Abstract
Objective
Less parental monitoring of adolescents’ diabetes self-care and more family conflict are each associated with poorer diabetes outcomes. However, little is known about how these two family factors relate with one another in the context of self-care and glycemic control. Diabetes self-care was evaluated as a mediator of the associations among parental monitoring, family conflict, and glycemic control in early adolescents with type 1 diabetes.
Methods
Adolescent-parent dyads (n=257) reported on the frequency of parental monitoring, family conflict, and diabetes self-care. Hemoglobin A1c was abstracted from medical charts. Structural equation modeling was used for mediation analysis.
Results
A mediation model linking parental involvement and family conflict with A1c through diabetes self-care fit the data well (χ2 (130) = 191.70, p = 0.00, SRMR = 0.07, RMSEA = 0.04 (CI = 0.03 – 0.06), CFI = 0.92, TLI = 0.89). Monitoring and conflict were inversely correlated (β = −0.23, p<.05) and each demonstrated indirect associations with A1c (standardized indirect effects: −0.13 and 0.07, respectively) through their direct associations with self-care (β = 0.39, p<.001 and β = −0.19, p<.05, respectively). Conflict also was positively associated with higher A1c (β = 0.31, p<.01).
Conclusions
Elevated family conflict and less parental monitoring are risk factors for poorer glycemic control, and diabetes self-care is one mediator linking these variables. Interventions to promote parental monitoring of diabetes management during early adolescence may benefit from emphasizing strategies to prevent or reduce family conflict.
Keywords: Type 1 diabetes, adolescence, family conflict, self-care, parental monitoring
Type 1 diabetes is a chronic condition that impacts two of every 1000 youth in the United States (Liese et al., 2006). The complex, demanding regimen for diabetes self-care requires frequent blood glucose (BG) monitoring and management of insulin, nutritional intake, and physical activity (Silverstein et al., 2005). Common declines in adolescents’ self-care and disease control (Helgeson, Siminerio, Escobar, & Becker, 2009; Luyckx, Seiffge-Krenke, & Hampson, 2010) elevate the risk for complications, lead to greater healthcare utilization, and result in higher medical costs (Menzin, Langley-Hawthorne, Friedman, Boulanger, & Cavanaugh, 2001; Wagner et al., 2001).
Ideally, youth with diabetes and their parents complete diabetes management jointly (Anderson, 2004; Silverstein et al., 2005), and family interactions related to diabetes can impact youths’ diabetes self-care and glycemic outcomes (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997). Parental involvement in diabetes management can take the form of parental responsibility, in which parents complete particular tasks for youth, or of parental monitoring, in which parents supervise youths’ independent completion of self-care tasks. Both forms of involvement are associated with better glycemic control via their beneficial impact on adolescents’ self-care (Anderson et al., 1997; Ellis et al., 2007). In early adolescence, increasing autonomy is typical (Beyers, Goossens, Vansant, & Moors, 2003) and parents’ expectations for teens’ diabetes self-care tend to increase while their direct participation in diabetes management often decreases (Palmer et al., 2004; Wysocki et al., 1996). During this period, parental involvement may begin to transition from responsibility to monitoring. The shift in disease management responsibility from parents to youth is one factor associated with the common deterioration in diabetes outcomes in adolescence (Holmes et al., 2006; Grey, Boland, Yu, Sullivan-Bolyai, & Tamborlane, 1998; Palmer et al., 2004), although greater parental monitoring appears to minimize this risk (Ellis et al., 2007).
Conflict between teens and their parents about diabetes is another factor associated with worsening diabetes outcomes in adolescence. Diabetes-related conflict is of significant concern given that even minimally elevated conflict can negatively impact glycemic control (Hood, Anderson, Butler, & Laffel, 2007), in part by detracting from diabetes self-care (Hilliard, Guilfoyle, Dolan, & Hood, 2011). For example, some adolescents who are distressed, angry, or frustrated about family conflict may be less inclined or less able to complete diabetes tasks. Others might limit their completion of diabetes self-care tasks to avoid arguments (e.g., related to BG values).
While previous studies have identified family conflict and lower parental monitoring as independent risk factors for suboptimal self-care and ultimately poorer glycemic outcomes (Ellis et al., 2007; Hilliard et al., 2011), it remains unclear how these two important family characteristics are related to one another in the context of diabetes management and glycemic control. There are likely reciprocal relations between them, such that the amount and nature of parental monitoring may impact family conflict, and family conflict may impact how much and in what ways parents are involved in adolescents’ daily diabetes self-care.
Greater parental responsibility for and monitoring of diabetes self-care often are encouraged to improve adolescents’ diabetes management and glycemic outcomes (Anderson et al., 1997; Ellis et al., 2007). Yet, clinically it is apparent that some aspects of parent involvement in adolescents’ diabetes self-care, including parental monitoring, may inadvertently foster conflict. For example, frequent reminders or questions about diabetes self-care might irritate teens and lead to or exacerbate conflict. Among youth without diabetes, those with more family conflict want less parental supervision (Laird, Pettit, Dodge, & Bates, 2003) and this preference likely is mirrored in teens with diabetes, who often want less frequent or less intrusive parental involvement in their diabetes self-care (Hanna & Guthrie, 2001; Weinger, O’Donnell, & Ritholz, 2001).
Conflict also may detract from diabetes management and glycemic control through its impact on the amount and ways that parents participate in their adolescents’ self-care (Weinger et al., 2001). For example, in order to avoid conflict some parents might decrease their monitoring or oversight of diabetes management (Palmer et al., 2004). Parents who argue frequently with their teens about diabetes may refrain from asking questions or be hesitant to offer assistance with diabetes tasks. Other parents may increase or become more persistent in their monitoring to ensure their teens follow directions. Additionally, adolescents themselves may avoid discussions about diabetes with their parents as a means to avoid conflict. Any of these scenarios could detract from self-care and negatively impact glycemic control.
Given the developmentally normative changes in parent-youth relationships at the entry to adolescence and their potential impact on diabetes self-care and glycemic control, a better understanding of how family conflict and parental monitoring relate to each other and to diabetes self-care and glycemic outcomes is valuable. Thus, this paper aims to examine the associations among family conflict, parental monitoring, diabetes self-care, and glycemic control. More family conflict and less parental monitoring were hypothesized to be correlated and to each have a direct association with poorer glycemic control. Given previous evidence of self-care as a mediator of the separate relations of parental monitoring (Ellis et al., 2007) and family conflict (Hilliard et al., 2011) with glycemic control, poorer self-care was hypothesized to mediate these associations. Confirmation of this model would indicate that interventions to improve adolescents’ glycemic control should address multiple aspects of the parent-adolescent relationship related to diabetes and directly address teens’ diabetes self-care behaviors.
METHODS
Participants
Early adolescents (11 to 14 years old) and their parents (one per dyad) enrolled in a randomized clinical trial (RCT) at two mid-Atlantic children’s hospitals. Participation in the RCT entailed completion of four brief sessions of behavioral intervention or diabetes information in conjunction with quarterly diabetes clinic visits over the course of 1–1½ years. The data for this study were drawn from the baseline assessments.
Eligibility requirements for the RCT included diabetes duration of at least 1 year, absence of severe complications or other medical diagnoses, and English fluency. The sample consisted of 257 dyads composed of one adolescent with type 1 diabetes (49% female) and one parent (91% mothers). Mean age at baseline was 12.8 years (SD = 1.2), mean illness duration was 5.1 years (SD = 3.1), and mean A1c was 8.8% (SD = 1.6). Two-thirds (64%) of the sample were prescribed an intensive insulin basal/bolus [BB] regimen (i.e., received 4 or more daily insulin injections or continuous subcutaneous insulin infusion [CSII; insulin pump]). Participant characteristics are summarized in Table 1.
Table 1.
Participant characteristics
| % or M±SD | Adolescent- report |
Parent- report |
|
|---|---|---|---|
| Youth Age (years) | 12.8 ±1.2 | ||
| Youth Gender, % female | 49.4 | ||
| Family Structure, % unmarried | 23.3 | ||
| Youth Ethnicity, % non-white | 30.4 | ||
| Hollingshead SES, % in 2 highest social classes | 53.6 | ||
| Diabetes Duration (years) | 5.1 ± 3.1 | ||
| Insulin regimen: CSII (%) | 44.0 | ||
| BB injections (%) | 20.2 | ||
| Conventional 2–3 injections (%) | 35.0 | ||
| Diabetes Management (DBRS) | 0.64 ± 0.13 | 0.67 ± 0.11 | |
| Average frequency of BG checks/day (DI) | 4.3 ± 1.5 | 4.3 ± 1.5 | |
| Parent Monitoring (PMDC) | 77.1 ± 8.5 | 78.0 ± 7.9 | |
| % of BG checks observed/discussed/day (DI) | 57.7 ± 21.8 | 60.0 ± 23.6 | |
| Diabetes-Related Family Conflict – Revised (DFCS-R) |
28.2 ± 11.2 | 25.9 ± 7.0 | |
| General Family Conflict (FES) | 56.2 ± 23.6 | 46.2 ± 11.7 | |
| A1c (%) | 8.8±1.6 |
Procedure
Appropriate institutional review boards from each hospital approved the RCT from which the present data were drawn. Eligible families were identified based on clinic lists, were mailed an informational letter, and received a follow-up telephone call from trained research assistants. At a regularly scheduled diabetes clinic visit, each participant and his/her parent provided consent and assent, completed self-report questionnaires, and participated in individual diabetes interviews. A second diabetes interview was completed over the telephone within two weeks of in-person data collection. Completion of questionnaires took approximately 45 minutes and each interview was approximately 15 minutes. Families received a $25 gift card in appreciation of their time. Of the 404 eligible families successfully contacted, 285 consented to participate (71%). Those who declined consent primarily cited lack of interest or time as the reason. Completed baseline data reported here were provided by 257 parent-adolescent dyads (89%).
Measures
Diabetes self-care
The completion of diabetes self-care tasks was assessed in two ways. First, parents and adolescents individually completed the 24-hour diabetes interviews (DI; Holmes et al., 2006, adapted from Johnson, Silverstein, Rosenbloom, Carter, & Cunningham, 1986). The DI is a diary-like interview in which parents and adolescents separately describe the completion of diabetes self-care tasks, such as blood glucose (BG) monitoring and insulin administration, over the previous 24 hours. Parents and adolescents completed the DI on two occasions over two weeks. Each reporter’s responses were averaged together across the two DIs to create a youth-report variable and a parent-report variable for self-care. The frequency of BG checks reported over two days by parent and adolescent were tallied separately. For the current study, frequency of BG monitoring was selected as a proxy measure of diabetes self-care because prescribed insulin doses can vary across patients and different insulin regimens, while recommendations for BG monitoring frequency are more consistent. The frequency of BG monitoring has been used previously as a proxy for diabetes self-management and is linked with glycemic control (Hilliard et al., 2011). The 24-hour methodology is a reliable, valid, “well-established” measure of diabetes self-care behavior (Freund, Johnson, Silverstein, & Thomas, 1991; Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008).
Parents and adolescents also completed the Diabetes Behavior Rating Scale (DBRS; Iannotti, 2006). The DBRS asks respondents to rate the frequency with which routine diabetes care behaviors (e.g., BG monitoring, insulin administration and adjustments) occurred over the previous week on a 5-point Likert scale ranging from ‘never’ to ‘always.’ Two forms exist, one for adolescents who are on a regimen of multiple daily insulin injections (36 items) and the other for adolescents who are on an insulin pump (37 items). Scores are represented as a proportion of tasks completed (scored between 0 and 1); higher scores indicate more frequent engagement in self-care activities. The DBRS has good internal consistency, test-retest reliability, and content validity (Iannotti, 2006). The internal reliability estimates in this sample were adequate (injection regimen form: α = 0.79, parent and teen; insulin pump regimen form: teen α = 0.82; parent α = 0.76).
Parental monitoring
Parental monitoring of adolescents’ diabetes self-care was assessed in two ways. First, the DI (Holmes et al., 2006; described above) was modified to assess the percentage of BG checks that parents observed or discussed with their teens over the previous 24 hours. A percentage was calculated that represents the proportion of completed BG checks that were monitored by a parent. Percentages, rather than frequencies, were used to correct for individual differences in the total number of checks completed.
Parents and adolescents also completed the Parental Monitoring of Diabetes Care scale (PMDC; Ellis et al., 2007; Ellis et al., 2008). Respondents rated the frequency with which parents typically monitor 18 specific tasks of diabetes management on a 5-point Likert scale ranging from ‘less than once a week’ to ‘more than once a day.’ The total score can range from 18 to 90; higher scores indicate more frequent parental monitoring. The PMDC has good psychometric properties for adolescent- and parent-completed forms (Ellis et al., 2007; Ellis et al., 2008). Estimates of the internal reliability of scores from this sample were adequate (adolescent and parent α = 0.75).
Family conflict
Diabetes-specific parent-teen conflict was measured using the Diabetes Family Conflict Scale - Revised (DFCS-R; Hood et al., 2007). Parents and adolescents independently rated the frequency of conflict about 19 specific diabetes management tasks on a 5-point Likert scale ranging from ‘never’ to ‘almost always.’ The 2007 version of the DFCS-R uses a 3-point Likert scale; however, to facilitate comparison with earlier findings, the current study used a 5-point scale. The total score for the DFCS-R can range from 19 to 95; higher scores indicate greater diabetes-related conflict. The DFCS-R has strong psychometric properties (Hood et al., 2007). The internal reliability of scores in this sample was excellent (teen α = 0.96; parent α = 0.89).
General family conflict was measured using the Conflict subscale of the Family Environment Scale (FES; Moos & Moos, 2002). Parents and adolescents completed the 9-item measure about the occurrence of conflict behaviors (e.g., temper outbursts, anger at home). Conflict scores use a true/false format and can range from 0 to 9; higher scores indicate more general conflict. The FES Conflict scale historically demonstrates modest yet sufficient reliability in adolescent- and parent-completed forms (Boyd, Gullone, Needleman, & Burt, 1997; Loveland-Cherry, Youngblut, & Leidy, 1989; Moos & Moos, 2002). The internal reliability of scores in this sample was adequate (teen α = 0.74; parent α = 0.76).
Medical information
Medical data included date of diagnosis and prescribed insulin regimen and were obtained through reviews of medical charts. Prescribed insulin regimens were categorized as conventional injections (2–3 fixed injections per day, scored as ‘1’), basal/bolus injections [BB] (combination of 4 or more insulin injections per day, scored as ‘2’), or continuous subcutaneous insulin infusion [CSII], known as an insulin pump (a medical device worn on the body, scored as ‘3’). To measure glycemic control, hemoglobin A1c (A1c) was analyzed via blood assay (DCA 2000, Bayer Inc.; Tarrytown, NY, USA) during a diabetes clinic visit. Hemoglobin A1c values were abstracted from medical records.
Demographic information
Parents completed a background information questionnaire developed by the research team that included information about youth age, gender (1 = male, 2 = female), and ethnicity (1 = Caucasian, 2 = non-Caucasian) and caregiver marital status (1 = married, 2 = unmarried). Socio-economic status (SES) was calculated with the Hollingshead Four Factor Index (Hollingshead, 1975). Parental education level and occupation were categorized on a scale from 1–5; a score of 1 indicated the highest social class and 5 indicated the lowest social class.
Data Analytic Plan
Descriptive analyses were conducted with SPSS Version 19 (SPSS, Inc., 2010). Structural equation modeling (SEM) analyses were conducted with MPlus Version 6 (Muthén & Muthén, 2008–2010). Full information maximum likelihood procedure was used to include all participants; those with missing data were presumed to have data missing at random. A two-stage modeling approach was used (Kline, 2005).
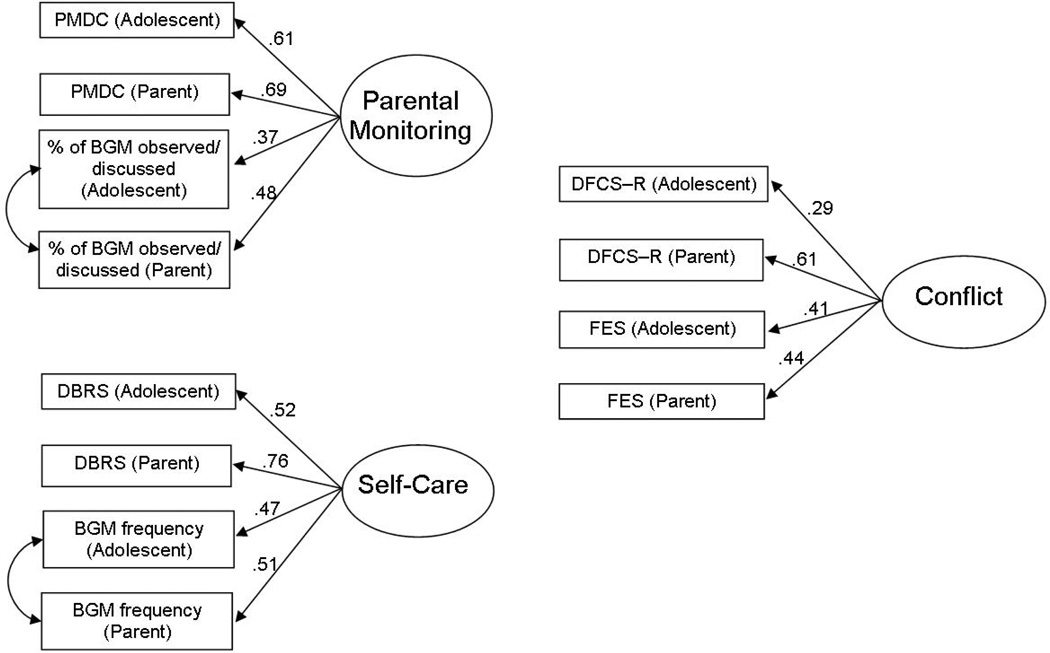
First, the fit and indicator factor loadings of each latent variable were examined (Figure 1). Parent- and adolescent-reported scores on each measure were used as indicators of the latent variables. Inclusion of ratings from multiple reporters allowed for the analysis of family-level data, accounted for within-family non-independence, reduced rater bias, and more accurately measured each construct (Kenny, 1995). Since each latent variable was comprised of indicators from within one measure, shared variance between subscales was expected and covariances for errors were added according to MPlus modification indices (Kline, 2005; MacCallum & Austin, 2002).
Figure 1.
Latent variable measurement models.
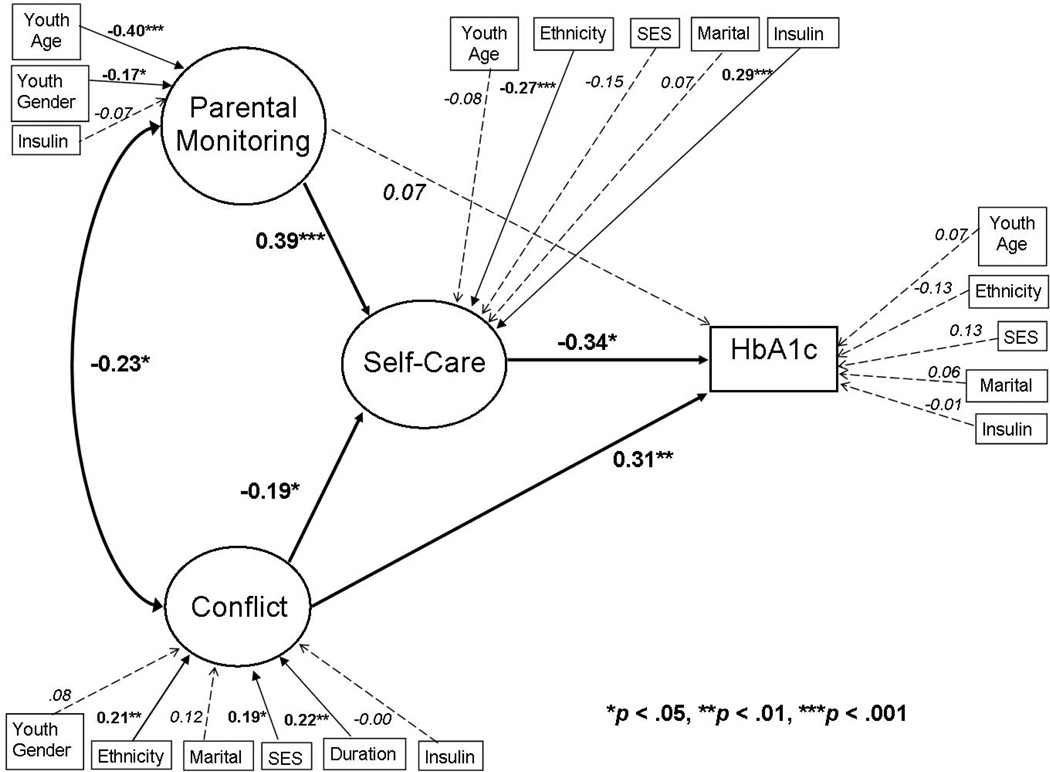
Second, the hypothesized mediation model was evaluated through examination of model fit and standardized path loadings (Kline, 2005; MacCallum & Austin, 2002) (Figure 2). To account for the contextual factors that are theorized to impact the constructs of interest, correlated medical and demographic variables were included in the model. In structural equation modeling, mediation is tested through an analysis of direct and indirect effects, or path coefficients, among the latent and observed variables in the model (MacKinnon, 2008). The mediated, or indirect, effect is calculated in MPlus as the product of the direct effects (standardized coefficients) among the independent, mediating, and dependent variables (Kline, 2005).
Figure 2.
Standardized path coefficients.
Note: Demographic and medical variables with significant bivariate correlations were included.
Empirically established indices of model fit were used in the SEM analysis (Hu & Bentler, 1998; Hu & Bentler, 1999). Favorable fit indices include: a Chi-square (χ 2) value closer to zero with a p value greater than 0.05, a Root Mean Square Error of Approximation (RMSEA) index less than 0.06, a Standardized Root Mean Square Residual (SRMR) index less than 0.10, a Tucker Lewis Index (TLI) value greater than 0.90, and a Comparative Fit Index (CFI) value greater than 0.90. Standardized path coefficients were examined. Effect sizes were described according to Cohen (1988): standardized betas of 0.2 represent a small effect size, 0.5 a medium effect size, and 0.8 a large effect size.
RESULTS
Bivariate Correlations
Bivariate correlations between observed measures, including demographic and medical covariates, were examined (see Table 2). First, correlations between parent- and adolescent-reports on the measures within each construct were examined. Significant correlations were found for the measures of parental monitoring (r = 0.41 for PMDC and r = 0.35 for percentage of BG checks monitored), diabetes-related family conflict (r = 0.19 for DFCS and r = 0.22 for FES), and diabetes self-care (r = 0.39 for DBRS and r = 0.79 for BG check frequency).
Table 2.
Correlation matrix.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Age | |||||||||||||||||||
| 2 Gend | −.03 | ||||||||||||||||||
| 3 Dur | .08 | −.07 | |||||||||||||||||
| 4 Ethn | .00 | .03 | .00 | ||||||||||||||||
| 5 Marit | .01 | −.02 | −.03 | .27 | |||||||||||||||
| 6 SES | −.06 | −.18 | −.01 | .34 | .42 | ||||||||||||||
| 7 Insulin | .04 | −.09 | −.27 | .32 | .25 | .32 | |||||||||||||
| 8 PM(A) | −.13 | −.08 | .04 | −.14 | −.06 | −.10 | −.16 | ||||||||||||
| 9 PM(P) | −.29 | −.11 | −.08 | −.09 | −.08 | −.01 | −.05 | .41 | |||||||||||
| 10%BG(A) | −.21 | −.05 | −.05 | −.09 | −.04 | .02 | −.08 | .19 | .26 | ||||||||||
| 11 %BG(P) | −.27 | −.10 | .04 | .11 | .04 | .09 | .12 | .30 | .33 | .35 | |||||||||
| 12 DF(A) | −.09 | −.05 | .09 | .12 | .07 | .23 | .06 | .01 | .04 | .05 | .16 | ||||||||
| 13 DF(P) | .10 | .04 | .18 | .27 | .22 | .24 | .09 | −.19 | −.20 | −.11 | −.01 | .19 | |||||||
| 14 FES(A) | .08 | −.25 | .18 | −.15 | .14 | .14 | .01 | −.30 | −.16 | −.10 | −.11 | .12 | .20 | ||||||
| 15 FES(P) | −.02 | .04 | −.09 | −.02 | .09 | .05 | .14 | −.14 | −.16 | −.05 | .08 | .10 | .26 | .22 | |||||
| 16 DB(A) | −.09 | .03 | −.03 | −.25 | −.12 | −.22 | −.26 | .27 | .13 | .21 | .06 | −.11 | −.22 | −.02 | .00 | ||||
| 17 DB(P) | −.14 | .03 | −.04 | −.39 | −.11 | −.20 | −.24 | .23 | .27 | .13 | .03 | −.14 | −.27 | −.15 | −.09 | .39 | |||
| 18 BGf(A) | −.19 | .05 | .10 | −.17 | −.13 | −.22 | −.32 | .30 | .12 | .18 | .02 | −.07 | −.14 | .05 | −.09 | .26 | .35 | ||
| 19 BGf(P) | −.27 | −.07 | .05 | −.18 | −.16 | −.20 | −.35 | .32 | .22 | .19 | .09 | −.03 | −.20 | .04 | −.14 | .27 | .38 | .79 | |
| 20 A1c | .13 | −.01 | .05 | .21 | .22 | .31 | .19 | −.21 | −.20 | −.00 | .13 | .17 | .38 | .04 | .16 | −17 | −.30 | −.29 | −.33 |
Note: p< .05, p< .01. A = adolescent-report, P = parent-report. PM = Parent Monitoring of Diabetes Care Scale. %BG = percent of blood glucose monitoring events observed/discussed by parent from 24-hour diabetes interview. DF = Diabetes Family Conflict Scale - Revised. FES = Family Environment Scale. DB = Diabetes Behavior Rating Scale. BGMf = frequency of blood glucose monitoring from 24-hour diabetes interview. A1c = hemoglobin A1c, glycemic control. Gend = gender. Dur = duration of diabetes diagnosis. Ethn = ethnicity. Marit = caregiver marital status. SES = Socio-economic status, 1=highest social class and 5=lowest class.
Next, bivariate correlations of the individual psychosocial measures were examined. Diabetes-related conflict scores were negatively correlated with diabetes self-care ratings (six significant rs ranged from −0.27 to −0.14) and parental monitoring (four significant rs ranged from −0.30 to −0.16), and were positively correlated with A1c (three significant rs ranged from 0.16 to 0.38). Parental monitoring and diabetes self-care scales were generally positively correlated with each other (eleven significant rs ranged from 0.13 to 0.32) and negatively correlated with A1c (rs = −0.20 and −0.21, for adolescent- and parent-rated PMDC, respectively).
Finally, correlations with demographic and medical variables were examined. Diabetes-related conflict scores were positively correlated with diabetes duration (r = 0.18) and SES category (rs = 0.23 to 0.24), indicating more conflict under lower socio-economic condition. Higher diabetes-related conflict was associated with non-Caucasian ethnicity (r = 0.27) and unmarried caregivers (r = 0.22), while higher general conflict was correlated with male gender (r = −0.25) and less intensive insulin regimens (r = 0.14). Parental monitoring was inversely correlated with age (r = −0.21 to −0.29), and greater monitoring was associated with a more intensive insulin regimen (r = −0.16). Diabetes self-care was negatively correlated with age (r = −0.14 to −0.27) and SES category (r = −0.20 to −0.22), indicating poorer self-care under lower socio-economic conditions. Poorer self-care was associated with unmarried caregivers (r = −0.13 to −0.16), non-Caucasian ethnicity (r = −0.17 to −0.39), and less intensive insulin regimens (r = −0.24 to −0.35), Glycemic control, or A1c, was positively correlated with age (r = 0.13) and SES category (r = 0.31), indicating higher A1c under lower socio-economic conditions. Higher A1c was associated with non-Caucasian ethnicity (r = 0.21), unmarried caregivers (r = 0.22), and less intensive insulin regimens (r = 0.19).
Latent Variable Measurement
Three measurement models for latent variables were individually constructed and tested (Figure 1) for use in the structural equation model. The latent variable that represents parental monitoring used four indicators: parent- and adolescent-reported frequency of parental monitoring behavior on the PMDC and parent- and adolescent-reported percentage of BG checks that parents observed and/or discussed with their adolescents from the DI. With the inclusion of one covariance for errors, this model had a good fit [χ 2 (1) = 0.01, p = .92, SRMR = .00, RMSEA = .00 (CI = .00 - .06), CFI = 1.00, TLI = 1.06]. The latent variable for diabetes self-care was constructed with four indicators: parent- and adolescent-report of general diabetes self-care from the DBRS and parent- and adolescent-report of blood glucose monitoring frequency from the DI. With the inclusion of one covariance for errors, this model also had a good fit [χ 2 (1) = 0.35, p = .56, SRMR = .00, RMSEA = .00 (CI = .00 - .14), CFI = 1.00, TLI = 1.01]. The latent variable for family conflict was constructed from four indicators: parent- and adolescent-report of diabetes-specific conflict from the DFCS-R and parent- and adolescent-report of general family conflict from the FES. This model also had a good fit [χ 2 (2) = 0.95, p = .62, SRMR = .02, RMSEA = .00 (CI = .00 - .10), CFI = 1.00, TLI = 1.11]. For all three latent variables, all indicators had significant factor loadings (p<.05; see Figure 1).
Mediation Model
Next, a structural equation model of diabetes self-care as a mediator of the associations among parental monitoring, family conflict, and A1c was examined (Figure 2). Demographic and medical variables with significant bivariate correlations were included as independent variables related to each latent variable and as covariates of the dependent variable, A1c. Per Mplus modification indices, gender also was included as an independent variable related to the parental monitoring latent variable. This model demonstrated a good fit [χ 2 (130) = 191.70, p = 0.00, SRMR = 0.07, RMSEA = 0.04 (CI = 0.03 – 0.06), CFI = 0.92, TLI = 0.89]. The standardized path coefficients for this model supported the mediation hypothesis and are displayed in Figure 2. Parental monitoring had an inverse association with family conflict (β = −0.23, p<.05). Parental monitoring was associated with diabetes self-care (β = 0.39, p<.001) with a small-medium effect size, and family conflict was inversely associated with self-care (β = −0.19, p<.05) with a small effect size. Diabetes self-care was inversely associated with A1c (β = −0.34, p<.05) with a small effect size. The total standardized indirect effect of parental monitoring through self-care on A1c was −0.13, and the total indirect effect of family conflict through self-care on A1c was 0.07. In addition to the indirect effects on A1c through self-care, family conflict also had a direct association with glycemic control (β = 0.31, p<.01) with a small effect size.
Demographic and medical variables also were significantly associated with the primary constructs of interest (Figure 2). Parental monitoring was negatively associated with adolescent age (β =−0.40, p<.001) and less monitoring was associated with female gender (β =−0.17, p<.05). Family conflict was positively associated with diabetes duration (β = 0.22, p<.01) and higher SES category (β=0.19, p<.05), indicating more conflict under lower socio-economic conditions. More conflict was associated with non-Caucasian ethnicity (β = 0.21, p<.01). Poorer self-care was linked with non-Caucasian ethnicity (β = −0.27, p<.001) and less intensive insulin regimens (β = −0.29, p<.001). There were no significant covariates of A1c.
DISCUSSION
Parental monitoring was inversely related to family conflict, and diabetes self-care mediated the associations of each of these key family constructs with glycemic control in early adolescents with type 1 diabetes. Family conflict also demonstrated a direct association with poorer glycemic control. While each of these links is consistent with previous research (Anderson et al., 1997; Ellis et al., 2007; Hilliard et al., 2011), this study is the first to examine how these two important family factors simultaneously relate to one another and to diabetes management and glycemic control. This constellation of associations among family conflict, parental monitoring, diabetes self-care, and glycemic control suggests that examinations of either family factor in isolation may be too narrow to fully understand the multidimensional relations that exist among family interactions and adolescents’ diabetes outcomes.
Simultaneous examination of parental monitoring and conflict revealed an inverse relation between these variables with sizable common variance (β = −0.23). In this early adolescent age range, elevations in conflict do not appear to co-occur with more parental monitoring. Prevention and intervention programs may be able to capitalize on the relatively low levels of conflict accompanying parental monitoring in early adolescence by encouraging parental monitoring without necessarily increasing the risk for conflict. Promotion of parental monitoring in early adolescence has the potential to set the stage for ongoing parental monitoring later, with the goal of preventing both conflict and deterioration in diabetes outcomes in older adolescence.
The model also revealed unique indirect relations of parental monitoring and family conflict with glycemic control through diabetes self-care. The standardized effect size of the direct association between parental monitoring and self-care is small (β = −0.39) yet roughly double that of family conflict (β = −0.19). Thus, the beneficial role of parental monitoring may outweigh the risks associated with conflict in direct relation to diabetes self-care. In addition to associations with self-care, these data model and measure the associations of each latent construct with glycemic control. Family conflict demonstrated both direct (β = 0.31) and indirect links with glycemic control through self-care (total indirect effect = 0.07). Parental monitoring demonstrated an indirect association with glycemic control through self-care (total indirect effect = 0.13), yet there was no evidence of a significant direct relation. While parental monitoring displays a stronger direct link with diabetes self-care than does family conflict, family conflict displays a relatively stronger direct association with glycemic control. The distinct associations of parental monitoring and family conflict with diabetes self-care and glycemic control indicate that both factors have unique yet interrelated roles in the context of diabetes outcomes.
The relations among family factors, diabetes self-care, and glycemic control reported in this study were evident even at relatively low levels of conflict and relatively high levels of parental monitoring, with scores at the lower (diabetes-related family conflict; range: 19–95) and upper (parental monitoring; range: 18–90) ends of the measures’ possible ranges, similar to other samples (Ellis et al., 2007; Hood et al., 2007). The effect sizes in this study were modest and the associations may be even more pronounced in samples that report higher levels of conflict, less frequent parental monitoring, or with youth in poorer glycemic control.
In addition to parental monitoring and diabetes self-care, other factors beyond these primary constructs may mediate or impact the direct relation between family conflict and glycemic control. Although unmeasured in this study, previous research suggests that youth without diabetes exposed to family conflict report more emotional difficulties, such as stress and depression, and exhibit physiologic signs of stress (Repetti, Taylor, & Seeman, 2002), all of which are risk factors with implications for glycemic control (Golden, 2007; Grey, Whittemore, & Tamborlane, 2002; Lloyd, Smith, & Weinger, 2005). For some families, conflict also may occur in response to higher A1c values due to assumptions about mismanagement (Weinger et al., 2001) or as the result of behavior problems related to chronically higher BG levels (Holmes et al., 2006). Longitudinal analyses are needed to investigate potential causal or bidirectional pathways among family conflict, mediating processes, and diabetes outcomes.
Demographic and medical variables also should be considered in the context of study constructs. Parental monitoring was inversely associated with youth age, consistent with previous literature (Holmes et al., 2006; Palmer et al., 2004), and was linked with gender, which has not been reported previously. Multiple factors likely influence the degree to which parents maintain their level of monitoring as their children progress through adolescence. Older teens typically require less assistance with diabetes self-care tasks and they and their parents may perceive less need for oversight. Older adolescents also may complete more of their diabetes self-care away from home, which poses a different challenge for parental monitoring (Hanna & Decker, 2010; Palmer et al., 2004; Palmer et al., 2010). Prior literature demonstrates fewer behavior problems and better diabetes self-care in girls than boys (Naar-King et al., 2006) which may contribute to this study’s finding of lower parental monitoring of girls, since parents of adolescent girls may perceive less need for close monitoring.
Lower SES and non-Caucasian ethnicity were associated with higher conflict, and non-Caucasian ethnicity was associated with poorer self-care. These demographic variables often co-occur and may represent a combination of limited access to resources and other barriers to diabetes self-care (Swift, Chen, Hershberger, & Holmes, 2006), worthy of examination yet beyond the scope of this investigation. In some situations, intensive programs that target multiple aspects of the family system may be necessary to efficaciously impact the various factors related to diabetes self-care and glycemic control (Ellis et al., 2005; Wysocki et al., 2008).
Finally, youth with more intensive insulin regimens engaged in more frequent BG checks and reported better overall self-care. This favorable association may reflect clinic practices to transition youth with better diabetes self-care to more intensive basal bolus insulin injections or pump regimens. Although not measured in this study, better self-care among youth on the insulin pump also may be attributable in part to reduced barriers to self-care; for example, youth on the insulin pump report perceptions of greater convenience and flexibility (Ritholz et al., 2007) despite the visibility of the device and diligence needed to operate it.
In addition to the conceptual contributions of this study, the methodological strengths also represent an expansion of previous research. The use of latent variables and structural equation modeling accounted for the simultaneous interrelations among key family constructs and diabetes outcomes. Construct measurement was strengthened and bias was reduced by the creation of latent variables and use of data from multiple assessment methods (biological, self-report, and diary-like interviews) and sources (parent and adolescent dyads). Both parent and adolescent reports were included as separate indicators of the constructs in the measurement model to account for measurement error. Latent variable modeling estimated and compensated for measurement error, improved construct estimation, and accounted for non-independence between reporters (Kenny, 1995). The study was also strengthened by a relatively large sample with greater diversity than is often reported in research samples in type 1 diabetes, including nearly one-third non-Caucasian participants, across two sites spanning urban, suburban and rural areas.
Study limitations included the use of cross-sectional data, which precludes making causal inferences about the relations among the constructs. Further, because participants agreed to take part in a larger intervention trial, unknown characteristics may differentiate them from other families and could limit generalizability. Finally, mothers were the primary parent reporters, despite growing evidence for the role of fathers in monitoring adolescents’ diabetes management (Palmer et al., 2010).
Nevertheless, a combination of higher family conflict and lower parental monitoring are related to less frequent or less effective diabetes self-care and poorer glycemic control in early adolescence. These data have the potential to build upon earlier successful behavioral interventions and recommendations for optimizing how parents participate in adolescents’ diabetes self-care (e.g., Anderson, 2004; Ellis et al., 2005; Grey et al., 1998; Silverstein et al., 2005; Wysocki et al., 2008) by emphasizing both the quality of family interactions and importance of parental monitoring. In addition to encouraging parental monitoring of diabetes self-care within the family at the start of adolescence, diabetes clinicians may benefit from assessment of family conflict that may be linked with suboptimal diabetes self-care and poorer glycemic control.
These findings can inform the development, refinement, and delivery of adherence-promotion interventions that address relevant family dimensions. Interventions that take a multidimensional approach to increase parental monitoring, that emphasize efforts to reduce conflict such as via coping skills training, and that specifically target diabetes self-care have the potential to be particularly beneficial (Anderson, Brackett, Ho, & Laffel, 1999; Ellis et al., 2005; Grey, Boland, Davidson, & Tamborlane, 2000; Wysocki et al., 2008). To expand upon these well-supported family-focused interventions, the current study suggests that both family conflict and parental monitoring are crucial elements of adolescent diabetes self-care and that enhancement of both aspects of family relations has the potential to significantly improve adolescents’ diabetes self-care and may ultimately impact glycemic outcomes.
Future research could benefit from longitudinal analyses, which can examine the ways in which relations among family factors, diabetes self-care, and glycemic control evolve from early adolescence into later adolescence and then into early adulthood. Longitudinal research would allow for evaluation of bidirectional associations to determine whether and to what degree family relations influence diabetes outcomes and vice versa. In order to promote optimal diabetes outcomes during this challenging developmental period, interventions that build upon the earlier works of Anderson et al. (1999), Ellis et al. (2005), Grey et al. (2000), and Wysocki et al. (2008) are clearly needed. Specifically, further clinical research could determine the most effective intervention strategies and most salient treatment components to promote parental monitoring without increasing or creating family conflict. The overarching goal of such interventions should be to slow or reverse the trajectory of deteriorations in diabetes self-care and glycemic control commonly found in adolescence (Helgeson et al., 2009; Luyckx et al., 2010) by enhancing relevant aspects of family relationships at a critical point in development.
Acknowledgments
This work was supported by NIH/NIDDK 5R01DK070917-04 awarded to CH.
REFERENCES
- Anderson BJ. Family conflict and diabetes management in youth: Clinical lessons from child development and diabetes research. Diabetes Spectrum. 2004;17:22–26. [Google Scholar]
- Anderson BJ, Brackett J, Ho J, Laffel LMB. An office-based intervention to maintain parent-adolescent teamwork in diabetes management: Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22:713–721. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Beyers W, Goossens L, Vansant I, Moors E. A structural model of autonomy in middle and late adolescence: Connectedness, separation, detachment, and agency. Journal of Youth and Adolescence. 2003;32:351–365. [Google Scholar]
- Boyd CP, Gullone E, Needleman GL, Burt T. The family environment scale: Reliability and normative data for an adolescent sample. Family Process. 1997;36:369–373. doi: 10.1111/j.1545-5300.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power for the behavioral sciences. 2nd Edition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control. Diabetes Care. 2005;28:1604–1610. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Podolski C, Frey M, Naar-King S, Wang B, Molktz K. The role of parental monitoring on adolescent health outcomes: Impact on regimen adherence in youth with type 1 diabetes. Journal of Pediatric Psychology. 2007;32:907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Templin TN, Podolski CL, Frey MA, Naar-King S, Moltz K. The parental monitoring of diabetes care scale: Development, reliability and validity of a scale to evaluate parental supervision of adolescent illness management. Journal of Adolescent Health. 2008;42:146–153. doi: 10.1016/j.jadohealth.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Freund A, Johnson SB, Silverman J, Thomas J. Assessing daily management of childhood diabetes using 24-hour recall interviews: Reliability and stability. Health Psychology. 1991;10:200–208. doi: 10.1037//0278-6133.10.3.200. [DOI] [PubMed] [Google Scholar]
- Golden SH. A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Current Diabetes Reviews. 2007;3:252–259. doi: 10.2174/157339907782330021. [DOI] [PubMed] [Google Scholar]
- Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes has long-lasting effects on metabolic control and quality of life. Journal of Pediatrics. 2000;137:107–113. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- Grey M, Boland EA, Yu C, Sullivan-Bolyai S, Tamborlane WV. Personal and family factors associated with quality of life in adolescents with diabetes. Diabetes Care. 1998;21:909–914. doi: 10.2337/diacare.21.6.909. [DOI] [PubMed] [Google Scholar]
- Grey M, Whittemore R, Tamborlane W. Depression in type 1 diabetes in children: Natural history and correlates. Journal of Psychosomatic Research. 2002;53:907–911. doi: 10.1016/s0022-3999(02)00312-4. [DOI] [PubMed] [Google Scholar]
- Hanna KM, Decker CL. A concept analysis: Assuming responsibility for self-care among adolescents with type 1 diabetes. Journal for Specialists in Pediatric Nursing. 2010;15:99–110. doi: 10.1111/j.1744-6155.2009.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna KM, Guthrie D. Parents’ and adolescents’ perceptions of helpful and nonhelpful support for adolescents’ assumption of diabetes management responsibility. Issues in Comprehensive Pediatric Nursing. 2001;24:209–223. doi: 10.1080/014608601753260317. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A 4-year longitudinal study. Journal of Pediatric Psychology. 2009;34:254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard ME, Guilfoyle SM, Dolan LM, Hood KK. Prediction of adolescents’ glycemic control 1 year after diabetes-specific family conflict. Archives of Pediatrics and Adolescent Medicine. 2011;165:624–629. doi: 10.1001/archpediatrics.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Unpublished manuscript. Yale University; New Haven, CT: 1975. [Google Scholar]
- Holmes CS, Chen R, Streisand R, Marschall DE, Souter S, Swift EE, …Peterson CC. Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. Journal of Pediatric Psychology. 2006;31:770–784. doi: 10.1093/jpepsy/jsj083. [DOI] [PubMed] [Google Scholar]
- Hood KK, Anderson BJ, Butler DA, Laffel LMB. Updated and revised diabetes family conflict scale. Diabetes Care. 2007;30:1764–1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler P. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Iannotti R. Assessing regimen adherence of adolescents with type 1 diabetes. Diabetes Care. 2006;29:2263–2267. doi: 10.2337/dc06-0685. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Silverstein J, Rosenbloom A, Carter R, Cunningham W. Assessing daily management in childhood diabetes. Health Psychology. 1986;5:545–564. doi: 10.1037/0278-6133.5.6.545. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2nd edition. New York, NY: Guilford Press; 2005. [Google Scholar]
- Kenny DA. The effect of nonindependence on significance testing in dyadic research. Personal Relationships. 1995;2:67–75. [Google Scholar]
- Laird RD, Pettit GS, Dodge KA, Bates JE. Change in parents’ monitoring knowledge: Links with parenting, relationship quality, adolescent beliefs, and antisocial behavior. Social Development. 2003;12:401–419. doi: 10.1111/1467-9507.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese AD, D’Agostino RB, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, Williams DE. The burden of diabetes mellitus among US youth: Prevalence estimates from the SEARCH for diabetes in youth study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- Lloyd C, Smith J, Weinger K. Stress and diabetes: A review of the links. Diabetes Spectrum. 2005;18:121–127. [Google Scholar]
- Loveland-Cherry CJ, Youngblut JM, Leidy NWK. A psychometric analysis of the Family Environment Scale. Nursing Research. 1989;38:262–266. [PubMed] [Google Scholar]
- Luyckx K, Seiffge-Krenke I, Hampson SE. Glycemic control, coping, and internalizing and externalizing symptoms in adolescents with type 1 diabetes: A cross-lagged longitudinal approach. Diabetes Care. 2010;33:1424–1429. doi: 10.2337/dc09-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Annual Review of Psychology. 2000;51:201–226. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- Menzin J, Langley-Hawthorne C, Friedman M, Boulanger L, Cavanaugh R. Potential short-term economic benefits of improved glycemic control. Diabetes Care. 2001;24:51–55. doi: 10.2337/diacare.24.1.51. [DOI] [PubMed] [Google Scholar]
- Moos, Moos . Family Environment Scale Manual. Palo Alto, CA: Mindgarden, Inc; 2002. [Google Scholar]
- Naar-King S, Idalski A, Ellis D, Frey M, Templin T, Cunningham PB, Cakan N. Gender differences in adherence and metabolic control in urban youth with poorly controlled type 1 diabetes: The mediating role of mental health symptoms. Journal of Pediatric Psychology. 2006;31:793–802. doi: 10.1093/jpepsy/jsj090. [DOI] [PubMed] [Google Scholar]
- Palmer DL, Berg CA, Wiebe DJ, Beveridge RM, Korbel CD, Upchurch R, Donaldson DL. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. Journal of Pediatric Psychology. 2004;29:35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- Palmer DL, Osborn P, King PS, Berg CA, Butler J, Butner J, Wiebe DJ. The structure of parental involvement and relations to disease management for youth with type 1 diabetes. Journal of Pediatric Psychology. 2010;36:596–605. doi: 10.1093/jpepsy/jsq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology. 2008;33:916–936. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care. 2004;30:549–554. doi: 10.2337/dc06-1755. [DOI] [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Clark N. Care of children and adolescents with type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Swift EE, Chen R, Hershberger A, Holmes CS. Demographic risk factors, mediators, and moderators in youths’ diabetes metabolic control. Annals of Behavioral Medicine. 2006;32:39–49. doi: 10.1207/s15324796abm3201_5. [DOI] [PubMed] [Google Scholar]
- Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. Journal of the American Medical Association. 2001;285:182–189. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]
- Weinger K, O’Donnell KA, Ritholz MD. Adolescent views of diabetes-related parent conflict and support: A focus group analysis. Journal of Adolescent Health. 2001;29:330–336. doi: 10.1016/s1054-139x(01)00270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Sadler M, White NH. Randomized, controlled trial of behavioral family systems therapy for diabetes: Maintenance and generalization of effects on parent-adolescent communication. Behavior Therapy. 2008;39:33–46. doi: 10.1016/j.beth.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Taylor A, Hough BS, Linscheid TR, Yeates KO, Naglieri JA. Deviation from developmentally appropriate self-care autonomy: Association with diabetes outcomes. Diabetes Care. 1996;19:119–125. doi: 10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]