Abstract
New subunit vaccine formulations with increased potency are of interest to improve immune responses against poorly immunogenic antigens, avoid vaccine shortages in pandemic situations, and to promote dose-sparing of potent adjuvant molecules that can cause unacceptable side effects in prophylactic vaccination. Here we report strong class-switched, high avidity humoral immune responses elicited by a vaccine system based on poly(lactide-co-glycolide) micro- or nano-particles enveloped by PEGylated phospholipid bilayers, with protein antigens covalently anchored to the lipid surface and lipophilic adjuvants inserted in the bilayer coating. Strikingly, these particles elicited high endpoint antigen-specific IgG titers (>106) sustained for over 100 days after two immunizations with as little as 2.5 ng of antigen. At such low doses, the conventional adjuvant alum or the molecular adjuvants monophosphoryl lipid A (MPLA) or α-galactosylceramide (αGC) failed to elicit responses. Co-delivery of antigen with MPLA or αGC incorporated into the particle bilayers in a pathogen-mimetic fashion further enhanced antibody titers by ~12-fold. MPLA provided the highest sustained IgG titers at these ultra-low antigen doses, while αGC promoted a rapid rise in serum IgG after one immunization, which may be valuable in emergencies such as disease pandemics. The dose of αGC required to boost the antibody response was also spared by particulate delivery. Lipid-enveloped biodegradable micro- and nano-particles thus provide a potent dose-sparing platform for vaccine delivery.
Keywords: vaccine, adjuvant, microparticle, nanoparticle, lipid membranes, biomimicry
1. Introduction
The immune system has evolved to respond strongly to antigens encountered in micro- or nano-particulate form, likely reflecting the intrinsic particulate nature of foreign microbes. B-lymphocytes are strongly activated by particles displaying repeat copies of antigens capable of crosslinking B-cell receptors [1–3], and particulate delivery also allows antigens to be processed and loaded onto class I MHC molecules, enhancing CD8+ T-cell responses [4–6]. These findings, combined with the desire to control the duration of exposure to antigen via controlled release, have motivated extensive studies of biodegradable polymer micro- or nano-particles as potential vaccine delivery materials [7–10]. Such systems improve immune responses not only due to their ability to control the release rate of their components, but also due to the inherent potency of degradable particles as materials for vaccine delivery [11–13], particularly PLGA [14, 15]. Additionally, particles can co-deliver immunostimulatory molecules on the same particle, targeting multiple classes of molecules to the same intracellular compartment [16–19].
However, these technologies have failed to move into the clinic in part due to the challenges of low antigen encapsulation efficiency and denaturation of protein antigen during the encapsulation process [8, 10]. In order to avoid antigen denaturation, strategies based on the binding of antigens to the surfaces of particles post-synthesis have been pursued. This approach has the additional benefit of mimicking multivalent antigen display on natural pathogens. Examples of this approach include adsorption of antigens to charged PLGA particles [20, 21] or covalent coupling of protein to reactive groups on particle surfaces [22, 23].
We sought to combine this concept of antigen surface-display with a strategy for creating degradable particles whose surfaces could mimic microbial pathogens in their structure and surface chemistry. To this end, we began exploring lipid-enveloped micro- and nano-particles composed of a biodegradable PLGA polymer core surrounded by a self-assembled phospholipid membrane. We recently reported on the nanoscale structure of lipid membrane assemblies formed when lipids are used as surfactants in emulsion/solvent evaporation syntheses of PLGA particles [24]. For a range of compositions, PLGA particles were prepared with a two-dimensionally fluid surface phospholipid bilayer surface that tightly envelopes the polymer core.
Here we report on in vivo testing of this lipid-coated particle system for delivery of protein antigens with or without co-delivered danger signals displayed in a native lipid context. The model protein antigen ovalbumin (ova) was conjugated to PEGylated lipids incorporated in the particle lipid shells. We also incorporated lipophilic molecular danger signals into the surface bilayers of these particles, focusing primarily on monophosphoryl lipid A (MPLA) and α-galactosylceramide (αGC). MPLA is a nontoxic lipopolysaccharide derivative that binds to Toll-like receptor 4, which is already in use in human vaccines including the papillomavirus vaccine Cervarix™ recently approved in the United States [25]. αGC is a synthetic glycolipid that can be loaded into non-classical MHC CD1d molecules by antigen presenting cells; αGC/CD1d complexes stimulate invariant natural killer T cells (NKT cells) through their conserved T-cell receptors [26]. αGC is in clinical development as a drug against cancer and autoimmunity, but has been recognized as a candidate vaccine adjuvant as well, in part due to the recently discovered role for NKT cells in promoting humoral immune responses [17]. Lipopolysaccharide and its derivatives, including MPLA, have been used as membrane-incorporated components in liposomal vaccines for many years [18, 27, 28], whereas this report provides one of the first applications of this concept to αGC.
In analyzing immune responses elicited by this lipid-coated particle system, we particularly explored immunizations using limiting (down to sub-nanogram) doses of both the antigen and molecular adjuvant molecules. Such an analysis is useful in several contexts. First, dose sparing of antigen is of significant interest in the setting of seasonal influenza vaccines, where production issues have in the past led to vaccine shortages, as well as in bioterrorism and pandemic vaccine development settings, where rapid deployment of limited vaccine stocks may be critical [29–34]. Second, dose sparing of molecular adjuvants such as MPLA and αGC lowers the likelihood of reactogenicity or systemic side effects that can block clinical translation of promising adjuvant candidates for prophylactic vaccines [35]. Lastly, dose titration is a powerful strategy for comparing potency of candidate vaccines in mice, allowing important differences in vaccine potency to be revealed that may be missed by immunizations with high antigen doses [36]. These quantitative features of vaccination are infrequently characterized in small-animal models but may be relevant for predicting the performance of candidate particle-based vaccines in non-human primates and humans.
2. Materials and methods
2.1. Materials
PLGA with a 50:50 lactide:glycolide ratio was purchased from Lakeshore Biomaterials (Birmingham, Alabama). The lipids 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phospho-(1'-rac-glycerol) (DOPG), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)2000] (mal-PEG2k-PE) were purchased from Avanti Polar Lipids (Alabaster, Alabama). Carboxyfluorescein succinimidyl ester (CFSE) was from Invitrogen (Carlsbad, CA). MPLA was purchased from Sigma Aldrich (St. Louis, Missouri), rhodamine-conjugated Pam3Cys was purchased from Invivogen (San Diego, California), and αGC was purchased from Toronto Research Chemicals Inc (North York, Ontario, Canada). Aluminum hydroxide adjuvant (Imject) and n-succinimidyl s-acetyl(thiotetraethylene glycol) (SAT(PEG)4) were purchased from Pierce Biotechnology. Solvents were from Sigma-Aldrich and used as received.
2.2. Lipid-enveloped particle synthesis
Lipid bilayer-enveloped microparticles were synthesized as previously reported [24]. Briefly, lipid (DOPC:DOPG:mal-PEG2k-PE 72:18:10 molar ratio) and polymer were co-dissolved in dichloromethane (DCM) and this organic phase was dispersed into distilled deionized ultrapure water (DDI water) by homogenization. After evaporation of DCM by stirring the emulsion for 12 hrs, solid PLGA particles with lipid bilayer coatings were recovered by centrifugation. Larger microspheres were separated from particles <1 µm by two sequential steps of centrifugation for 1 minute at 1,100 RCF. To prepare lipid-enveloped nanoparticles, we adapted a procedure published by Wassel et al. for the synthesis of poly(vinyl alcohol)-stabilized PLGA particles [37]. PLGA (30 mg) was co-dissolved in 1 mL DCM with 1.3 mg of DOPC, 0.34 mg of DOPG, and 0.62 mg of mal-PEG2k-PE to form the organic phase. An internal aqueous phase of 200 µL DDI water was dispersed in the organic phase by sonication for 1 minute on ice using a Misonix XL2000 Probe Tip Sonicator (Farmingdale, NY) at 7 Watts output power. The resulting solution was immediately dispersed in 6 mL DDI water by sonication for 5 minutes on ice using the Misonix XL2000 at 12 Watts output power. DCM was evaporated overnight at ambient temperature and pressure while agitating the solution on an orbital shaker.
To purify polymer-core nanoparticles from free liposomes, particles were layered over a cushion of 30% sucrose in ultrapure water and centrifuged at 13,000 g for 5 minutes. The liposome-containing solution retained above the sucrose gradient was discarded, and the particles that formed a pellet below the sucrose gradient were retained. Self-assembly of lipids on particle surfaces was confirmed using electron microscopy. Particle size was determined using a Horiba Partica LA-950V2 Laser Diffraction Particle Size Analysis System, and confirmed using scanning electron microscopy and optical microscopy of microparticles.
2.3. Antigen conjugation to lipid-enveloped particles
To load lipid-enveloped particles with surface-displayed antigens, thiolated proteins were linked to the lipid surfaces via the maleimide terminus of mal-PEG2k-PE. As a model protein antigen, purified ovalbumin (ova, Worthington Biochemical, Lakewood, New Jersey) was passed through a Detoxi-Gel endotoxin removal affinity column (Pierce Biotechnology, Rockford, Illinois), and the resulting protein solution contained no endotoxin detectable by the Limulus Amebocyte Lysate assay (Lonza, Basel, Switzerland). Ova was modified with the heterobifunctional cross-linker SAT(PEG)4 (Pierce Biotechnology, Rockford, Illinois) by adding a 10-fold molar excess of the crosslinker (2.2 mM) to ova solution (0.22 mM or 10 mg/mL) and incubating on a revolving rotator for 30 minutes at room temperature. To quench NHS groups on unreacted SAT(PEG)4 molecules, 25 mM glycine was added, and protein was incubated for an additional 15 minutes rotating at room temperature. Quenched SAT(PEG)4 was removed by buffer exchange with a 7000 MWCO desalting spin column (Pierce Biotechnology Rockford, Illinois) and stored for up to 16 hours at 4 °C. Sulfhydryl groups on SAT(PEG)4 –modified ova were deprotected by adding 50 mM hydroxylamine and 2.5 mM EDTA (pH = 7.4) and rotating for 2 hours at room temperature followed by a second buffer exchange into 10 mM EDTA (pH = 7.4). Particles (70 mg/mL) were then incubated with protein (5 mg/mL) for 4 hrs at 25°C before washing with sterile saline to remove unbound antigen. Buffers and products of the synthesis contained no detectable endotoxin. An analogous procedure was used to couple green fluorescent protein (GFP) or fluorescein isothiocyanate (FITC)-labeled ova, and the resulting particles were visualized using a Zeiss LSM510 confocal fluorescence microscope. The particles were generally used within 12 hrs after preparation, and were stored at 4°C until use.
The dose of protein carried by lipid-enveloped particles was determined by several independent experiments: (i) Microparticles were analyzed by flow cytometry using Reference Standard Microparticles (Bangs Labs, Fishers, IN) to estimate the number of protein molecules carried by each particle. (ii) Additionally, protein was stripped from particles using 1% Tween-20 or Triton X-100 and the released protein was quantitated by direct fluorescence measurements (in the case of fluorescent protein) or by enzyme-linked immunosorbent assay (ELISA). (iii) A bicinchoninic acid (BCA) protein assay (Sigma-Aldrich, St. Louis, Missouri) comparing ova-conjugated and blank particles stripped with Tween served as a third independent test of protein dose. Because microparticles were large enough to be counted by optical microscopy, the dose of ova measured by ELISA could also be translated into a per-particle protein quantity.
2.4. Post-insertion of lipophilic danger signals into particle membranes
To incorporate danger signals in the antigen-bearing particles, lipophilic Toll-like receptor agonists or the NK T-cell agonist αGC were introduced into the particle membranes via a post-insertion method, similar to strategies utilized for functionalization of liposomes with lipid-conjugated proteins and other ligands [38–40]. In a typical experiment, 0.7 nanomoles of each ligand (1.3 µg MPLA, 0.6 µg αGC, and/or 1.8 µg Pam3Cys from stock solutions of 2.1 mg/mL, 1.0 mg/mL, and 2.9 mg/mL in DMSO, respectively) were added to 0.1 mg of antigen-conjugated particles in 200 µL PBS, and no additional washes were performed. This post-insertion approach allowed us to compare adjuvant-containing and adjuvant-free particles derived from a single source formulation.
2.5 In vitro bioactivity of TLR agonist-loaded particles
Bone marrow-derived dendritic cells (DCs) were prepared from C57Bl/6 mice as previously described [41]. DCs at day 7 of culture in a 48-well plate containing 106 BMDCs/well in 1 mL of media were pulsed overnight with lipid-enveloped PLGA nanoparticles containing 10 mole% or 1 mole% MPLA (relative to lipid), or no MPLA. The total adjuvant dose per well was 70 µg MPLA in the 10% case and 7 µg MPLA in the 1% case. Control cells were given equivalent quantities of MPLA alone (70, 7, or 0 µg) in complete RPMI media. Cells were blocked with anti-mouse CD16/32 and stained with fluorescent antibodies against MHC Class II or CD80 and then analyzed by flow cytometry to detect upregulation of these maturation markers.
Responses of naïve CD4+ or CD8+ T-cells transgenically expressing T cell receptors specific for ova-derived peptides were assessed by in vitro co-culture of OT-II or OT-I primary T-cells with particle-pulsed DCs. Primary dendritic cells were isolated from spleens of C57Bl/6 mice by digesting spleens with collagenase and isolating DCs using a CD11c+ magnetic bead isolation kit (Miltenyi Biotec). In parallel, naïve CD4+ T-cells or CD8+ T-cells were isolated from OT-II or OT-I TCR-transgenic mice, respectively (Jackson Laboratories), and labeled with CFSE to trace cell division following the manufacturer’s instructions [42]. Ova-loaded particles with post-inserted MPLA or soluble ova/MPLA were added to splenic DCs (12,500 cells/well) at titrated cell:antigen ratios (starting from 40:1 particles:DC, corresponding to 6.2 µg particles), and incubated for 3 hr in a total volume of 150 µL/well at 37 °C and 5% CO2. CFSE-labeled OT-I or OT-II cells (50,000 cells/well) were then added to DCs in a volume of 50 µL complete RPMI media. This total culture volume of 200 µL/well was incubated for 3 days at 37 °C and 5% CO2 to allow proliferation of T-cells, and CFSE dilution was then measured by flow cytometry. The percentage of divided cells was determined by setting gates defining undivided cells from negative (unstimulated) control cells, and calculating the fraction of cells with CFSE outside this unstimulated gate.
2.6 Animal studies
Female BALB/c or C57Bl/6 mice 6–7 weeks of age were purchased from Jackson Laboratories and cared for under local, state, and NIH care and use guidelines. Animals were immunized subcutaneously (s.c.) at the tail base with 100 uL particles or soluble protein in sterile saline, followed by a contralateral boost of the same formulation 2 or 3 weeks later. The dose of antigen per particle was fixed and dose titrations were made by injecting different numbers of particles. Experiments comparing different particle compositions generally employed a single source batch of antigen-conjugated particles to control for any possible variations in particles from batch to batch. Alum-adjuvanted control immunizations were performed with ova mixed with 100 µL alum. Weekly samples of 50–80 uL of blood were obtained by retro-orbital or submandibular bleeding for analysis of serum antibody titers.
2.7 Cellular response measurements
Cellular responses following immunization were assessed by isolation of splenocytes from immunized mice 7 days post boosting (or control naïve mice) and restimulation of the cells in round-bottom plates (106 cells/well in RPMI medium with 10% FCS in triplicate) with 10 µM class I- or class II-restricted immunodominant peptides from ova (SIINFEKL or ISQAVHAAHAEINEAGR, respectively) for 24–48 hrs. Concentrations of cytokines in the culture supernatants were analyzed by the flow cytometry-based Cytokine Bead Array kit (Becton Dickinson Th1/Th2/Th17 kit) according to the manufacturer’s instructions. In parallel, lymphocytes from spleens of immunized animals were stained with antibodies to CD8 and peptide-MHC tetramers (phycoerythrin-conjugated SIINFEKL/H-2Kb tetramer, Beckman Coulter) and analyzed by flow cytometry to determine the frequency of ova-specific CD8+ T-cells.
2.8 Antibody titer measurements
Total IgG titers from sera were measured using an ELISA by adsorbing ova to flat-bottom transparent 96-well plates at room temperature overnight, blocking overnight with bovine serum albumin, adding serially-diluted serum (starting from a minimal dilution of 200X) for 2 hr, and then detecting bound ova-specific IgG antibody using HRP-labeled anti-mouse IgG (Bio-Rad). Plates were washed between each step using 0.05% Tween-20 in PBS. HRP developed with tetramethylbenzidine was measured using a Molecular Devices SpectraMax Microplate Reader. Monoclonal mouse anti-ova IgG1 (clone OVA-14, Sigma-Aldrich, St. Louis, Missouri) was included as a standard reference in each assay. Endpoint titers were defined as the highest dilution at which immunized serum ELISA signal exceeded the average + 2 standard deviations of pre-immune sera analyzed in parallel. To interpret our titer values in more physiological terms, we used OVA-14 as a standard to determine the concentration of ova-specific IgG as equivalents of this monoclonal antibody.
Isotype titers from sera were measured using an ELISA with identical methods to those described above for total IgG titers except that ova-specific IgG1 antibody was detected using HRP-labeled goat anti-mouse IgG1 (Alpha Diagnostics) and ova-specific IgG2A antibody was detected using HRP-labeled goat anti-mouse IgG2A (Alpha Diagnostics).
The avidity of IgG responses to immunization was measured using an ELISA analysis of serum binding in the presence of urea using a commonly reported procedure from the literature [43]. Serum titer analysis was conducted in duplicate assay plates until serum adsorption was complete. At this point, one plate was incubated in the presence of 6M urea for 10 min followed by washing and detection of bound IgG on both plates as above. Avidity indices were defined as the serum dilution of urea-treated samples where the ELISA absorbance was 0.5 divided by the dilution of untreated samples giving the same absorbance.
2.9 Statistical analysis
Statistical analyses were carried out using GraphPad Prism 5.0c software. For comparisons of two samples, Student’s t-test was used to determine statistical significance and a P value less than 0.05 was considered significant. One-way ANOVA was applied for comparisons of multiple groups; Two-way ANOVA was used to determine statistical significance in longitudinal studies. For ANOVA analyses, Bonferroni post-tests were used to make comparisons of individual pairs of conditions.
3. Results
3.1. Synthesis of antigen- and danger signal-displaying lipid-enveloped microparticles and nanoparticles
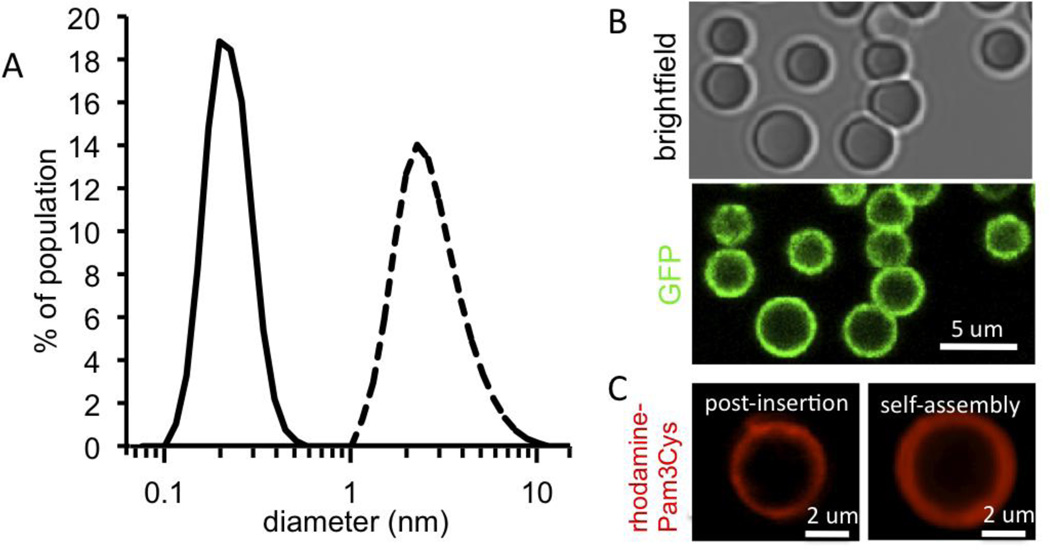
We recently showed that synthesis of PLGA micro- or nano-particles employing phospholipids as stabilizing agents in the emulsion process leads to the self-assembly of fluid bilayer surface coatings on these particles [24]. We hypothesized that these lipid-enveloped particles could be effective agents for vaccine delivery, by co-displaying anchored antigen and lipid-embedded adjuvant molecules together on the two-dimensionally diffusing lipid bilayer surfaces. We prepared particles where the lipid coating was comprised of mal-PEG2k-PE:DOPC:DOPG in a 10:72:18 mol ratio, and conjugated thiolated protein antigens to the particles via the maleimide-PEG tethers, followed by the introduction of lipophilic adjuvant molecules via post-insertion into the lipid coatings (Supplementary Fig. 1). By changing the lipid:polymer ratio and the method of dispersion, lipid-enveloped particles with micron or submicron size distributions were obtained, having mean diameters of 2.66±1.20 µm or 212±59.2 nm, respectively (Fig. 1A). Using confocal microscopy, we directly visualized the conjugation of substantial quantities of fluorescent antigens, such as GFP (Fig. 1B) or fluorescent ova (not shown). Similar to prior studies using post-insertion strategies to introduce lipid-conjugated proteins, peptides, or PEG into liposomal structures [38–40, 44, 45], we found that lipophilic molecular danger signals such as Pam3Cys (Toll-like receptor 2 agonist), monophosphoryl lipid A (TLR 4 agonist), or α-galactosylceramide (αGC, an invariant NK T-cell ligand) readily incorporated into the coatings of the antigen-conjugated particles. Surface loading of these ligands achieved by self-assembly during particle synthesis (ligands co-dissolved in DCM with lipids) was indistinguishable from results obtained when the ligands were added by post-insertion (illustrated in Fig. 1C for fluorescently-tagged Pam3Cys); we thus used the post-insertion approach for immunization studies. The quantity of antigen conjugated to the particles was determined by solubilizing the lipid surface coating with detergents and measuring the released protein by ELISA, BCA protein assay, or direct fluorescence (for GFP).
Figure 1. Synthesis of lipid-enveloped micro- or nano-particles with surface-displayed antigen and molecular adjuvants.
(A) Light scattering analysis of purified particle size distributions for microparticles (dashed line) or nanoparticles (solid line) synthesized by homogenization or sonication, respectively, to disperse lipid/polymer emulsion during particle synthesis. (B) Confocal imaging of lipid-enveloped microparticles bearing ~7×104 green fluorescent protein molecules per particle (green, GFP intrinsic fluorescence). (C) Confocal imaging of microparticles modified with rhodamine-labeled Pam3Cys (red fluorescence, lipid-like TLR-2 agonist) incorporated via post-insertion or through self-assembly during particle synthesis.
These measurements were in general agreement, and gave a typical conjugation level of 0.42±0.014 µg protein per mg microparticles, corresponding to 7×104 ova molecules per microparticle. This conjugation level was also similar to the per-particle loading measured by quantitative flow cytometry, and correlated with bright protein fluorescence that could be detected on particle surfaces by confocal microscopy (Fig. 1B). A key advantage of this surface antigen display strategy, as noted by others [22], is the ability to perform the conjugation under mild aqueous conditions and avoid exposure of potentially fragile antigens to harsh processing conditions commonly employed for encapsulation strategies.
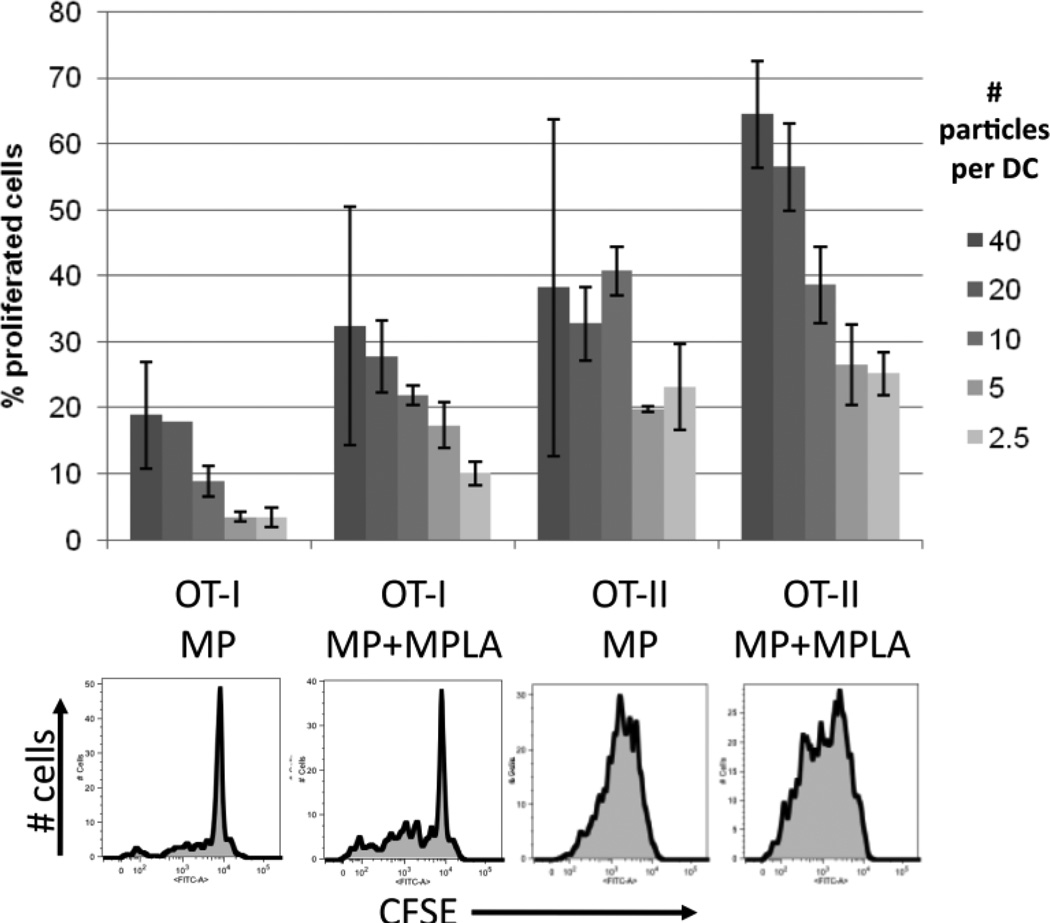
To assess the functional incorporation of adjuvant molecules in these lipid-enveloped particles and their potential for promoting cellular responses, we measured activation of dendritic cells (DCs) by MPLA-carrying nanoparticles and priming of naïve ova-specific CD4+ (OT-II) or CD8+ (OT-I) T-cells by DCs exposed to particles or soluble ovalbumin. Bone marrow-derived DCs incubated with MPLA-decorated nanoparticles upregulated the maturation markers class II MHC and CD80 to a similar or greater extent than DCs incubated with soluble MPLA (Supplementary Fig. 2). Notably, DCs cultured with particles lacking MPLA showed the same basal levels of MHC II/CD80 expression as cells incubated with medium alone, confirming the lack of endotoxin contamination in the materials. When primary splenic DCs were pulsed with titrated doses of ova-conjugated particles and mixed with CFSE-labeled naïve OT-I or OT-II T-cells, T-cell proliferation was triggered in both CD4+ and CD8+ T-cells (Fig. 2), while no proliferation was observed in controls where DCs were exposed blank particles or medium (data not shown). The particles triggered cross-presentation of ova to prime the OT-I cells at total ova doses of only 1 ng protein per well (or less), but DCs pulsed with 10,000-fold higher doses of soluble ova showed minimal OT-I proliferation, even in the presence of MPLA (not shown). Notably, addition of MPLA to the particles enhanced the response of both the OT-I and OT-II cells relative to particles displaying antigen alone (Fig. 2).
Figure 2. Priming of naïve CD4+ or CD8+ T-cells by antigen-conjugated lipid-enveloped particles.
Primary splenic DCs were incubated with ova-conjugated microparticles (with or without post-inserted MPLA) for 3 hrs, then co-cultured with naïve CFSE-labeled OT-I (CD8+) or OT-II (CD4+) T-cells. Proliferation of T-cells was assessed after 3 days by flow cytometry. Shown are representative flow histograms (10:1 particle:DC ratio) and mean percentages of proliferated cells from triplicate wells (± St. dev.). The maximum particle:DC ratio (40:1) corresponds to a total dose of 2.6 ng ova in the 200 µL culture.
3.2. Extreme dose-sparing antibody responses elicited by particle immunization
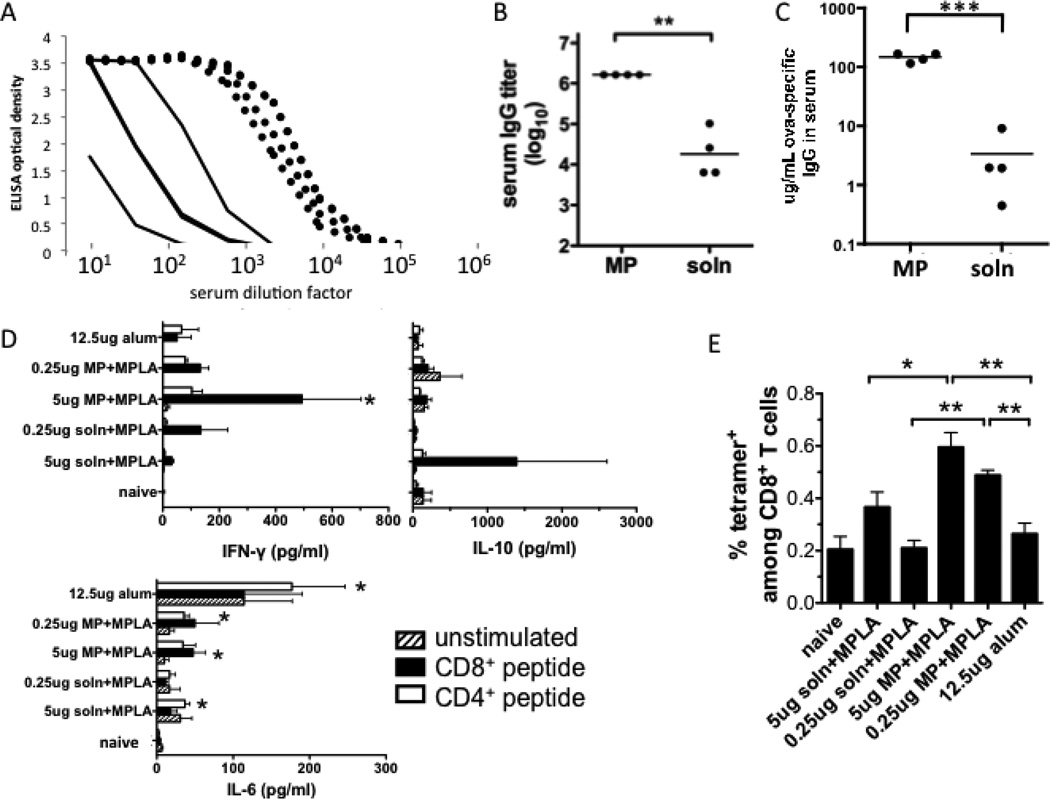
For in vivo studies, we first confirmed that our particulate antigen delivery system could augment the serum antibody titer elicited by protein immunization, similarly to previous reports of particle vaccines loaded with antigen [46–48] including the model antigen ova [7, 11, 12]. BALB/c mice were immunized s.c. with a modest dose of ova (0.5 µg) and boosted after 3 weeks with the same dose, either in soluble form or bound to lipid-coated microparticles (1.2 mg particles/dose). Ova delivered on lipid-enveloped particles elicited substantially higher levels of serum anti-ova IgG compared to soluble antigen, as revealed by ELISA serial dilution analysis of sera from individual immunized mice (Fig. 3A), endpoint anti-ova IgG titers (Fig. 3B), or total anti-ova IgG concentrations determined by calibrating against an anti-ova monoclonal antibody standard (Fig. 3C). Ova particle vaccination generated a mean of 150 µg/mL ova-specific IgG in serum, a 45-fold increase relative to the control soluble ova immunization (P < 0.0001). We verified that SAT(PEG)4 modification of ova for particle coupling had no significant influence on the protein’s immunogenicity (Supplementary Fig. 3).
Figure 3. Serum IgG responses to particle-delivered or soluble ova at a modest but conventional dose of 0.5 µg ova.
(A–C) BALB/c mice were immunized s.c. with 500 ng of ova in solution or displayed on lipid-coated microparticles and boosted on day 21 with the same formulations. Shown are analyses of sera collected on day 28: (A) Total anti-ova IgG ELISA on serum from mice immunized with ova-particles (dotted lines) or ova solution (solid lines); (B) Endpoint total IgG titers (**, P < 0.01) (C) total ova-specific IgG concentration in sera (***, P<0.0001). (D, E) C57Bl/6 mice were immunized on day 0 and day 21 s.c. with indicated doses of ova with alum, ova-particles with MPLA, or ova solution mixed with MPLA (2.5 µg MPLA in all cases). 7 days after boosting, splenocytes were collected and restimulated ex vivo with immunodominant CD8+ or CD4+ ova peptides for analysis of cytokine production (D) and frequencies of ova-specific CD8+ T-cells in spleens were analyzed by peptide-MHC tetramer staining and flow cytometry (E). (D, *, P < 0.05 relative to naïve mice; E, *, P < 0.05; **, P < 0.01).
We also assessed the T-cell responses to lipid-enveloped particle immunization, comparing to soluble ova immunization in the presence of MPLA or ova mixed with the traditional adjuvant alum. Lymphocytes from mice given a prime followed by a boost on day 21 were analyzed 7 days after the boost. Splenocytes from immunized mice produced cytokines in response to ex vivo restimulation with immunodominant MHC class I- or class II-restricted peptides: Mice immunized with soluble ova + MPLA produced some IL-10, and a lum-immunized mice produced IL-6. Notably however, only ova-particle immunized mice showed production of statistically significant quantities of the Th1 cytokine IFN-γ in response to ex vivo restimulation (Fig. 3D). Other cytokines assayed (IL-17, IL-4, and TNF-α) were at background levels for all groups. Analysis of the frequency of ova-specific CD8+ T-cells in spleens by peptide-MHC tetramer staining on day 7 following boosting showed no detectable response above background for alum-immunized animals even when twice as much antigen was used, while ova-particle immunization elicited easily detectable T-cell responses significantly greater than soluble ova immunization at both doses of antigen tested (Fig. 3E). Thus, lipid-enveloped particle delivery of antigen substantially enhanced both humoral and cellular responses at modest antigen doses relative to soluble antigen.
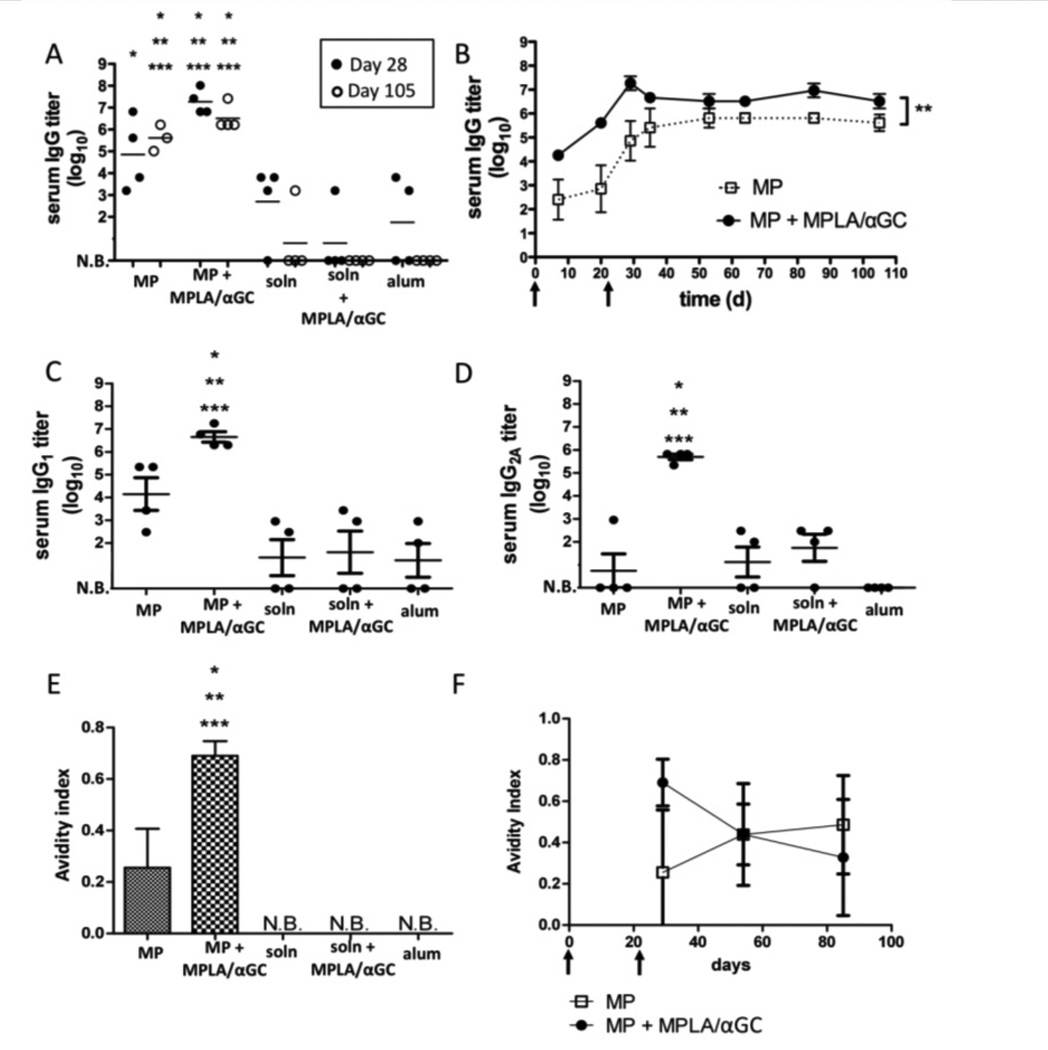
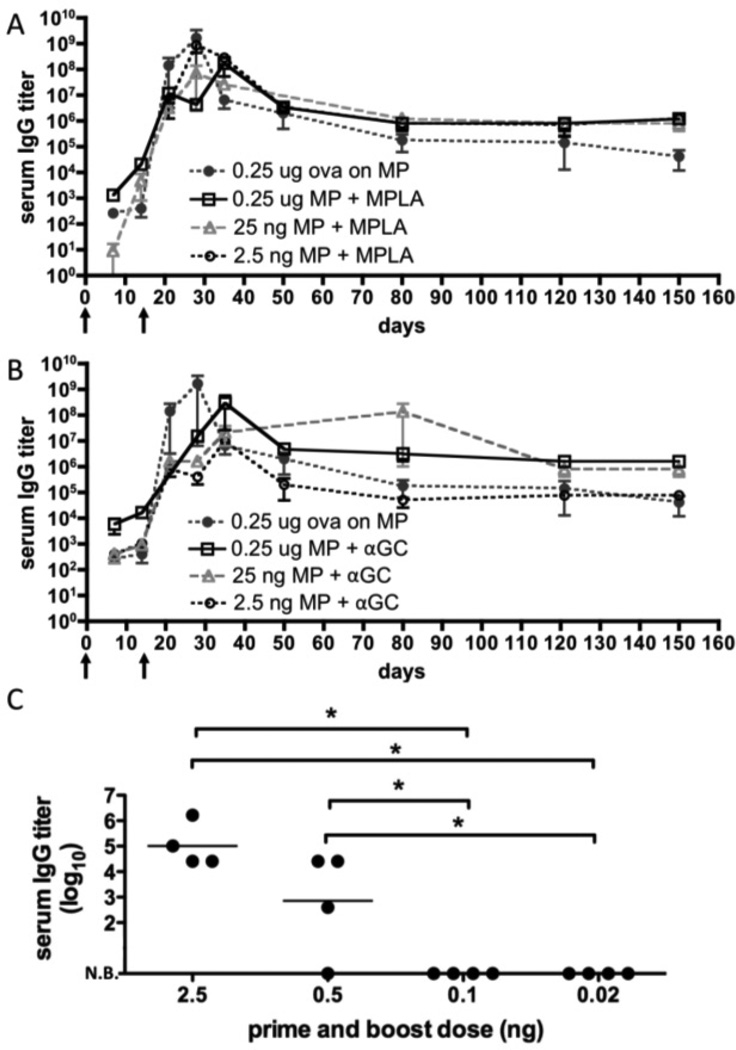
Immunization with microgram doses of protein antigens is common in murine studies [9, 12, 49], but providing saturating amounts of antigen may obscure the comparative potency of vaccines. In addition, strategies to dose-spare recombinant protein antigens are of interest for responding to both seasonal and potential pandemic diseases [29–34]. Thus, we next asked whether lipid-enveloped microparticles could potentiate antibody responses at a 50-fold lower dose of antigen, and directly compared the effectiveness of these lipid-enveloped PLGA particles with two licensed adjuvants, alum and MPLA. We found that a priming immunization with 10 ng of antigen followed by boosting with the same dose 3 weeks later elicited substantial anti-ova IgG titers using lipid-enveloped particles as a delivery vehicle (Fig. 4A). Anti-ova titers were increased 12-fold (P = 0.0053) by co-displaying antigen together with the lipid-like adjuvants MPLA and αGC in the bilayers surrounding the particles (Figs. 4A and B), but serum titers were maintained for at least 12 weeks regardless of whether these molecular adjuvants were included. In contrast, at this dose only a subset of mice responded when immunized with ova solution or ova mixed with the conventional vaccine adjuvant alum (even following boosting), and this response was not sustained in a majority of the mice (Fig. 4A).
Figure 4. Serum IgG responses elicited by lipid-coated particles vs. conventional adjuvants at limiting antigen doses.
Groups of BALB/c mice (n = 4) were immunized s.c. with 10 ng ova displayed on microparticles (“MP”), dissolved in saline (“soln”), mixed with alum, or mixed with MPLA and αGC; animals were boosted on day 21 with the same formulations. In both the particle-displayed and soluble adjuvant cases, equimolar quantities of 1.3 µg MPLA and 600 ng αGC were used. (A) Post-boost peak (day 28) and late (day 105) endpoint titers from individual mice. (B) Mean endpoint titers (±SEM) for particle immunizations over time (**, P=0.0053). (C, D) Endpoint IgG1 (C) and IgG2A titers at day 28. (E, F) Avidity of ova-specific IgG in each group measured at day 28 for all groups (E) or for the particle-immunized groups over time (F). (N.B.: No binding detected. *, **, *** in panels A, C–E: P < 0.05 relative to soln+MPLA/αGC, soln, or alum at the same time point, respectively).
Ova mixed with soluble MPLA+αGC was also unable to elicit detectable titers at this low antigen dose (Fig. 4A). Particle immunization with or without the molecular adjuvant molecules increased IgG1 antibody responses, but the presence of MPLA and αGC specifically boosted the Th1-like IgG2A antibody response (Fig. 4C, D). Notably, measurement of the avidity of the IgG elicited by particle immunization showed that particle immunization promoted antibody responses that could still be detected following urea washes, whereas soluble ova immunizations showed no binding under these conditions (Fig. 4E); this enhanced avidity was stable over at least 3 months post immunization (Fig. 4F). Thus, antigen delivery using lipid-coated particles was substantially more potent than either alum or soluble TLR agonist/NKT ligand danger signals for adjuvanting the humoral response, and particulate antigen delivery exhibited synergy with co-incorporated molecular danger signals. BALB/c and C57Bl/6 mice responded with similar titers of serum IgG (Supplementary Fig. 4). We confirmed that antibody responses elicited at such low antigen doses were not confined to ova by repeating immunizations using GFP as an immunogen, which also elicited substantial IgG titers at doses as low as 10 ng (Supplementary Fig. 5).
These results prompted us to explore the dose-sparing capability of lipid-enveloped particle vaccines more completely. We tested the ability of particles co-displaying antigen and individual molecular danger signals (MPLA or αGC) to elicit sustained antibody titers using antigen doses ranging from 250 ng down to 2.5 ng (Fig. 5). Ova-displaying microparticles co-delivering MPLA elevated antibody titers modestly compared to particles lacking MPLA, but this elevated antibody titer was maintained for ova doses as low as 2.5 ng (Fig. 5A). By contrast, αGC-bearing particles induced higher titers shortly after a single immunization, but appeared to be slightly less potent in terms of inducing sustained high antibody titers, compared to particles carrying MPLA (Fig. 5B). Using MPLA-loaded particles as an optimal carrier for dose-sparing, we reduced the dose 5-fold from 2.5 ng to 0.5 ng, and only a fraction of mice had detectable ova-specific serum IgG 2 weeks post-boost (Fig. 5C). No ova-specific IgG was detected in mice immunized with 0.1 ng or 20 pg ova. Thus, 2.5 ng of ova was approximately the lowest antigen dose eliciting robust IgG responses following MPLA-adjuvanted particle delivery. This is 1000-fold lower than doses typically used in soluble protein immunizations.
Figure 5. IgG responses following dose sparing immunizations with lipid-coated particle immunogens.
Groups of C57Bl/6 mice (n = 3) were immunized with lipid-coated microparticles delivering the indicated dose of ova and boosted on day 14. The particle-only conditions (black circles) carried ova alone; otherwise, 13 µg MPLA or 6 µg αGC were added via the post-insertion method to the antigen-loaded particles. Shown are mean endpoint titers (±SEM) for dose titrations of particles carrying ova and (A) MPLA or (B) αGC. (C) Groups of BALB/c mice (n = 4) were immunized with diminishing doses of ova co-displayed with 1.3 µg MPLA and boosted on day 14 to determine the minimum dose capable of eliciting measurable antibody responses. Post-boost peak (day 28) endpoint titers are shown for individual mice (*, P < 0.05).
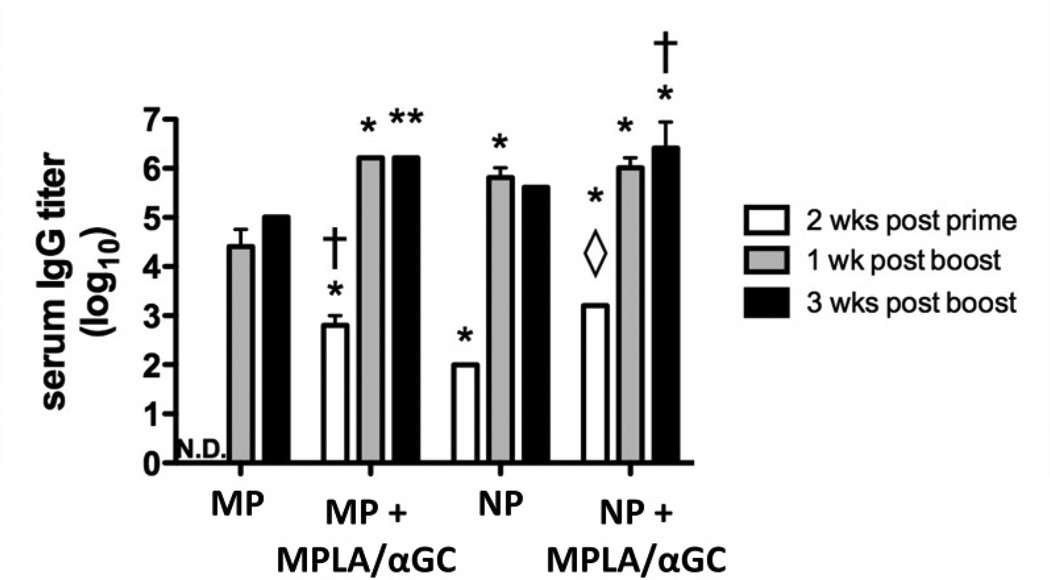
Nanoparticles have been proposed as potentially superior vaccine delivery agents relative to microparticles, though conflicting data exist in the literature in studies where particle size has been evaluated explicitly [22, 23, 50–52]. To determine whether particle size is an important parameter in this lipid-enveloped delivery system, we tested whether microparticles (mean diameter 2.66 ± 1.20 µm) or nanoparticles (mean diameter 212 ± 59.2 nm) were more potent in a dose-sparing immunization with 10 ng ova. Nanoparticles consistently elicited measurable titers following a single immunization even in the absence of added danger signal molecules; however, when particles co-displayed ova and MPLA/αGC, nanoparticles and microparticles elicited comparable antibody titers (Fig. 6). In addition, nanoparticles and microparticles elicited similar IgG1 and IgG2a titers (data not shown). Thus, in the limiting case of a single low dose without adjuvant, nanoparticles may provide a better dose-sparing vaccine delivery platform.
Figure 6. Comparison of adjuvant effect of lipid-enveloped microparticles vs. nanoparticles.
Groups of BALB/c mice (n = 3) were immunized s.c. with 10 ng ova displayed on microparticles (“MP”) or nanoparticles (“NP”), and boosted on day 14. For comparison, particles from the same syntheses were loaded with 1.3 µg MPLA and 600 ng αGC via the post-insertion method. Bars show mean endpoint titers ±SEM. N.D., no antigen-specific IgG detected above background (*, P < 0.001 vs. MP; **, P < 0.01 vs. MP; ◊, P < 0.01 vs. NP; †, P < 0.05 vs. NP; all comparisons made by Bonferroni post-tests at the same time point).
3.3 Roles for MPLA/αGC in enhancing lipid-enveloped vaccine delivery
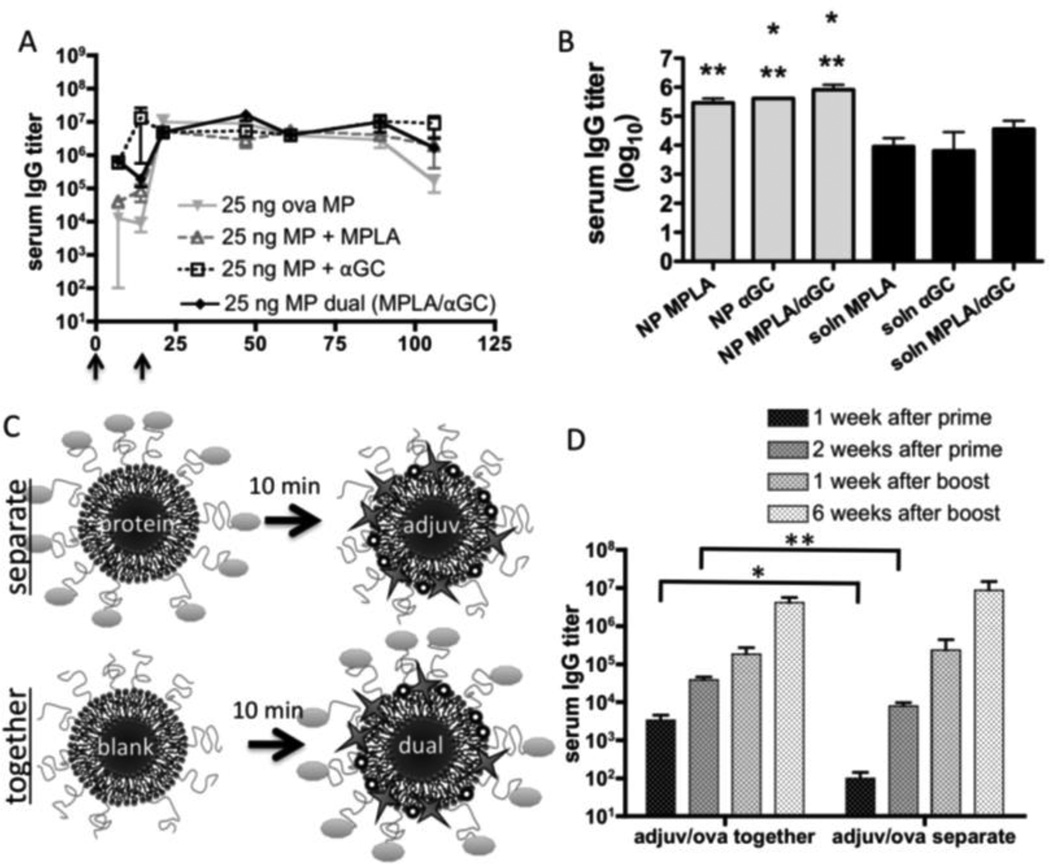
Because MPLA and αGC promote immunity through distinct but interacting cell subsets, it has been proposed that these adjuvant molecules could have synergistic effects [53]. Therefore, we compared the humoral responses following immunization with particles carrying MPLA alone, αGC alone, or the combination of these two ligands to determine if synergy between these adjuvants could be detected. We found that MPLA and αGC together could promote early titers comparable to αGC alone, and could sustain long-term titers at similar levels as individual adjuvants, but we did not observe further enhancement of titers above what was seen with individual adjuvant molecules (Fig. 7A). To determine whether co-loading of the two adjuvant molecules onto particles might interfere with potential synergy by directing them to the same antigen-presenting cells, we directly compared vaccination with MPLA/αGC loaded onto the antigen-bearing particles vs. the same doses of adjuvant molecules injected in soluble form, 10 minutes prior to injection of the antigen-bearing particles at the same site (Fig. 7B). MPLA/αGC co-loaded with antigen on particles showed 5–10-fold enhanced IgG titers compared to the soluble adjuvants injected separately from the antigen-loaded particles. No substantial synergy for the co-delivered adjuvants was seen compared to MPLA or αGC alone in either mode of delivery.
Figure 7. Analysis of synergy between MPLA and αGC in particle vaccine responses.
Groups of BALB/c mice (n = 4) were immunized s.c. with ova displayed on microparticles and boosted on day 21; endpoint total IgG titers were determined and shown are means ± SEM. (A) Mice were immunized with 25 ng ova and 1.3 µg MPLA and/or 600 ng αGC co-loaded onto microparticles. (B) Mice were immunized with 10 ng ova-conjugated nanoparticles co-loaded with MPLA, αGC, or both adjuvants, and compared to mice given the same doses of the adjuvant molecules injected in soluble form 10 min before injection of the antigen-loaded particles at the same site. Titers were assessed on day 28 (*, P < 0.05 vs. soln MPLA; **, P < 0.05 vs. soln αGC). (C, D) Mice were immunized with microparticles displaying 10 ng ova, followed 10 minutes later by microparticles displaying 1.3 µg MPLA and 600 ng αGC injected at the same site (C, “separate”). For comparison, mice received an equivalent number of blank microparticles, followed 10 minutes later by microparticles co-displaying 10 ng ova, 1.3 µg MPLA, and 600 ng αGC (C, “together”). *, P = 0.0284; **, P = 0.0070.
We next tested whether the enhancement of antibody responses elicited by MPLA/αGC in lipid-enveloped particle immunization depended on co-delivery of these adjuvant molecules on the same particle as the antigen. As illustrated schematically in Fig. 7C, mice were immunized with ova-loaded microparticles, followed 10 minutes later by an injection of adjuvant-loaded microparticles at the same site (“separate”), to avoid lipid component exchange between particles. For particles co-displaying antigen and adjuvant molecules, we first injected blank particles, followed 10 minutes later by antigen/MPLA/αGC particles (“together”), to compare immunizations with equal total quantities of particles present. We found that prior to the booster immunization, co-display of antigen and adjuvant molecules on the same particle significantly elevated titers (34-fold increase one week after prime, P=0.028; 4.8-fold increase two weeks after prime, P=0.007)). However, no significant difference was seen after boosting (Fig. 7D). We conclude that co-delivery of antigen and adjuvant on the same particle was only important during the primary humoral response of naïve animals.
3.4 Dose sparing of molecular danger signals for antibody response by particle delivery
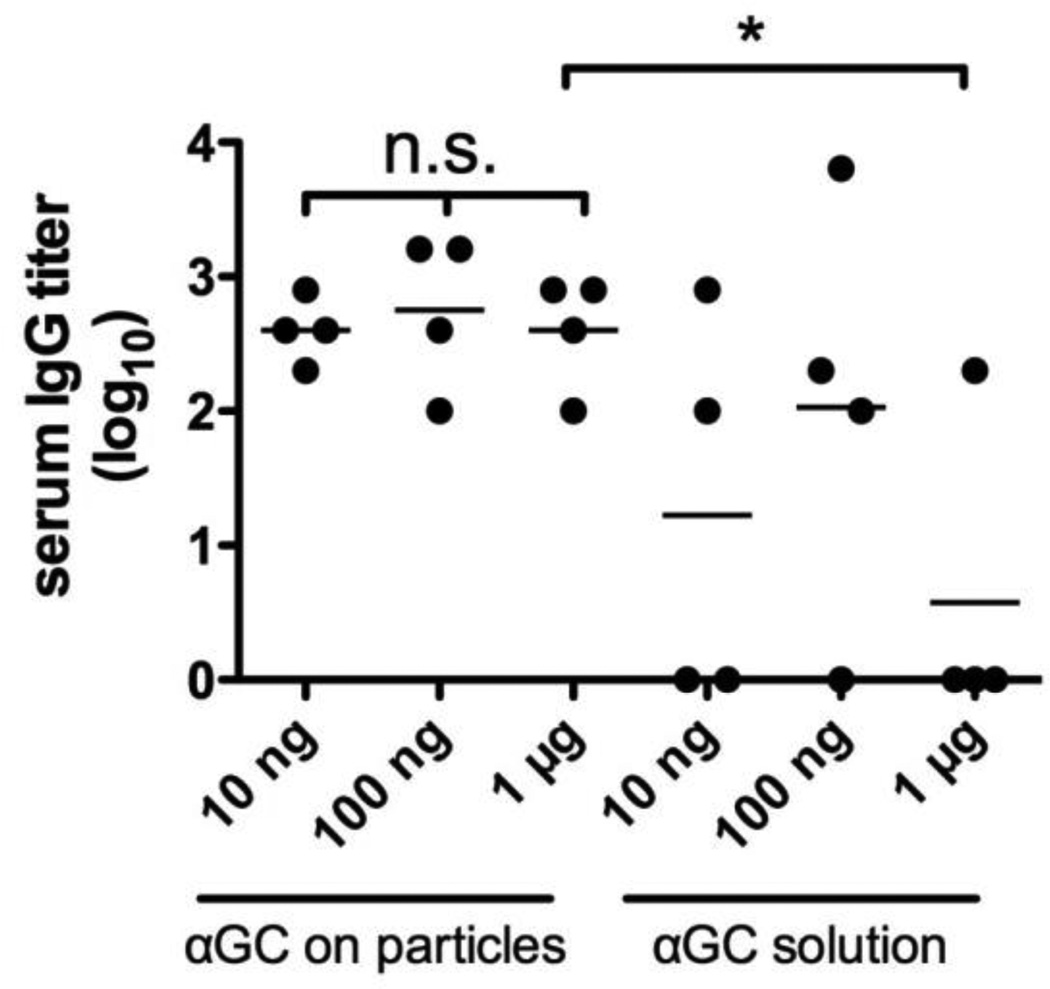
We finally investigated the effect of αGC dose on antibody responses to a limited dose of only 2 ng of ova displayed on microparticles. To our knowledge, there have been no reports thus far on αGC delivery as an adjuvant for a particulate vaccine; however, a report by Thapa et al. in which αGC was used as a stand-alone drug without an antigen provided evidence that nanoparticle-encapsulated αGC could be used to repeatedly stimulate NK T-cells, whereas αGC in solution induced anergy after a single αGC injection [54]. We measured antibody titers 14 days following priming with 2 ng ova co-loaded onto microparticles with 10 ng, 100 ng, or 1 µg αGC. Alternatively, the same dose of αGC in solution was injected 10 minutes prior to injection of particles displaying 2 ng ova alone. The resulting IgG titers, shown in Fig. 8, revealed that all 3 doses of αGC delivered on the antigen-bearing particles elicited IgG titers from all mice following a single immunization, and the differences between groups did not reach statistical significance in any pairing of conditions (e.g., 100 ng vs. 1 µg soluble αGC: P=0.34). Notably, only a fraction of mice had detectable titers against 2 ng of antigen when αGC was delivered separately from the antigen-loaded particles in soluble form. We conclude that co-delivery of αGC with antigen together on lipid-enveloped microparticles enhances the potency of this adjuvant molecule relative to soluble delivery at the same injection site, and particle delivery further allows for at least 100-fold dose sparing of this potent immunostimulatory ligand.
Figure 8. Dose sparing of molecular adjuvants by lipid-coated particles.
Groups of BALB/c mice (n = 4) were immunized s.c. with 2 ng ova displayed on microparticles co-loaded with the indicated quantities of αGC via the post-insertion method (αGC on particles). A second group of mice was immunized by injecting the indicated doses of αGC followed 10 min later by 2 ng ova-microparticles at the same site (αGC solution). Shown are endpoint total IgG titers for individual mice two weeks after a single immunization (*, P < 0.05).
4. Discussion
The development of new adjuvants capable of potentiating immune responses against recombinant protein antigens is an important goal for a broad range of candidate vaccines [13, 55]. Particulate vaccine delivery systems have been studied as a way of delivering adjuvant molecules, and as an intrinsically potent method of vaccine delivery that mimics the particulate nature of foreign pathogens. Many studies have focused on the potency of particles in eliciting CD8+ T cell responses [4–6], but antigen-bearing particles can also strongly engage B lymphocytes [1–3], promoting increased antibody production compared to antigen solutions [7, 11, 12, 56].
Here we tested an approach where degradable polymer particles were “enveloped” by a functionalized phospholipid bilayer. The bilayer coating provided a facile means for anchoring antigens to the particle surfaces (via reactive lipid headgroups) and also allowed for a biomimetic presentation of membrane-incorporated adjuvant molecules. We hypothesized that this pathogen-mimetic surface structure would enhance immune responses to delivered antigens. Because most existing vaccines are thought to provide protection via the generation of neutralizing antibodies [57–59], we focused our analysis on the humoral immune response. In line with prior studies, we found that particle-based delivery of vaccine antigen could significantly improve antibody responses to typical doses of protein antigen. We detected antibody by ELISA in million-fold diluted sera, corresponding to >100 µg/mL ova-specific IgG when normalized to a commercially available monoclonal antibody standard. However, the most striking results were observed when the dose-sparing capacity of this particle-based delivery system was examined. We observed strong and sustained titers using a prime-boost regimen of a few nanograms of antigen displayed on particles. Neither the conventional adjuvant alum, nor protein solutions mixed with potent adjuvant molecules such as TLR agonists or NKT agonists, were effective at these ultra-low antigen doses.
We also observed that membrane-incorporating adjuvant molecules co-delivered by lipid-enveloped particles could further enhance this dose-sparing capacity. Both MPLA and αGC helped to raise and maintain antibody responses, but our results suggest that each may be ideal for a different infectious disease application. MPLA-adjuvanted particles elicited lower initial titers (prior to boosting), but sustained the antibody response at the lowest doses (2.5 ng antigen) for over 150 days; such a response could help provide lifelong immunity to a disease that poses a constant hazard. By contrast, αGC-carrying particles elicited higher early titers shortly after immunization, but did not sustain long-term titers at doses as low as MPLA. Thus, αGC may be better suited in pandemic or bioterrorism scenarios in which immunity must be acquired quickly to address an immediate danger.
We initially chose to test both MPLA and αGC due to their potential for cross-talk and synergy as vaccine adjuvants [60, 61]. MPLA activates dendritic cells or B-cells through Toll like receptor-4 [62], while αGC is a glycolipid that can be loaded into the cleft of non-classical CD1d MHC molecules on dendritic cells; αGC/CD1d complexes trigger activation of invariant natural killer T (iNKT) cells [60]. Recent studies have shown that iNKT cells can provide CD4 T-cell-independent help for antibody responses [17, 63, 64]. Because of this non-classical helper activity and the fact that αGC does not employ the same MyD88-dependent signaling pathway used by MPLA [26, 60], these adjuvant molecules could potentially synergize in promoting vaccine responses. Indeed, Silk et al. have shown that following i.v. injection of soluble antigen, αGC, and MPLA, immune responses are amplified relative to immunizations with each of the adjuvant molecules alone with antigen [61]. However, i.v. immunization primes immune responses primarily in the spleen, and to our knowledge the same combinations have not yet been demonstrated to show synergy following traditional parenteral immunization. Here, the combination of MPLA and αGC on the same particle in s.c. immunizations did not dramatically elevate titers compared to the use of each adjuvant on its own, despite the divergent cell subsets and mechanisms through which these adjuvants act. However, combining these two ligands did allow for each feature of the response unique to the individual ligands to be achieved by a single vaccine that elicited both rapid early titer increases and sustained high titers.
Dose sparing has important practical implications, as vaccines requiring high doses of antigen suffer from high production costs and a risk of vaccine shortages [29–34]. In addition, the potency implied by highly dose-sparing formulations may be especially relevant for weakly immunogenic antigens such as recombinant HIV envelope glycoproteins, which have required high doses of antigen to elicit measurable antibody responses in animal models and human HIV vaccine trials [65–68]. In mouse models of vaccination, even highly immunogenic model antigens such as ova are rarely used at doses below 1 µg [69–72] even in dose-response studies [73], and doses as high as 500 µg are common for model antigens [9, 12, 49]. Few studies of any vaccine have reported humoral immune responses to doses of subunit vaccine antigens as low as those reported here to our knowledge. In the late 1980’s, liposomes with surface-conjugated antigens were reported to elicit weak antibody responses to doses as low as 40 ng of tetanus toxoid, although the longevity of these responses was not analyzed [74, 75]. Dose sparing of adjuvants such as TLR agonists by encapsulation in degradable polymer particles has been observed by others in the context of cellular immunity [35], and here we have observed that particles also have adjuvant-sparing capabilities in the context of humoral responses. Lipid-coated particles co-displaying antigen and αGC achieved similar titers over a wide range of doses of αGC down to 10 ng. Thus, antigen surface-display allowed for dramatic dose sparing not only of antigen, but of adjuvant as well.
Conflicting evidence exists for whether adjuvants and antigens must access the same intracellular compartment by being carried on the same particle or covalently linked together. Some studies of particle-based vaccines show that co-delivery of these molecules on the same particle is required for the adjuvant to take effect [16–19], while others have shown equivalent antibody responses regardless of whether antigen is displayed with adjuvant on the same particle, or antigen-only particles are mixed with adjuvant-only particles prior to immunization [20, 76]. We designed our study to minimize the possibility that antigen-only and adjuvant-only particles could exchange lipid molecules in solution or at the injection site by injecting blank or antigen-only particles 10 minutes prior to injection at the same site with co-loaded or adjuvant-only particles. We thus ensured that antigen and adjuvant were truly delivered on different particle populations in the test case, or co-loaded on the same particles in the control case. We found that co-loading of antigen and adjuvant was significantly advantageous after a single immunization, but that after a booster immunization, it did not matter whether antigen and adjuvant were co-loaded or delivered on separate particle populations. We further showed that αGC could be equally effective in solution injected at the same site as antigen-displaying particles. Thus, only the initial humoral response of antigen-naïve mice depended on co-loading of antigen and adjuvant.
Both microparticles and nanoparticles have been tested as vaccine carriers, but few studies have directly tested the effect of particle size within a single system. Where this comparison has been carried out, the results have varied system to system, with optimal responses seen for nano-scale [23, 77, 78], micro-scale [79, 80], or intermediate-scale particle diameters [81, 82]. Here, we found that microparticles and nanoparticles were both viable vehicles for dose-sparing delivery in a boosted vaccine regimen, but nanoparticles promoted higher titers after a single immunization, particularly in the absence of an adjuvant.
While our studies here focused on particles freshly prepared and used within ~12 hrs, we have also shown in prior studies that lipid-enveloped PLGA micro- and nano-particles retain their original size distributions following lyophilization/reconstitution, and retain intact their surface nanoscale lipid coating organization following lyophilization/reconstitution [24]. We also confirmed in the present work that functionalized lipid headgroups remained surface-accessible following lyophilization (data not shown). The ability to lyophilize these formulations will substantially enhance their potential for long-term storage, and the immunogenicity of such freeze-dried formulations is a topic for future work.
5.Conclusion
Lipid-enveloped PLGA micro- and nano-particles were surface-modified with incorporated lipophilic molecular adjuvants and lipid-anchored protein antigens. These antigen-displaying particles elicited strong antibody titers at antigen doses of a few nanograms: far below the conventional doses used in mice, even in dose-sparing formulations such as intradermal immunizations. Co-display of adjuvants on particles further enhanced antibody responses: the TLR4 agonist MPLA sustained titers for over 150 days at the lowest doses, and the NKT agonist αGC promoted rapid IgG production after a single immunization, which may prove particularly useful in the context of a disease pandemic. The materials chosen for this vaccine platform are well suited for future clinical studies because of precedent for their use in humans (the polymer and lipid components are available from their manufacturers in GMP-compliant form), and one of the adjuvants we tested, MPLA, is already in use in human vaccines. The particles also offer the potential for controlled release of drugs from the polymer core, an aspect of significant interest for future work. Through dramatic dose sparing, this technology may facilitate protective responses with weakly immunogenic subunit vaccines, lower the cost of vaccine manufacture, and reduce the risk of seasonal or pandemic vaccine shortages.
Supplementary Material
Acknowledgments
This work was supported in part by the Ragon Institute of MGH, MIT, and Harvard, the Gates Foundation, the NIH (AI073165 and U19AI091693), and the Human Frontier Science Program. A.B. was supported by graduate fellowships from the NSF and the Hertz Foundation. D.J.I. is an investigator of the Howard Hughes Medical Institute. We gratefully acknowledge the staff of MIT’s Division of Comparative Medicine, particularly Santina Caruso, Elizabeth Horrigan, Ricardo Moreno, and Natasha Pogue for technical and veterinary assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The Influence of Antigen Organization on B-Cell Responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 2.Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. Journal of Clinical Investigation. 2001;108:415–423. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dintzis HM, Dintzis RZ, Vogelstein B. Molecular Determinants of Immunogenicity - Immunon Model of Immune-Response. Proceedings of the National Academy of Sciences of the United States of America. 1976;73:3671–3675. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamvrellis A, Leong D, Hanley JC, Xiang SD, Mottram P, Plebanski M. Vaccines that facilitate antigen entry into dendritic cells. Immunology and Cell Biology. 2004;82:506–516. doi: 10.1111/j.0818-9641.2004.01271.x. [DOI] [PubMed] [Google Scholar]
- 5.Kovacsovicsbankowski M, Clark K, Benacerraf B, Rock KL. Efficient Major Histocompatibility Complex Class-I Presentation of Exogenous Antigen Upon Phagocytosis by Macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, Edelson RL, Saltzman WM, Hanlon DJ. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garinot M, Fievez V, Pourcelle V, Stoffelbach F, des Rieux A, Plapied L, Theate I, Freichels H, Jerome C, Marchand-Brynaert J, Schneider YJ, Preat V. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. Journal of Controlled Release. 2007;120:195–204. doi: 10.1016/j.jconrel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Jiang WL, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Advanced Drug Delivery Reviews. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Maloy KJ, Donachie AM, Ohagan DT, Mowat AM. Induction of Mucosal and Systemic Immune-Responses by Immunization with Ovalbumin Entrapped in Poly(Lactide-Co-Glycolide) Microparticles. Immunology. 1994;81:661–667. [PMC free article] [PubMed] [Google Scholar]
- 10.O'Hagan DT, Singh M, Gupta RK. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Advanced Drug Delivery Reviews. 1998;32:225–246. [PubMed] [Google Scholar]
- 11.Bal SM, Slutter B, van Riet E, Kruithof AC, Ding Z, Kersten GF, Jiskoot W, Bouwstra JA. Efficient induction of immune responses through intradermal vaccination with N-trimethyl chitosan containing antigen formulations. J Control Release. 2010;142:374–383. doi: 10.1016/j.jconrel.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Prokop A, Kozlov E, Carlesso G, Davidson JM. Hydrogel-based colloidal polymeric system for protein and drug delivery: Physical and chemical characterization, permeability control and applications. Filled Elastomers Drug Delivery Systems. 2002;160:119–173. [Google Scholar]
- 13.Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: current challenges and future approaches. J Pharm Sci. 2009;98:1278–1316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babensee JE, Paranjpe A. Differential levels of dendritic cell maturation on different biomaterials used in combination products. Journal of Biomedical Materials Research Part A. 2005;74A:503–510. doi: 10.1002/jbm.a.30429. [DOI] [PubMed] [Google Scholar]
- 15.Bennewitz NL, Babensee JE. The effect of the physical form of poly(lactic-co-glycolic acid) carriers on the humoral immune response to co-delivered antigen. Biomaterials. 2005;26:2991–2999. doi: 10.1016/j.biomaterials.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Saltzman WM, Mellman I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27:3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, Besra GS, Cerundolo V, Batista FD. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friede M, Muller S, Briand JP, Vanregenmortel MHV, Schuber F. Induction of Immune-Response against a Short Synthetic Peptide Antigen Coupled to Small Neutral Liposomes Containing Monophosphoryl Lipid-A. Molecular Immunology. 1993;30:539–547. doi: 10.1016/0161-5890(93)90028-a. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser-Schulz G, Heit A, Quintanilla-Martinez L, Hammerschmidt F, Hess S, Jennen L, Rezaei H, Wagner H, Schatzl HM. Polylactide-coglycolide microspheres coencapsulating recombinant tandem prion protein with CpG-oligonucleotide break self-tolerance to prion protein in wild-type mice and induce CD4 and CD8 T cell responses. Journal of Immunology. 2007;179:2797–2807. doi: 10.4049/jimmunol.179.5.2797. [DOI] [PubMed] [Google Scholar]
- 20.Singh M, Ott G, Kazzaz J, Ugozzoli M, Briones M, Donnelly J, O'Hagan DT. Cationic microparticles are an effective delivery system for immune stimulatory CpG DNA. Pharmaceutical Research. 2001;18:1476–1479. doi: 10.1023/a:1012269226066. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Briones M, Ott G, O'Hagan D. Cationic microparticles: A potent delivery system for DNA vaccines. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:811–816. doi: 10.1073/pnas.97.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IFC, Plebanski M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. Journal of Immunology. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 23.Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neill CP, Lee LK, Swartz MA, Hubbell JA. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnology. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 24.Bershteyn A, Chaparro J, Yau R, Kim M, Reinherz E, Ferreira-Moita L, Irvine DJ. Polymer-supported lipid shells, onions, and flowers. Soft Matter. 2008;4:1787–1791. doi: 10.1039/b804933e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burgess S. FDA News Release. U. S. Food and Drug Administration; 2009. Oct 16, FDA Approves New Vaccine for Prevention of Cervical Cancer. [Google Scholar]
- 26.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nature Reviews Immunology. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 27.Glenn GM, Rao M, Richards RL, Matyas GR, Alving CR. Murine IgG subclass antibodies to antigens incorporated in liposomes containing lipid A. Immunol Lett. 1995;47:73–78. doi: 10.1016/0165-2478(95)00069-h. [DOI] [PubMed] [Google Scholar]
- 28.Tamauchi H, Tadakuma T, Yasuda T, Tsumita T, Saito K. Enhancement of immunogenicity by incorporation of lipid A into liposomal model membranes and its application to membrane-associated antigens. Immunology. 1983;50:605–612. [PMC free article] [PubMed] [Google Scholar]
- 29.Fedson DS. Preparing for pandemic vaccination: An international policy agenda for vaccine development. Journal of Public Health Policy. 2005;26:4–29. doi: 10.1057/palgrave.jphp.3200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Germann TC, Kadau K, Longini IM, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. New England Journal of Medicine. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 32.Russell PK. Vaccines in civilian defense against bioterrorism. Emerging Infectious Diseases. 1999;5:531–533. doi: 10.3201/eid0504.990413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sloan FA, Berman S, Rosenbaum S, Chalk RA, Giffin RB. The fragility of the US vaccine supply. New England Journal of Medicine. 2004;351:2443–2447. doi: 10.1056/NEJMsb033394. [DOI] [PubMed] [Google Scholar]
- 34.Yamada T. Poverty, Wealth, and Access to Pandemic Influenza Vaccines. New England Journal of Medicine. 2009;361:1129–1131. doi: 10.1056/NEJMp0906972. [DOI] [PubMed] [Google Scholar]
- 35.Diwan M, Elamanchili P, Cao M, Samuel J. Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Curr Drug Deliv. 2004;1:405–412. doi: 10.2174/1567201043334597. [DOI] [PubMed] [Google Scholar]
- 36.O'Hagan D, Singh M, Ugozzoli M, Wild C, Barnett S, Chen M, Schaefer M, Doe B, Otten GR, Ulmer JB. Induction of potent immune responses by cationic microparticles with adsorbed human immunodeficiency virus DNA vaccines. J Virol. 2001;75:9037–9043. doi: 10.1128/JVI.75.19.9037-9043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wassel RA, Grady B, Kopke RD, Dormer KJ. Dispersion of super paramagnetic iron oxide nanoparticles in poly(d,l-lactide-co-glycolide) microparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2007;292:125–130. [Google Scholar]
- 38.Iden DL, Allen TM. In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochimica Et Biophysica Acta-Biomembranes. 2001;1513:207–216. doi: 10.1016/s0005-2736(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 39.Ishida T, Iden DL, Allen TM. A combinatorial approach to producing sterically stabilized (Stealth) immunoliposomal drugs. Febs Letters. 1999;460:129–133. doi: 10.1016/s0014-5793(99)01320-4. [DOI] [PubMed] [Google Scholar]
- 40.Takasaki J, Ansell SM. Micelles as intermediates in the preparation of protein-liposome conjugates. Bioconjugate Chemistry. 2006;17:438–450. doi: 10.1021/bc050051r. [DOI] [PubMed] [Google Scholar]
- 41.Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials. 2008;29:3671–3682. doi: 10.1016/j.biomaterials.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 42.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 43.Yue Y, Xu W, Hu L, Jiang Z, Xiong S. Enhanced resistance to coxsackievirus B3-induced myocarditis by intranasal co-immunization of lymphotactin gene encapsulated in chitosan particle. Virology. 2009;386:438–447. doi: 10.1016/j.virol.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Shmeeda H, Mak L, Tzemach D, Astrahan P, Tarshish M, Gabizon A. Intracellular uptake and intracavitary targeting of folate-conjugated liposomes in a mouse lymphoma model with up-regulated folate receptors. Molecular Cancer Therapeutics. 2006;5:818–824. doi: 10.1158/1535-7163.MCT-05-0543. [DOI] [PubMed] [Google Scholar]
- 45.Yoshina-Ishii C, Miller GP, Kraft ML, Kool ET, Boxer SG. General method for modification of liposomes for encoded assembly on supported bilayers. Journal of the American Chemical Society. 2005;127:1356–1357. doi: 10.1021/ja043299k. [DOI] [PubMed] [Google Scholar]
- 46.Jung T, Kamm W, Breitenbach A, Hungerer KD, Hundt E, Kissel T. Tetanus toxoid loaded nanoparticles from sulfobutylated poly(vinyl alcohol)-graft-poly(lactide-co-glycolide): evaluation of antibody response after oral and nasal application in mice. Pharm Res. 2001;18:352–360. doi: 10.1023/a:1011063232257. [DOI] [PubMed] [Google Scholar]
- 47.Shahin R, Leef M, Eldridge J, Hudson M, Gilley R. Adjuvanticity and protective immunity elicited by Bordetella pertussis antigens encapsulated in poly(DL-lactide-co-glycolide) microspheres. Infect Immun. 1995;63:1195–1200. doi: 10.1128/iai.63.4.1195-1200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomasin C, Corradin G, Men Y, Merkle HP, Gander B. Tetanus toxoid and synthetic malaria antigen containing poly(lactide)/poly(lactide-co-glycolide) microspheres: Importance of polymer degradation and antigen release for immune response. Journal of Controlled Release. 1996;41:131–145. [Google Scholar]
- 49.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lmphoid tissues. Journal of Experimental Medicine. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer HC, Chan WCW. Nanotoxicity: the growing need for in vivo study. Current Opinion in Biotechnology. 2007;18:565–571. doi: 10.1016/j.copbio.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Grgacic EVL, Anderson DA. Virus-like particles: Passport to immune recognition. Methods. 2006;40:60–65. doi: 10.1016/j.ymeth.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: From dendritic cell homing to vaccine design. Seminars in Immunology. 2008;20:147–156. doi: 10.1016/j.smim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 53.Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, Besra GS, Platt FM, Cerundolo V. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thapa P, Zhang GD, Xia CF, Gelbard A, Overwijk WW, Liu CW, Hwu P, Chang DZ, Courtney A, Sastry JK, Wang PG, Li C, Zhou DP. Nanoparticle formulated alpha-galactosylceramide activates NKT cells without inducing anergy. Vaccine. 2009;27:3484–3488. doi: 10.1016/j.vaccine.2009.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lore K, Karlsson Hedestam GB. Novel adjuvants for B cell immune responses. Curr Opin HIV AIDS. 2009;4:441–446. doi: 10.1097/COH.0b013e32832da082. [DOI] [PubMed] [Google Scholar]
- 56.Kipper MJ, Wilson JH, Wannemuehler MJ, Narasimhan B. Single dose vaccine based on biodegradable polyanhydride microspheres can modulate immune response mechanism. Journal of Biomedical Materials Research Part A. 2006;76A:798–810. doi: 10.1002/jbm.a.30545. [DOI] [PubMed] [Google Scholar]
- 57.Burton DR. Antibodies, viruses and vaccines. Nature Reviews Immunology. 2002;2:706–713. doi: 10.1038/nri891. [DOI] [PubMed] [Google Scholar]
- 58.Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatric Infectious Disease Journal. 2001;20:63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Plotkin SA. Correlates of vaccine-induced immunity. Clinical Infectious Diseases. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 60.Kim S, Lalani S, Parekh VV, Wu L, Van Kaer L. Glycolipid ligands of invariant natural killer T cells as vaccine adjuvants. Expert Rev Vaccines. 2008;7:1519–1532. doi: 10.1586/14760584.7.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, Mathew B, Schmidt RR, Lunt SJ, Williams KJ, Stratford IJ, Harris AL, Cerundolo V. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114:1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh M, O'Hagan DT. Recent advances in vaccine adjuvants. Pharmaceutical Research. 2002;19:715–728. doi: 10.1023/a:1016104910582. [DOI] [PubMed] [Google Scholar]
- 63.Leadbetter E, Brigl M, Illarionov P, Cohen N, Luteran M, Pillai S, Besra G, Brenner M. NK T cells provide lipid antigen-specific cognate help for B cells. Proceedings of the National Academy of Sciences. 2008;105:8339. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banerjee K, Andjelic S, Klasse PJ, Kang Y, Sanders RW, Michael E, Durso RJ, Ketas TJ, Olson WC, Moore JP. Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo. Virology. 2009;389:108–121. doi: 10.1016/j.virol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilbert PB, Peterson ML, Follmann D, Hudgens MG, Francis DP, Gurwith M, Heyward WL, Jobes DV, Popovic V, Self SG, Sinangil F, Burke D, Berman PW. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. Journal of Infectious Diseases. 2005;191:666–677. doi: 10.1086/428405. [DOI] [PubMed] [Google Scholar]
- 67.Graham BS, McElrath MJ, Connor RI, Schwartz DH, Gorse GJ, Keefer MC, Mulligan MJ, Matthews TJ, Wolinsky SM, Montefiori DC, Vermund SH, Lambert JS, Corey L, Belshe RB, Dolin R, Wright PF, Korber BT, Wolff MC, Fast PE, Grp AVE, Grp HIP. Analysis of intercurrent human immunodeficiency virus type 1 infections in phase I and II trials of candidate AIDS vaccines. Journal of Infectious Diseases. 1998;177:310–319. doi: 10.1086/514209. [DOI] [PubMed] [Google Scholar]
- 68.Grundner C, Li YX, Louder M, Mascola J, Yang XZ, Sodroski J, Wyatt R. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology. 2005;331:33–46. doi: 10.1016/j.virol.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 69.Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. Journal of Leukocyte Biology. 2002;72:101–106. [PubMed] [Google Scholar]
- 70.Bruce MG, Ferguson A. The Influence of Intestinal Processing on the Immunogenicity and Molecular-Size of Absorbed, Circulating Ovalbumin in Mice. Immunology. 1986;59:295–300. [PMC free article] [PubMed] [Google Scholar]
- 71.Klinman DM, Barnhart KM, Conover J. CpG motifs as immune adjuvants. Vaccine. 1999;17:19–25. doi: 10.1016/s0264-410x(98)00151-0. [DOI] [PubMed] [Google Scholar]
- 72.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nature Immunology. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 73.Uchida T, Martin S, Foster TP, Wardley RC, Grimm S. Dose and Load Studies for Subcutaneous and Oral Delivery of Poly(Lactide-Co-Glycolide) Microspheres Containing Ovalbumin. Pharmaceutical Research. 1994;11:1009–1015. doi: 10.1023/a:1018987404751. [DOI] [PubMed] [Google Scholar]
- 74.Davis D, Gregoriadis G. Liposomes as Adjuvants with Immunopurified Tetanus Toxoid - Influence of Liposomal Characteristics. Immunology. 1987;61:229–234. [PMC free article] [PubMed] [Google Scholar]
- 75.Davis D, Davies A, Gregoriadis G. Liposomes as Adjuvants with Immunopurified Tetanus Toxoid - the Immune-Response. Immunology Letters. 1987;14:341–348. doi: 10.1016/0165-2478(87)90016-2. [DOI] [PubMed] [Google Scholar]
- 76.Kazzaz J, Singh M, Ugozzoli M, Chesko J, Soenawan E, O'Hagan DT. Encapsulation of the immune potentiators MPL and RC529 in PLG microparticles enhances their potency. Journal of Controlled Release. 2006;110:566–573. doi: 10.1016/j.jconrel.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 77.Chen YS, Hung YC, Lin WH, Huang GS. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology. 2010;21 doi: 10.1088/0957-4484/21/19/195101. [DOI] [PubMed] [Google Scholar]
- 78.Nixon DF, Hioe C, Chen PD, Bian ZN, Kuebler P, Li ML, Qiu H, Li XM, Singh M, Richardson J, McGee P, Zamb T, Koff W, Wang CY, OHagan D. Synthetic peptides entrapped in microparticles can elicit cytotoxic T cell activity. Vaccine. 1996;14:1523–1530. doi: 10.1016/s0264-410x(96)00099-0. [DOI] [PubMed] [Google Scholar]
- 79.Eldridge JH, Staas JK, Meulbroek JA, Tice TR, Gilley RM. Biodegradable and Biocompatible Poly(Dl-Lactide-Co-Glycolide) Microspheres as an Adjuvant for Staphylococcal Enterotoxin-B Toxoid Which Enhances the Level of Toxin-Neutralizing Antibodies. Infection and Immunity. 1991;59:2978–2986. doi: 10.1128/iai.59.9.2978-2986.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tabata Y, Inoue Y, Ikada Y. Size effect on systemic and mucosal immune responses induced by oral administration of biodegradable microspheres. Vaccine. 1996;14:1677–1685. doi: 10.1016/s0264-410x(96)00149-1. [DOI] [PubMed] [Google Scholar]
- 81.Nakaoka R, Inoue Y, Tabata Y, Ikada Y. Size effect on the antibody production induced by biodegradable microspheres containing antigen. Vaccine. 1996;14:1251–1256. doi: 10.1016/s0264-410x(96)00016-3. [DOI] [PubMed] [Google Scholar]
- 82.Kanchan V, Panda AK. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 2007;28:5344–5357. doi: 10.1016/j.biomaterials.2007.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.