinc plays critical roles in various biological processes in the body [123–127]. Early evidence has indicated that zinc deficiency can cause body growth retardation, delayed sexual maturation, depressed immune response, and cause abnormal cognitive functions. Zinc supplementation prevents these adverse effects.
The relationship of zinc and tumor has been intensively investigated in experimental and human studies over decades. Although one early report demonstrated that dietary zinc deficiency inhibited tumor growth in immunocompetent mice and rats [128]; however, the majority of reports have demonstrated that zinc deficiency increases the growth and/or incidence of carcinogen-induced gastrointestinal tumors in immuno-competent mice and rats [129–145], consistent with the recent findings [128,146,147]. Furthermore, zinc supplementation can inhibit the growth of human prostate cancer PC-3 cells-induced tumor in nude mice, compared to zinc deficient nude mice [148]. All these animal studies are based on carcinogen-induced and xerograft tumor models. Recently, the protective role of dietary zinc in prostate cancer has been investigated in TRAMP (transgenic adenocarcinoma of the mouse prostate) mouse model [149]. The results showed that tumor weights were significantly higher when the dietary zinc intake was either deficient or high in comparison to normal zinc intake level, suggesting that an optimal dietary zinc intake may play a protective role against prostate cancer. A large number of epidemiological and clinical studies demonstrate that zinc deficiency is associated with increased risk of some cancers, such as prostate, lung, esophageal, and oral cancers [150–153]. These data from experimental animal and human studies have strongly suggested that zinc may play a protective role in the development and progression of cancer; however, the exact mechanism of zinc action within the tumor microenvironment has not been fully understood. A large number of studies have demonstrated that zinc deficiency induces inflammatory cytokines and oxidative stress, apoptosis and cellular dysfunction, disrupts DNA-protein interaction, and depresses the function of T cell mediated immune response [153,154]. Zinc supplementation reverses these adverse effects, suggesting that the anti-tumor effect of zinc could be potentially due to its differential regulation of host immune cells and cancer cells within tumor microenvironment; however, how zinc accomplishes this task has not been investigated. Zinc has been implicated as a chemo-preventive agent, but the role of zinc in conditioning the tumor microenvironment is not known and requires more mechanistic investigation.
9.1. The inhibitory effect of zinc on cancer cell growth
A large number of cell culture studies have demonstrated that the physiological level of zinc could exhibit anti-tumor effects by inhibiting cell cycle, cell growth and proliferation, and cancer cell invasion by regulating oncogenic signaling pathways such as NF-κB, AP-1, Notch-1, and PI3K/Akt [155,156]. Moreover, zinc is also known for induction of mitochondria-mediated apoptosis, cytotoxicity, up-regulation of zinc-dependent activities of tumor suppressors (such as p21, p53, and A20) and stabilization of protein-DNA binding integrity in many different normal and cancer cell lines (such as pancreatic cancer cells, prostate cancer cells, and liver cancer cells) [157–163]. The loss of zinc could be responsible for the development and progression of human malignancies, and thus suggesting that retention of the normal levels of cellular zinc might exhibit cytotoxic effects on the malignant cells. Exposure of the human pancreatic cancer Panc1 cells to a physiological concentration of 2.5–10 µM zinc has been reported to increase the cellular level of zinc, which resulted in 60%–80% inhibition of cell proliferation, compared to the zinc deficient condition. This cytotoxic effect indicates that incorporation of zinc into malignant pancreatic cells could exhibit anti-tumor effects [164].
9.2. The effect of zinc on Th1/Th2 cells and T-bet activity
One early study showed that zinc is essential for IL-2 mediated T cell activation [165]. Our previous cell culture studies revealed that zinc increases IL-2 and IFN-γ cytokines and mRNAs in HUT-78 (Th0 malignant leukemia) and D1.1 (Th1 malignant leukemia) cells after PMA/PHA and PMA/ionomycin D stimulation, compared to zinc deficient condition [166,167]. Studies in our experimental human model, where we induced a mild deficiency of zinc by dietary restriction, showed that ex vivo production of IL-2, and IFN-γ in isolated peripheral blood mononuclear cells (PBMC) was decreased and was normalized following zinc supplementation. Production of IL-4, IL-5 and IL-6 was not affected in zinc deficiency [168]. Our previous clinical observation demonstrated that 50% of patients with newly diagnosed head and neck cancer had zinc deficiency. The tumor size and overall stage of the disease was correlated with baseline zinc status. These patients also had decreased production of Th1 cytokines, but not Th2 cytokines [169–171]. Our recent study also demonstrates that zinc-dependent IL-2 and IFN-γ productions are associated with the up-regulation of T-bet gene expression via increased intracellular free zinc in HUT-78 cells [167]. This suggests that zinc plays an important role in Th1 phenotype and function.
9.3. The effect of zinc on T cell subpopulations
In our experimental human studies, zinc deficiency was associated with a decrease in the CD4+/CD8+ ratio, which was normalized following zinc supplementation [168]. Our studies showed that naive T cells (CD4+CD45RA+) newly exported from the thymus were affected in zinc deficiency. A significant decrease in the ratio of CD4+CD45RA+/CD4+CD45RO+ cells was observed at the end of zinc depletion phase, which was normalized following zinc supplementation. Inasmuch as maturation of the T cell is dependent upon intact thymic functions, one may expect a decrease in the T cell population if thymic function is abnormal [172]. We have demonstrated that thymulin, an important thymic hormone, is zinc dependent. Zinc deficiency results in the reduction of thymulin activity. Therefore, zinc appears to play an essential role in T cell activation, which could be very important within the tumor microenvironment although such studies have not been done to-date.
9.4. The role of zinc on Tregs, TAM, DCs, Th17, and MDSCs
As an essential immune responsive mediator, the role of zinc in the regulation of Treg, TAM, DC, Th17, and MDSC function in tumor development and progression has not been fully elucidated. However, emerging evidence suggest that zinc is required to maintain the normal functions of macrophages and DCs. For example, zinc deficiency has been found to cause either bacterial phagocytic dysfunction in alveolar macrophages from alcohol-fed rats or DC disintegration and immaturation in mice and rats. Zinc supplementation reverses this adverse effect [173–175]. These results suggest that zinc may play a protective role in tumor development and progression although further in-depth investigation is required for understanding of its molecular roles in the tumor microenvironment.
9.5. The role of zinc on inflammation and oxidative stress
A large number of cell culture and animal studies have demonstrated that zinc deficiency increases oxidative stress and inflammatory responses, which are known as key contributors of chemo- and radiotherapy resistance. For example, zinc deficiency causes increased productions of ROS and inflammatory cytokines such as TNF-α, IL-1β, IL-8, VCAM, and MCP-1 in both non-malignant (such as vascular endothelial, lung, and prostate cells) and malignant cells (such as prostate cancer cells, colon cancer cells, promyelocytic and monocytic leukemia cells) [155,173–179]. Zinc supplementation reverses these adverse effects, which suggests that zinc could be useful as an anti-oxidant and anti-inflammatory agent.
Some early cell culture and animal studies have shown that zinc could function as an anti-oxidant agent as a site-specific antioxidant in the body by several mechanisms [180–183]. First, zinc competes with iron and copper ions, which catalyze the production of OH from H2O2, for binding to cell membranes and some proteins, by displacing these redox-active metals. Secondly, it binds to sulfhy-dryl (SH) groups protecting them from oxidation [181]. Thirdly, zinc has been shown to increase the activities of glutathione (GSH), catalase, and superoxide dismutase (SOD, a ROS scavenger), and could decrease the activities of inducible nitric oxide synthase (iNOS) and NADPH oxidase and lipid peroxidation products [184,185]. Fourthly, zinc is known to induce the expression of metallothionein (MT) protein, which is very rich in cysteine and an excellent scavenger of •OH [186,187]. NF-κB mediated inflammatory response has been recognized as one of the major sources of oxidative stress. Recently, it has been considered that the inhibition of NF-κB-mediated inflammatory response by zinc contributes to the repression of oxidative stress.
One clinical trial of 30 mg zinc supplementation on oxidative stress was conducted in 56 patients with type 2 diabetes mellitus [183]. Following 6 months of zinc supplementation, plasma zinc increased and plasma TBARS, a lipid peroxidation marker significantly decreased, whereas the placebo group showed no such changes [183]. Increased plasma lipid peroxidation by-products and decreased erythrocyte SOD were reported to be associated with decreased zinc status in children with chronic giardiasis [182]. Our earlier studies have clearly demonstrated that following zinc supplementation, oxidative stress as assessed by the generation of lipid peroxidation and DNA oxidation by-products, inflammatory cytokines, and ex vivo TNF-α induced activation of NF-κB in isolated PMNC, were significantly decreased compared to placebo supplemented normal human subjects [188]. Our recent studies in collaboration with Dr. Prasad in elderly subjects and sickle cell disease (SCD) patients confirmed that zinc supplementation significantly increased plasma zinc level and decreased plasma oxidative stress bio-markers, and inflammatory cytokines along with decreased rate of infection [189]. These data strongly suggest that zinc certainly functions as an anti-oxidant and anti-inflammatory agent, which could play a protective role in the development and progression of cancers; however, further definitive clinical trial in human cancer patients or in population with high-risk for the development of cancer are warranted especially within the context of tumor microenvironment.
9.6. The differential role of zinc in the regulation of NF-κB activation
As indicated above that zinc plays an important role in the regulation of NF-κB activation; however, the regulation of NF-κB activation by zinc appears to be cell type specific. For example, zinc has been found to be required for NF-κB DNA binding activity either by human placenta-purified proteins, recombinant NF-κB p50, or human Th0 cell line-derived nuclear protein extracts [190–192]. Inasmuch as NF-κB binds to the promoter enhancer area of IL-2 and IL-2Rα genes, we investigated the effect of zinc deficiency on activation of NF-κB and its binding to DNA in HUT-78, a Th0 malignant human lymphoblastoid cell line. We showed that in low zinc HUT-78 cells, phosphorylated IκB, IKK, and ubiquitinated IκB and binding of NF-κB to DNA were all significantly decreased, which was consistent with decreased production of IL-2 and IL-2Rα, and IFN-γ, in comparison to HUT-78 cells with normal zinc levels [191]. Zinc increased the translocation of NF-κB from cytosol to the nucleus. We also demonstrated that binding of recombinant NF-κB (p50)2 to DNA in HUT-78 cells was zinc specific. Inhibition of NF-κB activation by anti-sense p105 (p50 precursor) mRNA abrogates the zinc-mediated IL-2 and IL-2Ra production in HUT-78 cells. These results suggest that zinc plays an important role in the regulation of the productions of IL-2 and IL-2Rα via the activation of NF-κB in HUT-78 cells. Such results are provocative although the role of these cytokines production affected by zinc must be investigated thoroughly within the context of tumor microenvironment.
Interestingly, a number of in vitro studies have shown that zinc inhibits LPS-, ROS-, or TNF-α-induced NF-κB activation in endothelial cells, pancreatic cells, cancer cells, and PMNC [155,176–178, 189,193,194], consistent with decreased expression of inflammatory cytokines, oxidative stress, anti-apoptotic protein C-1AP2, and activated C-Jun NH2-terminal kinases, which promotes apoptotic pathways [195,196]. Thus, anti-inflammatory and anti-oxidant functions of zinc are associated with its inhibition of NF-κB activation in these non-T helper cells. There are inhibitors of NF-κB activation that are different from IκB protein. One such endogenous inhibitor of NF-κB activation is A20 (also known as TNF-α induced protein 3, TNFAIP3), a cytoplasmic zinc finger-transactivating factor that plays a key role in the negative regulation of inflammation via inhibition of IL-1β- and TNF-a-induced NF-κB activation [197–199]. A20 has been found to inhibit NF-κB activation through the down-regulation of TNF-receptor associated factor (TRAF) signaling pathway, resulting in the inactivation of IKK, a NF-κB activating kinase.
The inhibitory role of zinc in NF-κB-mediated inflammation has been considered to be associated with A20 in non-T helper cells. Our recent studies have shown that zinc increases A20 and A20-TRAF1 complex, and decreases NF-κB activation, IKK, and the production of inflammatory cytokines (TNF-α, IL-1β, IL-8, MCP-1, and VCAM) and oxidative stress in HL-60, TPH-1, and vascular endothelial cells, compared to zinc deficient cells [155,176,188]. Silencing of A20 by its anti-sense RNA (siRNA) increased TNF-α and IL-1β production in zinc-sufficient cells [155], suggesting that zinc down-regulates the production of inflammatory cytokines via A20 pathway, which is due to deregulation of NF-κB activity. Therefore, zinc differentially regulates NF-κB activation through a cell lineage-dependent mechanism, which may contribute to its protective role in host immune cells within the tumor microenvironment.
10. Perspectives and conclusions
The traditional approach such as cytotoxic chemotherapeutics or even targeted therapeutics for cancer treatment by targeting cancer cells alone may not produce optimal therapeutic outcomes. Recent advances suggest that the tumor microenvironment may play a pivotal role in tumor initiation, progression, invasion and metastasis, and thus targeting the tumor microenvironment is expected to produce results that would shift paradigms of cancer therapeutics leading to improved clinical outcomes. Thus, targeting the tumor microenvironment provides a novel strategy for cancer treatment although the specific mechanisms and functions of each component of the tumor microenvironment are not fully understood, and thus require in-depth molecular investigations. Since NF-κB plays an important role in cancer development and progression, further understanding of the role of NF-κB in the deregulation of the immunological processes within the tumor microenvironment are critical. Accumulating evidence suggests that NF-κB may have different regulatory effects on tumor associated immune cells such as Th1, Th2, Th17, Tregs, DCs, MDSCs, and TAMs in the tumor microenvironment. Furthermore, it is tempting to speculate that zinc could serve as an important agent for conditioning the tumor microenvironment to optimize antitumor effects of the immune system since zinc is known to differentially regulate NF-κB activation through a cell-specific manner and is known to play an important role in Th1/Th2 polarization. Therefore, zinc could be useful in conditioning the tumor microenvironment for improving therapeutic outcome of cancer patients treated with conventional therapeutics. Moreover, any approach by which the tumor microenvironment could be conditioned would become useful for optimizing cancer therapy using existing conventional or targeted therapy.
Acknowledgements
We thank Puschelberg and Guido foundations for their generous financial contribution.
Grant support: National Cancer Institute, NIH grants 5R01CA131151, 3R01CA131151-02S1, and 5R01CA132794 (F.H. Sarkar), and DOD Exploration-Hypothesis Development Award PC101482 (B Bao).
References
- 1.Alvaro T, de la Cruz-Merino L, Henao-Carrasco F, Villar Rodríguez JL, Vicente Baz D, Codes Manuel de Villena M, Provencio M. Tumor microenvironment and immune effects of antineoplastic therapy in lymphoproliferative syndromes. J. Biomed. Biotechnol. 2010 doi: 10.1155/2010/846872. (2010) [Electronic publication ahead of print, 2010 Aug 12]. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Cretu A, Brooks PC. Impact of the non-cellular tumor microenvironment on metastasis: potential therapeutic and imaging opportunities. J. Cell. Physiol. 2007;213:391–402. doi: 10.1002/jcp.21222. [DOI] [PubMed] [Google Scholar]
- 4.Oluwadara O, Giacomelli L, Brant X, Christensen R, Avezova R, Kossan G, Chiappelli F. The role of the microenvironment in tumor immune surveillance. Bioinformation. 2010;5:285–290. doi: 10.6026/97320630005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piersma SJ. Immunosuppressive tumor microenvironment in cervical cancer patients. Cancer Microenviron. 2011;4:361–375. doi: 10.1007/s12307-011-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sautes-Fridman C, Cherfils-Vicini J, Damotte D, Fisson S, Fridman WH, Cremer I, eu-Nosjean MC. Tumor microenvironment is multifaceted. Cancer Metastasis Rev. 2011;30:13–25. doi: 10.1007/s10555-011-9279-y. [DOI] [PubMed] [Google Scholar]
- 7.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration invasion and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 9.Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O'Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J. Clin. Immunol. 2003;23:147–161. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 12.Frucht DM, Fukao T, Bogdan C, Schindler H, O'Shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556–560. doi: 10.1016/s1471-4906(01)02005-1. [DOI] [PubMed] [Google Scholar]
- 13.Fukao T, Frucht DM, Yap G, Gadina M, O'Shea JJ, Koyasu S. Inducible expression of Stat4 in dendritic cells and macrophages and its critical role in innate and adaptive immune responses. J. Immunol. 2001;166:4446–4455. doi: 10.4049/jimmunol.166.7.4446. [DOI] [PubMed] [Google Scholar]
- 14.Grohmann U, Belladonna ML, Bianchi R, Orabona C, Ayroldi E, Fioretti MC, Puccetti P. IL-12 acts directly on DC to promote nuclear localization of NF-kappaB and primes DC for IL-12 production. Immunity. 1998;9:315–323. doi: 10.1016/s1074-7613(00)80614-7. [DOI] [PubMed] [Google Scholar]
- 15.Cui G, Florholmen J. Polarization of cytokine profile from Th1 into Th2 along colorectal adenoma-carcinoma sequence: implications for the biotherapeutic target? Inflamm. Allergy Drug Targets. 2008;7:94–97. doi: 10.2174/187152808785107589. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 17.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 18.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper1 cells. J. Exp. Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinigaglia F, D'Ambrosio D, Panina-Bordignon P, Rogge L. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol. Rev. 1999;170:65–72. doi: 10.1111/j.1600-065x.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 20.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Apilado R, Coleman J, Ben-Sasson S, Tsang S, Hu-Li J, Paul WE, Huang H. Interferon gamma stabilizes the T helper cell type 1 phenotype. J. Exp. Med. 2001;194:165–172. doi: 10.1084/jem.194.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 23.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O'Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 25.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Thakur A, Schalk D, Sarkar SH, Al-Khadimi Z, Sarkar FH, Lum LG. A Th1 cytokine enriched microenvironment enhances tumor killing by activated T cells armed with bispecific antibodies and inhibits the development of myeloid-derived suppressor cells. Cancer Immunol. Immunother. 2011 doi: 10.1007/s00262-011-1116-1. [Oct 5, Electronic publication ahead of print]. PMID: 21971587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Visser L, Blokzijl T, Harms G, Atayar C, Poppema S, van den BA. The CD4+ Lab. Invest. 2008;88:482–490. doi: 10.1038/labinvest.2008.24. [DOI] [PubMed] [Google Scholar]
- 29.Poppema S, Lai R, Visser L, Yan XJ. CD45 (leucocyte common antigen) expression in T and B lymphocyte subsets. Leuk. Lymphoma. 1996;20:217–222. doi: 10.3109/10428199609051610. [DOI] [PubMed] [Google Scholar]
- 30.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin's lymphoma: increasing evidence of the importance of the microenvironment. J. Clin. Oncol. 2011;29:1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 31.Berzofsky JA, Terabe M. A novel immunoregulatory axis of NKT cell subsets regulating tumor immunity. Cancer Immunol. Immunother. 2008;57:1679–1683. doi: 10.1007/s00262-008-0495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J. Intern. Med. 2011;269:64–73. doi: 10.1111/j.1365-2796.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trzonkowski P, Szmit E, Mysliwska J, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of CTL and NK cells in humans-impact of immuno-senescence. Clin. Immunol. 2006;119:307–316. doi: 10.1016/j.clim.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int. J. Biol. Sci. 2011;7:651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beissert S, Schwarz A, Schwarz T. Regulatory T cells. J. Invest. Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 37.Dieckmann D, Plottner H, Dotterweich S, Schuler G. Activated CD4+ CD25+ T cells suppress antigen-specific CD4+ and CD8+ T cells but induce a suppressive phenotype only in CD4+ T cells. Immunology. 2005;115:305–314. doi: 10.1111/j.1365-2567.2005.02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin. Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, Thompson TC, Old LJ, Wang RF. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin. Cancer Res. 2007;13:6947–6958. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 40.Yokokawa J, Cereda V, Remondo C, Gulley JL, Arlen PM, Schlom J, Tsang KY. Enhanced functionality of CD4+CD25(high)FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin. Cancer Res. 2008;14:1032–1040. doi: 10.1158/1078-0432.CCR-07-2056. [DOI] [PubMed] [Google Scholar]
- 41.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem. Biophys. Res. Commun. 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 42.Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, Zou W. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J. Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 43.Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN, Shi HZ. Generation and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusion. J. Immunol. 2010;185:6348–6354. doi: 10.4049/jimmunol.1001728. [DOI] [PubMed] [Google Scholar]
- 46.Gnerlich JL, Mitchem JB, Weir JS, Sankpal NV, Kashiwagi H, Belt BA, Porembka MR, Herndon JM, Eberlein TJ, Goedegebuure P, Linehan DC. Induction of Th17 cells in the tumor microenvironment improves survival in a murine model of pancreatic cancer. J. Immunol. 2010;185:4063–4071. doi: 10.4049/jimmunol.0902609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derhovanessian E, Adams V, Hahnel K, Groeger A, Pandha H, Ward S, Pawelec G. Pretreatment frequency of circulating IL-17+ CD4+ T-cells but not Tregs correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int. J. Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]
- 50.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J. Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J. Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 53.Young LJ, Wilson NS, Schnorrer P, Mount A, Lundie RJ, La Gruta NL, Crabb BS, Belz GT, Heath WR, Villadangos JA. Dendritic cell preactivation impairs MHC class II presentation of vaccines and endogenous viral antigens. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17753–17758. doi: 10.1073/pnas.0708622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Csomor E, Bajtay Z, Sandor N, Kristof K, Arlaud GJ, Thiel S, Erdei A. Complement protein C1q induces maturation of human dendritic cells. Mol. Immunol. 2007;44:3389–3397. doi: 10.1016/j.molimm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 55.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 56.Zitvogel L, Terme M, Borg C, Trinchieri G. Dendritic cell-NK cell cross-talk: regulation and physiopathology. Curr. Top. Microbiol. Immunol. 2006;298:157–174. doi: 10.1007/3-540-27743-9_8. [DOI] [PubMed] [Google Scholar]
- 57.Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor immune responses. II. Dendritic cells grown from bone marrow precursors, but not mature DC from tumor-bearing mice are effective antigen carriers in the therapy of established tumors. Cell. Immunol. 1996;170:111–119. doi: 10.1006/cimm.1996.0140. [DOI] [PubMed] [Google Scholar]
- 58.Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–2147. [PubMed] [Google Scholar]
- 59.Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J. Immunother. 2001;24:431–446. doi: 10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8(+) T cells. J. Clin. Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frey AB. Myeloid suppressor cells regulate the adaptive immune response to cancer. J. Clin. Invest. 2006;116:2587–2590. doi: 10.1172/JCI29906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: Recruitment phenotype, properties, and mechanisms of immune suppression. Semin. Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lingen MW. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch. Pathol. Lab. Med. 2001;125:67–71. doi: 10.5858/2001-125-0067-ROLAEC. [DOI] [PubMed] [Google Scholar]
- 67.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 68.Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, Suzuki N, Inoue M, Soma G, Nagasue N. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer. Res. 2003;23:5015–5022. [PubMed] [Google Scholar]
- 69.Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, Thompson TC. Reduced infiltration of tumor-associated macrophages in human prostate cancer: association with cancer progression. Cancer Res. 2000;60:5857–5861. [PubMed] [Google Scholar]
- 70.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J. Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 71.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J. Exp. Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 73.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 74.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, Shibata T, Takami T. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J. Leukoc. Biol. 2008;83:1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 76.Mantovani A, Locati M. Orchestration of macrophage polarization. Blood. 2009;114:3135–3136. doi: 10.1182/blood-2009-07-231795. [DOI] [PubMed] [Google Scholar]
- 77.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 78.Mantovani A, Sica A. Macrophages innate immunity and cancer: balance tolerance and diversity. Curr. Opin. Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 79.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J. Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 80.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 81.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Tumor immunity: a balancing act between T cell activation macrophage activation and tumor-induced immune suppression. Cancer Immunol. Immunother. 2005;54:1137–1142. doi: 10.1007/s00262-005-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barnes PJ. Nuclear factor-kappa B. Int. J. Biochem. Cell Biol. 1997;29:867–870. doi: 10.1016/s1357-2725(96)00159-8. [DOI] [PubMed] [Google Scholar]
- 84.Perkins ND. Achieving transcriptional specificity with NF-kappa B. Int. J. Biochem. Cell Biol. 1997;29:1433–1448. doi: 10.1016/s1357-2725(97)00088-5. [DOI] [PubMed] [Google Scholar]
- 85.Baldwin AS. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 86.Dong G, Loukinova E, Chen Z, Gangi L, Chanturita TI, Liu ET, Van WC. Molecular profiling of transformed metastatic murine squamous carcinoma cells by differential display cDNA microarray reveals altered expression of multiple genes related to growth apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res. 2001;61:4797–4808. [PubMed] [Google Scholar]
- 87.Wickremasinghe MI, Thomas LH, Friedland JS. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-kappa B-dependent network. J. Immunol. 1999;163:3936–3947. [PubMed] [Google Scholar]
- 88.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 89.Muzio M, Bosisio D, Polentarutti N, D'amico G, Stoppacciaro A, Mancinelli R, van't VC, Penton-Rol G, Ruco LP, Allavena P, Mantovani A. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 90.Grazia CM, Sutterwala FS, Trinchieri G, Mosser DM, Ma X. Suppression of Il-12 transcription in macrophages following Fc gamma receptor ligation. J. Immunol. 2001;166:4498–4506. doi: 10.4049/jimmunol.166.7.4498. [DOI] [PubMed] [Google Scholar]
- 91.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol. Cell. Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang H, Zhang Y, Wu M, Li J, Zhou W, Li G, Li X, Xiao B, Christadoss P. Suppression of ongoing experimental autoimmune myasthenia gravis by transfer of RelB-silenced bone marrow dentritic cells is associated with a change from a T helper Th17/Th1 to a Th2 and FoxP3+ regulatory T-cell profile. Inflamm. Res. 2010;59:197–205. doi: 10.1007/s00011-009-0087-6. [DOI] [PubMed] [Google Scholar]
- 93.Carreno LJ, Riedel CA, Kalergis AM. Induction of tolerogenic dendritic cells by NF-kappaB blockade and Fcgamma receptor modulation. Methods Mol. Biol. 2011;677:339–353. doi: 10.1007/978-1-60761-869-0_22. [DOI] [PubMed] [Google Scholar]
- 94.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 95.Yoshimoto T, Nagase H, Ishida T, Inoue J, Nariuchi H. Induction of interleukin-12 p40 transcript by CD40 ligation via activation of nuclear factor-kappaB. Eur. J. Immunol. 1997;27:3461–3470. doi: 10.1002/eji.1830271247. [DOI] [PubMed] [Google Scholar]
- 96.Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol. Today. 2000;21:479–483. doi: 10.1016/s0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 97.Lee NS, Barber L, Kanchwala A, Childs CJ, Kataria YP, Judson MA, Mazer MA, Arce S. Low levels of NF-kappaB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin. Vaccine Immunol. 2011;18:223–234. doi: 10.1128/CVI.00469-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang MC, Liao JJ, Bonasera S, Longo DL, Goetzl EJ. Nuclear factor-kappaB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 2008;22:2142–2150. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- 99.Mora A, Youn J, Keegan A, Boothby M. NF-kappa B/Rel participation in the lymphokine-dependent proliferation of T lymphoid cells. J. Immunol. 2001;166:2218–2227. doi: 10.4049/jimmunol.166.4.2218. [DOI] [PubMed] [Google Scholar]
- 100.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 101.Aronica MA, Mora AL, Mitchell DB, Finn PW, Johnson JE, Sheller JR, Boothby MR. Preferential role for NF-kappa B/Rel signaling in the type 1 but not type 2 T cell-dependent immune response in vivo. J. Immunol. 1999;163:5116–5124. [PubMed] [Google Scholar]
- 102.Hilliard B, Samoilova EB, Liu TS, Rostami A, Chen Y. Experimental autoimmune encephalomyelitis in NF-kappa B-deficient mice: roles of NF-kappa B in the activation and differentiation of autoreactive T cells. J. Immunol. 1999;163:2937–2943. [PubMed] [Google Scholar]
- 103.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J. Exp. Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nat. Immunol. 2001;2:45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 105.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 106.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 107.Snapper CM, Zelazowski P, Rosas FR, Kehry MR, Tian M, Baltimore D, Sha WC. B cells from p50 NF-kappa Bknockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 1996;156:183–191. [PubMed] [Google Scholar]
- 108.Tumang JR, Owyang A, Andjelic S, Jin Z, Hardy RR, Liou ML, Liou HC. c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur. J. Immunol. 1998;28:4299–4312. doi: 10.1002/(SICI)1521-4141(199812)28:12<4299::AID-IMMU4299>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 109.Deenick EK, Elford AR, Pellegrini M, Hall H, Mak TW, Ohashi PS. c-Rel but not NF-kappaB1 is important for T regulatory cell development. Eur. J. Immunol. 2010;40:677–681. doi: 10.1002/eji.201040298. [DOI] [PubMed] [Google Scholar]
- 110.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 111.Milkova L, Voelcker V, Forstreuter I, Sack U, Anderegg U, Simon JC, Maier-Simon C. The NF-kappaB signalling pathway is involved in the LPS/IL-2-induced upregulation of FoxP3 expression in human CD4+CD25high regulatory T cells. Exp. Dermatol. 2010;19:29–37. doi: 10.1111/j.1600-0625.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- 112.Hori S. c-Rel: a pioneer in directing regulatory T-cell lineage commitment? Eur. J. Immunol. 2010;40:664–667. doi: 10.1002/eji.201040372. [DOI] [PubMed] [Google Scholar]
- 113.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O'Shea JJ, Pesu M, Borie DC, Changelian PS. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat. Rev. Drug Discov. 2004;3:555–564. doi: 10.1038/nrd1441. [DOI] [PubMed] [Google Scholar]
- 115.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 116.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 117.Luedde T, Beraza N, Kotsikoris V, van LG, Nenci A, De VR, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 118.Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 119.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 120.Mancino A, Lawrence T. Nuclear factor-kappaB and tumor-associated macro-phages. Clin. Cancer Res. 2010;16:784–789. doi: 10.1158/1078-0432.CCR-09-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biswas SK, Tergaonkar V. Myeloid differentiation factor 88-independent Tolllike receptor pathway: sustaining inflammation or promoting tolerance? Int. J. Biochem. Cell Biol. 2007;39:1582–1592. doi: 10.1016/j.biocel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 122.Oshima H, Oshima M, Inaba K, Taketo MM. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO J. 2004;23:1669–1678. doi: 10.1038/sj.emboj.7600170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Prasad AS, Miale A, Jr, Farid Z, Sandstead HH, Schulert AR. Zinc metabolism in patients with the syndrome of iron deficiency anemia hepatosplenomegaly, dwarfism, and hypognadism. J. Lab. Clin. Med. 1963;61:537–549. [PubMed] [Google Scholar]
- 124.Prasad AS, Meftah S, Abdallah J, Kaplan J, Brewer GJ, Bach JF, Dardenne M. Serum thymulin in human zinc deficiency. J. Clin. Invest. 1988;82:1202–1210. doi: 10.1172/JCI113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Prasad AS. Zinc in growth and development and spectrum of human zinc deficiency. J. Am. Coll. Nutr. 1988;7:377–384. doi: 10.1080/07315724.1988.10720255. [DOI] [PubMed] [Google Scholar]
- 126.Prasad AS. Zinc: an overview. Nutrition. 1995;11:93–99. [PubMed] [Google Scholar]
- 127.Prasad AS. Zinc deficiency. BMJ. 2003;326:409–410. doi: 10.1136/bmj.326.7386.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pories WJ, DeWys WD, Flynn A, Mansour EG, Strain WH. Implications of the inhibition of animal tumors by dietary zinc deficiency. Adv. Exp. Med. Biol. 1977;91:243–257. doi: 10.1007/978-1-4684-0796-9_17. [DOI] [PubMed] [Google Scholar]
- 129.Chadha VD, Garg ML, Dhawan D. Influence of extraneous supplementation of zinc on trace elemental profile leading to prevention of dimethylhydrazine-induced colon carcinogenesis. Toxicol. Mech. Methods. 2010;20:493–497. doi: 10.3109/15376516.2010.511300. [DOI] [PubMed] [Google Scholar]
- 130.Chadha VD, Dhawan D. (65)Zn kinetics as a biomarker of DMH induced colon carcinogenesis. Hell. J. Nucl. Med. 2010;13:257–260. [PubMed] [Google Scholar]
- 131.Dani V, Goel A, Vaiphei K, Dhawan DK. Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicol. Lett. 2007;171:10–18. doi: 10.1016/j.toxlet.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 132.Fong LY, M P. Newberne, Nitrosobenzylmethylamine, zinc deficiency and oesophageal cancer. IARC Sci. Publ. 1978:503–513. [PubMed] [Google Scholar]
- 133.Fong LY, Sivak A, Newberne PM. Zinc deficiency and methylbenzylnitrosamine-induced esophageal cancer in rats. J. Natl. Cancer Inst. 1978;61:145–150. doi: 10.1093/jnci/61.1.145. [DOI] [PubMed] [Google Scholar]
- 134.Fong LY, Lee JS, Chan WC, Newberne PM. Zinc deficiency and the induction of oesophageal tumors in rats by benzylmethylamine and sodium nitrite. IARC Sci. Publ. 1982:679–683. [PubMed] [Google Scholar]
- 135.Fong LY, Lee JS, Chan WC, Newberne PM. Zinc deficiency and the development of esophageal and forestomach tumors in Sprague-Dawley rats fed precursors of N-nitroso-N-benzylmethylamine. J. Natl. Cancer Inst. 1984;72:419–425. [PubMed] [Google Scholar]
- 136.Fong LY, Ng WL, Newberne PM. N-nitrosodimethylamine-induced foresto-mach tumours in male Sprague-Dawley rats fed a zinc-deficient diet. IARC Sci. Publ. 1984:543–546. [PubMed] [Google Scholar]
- 137.Fong LY, Lui CP, Ma-Tung L, Ng WL. Zinc-deficiency and the development of malignant lymphoma in rats given a single intragastric dose of N-methyl-N-nitrosourea. IARC Sci. Publ. 1987:261–263. [PubMed] [Google Scholar]
- 138.Fong LY, Lau KM, Huebner K, Magee PN. Induction of esophageal tumors in zinc-deficient rats by single low doses of N-nitrosomethylbenzylamine (NMBA): analysis of cell proliferation, and mutations in H-ras and p53 genes. Carcinogenesis. 1997;18:1477–1484. doi: 10.1093/carcin/18.8.1477. [DOI] [PubMed] [Google Scholar]
- 139.Fong LY, Farber JL, Magee PN. Zinc replenishment reduces esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in zinc-deficient rats. Carcinogenesis. 1998;19:1591–1596. doi: 10.1093/carcin/19.9.1591. [DOI] [PubMed] [Google Scholar]
- 140.Fong LY, Magee PN. Dietary zinc deficiency enhances esophageal cell proliferation and N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor incidence in C57BL/6 mouse. Cancer Lett. 1999;143:63–69. doi: 10.1016/s0304-3835(99)00191-3. [DOI] [PubMed] [Google Scholar]
- 141.Fong LY, Nguyen VT, Farber JL. Esophageal cancer prevention in zinc-deficient rats: rapid induction of apoptosis by replenishing zinc. J. Natl. Cancer Inst. 2001;93:1525–1533. doi: 10.1093/jnci/93.20.1525. [DOI] [PubMed] [Google Scholar]
- 142.Fong LY, Zhang L, Jiang Y, Farber JL. Dietary zinc modulation of COX-2 expression and lingual and esophageal carcinogenesis in rats. J. Natl. Cancer Inst. 2005;97:40–50. doi: 10.1093/jnci/dji006. [DOI] [PubMed] [Google Scholar]
- 143.Fong LY, Jiang Y, Farber JL. Zinc deficiency potentiates induction and progression of lingual and esophageal tumors in p53-deficient mice. Carcinogenesis. 2006;27:1489–1496. doi: 10.1093/carcin/bgl012. [DOI] [PubMed] [Google Scholar]
- 144.Ng WL, Fong LY, Ma L, Newberne PM. Dietary zinc deficiency and tumorigenesis: a scanning electron microscope study. J. Electron Microsc. 1984;33:344–348. (Tokyo) [PubMed] [Google Scholar]
- 145.Sun J, Liu J, Pan X, Quimby D, Zanesi N, Druck T, Pfeifer GP, Croce CM, Fong LY, Huebner K. Effect of Zinc Supplementation on N-nitrosomethylbenzylamine-induced forestomach tumor development and progression in tumor suppressor-deficient mouse strains. Carcinogenesis. 2011;32:351–358. doi: 10.1093/carcin/bgq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fong LY, Jiang Y, Rawahneh ML, Smalley KJ, Croce CM, Farber JL, Huebner K. Zinc supplementation suppresses 4-nitroquinoline 1-oxide-induced rat oral carci-nogenesis. Carcinogenesis. 2011;32:554–560. doi: 10.1093/carcin/bgr004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wan SG, Taccioli C, Jiang Y, Chen H, Smalley KJ, Huang K, Liu XP, Farber JL, Croce CM, Fong LY. Zinc deficiency activates S100A8 inflammation in the absence of COX-2 and promotes murine oral-esophageal tumor progression. Int. J. Cancer. 2011;129:331–345. doi: 10.1002/ijc.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Effect of zinc on prostatic tumorigenicity in nude mice. Ann. N. Y. Acad. Sci. 2003;1010:316–320. doi: 10.1196/annals.1299.056. [DOI] [PubMed] [Google Scholar]
- 149.Prasad AS, Mukhtar H, Beck FW, Adhami VM, Siddiqui IA, Din M, Hafeez BB, Kucuk O. Dietary zinc and prostate cancer in the TRAMP mouse model. J. Med. Food. 2010;13:70–76. doi: 10.1089/jmf.2009.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Allen JI, Bell E, Boosalis MG, Oken MM, McClain CJ, Levine AS, Morley JE. Association between urinary zinc excretion and lymphocyte dysfunction in patients with lung cancer. Am. J. Med. 1985;79:209–215. doi: 10.1016/0002-9343(85)90011-7. [DOI] [PubMed] [Google Scholar]
- 151.Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 1999;8:887–892. [PubMed] [Google Scholar]
- 152.Lekili M, Ergen A, Celebi I. Zinc plasma levels in prostatic carcinoma and BPH. Int. Urol. Nephrol. 1991;23:151–154. doi: 10.1007/BF02549712. [DOI] [PubMed] [Google Scholar]
- 153.Prasad AS, Beck FW, Snell DC, Kucuk O. Zinc in cancer prevention. Nutr. Cancer. 2009;61:879–887. doi: 10.1080/01635580903285122. [DOI] [PubMed] [Google Scholar]
- 154.Prasad AS, Kucuk O. Zinc in cancer prevention. Cancer Metastasis Rev. 2002;21:291–295. doi: 10.1023/a:1021215111729. [DOI] [PubMed] [Google Scholar]
- 155.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc-suppressed inflammatory cytokines by induction of A20-mediated inhibition of nuclear factor-kappaB. Nutrition. 2011;27:816–823. doi: 10.1016/j.nut.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 156.Baek SH, Kim MY, Mo JS, Ann EJ, Lee KS, Park JH, Kim JY, Seo MS, Choi EJ, Park HS. Zinc-induced downregulation of Notch signaling is associated with cytoplasmic retention of Notch1-IC and RBP-Jk via PI3k–Akt signaling pathway. Cancer Lett. 2007;255:117–126. doi: 10.1016/j.canlet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 157.Costello LC, Franklin RB. Zinc is decreased in prostate cancer: an established relationship of prostate cancer! J. Biol. Inorg. Chem. 2011;16:3–8. doi: 10.1007/s00775-010-0736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Feng P, Liang JY, Li TL, Guan ZX, Zou J, Franklin R, Costello LC. Zinc induces mitochondria apoptogenesis in prostate cells. Mol. Urol. 2000;4:31–36. [PubMed] [Google Scholar]
- 159.Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol. Cancer. 2008;725 doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ishii K, Usui S, Sugimura Y, Yoshida S, Hioki T, Tatematsu M, Yamamoto H, Hirano K. Aminopeptidase N regulated by zinc in human prostate participates in tumor cell invasion. Int. J. Cancer. 2001;92:49–54. [PubMed] [Google Scholar]
- 162.Ishii K, Usui S, Sugimura Y, Yamamoto H, Yoshikawa K, Hirano K. Inhibition of aminopeptidase N (AP-N) and urokinase-type plasminogen activator (uPA) by zinc suppresses the invasion activity in human urological cancer cells. Biol. Pharm. Bull. 2001;24:226–230. doi: 10.1248/bpb.24.226. [DOI] [PubMed] [Google Scholar]
- 163.Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Costello LC, Bernard AL, Desouki MM, Zou J, Zou J, Bagasra O, Johnson LA, Hanna N, Franklin RB. Decreased zinc and down regulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol. Ther. 2011;12 doi: 10.4161/cbt.12.4.16356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tanaka Y, Shiozawa S, Morimoto I, Fujita T. Role of zinc in interleukin 2 (IL-2)-mediated T-cell activation. Scand. J. Immunol. 1990;31:547–552. doi: 10.1111/j.1365-3083.1990.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 166.Bao B, Prasad AS, Beck FW, Godmere M. Zinc modulates mRNA levels of cyto-kines. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1095–E1102. doi: 10.1152/ajpendo.00545.2002. [DOI] [PubMed] [Google Scholar]
- 167.Bao B, Prasad AS, Beck FW, Bao GW, Singh T, Ali S, Sarkar FH. Intracellular free zinc up-regulates IFN-gamma and T-bet essential for Th(1) differentiation in Con-A stimulated HUT-78 cells. Biochem. Biophys. Res. Commun. 2011;407:703–707. doi: 10.1016/j.bbrc.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beck FW, Prasad AS, Kaplan J, Fitzgerald JT, Brewer GJ. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am. J. Physiol. 1997;272:E1002–E1007. doi: 10.1152/ajpendo.1997.272.6.E1002. [DOI] [PubMed] [Google Scholar]
- 169.Doerr TD, Prasad AS, Marks SC, Beck FW, Shamsa FH, Penny HS, Mathog RH. Zinc deficiency in head and neck cancer patients. J. Am. Coll. Nutr. 1997;16:418–422. doi: 10.1080/07315724.1997.10718707. [DOI] [PubMed] [Google Scholar]
- 170.Doerr TD, Marks SC, Shamsa FH, Mathog RH, Prasad AS. Effects of zinc and nutritional status on clinical outcomes in head and neck cancer. Nutrition. 1998;14:489–495. doi: 10.1016/s0899-9007(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 171.Prasad AS, Beck FW, Grabowski SM, Kaplan J, Mathog RH. Zinc deficiency: changes in cytokine production and T-cell subpopulations in patients with head and neck cancer and in noncancer subjects. Proc. Assoc. Am. Physicians. 1997;109:68–77. [PubMed] [Google Scholar]
- 172.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, Steinberg SM. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N. Engl. J. Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 173.Weismann K, Christophersen J, Kobayasi T. Ultrastructural changes of zinc deficient rat epidermis: an electron microscopic study. Acta Derm. Venereol. 1980;60:197–202. [PubMed] [Google Scholar]
- 174.Dvergsten CL, Johnson LA, Sandstead HH. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. III. Impaired dendritic differentiation of basket and stellate cells. Brain Res. 1984;318:21–26. doi: 10.1016/0165-3806(84)90058-0. [DOI] [PubMed] [Google Scholar]
- 175.Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am. J. Respir. Cell Mol. Biol. 2009;41:207–216. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bao B, Prasad AS, Beck FW, Fitzgerald JT, Snell D, Bao GW, Singh T, Cardozo LJ. Zinc decreases C-reactive protein lipid peroxidation and inflammatory cytokines in elderly subjects: a potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010;91:1634–1641. doi: 10.3945/ajcn.2009.28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage disrupts p53, NFkappa B, AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16770–16775. doi: 10.1073/pnas.222679399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J. Nutr. 2003;133:2543–2548. doi: 10.1093/jn/133.8.2543. [DOI] [PubMed] [Google Scholar]
- 179.Ho E, Song Y. Zinc and prostatic cancer. Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:640–645. doi: 10.1097/MCO.0b013e32833106ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bettger WJ. Zinc selenium site-specific versus general antioxidation. Can. J. Physiol. Pharmacol. 1993;71:721–724. doi: 10.1139/y93-108. [DOI] [PubMed] [Google Scholar]
- 181.Candan F, Gultekin F, Candan F. Effect of vitamin C and zinc on osmotic fragility and lipid peroxidation in zinc-deficient haemodialysis patients. Cell Biochem. Funct. 2002;20:95–98. doi: 10.1002/cbf.947. [DOI] [PubMed] [Google Scholar]
- 182.Demirci M, Delibas N, Altuntas I, Oktem F, Yonden Z. Serum iron, zinc and copper levels and lipid peroxidation in children with chronic giardiasis. J. Health Popul. Nutr. 2003;21:72–75. [PubMed] [Google Scholar]
- 183.Roussel AM, Kerkeni A, Zouari N, Mahjoub S, Matheau JM, Anderson RA. Antioxidant effects of zinc supplementation in Tunisians with type 2 diabetes mellitus. J. Am. Coll. Nutr. 2003;22:316–321. doi: 10.1080/07315724.2003.10719310. [DOI] [PubMed] [Google Scholar]
- 184.Dimitrova AA, Strashimirov DS, Russeva AL, ndreeva-Gateva PA, Lakova ET, Tzachev KN. Effect of zinc on the activity of Cu/Zn superoxide dismutase and lipid profile in Wistar rats. Folia Med. 2005;47:42–46. (Plovdiv) [PubMed] [Google Scholar]
- 185.Goel A, Dani V, Dhawan DK. Protective effects of zinc on lipid peroxidation antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem. Biol. Interact. 2005;156:131–140. doi: 10.1016/j.cbi.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 186.Kagi JH, Schaffer A. Biochemistry of metallothionein. Biochemistry. 1988;27:8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- 187.Prasad AS. Clinical immunological anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008;43:370–377. doi: 10.1016/j.exger.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 188.Prasad AS, Bao B, Beck FW, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic. Biol. Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 189.Bao B, Prasad AS, Beck FW, Snell D, Suneja A, Sarkar FH, Doshi N, Fitzgerald JT, Swerdlow P. Zinc supplementation decreases oxidative stress incidence of infection and generation of inflammatory cytokines in sickle cell disease patients. Transl. Res. 2008;152:67–80. doi: 10.1016/j.trsl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 190.Otsuka M, Fujita M, Aoki T, Ishii S, Sugiura Y, Yamamoto T, Inoue J. Novel zinc chelators with dual activity in the inhibition of the kappa B site-binding proteins HIV-EP1 and NF-kappa B. J. Med. Chem. 1995;38:3264–3270. doi: 10.1021/jm00017a011. [DOI] [PubMed] [Google Scholar]
- 191.Prasad AS, Bao B, Beck FW, Sarkar FH. Zinc activates NF-kappaB in HUT-78 cells. J. Lab. Clin. Med. 2001;138:250–256. doi: 10.1067/mlc.2001.118108. [DOI] [PubMed] [Google Scholar]
- 192.Zabel U, Schreck R, Baeuerle PA. DNA binding of purified transcription factor NF-kappa Affinity B specificity Zn2+ dependence and differential half-site recognition. J. Biol. Chem. 1991;266:252–260. [PubMed] [Google Scholar]
- 193.Connell P, Young VM, Toborek M, Cohen DA, Barve S, McClain CJ, Hennig B. Zinc attenuates tumor necrosis factor-mediated activation of transcription factors in endothelial cells. J. Am. Coll. Nutr. 1997;16:411–417. doi: 10.1080/07315724.1997.10718706. [DOI] [PubMed] [Google Scholar]
- 194.Ho E, Quan N, Tsai YH, Lai W, Bray TM. Dietary zinc supplementation inhibits NFkappaB activation and protects against chemically induced diabetes in CD1 mice. Exp. Biol. Med. 2001;226:103–111. doi: 10.1177/153537020122600207. (Maywood) [DOI] [PubMed] [Google Scholar]
- 195.Uzzo RG, Leavis P, Hatch W, Gabai VL, Dulin N, Zvartau N, Kolenko VM. Zinc inhibits nuclear factor-kappa B activation and sensitizes prostate cancer cells to cytotoxic agents. Clin. Cancer Res. 2002;8:3579–3583. [PubMed] [Google Scholar]
- 196.Uzzo RG, Crispen PL, Golovine K, Makhov P, Horwitz EM, Kolenko VM. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis. 2006;27:1980–1990. doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 197.Heyninck K, Beyaert R. The cytokine-inducible zinc finger protein A20 inhibits IL-1-induced NF-kappaB activation at the level of TRAF6. FEBS Lett. 1999;442:147–150. doi: 10.1016/s0014-5793(98)01645-7. [DOI] [PubMed] [Google Scholar]
- 198.Jaattela M, Mouritzen H, Elling F, Bastholm L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J. Immunol. 1996;156:1166–1173. [PubMed] [Google Scholar]
- 199.Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fraczek J, Kim TW, Xiao H, Yao J, Wen Q, Li Y, Casanova JL, Pryjma J, Li X. The kinase activity of IL-1 receptor-associated kinase 4 is required for interleukin-1 receptor/toll-like receptor-induced TAK1-dependent NFkappaB activation. J. Biol. Chem. 2008;283:31697–31705. doi: 10.1074/jbc.M804779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Verstrepen L, Bekaert T, Chau TL, Tavernier J, Chariot A, Beyaert R. TLR-4 IL-1R and TNF-R signaling to NF-kappaB: variations on a common theme. Cell. Mol. Life Sci. 2008;65:2964–2978. doi: 10.1007/s00018-008-8064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, Li Y, Pitti R, Totpal K, Yee S, Ross S, Vernes JM, Lu Y, Adams C, Offringa R, Kelley B, Hymowitz S, Daniel D, Meng G, Ashkenazi A. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell. 2011;19:101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 203.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 204.Imler JL, Hoffmann JA. Toll and Toll-like proteins: an ancient family of receptors signaling infection. Rev. Immunogenet. 2000;2:294–304. [PubMed] [Google Scholar]
- 205.Sasaki Y, Calado DP, Derudder E, Zhang B, Shimizu Y, Mackay F, Nishikawa S, Rajewsky K, Schmidt-Supprian M. NIK overexpression amplifies whereas ablation of its TRAF3-binding domain replaces BAFF:BAFF-R-mediated survival signals in B cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10883–10888. doi: 10.1073/pnas.0805186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1 beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 208.He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, Sieghart W, Peck-Radosavljevic M, Leffert HL, Karin M. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–1408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee H, Deng J, Xin H, Liu Y, Pardoll D, Yu H. A requirement of STAT3 DNA binding precludes Th-1 immunostimulatory gene expression by NF-kappaB in tumors. Cancer Res. 2011;71:3772–3780. doi: 10.1158/0008-5472.CAN-10-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jimenez F, Quinones MP, Martinez HG, Estrada CA, Clark K, Garavito E, Ibarra J, Melby PC, Ahuja SS. CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-kappa B. J. Immunol. 2010;184:5571–5581. doi: 10.4049/jimmunol.0803494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat. Rev. Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 213.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 214.Gerondakis S, Grumont R, Rourke I, Grossmann M. The regulation and roles of Rel/NF-kappa B transcription factors during lymphocyte activation. Curr. Opin. Immunol. 1998;10:353–359. doi: 10.1016/s0952-7915(98)80175-1. [DOI] [PubMed] [Google Scholar]
- 215.Senftleben U, Li ZW, Baud V, Karin M. IKKbeta is essential for protecting T cells from TNFalpha-induced apoptosis. Immunity. 2001;14:217–230. doi: 10.1016/s1074-7613(01)00104-2. [DOI] [PubMed] [Google Scholar]
- 216.Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, Kress CL, Bailly-Maitre B, Li X, Osterman A, Matsuzawa S, Terskikh AV, Faustin B, Reed JC. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 217.Romagnoli M, Desplanques G, Maiga S, Legouill S, Dreano M, Bataille R, Barille-Nion S. Canonical nuclear factor kappaB pathway inhibition blocks myeloma cell growth and induces apoptosis in strong synergy with TRAIL. Clin. Cancer Res. 2007;13:6010–6018. doi: 10.1158/1078-0432.CCR-07-0140. [DOI] [PubMed] [Google Scholar]
- 218.Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- 219.Sung B, Pandey MK, Ahn KS, Yi T, Chaturvedi MM, Liu M, Aggarwal BB. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltrans-ferase suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaB alpha kinase leading to potentiation of apoptosis. Blood. 2008;111:4880–4891. doi: 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kinoshita J, Fushida S, Harada S, Makino I, Nakamura K, Oyama K, Fujita H, Ninomiya I, Fujimura T, Kayahara M, Ohta T. PSK enhances the efficacy of doce-taxel in human gastric cancer cells through inhibition of nuclear factor-kappaB activation and survivin expression. Int. J. Oncol. 2010;36:593–600. doi: 10.3892/ijo_00000534. [DOI] [PubMed] [Google Scholar]
- 221.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 222.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death. Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 223.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 224.Hinz M, Loser P, Mathas S, Krappmann D, Dorken B, Scheidereit C. Constitutive NF-kappaB maintains high expression of a characteristic gene network including CD40, CD86, and a set of antiapoptotic genes in Hodgkin/Reed-Sternberg cells. Blood. 2001;97:2798–2807. doi: 10.1182/blood.v97.9.2798. [DOI] [PubMed] [Google Scholar]
- 225.Cheung AK, Ko JM, Lung HL, Chan KW, Stanbridge EJ, Zabarovsky E, Tokino T, Kashima L, Suzuki T, Kwong DL, Chua D, Tsao SW, Lung ML. Cysteine-rich intestinal protein 2 (CRIP2) acts as a repressor of NF-kappaB-mediated proangiogenic cytokine transcription to suppress tumor-igenesis and angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:8390–8395. doi: 10.1073/pnas.1101747108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60:5334–5339. [PubMed] [Google Scholar]
- 227.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Huber MA, Beug H, Wirth T. Epithelial-mesenchymal transition: NF-kappaB takes center stage. Cell Cycle. 2004;3:1477–1480. doi: 10.4161/cc.3.12.1280. [DOI] [PubMed] [Google Scholar]