Abstract
A full understanding of the development of the brain's functional network architecture requires not only an understanding of developmental changes in neural processing in individual brain regions but also an understanding of changes in inter-regional interactions. Resting state functional connectivity MRI (rs-fcMRI) is increasingly being used to study functional interactions between brain regions in both adults and children. We briefly review methods used to study functional interactions and networks with rs-fcMRI and how these methods have been used to define developmental changes in network functional connectivity. The developmental rs-fcMRI studies to date have found two general properties. First, regional interactions change from being predominately anatomically local in children to interactions spanning longer cortical distances in young adults. Second, this developmental change in functional connectivity occurs, in general, via mechanisms of segregation of local regions and integration of distant regions into disparate subnetworks.
Keywords: Functional connectivity, Graph theory, fMRI, Segregation, Integration
Humans undergo an enormous number of developmental changes from birth through adulthood. Not only do we learn to walk, talk and perform other “fundamental” functions, we also increase our ability to identify and control emotions, follow complex “rules”, coordinate precise movements, and attend to task demands for longer periods of time, among many other capacities. Concomitantly, and relatedly, our brains undergo notable changes: synapses form, elaborate, and are removed (Cowan et al. 1984; Huttenlocher 1979), exuberant axonal projections are pruned (Luo and O'Leary 2005), axons are myelinated (Yakovlev and Lecours 1967; Asato et al. 2010), and patterns of neural activity in response to various task demands change considerably, (discussed in more detail below) (Stiles 2008). While it is clear that the human brain undergoes significant developmental transformations, the nascent field of developmental cognitive neuroscience is only beginning to explore and characterize the extent of these changes.
The advent of human neuroimaging, particularly functional magnetic resonance imaging (fMRI), has made it possible for developmental cognitive neuroscientists to begin to investigate how the neural regions used in individual cognitive tasks change with age (Stiles et al. 2003). For example, in comparison with adults, children performing controldemanding tasks show less blood oxygenation level dependent (BOLD) activity in some regions and more activity in other regions (i.e., Tamm et al. 2002). This differential use of neural regions is seen in tasks as disparate as response inhibition (i.e., Luna et al. 2001; Tamm et al. 2002), working memory (i.e., Bunge and Wright 2007) and lexical processing (i.e., Schlaggar et al. 2002; Brown et al. 2005; Church et al. 2008), to name a few.
These age-related activity differences in the brain are thought to reflect both differential use of neural processing units and increased specialization of the component operations performed in individual processing units through development. This transformation is sometimes referred to as interactive specialization because regional developmental changes in neural processing do not occur in isolation. Rather, the developmental changes are thought to be the consequence of inter-regional interactions (Johnson 2000; Brown et al. 2005; Schlaggar and McCandliss 2007). For example, as the processing performed in one region becomes more specialized, there may be less need to use other processes. The importance of changing neural relationships is underscored by the knowledge that accomplishing complex tasks typically requires a large set of regions, and that interactions between regions are necessary for efficient functioning (e.g., Mesulam 1990; Poldrack 2010). Hence, a full understanding of neural development encompasses not only an understanding of how activity within brain regions changes with age, but also how the interactions between regions change with age.
This review focuses on such developmental changes as revealed by a relatively new method for studying interactions in the brain, called resting state functional connectivity magnetic resonance imaging (rs-fcMRI). First we describe the rs-fcMRI signal and common rs-fcMRI analysis techniques, including the measurement of brain networks. We then discuss developmental differences in network configuration and between-region relationships found using rs-fcMRI. Next, we consider the possible neurobiological changes that drive large-scale developmental effects. Then, we briefly explore how this approach to the investigation of network development may influence the study of developmental disorders. We end with a short discussion of the possible advantages and difficulties in performing developmental studies with rs-fcMRI data.
Resting State Functional Connectivity MRI Signal, Brain Networks, and Common Analysis Techniques
Resting State Functional Connectivity MRI (rs-fcMRI) Signal
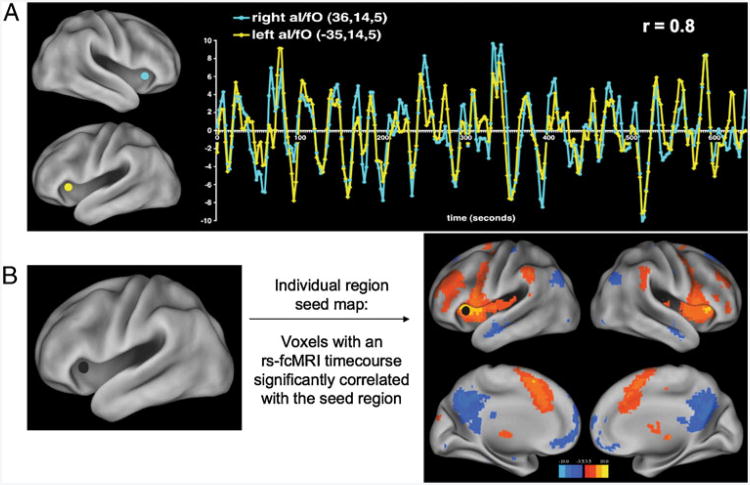
fMRI studies generally report differences in the brain's BOLD response to various task conditions (i.e., reading words as compared to reading nonwords). However, such task responses are only part of the BOLD signal; large, very slow BOLD signal fluctuations are known to occur in the range of 0.01 to 0.1 Hz. These slow, spontaneous fluctuations occur with or without subjects performing a task. For the types of analysis presented in this review, typically 5–10 min of fMRI data are acquired from subjects resting quietly in the MRI bore (i.e., the resting state). In 1995, Biswal and colleagues first reported that, at rest, low frequency BOLD signal fluctuations appear to define relationships between functionally related regions (Biswal et al. 1995). Specifically, the low-frequency timecourse of a region in somatomotor cortex was found to correlate well with timecourses in the contralateral somatomotor cortex, as well as to timecourses in bilateral ventral thalamus and bilateral supplementary motor areas. These correlations in timecourses are referred to as “functional connectivity”, and an example of these correlations can be found in Fig. 1a.
Fig. 1.
rs-fcMRI signal. a rs-fcMRI timecourses from left and right anterior insula/frontal operculum (aI/fO) regions, showing the high correlation or rs-fcMRI “connectivity” found between homotopic regions. b Left aI/fO seed map: the seed map uses the same type of correlations depicted in (a), but instead of determining the correlation between only the left and right aI/fO regions, the seed map shows all voxels with rs-fcMRI timecourses significantly correlated with the left aI/fO
Further research has shown that not only do motor regions show correlated resting state timecourses, but other groups of regions that often activate (or deactivate) at the same time in task settings possess correlated rs-fcMRI timecourses at rest. For example, visual processing regions in occipital cortex correlate strongly (Lowe et al. 1998), as do regions within the default mode network (Greicius et al. 2003) task control networks (Dosenbach et al. 2007; Seeley et al. 2007), attention networks (Fox et al. 2006), reading networks (Koyama et al. 2010), and memory networks (Hampson et al. 2006, 2010). A growing number of studies have utilized the rs-fcMRI signal to explore changes in brain networks over development, both typical (e.g., Fair et al. 2007, 2009; Kelly et al. 2009; Supekar et al. 2009; Stevens et al. 2009; Fransson et al. 2010) and atypical (e.g., Gozzo et al. 2009; Myers et al. 2010; Smyser et al. 2010), and in disease states (e.g., He et al. 2007; Church et al. 2009a; Cullen et al. 2009; Hampson et al. 2009; Jones et al. 2010).
An important aspect of these correlations is that they appear to be strongest between functionally related regions (Biswal et al. 1995; Lowe et al. 1998; Greicius et al. 2003; Fox et al. 2005; Dosenbach et al. 2007), even when those regions do not possess direct anatomical connections (Vincent et al. 2007). This observation has led to suggestions that the rs-fcMRI signal reflects the statistical history of coactivity between brain regions, and that this signal can therefore inform researchers about functional relationships within the brain (Dosenbach et al. 2007; Fair et al. 2007; Kelly et al. 2009). Consistent with this idea, recent work has demonstrated that visual perceptual learning (Lewis et al. 2009), repetition priming (Stevens et al. 2010) and memory training (Tambini et al. 2010) can modify rs-fcMRI signal between brain regions.
What is a Brain Network?
Having established a method to measure functional relationships within the brain, one must decide what relationships to study. Hundreds of rs-fcMRI (and fMRI) studies state that they are investigating “networks”, but the meaning of the term “network” varies significantly (detailed below). Networks are studied in a wide variety of fields, and an entire branch of mathematics, called graph theory, is devoted to the study of networks. Networks, from both an intuitive and a more formal graph theoretical perspective, are collections of items (or nodes) that possess pairwise relationships (called edges). It becomes immediately obvious how entities such as the Internet or transportation systems are well-defined networks in this sense.
The brain, of course, is also a network. With perfect knowledge, one could define a brain network composed of billions of interconnected neurons, with a (general) hierarchical arrangement of, for example, cortical neurons into columns, functional areas (e.g., V1, V2), and functional systems (e.g., visual or somatosensory systems) (Churchland and Sejnowski 1991). Just as economies may be described as interactions between people, between cities, or between nations, brain networks may be described as interactions between neurons, between functional areas, or between functional systems.
fMRI-based techniques can only deliver data at a macroscopic view of this network, since fMRI provides brain activity measurements at the level of the voxel (a cube typically measuring several millimeters per side). fMRI techniques are therefore restricted to describing brain networks at the upper levels of their hierarchy (i.e., functional areas, functional systems). To further complicate matters, the number (and locations) of functional areas (and even functional systems) in the human brain is poorly understood, and so researchers are currently unable to form clean networks corresponding to the brain's functional architecture. Lacking strong constraints, human brain networks are defined and measured in a variety of ways, including forming networks with nodes of voxels (e.g., Buckner et al. 2009; Sepulcre et al. 2010; Fransson et al. 2010), pre-defined anatomical parcellations of voxels (e.g., He et al. 2009), or pre-defined regions of interest obtained from fMRI studies (e.g., Dosenbach et al. 2007; Fair et al. 2009). While ultimately any region definition technique should be subject to anatomical constraints, current anatomical parcellation schemes underestimate the number of functional areas in the brain. For example, the cytoarchitec-tonic parcellation of human orbital and medial prefrontal cortex in Ongur et al. (2003) finds many more distinctions than are defined by the AAL parcellation (Tzourio-Mazoyer et al. 2002). Networks formed using regions of interest thought to reflect functional areas or systems should presumably represent the underlying functional network structure more faithfully, but our knowledge of this architecture is currently incomplete. Node definition is the critical underpinning of network properties and organization (i.e., Zalesky et al. 2010; Smith et al. in press), and a better understanding of the organization of functional areas and systems is a clear and pressing challenge in neuroimaging (Power et al. 2010b).
Though some authors study rs-fcMRI networks from the graph theoretic perspective, the word “network” has been applied in a number of other contexts in the neuroimaging literature. Sets of regions that activate or deactivate at the same time have been called “networks”, as have regions whose functional timecourses show some statistical dependency (e.g., Bitan et al. 2007; Saur et al. 2010). Groups of voxels that have correlated timecourses or shared covariance in the rs-fcMRI signal (methods detailed below) are also often referred to as networks. However, none of these examples are well-defined networks in a broader sense (i.e., nodes related by edges). From this point forward in the present review, when describing data, we will use the word “network” to denote well-defined networks and will avoid this terminology when describing non-graph-theoretic “networks”.
One exception to this rule, however, needs to be made: the “networks” (e.g., dorsal attention network, task control networks) referred to in the previous section were all initially defined by task-induced activations or deactivations in PET and fMRI studies. These groups of regions are not necessarily networks from the broader perspective, although in some cases they are explicitly treated as such (e.g., Dosenbach et al. 2007; Fair et al. 2008), making the “network” label correct in those situations. However, even when these groups of regions are not specifically treated as a network we will continue to use these labels, rather than creating unfamiliar labels, as they are widely accepted and recognized. To be clear, though, we believe the default mode “network” and task control “network” are subsets of potentially related regions within a much larger scale network of regions (Power et al. 2010b).
Common Analysis Techniques for rs-fcMRI Data
Methods used to study rs-fcMRI relationships have varied greatly. Some papers reviewed here have defined relationships of single brain regions to the rest of the brain using seed correlation maps, and others have defined relationships between groups of brain regions using a matrix-based approach. Each of these methodologies has been used to define “networks”, and here we review these methods and the relationships they describe.
Independent and Principal Components Analysis
Though none of the studies reviewed here have defined relationships with independent or principal component analyses (ICA/PCA), these methods are relatively popular tools for identifying “resting state networks” (Damoiseaux et al. 2006; Bullmore and Sporns 2009). These approaches employ dimensionality reduction techniques to partition voxels into groups with shared covariance. Nodes are not defined, nor are edges, and such methods do not utilize or produce networks in either an intuitive or graph-theoretic sense, though they are often labeled as networks. Such analyses are not addressed in this review.
Seed Map Analyses
The earliest rs-fcMRI studies (Biswal et al. 1995; Greicius et al. 2003; Fox et al. 2005) used seed correlation maps to define relationships between a single brain region (the seed) and the rest of the brain. In a typical seed-based analysis, researchers first delineate a particular region of interest, generally either a functionally defined region from a task-based fMRI study or an anatomically defined region. The rs-fcMRI timecourses of all voxels within the defined region are extracted and averaged together. This average timecourse is correlated with the rs-fcMRI timecourses of all other voxels in the brain to create a “seed map”. This map reveals the spatial locations of other brain regions whose timecourse correlates highly with the seed's. An example of the “map” produced by such correlations can be found in Fig. 1b. Seed map analysis yields a peculiar form of a network, in which only relationships to a single seed are defined, and relationships between non-seed regions are left undefined.
Region Matrix Analyses
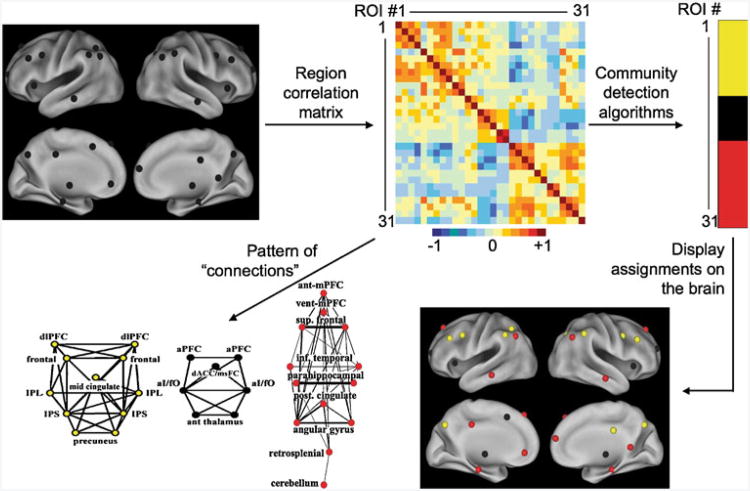
In contrast to the seed map approach, which finds voxels related to only a single seed, a region or seed matrix approach can be used to study the relationships between a defined set of regions. While on the surface, this approach may be thought of as “seed based” in that it utilizes seed regions, it is very different from the seed maps described above. Instead of starting with a single functionally- or anatomically-defined region of interest and correlating its timecourse with every other voxel, one starts with a group of regions either functionally or anatomically defined. The average timecourse of each region is then correlated with that of each other region to make a matrix of correlation values (see Fig. 2). Since the rs-fcMRI correlations used to define edges are a measure of similarity between two regions, each cell of the matrix will have a value. In an attempt to study biologically significant functional relationships, correlations above a given value may be labeled as connections or edges (see Dosenbach et al. 2007 and Fair et al. 2007 for examples). However, it should be noted that no one threshold is the “right” one and interpretations should be based on findings that are consistent across multiple thresholds. This regional matrix produced can rightly be described as a network since it describes a distinct set of nodes with defined connections or edges between them.
Fig. 2.
rs-fcMRI analyses using a region matrix approach to network definition. In contrast to a seed map analysis, this approach finds the relationships between a group of functionally or anatomically defined regions. The rs-fcMRI timecourse is extracted from each region, and the timecourse from each region is correlated with each other region to form a matrix. The correlation matrix can then be thresholded to define any correlation above a given value as an edge or connection, which can either be depicted visually (see example in bottom left) or entered into a community detection algorithm (see example on top right). The matrix, in that it includes nodes and edges, constitutes a network in the graph theoretical sense
Graph Theoretic Analyses of Region Matrices: Communities and Small-World Properties
In the course of this review, we will encounter several studies in which graph theoretic tools are applied to the aforementioned region matrices. Here we describe several graph theoretic tools and their meanings.
Many networks can be viewed as being composed of sub-networks. For example, a person's social network might consist of a group of friends, a group of coworkers, and a group of teammates, each with rather dense internal relationships, but few relationships between groups. These groupings of nodes, or sub-network structures, are called communities or modules. Communities have been found in a wide variety of complex networks, and tend to group nodes with shared characteristics (Newman 2010). Viewing networks in terms of communities can simplify and clarify the form and significance of the overall network structure. In functional brain networks, communities should identify brain regions with similar features or functions, which are potentially functional systems. Community detection tools such as modularity optimization algorithms (Newman and Girvan 2004; Newman 2006) or Infomap (Rosvall and Bergstrom 2008) can be applied to the region matrices described above to detect communities of brain regions. These algorithm-based community assignments are attractive because they are 1) quantitative, 2) objective, and 3) work in situations where the eye cannot (for example, when the relationships between large numbers of regions are in question).
In addition to providing the basis for dividing networks of nodes into communities, graph theory can be used to describe the properties of networks (Watts and Strogatz 1998). Network measures include the characteristic path length (the average number of connections it takes to travel from one node to another) and the average clustering coefficient (on average, how many of the nodes connected to a given node are also connected to one another). Until a decade ago, classic models of networks came in two predominant strains: random and regular networks. Random networks, in which edges are placed between nodes randomly, have short average path lengths but low clustering coefficients, affording them the ability to transfer information efficiently globally (though the whole graph) but not locally (to nearby nodes). Regular networks have nodes connected to nearby nodes in a regular, lattice-like pattern of edges. These networks have high clustering coefficients because each node is well-connected to nearby nodes, but they also have a long average path length. Thus regular networks have efficient local but not global information transfer. A critical discovery, made by Watts and Strogatz in 1998, is that a wide variety of real-world networks, which have been termed small world networks, enjoy the best of both worlds—a high clustering coefficient and a short path length, allowing for both globally and locally efficient information transfer (Watts and Strogatz 1998; Sporns and Honey 2006). These networks possess intermediate structures to the random and regular graphs, such that lattice-like portions of networks are connected by long-range shortcuts, facilitating both local and global efficiency. In other words, small world networks allow all nodes to share information with all other nodes, despite each node having only a small number of connections. Different networks are efficient to differing extents, and the small-world properties capture how well or poorly networks are suited to efficient processing. Note that here we are using efficiency to refer to the ease of information transfer (passing information from node to node); a mathematical definition of efficiency can be found in Latora and Marchiori 2001.
More comprehensive reviews of region matrix analysis techniques in the study of brain connectivity, both functional and structural, can be found in Rubinov and Sporns (2009) and Bullmore and Sporns (2009).
Mature Network Architecture Develops Via Segregation and Integration
Network Relationships Defined Using Region Matrix and Community Detection Methods
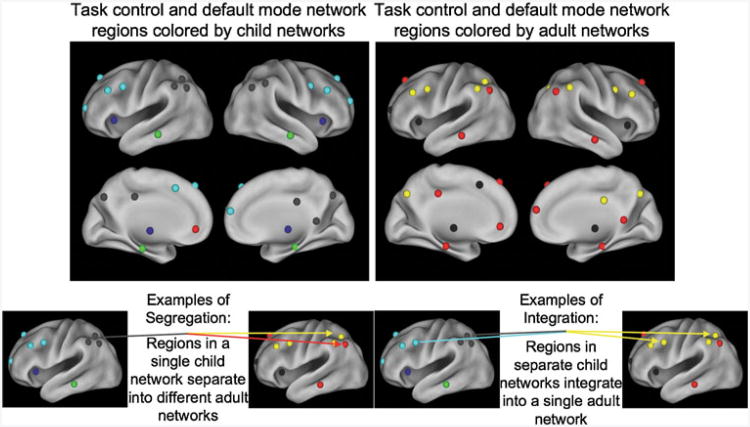
The methods described above (seed maps, region matrix analyses, and community detection techniques, network properties) have been used to describe the development of the brain's functional network architecture. Many of these analyses have focused on the task control and default mode networks. In adults, default mode regions were originally defined by the feature of decreased neural activity during attention-demanding tasks (Shulman et al. 1997; Raichle et al. 2001). Greicius and colleagues, in 2003, showed that brain regions sharing this characteristic of decreased activity during task also showed robust functional connectivity (Greicius et al. 2003). Multiple analyses have converged on a default mode network composed of a distributed set of brain regions including bilateral precu-neus, posterior cingulate, angular gyrus, inferior temporal, parahippocampal, superior frontal and medial prefrontal cortex regions, all shown in red in Fig. 3 (Fox et al. 2005; Greicius et al. 2003).
Fig. 3. Development of community structure from local to distributed communities via segregation and integration.
In contrast, task control regions show increased BOLD activity in a wide variety of tasks upon task initiation, with task maintenance, or with error commission (Dosenbach et al. 2006). Task control networks are also composed of a distributed set of regions and display correlated rs-fcMRI timecourses (Dosenbach et al. 2007). A fronto-parietal control network, which seems to be related to short-acting control like task instantiation and adjustment, includes regions in the precuneus, lateral inferior parietal sulcus, mid-cingulate cortex, and dorsolateral prefrontal cortex (depicted in yellow in Fig. 3). A cingulo-opercular network, which seems to be related to longer-acting task set instantiation and maintenance, includes regions in the anterior cingulate cortex, anterior prefrontal cortex, anterior insula/frontal operculum, and subcortical structures (shown in black in Fig. 3) (Dosenbach et al. 2006, 2007, 2008; Seeley et al. 2007).
The community structure of default mode and control regions develops from a local, anatomical organization into a distributed, functional organization. Fair et al. (2009) used community detection algorithms on matrices derived from default and task control regions, and found that communities were organized largely into lobar communities (e.g., frontal or parietal, see gray and light blue nodes in Fig. 3a) in children, whereas familiar functional systems were recovered in adult modules (e.g., the red default regions of Fig. 3b). This change occurred through segregation of the anatomically adjacent regions and integration of these regions in the distributed adult networks (Fig. 3). For example, while the left angular gyrus and lateral inferior parietal sulcus regions are located in the same module in children, they are separated into the default and fronto-parietal modules in adults (Fig. 3) (Fair et al. 2009). Likewise, the left lateral inferior parietal sulcus and dorsolateral prefrontal cortex regions are in separate modules in children (parietal and frontal, respectively), but integrate into the fronto-parietal module in adults (Fig. 3) (Fair et al. 2007, 2009).
Though we have discussed network development in terms of the task control and default networks, the observed developmental shift from local community organization to a distributed structure via segregation and integration is not restricted to these select sets of regions. We have also used graph theoretic methods, including modularity optimization and Infomap to define developmental changes in network organization of three larger sets of regions: 97 regions defined from a meta-analysis of both speaking and button press tasks (Power et al. 2009), 89 regions from a meta-analysis of single word reading studies (Vogel et al. 2009), and 265 regions defined from multiple meta-analyses and other fcMRI techniques (Power et al. 2010a). As with previous work (Fair et al. 2009), community detection algorithms show children have a preponderance of local relationships, while adults show communities composed of regions distributed across the brain.
Developmental Changes in Functional Relationships Observed with Support Vector Machines
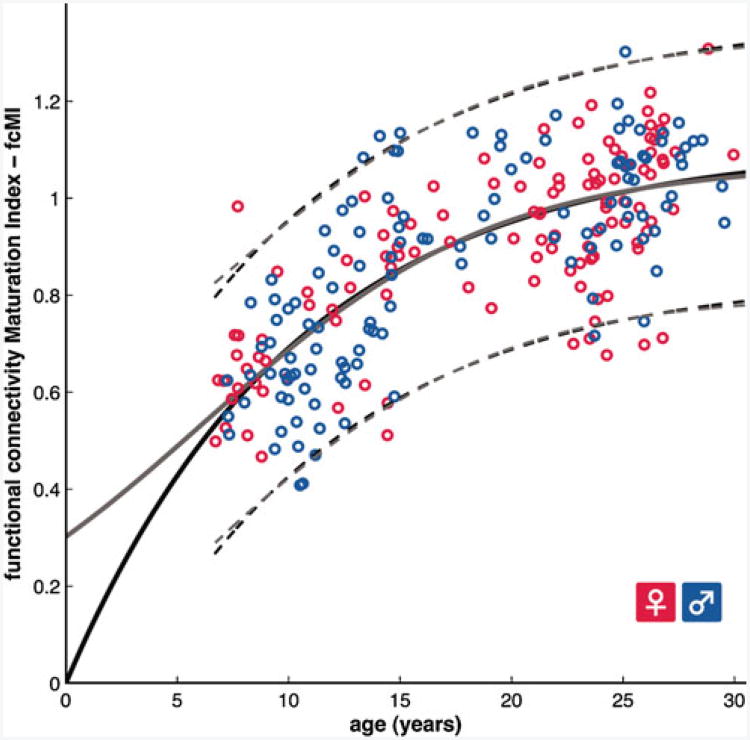
Development via integration and segregation can also be seen using a methodologically distinct analysis utilizing support vector machines. Dosenbach and colleagues used a support vector machine analysis to both determine whether children and adult rs-fcMRI scans can be separated into two groups by the machine and on what basis that separation is made. Support vector machine analyses learn to make group assignments using measurements (features) from many examples of each group. In this case the machine was given rs-fcMRI correlation values for region pairs (the features) for both children and adults (the assignment groups). When a new person is added, the machine can use its pattern of features to assign it to the child or adult group. The machine can also report which features (pair correlation values) were most useful in making the assignment. When dividing children and adults, the support vector machine was 91% accurate. In addition to classification, SVM regression was used to predict an individual's relative brain maturity on a functional connectivity maturation index (See Fig. 4). The functional maturation curve, derived from SVM regression, accounted for 55% of the sample variance and followed a classic nonlinear growth curve shape. For both the SVM classification and regression approaches, the features used by the SVM to make its determinations were predominately those that reflected segregation of networks (decreased correlations between anatomically local regions) with age (Dosenbach et al. 2010).
Fig. 4.
Functional brain maturation curve: 238 individual measures of brain maturity are shown as open circles (115 females in red), plotted by chronological age on the x-axis and rs-fcMRI brain maturation index on the y-axis. The data was fit to curves using information criteria analyses and form a non-linear shape typical of many growth curves. The maturation curves for two separate algorithms are shown in solid gray and black lines, while the 95% prediction limits are shown in the dashed lines. Figure from Dosenbach et al. 2010
Distance Based Comparisons of Child and Adult Regional Relationships
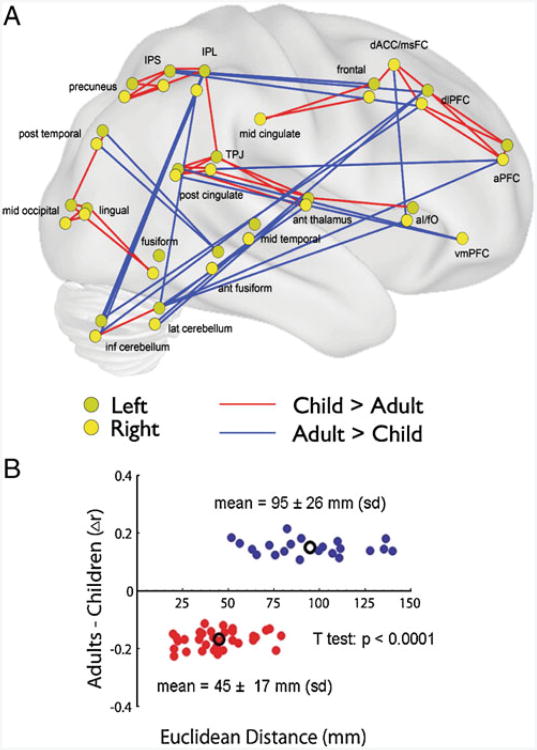
In addition to the aforementioned network analyses, developmental changes in rs-fcMRI defined relationships may be seen by directly comparing the correlation strength between region pairs using t-tests. If regions are developing via segregation of highly related anatomically proximal regions in children and integration of anatomically separate regions into distributed functional modules in adults, the rs-fcMRI correlational relationships between nearby regions should generally decrease with age and the correlations between distant regions should generally increase with age. In fact, when the rs-fcMRI correlation values of every pair of the task control regions described above are compared between children and adults (see Fig. 5), pairs showing significantly higher correlations in children were closer together (mean 45 mm apart in Euclidean distance) than pairs with significantly higher correlations in adults (mean 95 mm apart in Euclidean distance) (Fair et al. 2007). A similar result was seen in rs-fcMRI networks of 90 anatomically defined regions in 7–9 year old children and adults (Supekar et al. 2009). Pairs with higher correlation values in children were closer together (mean 54 mm “DTI wiring distance”) than those showing higher correlation values in adults (mean 63 mm “DTI wiring distance”) (Supekar et al. 2009). Thus, in a variety of matrix-based studies, higher correlations are seen at longer distances in adults than in children, consistent with a developmental trajectory of local segregation and distributed integration.
Fig. 5.
Direct comparison of region-pair correlations between children and adults. a Connections that get significantly stronger with age (shown in red) are between generally nearby regions. Connections that get significantly weaker with age (shown in blue) are between generally distant regions. Note that regions from both hemispheres are reflected onto a single surface, with the left hemisphere regions displayed in a darker yellow. (Figure adapted from Fair et al. 2007). b Plot of difference between adult and child correlation values by Euclidean distance for each of the pairwise connections shown in panel a. The mean distance for correlations greater in children than adults (red regions) is significantly shorter than the mean distance for correlations greater in adults than children. (Figure adapted from Fair et al. 2007)
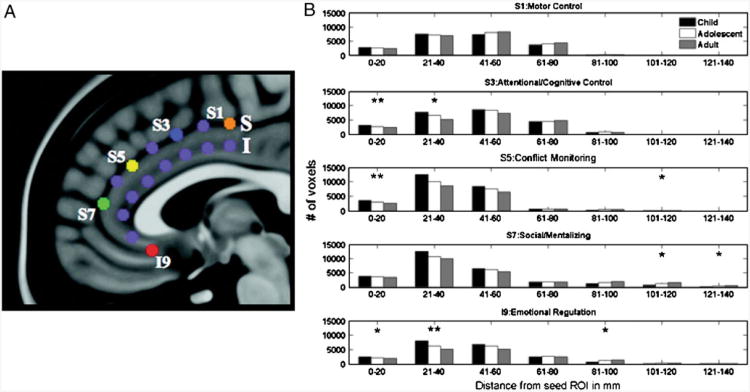
Kelly et al. (2009) took a different approach to directly compare rs-fcMRI regional relationships in children and adults (see Fig. 6). They started with five seed regions in the anterior cingulate cortex and calculated voxelwise seed maps for each of these five regions in children (8–13 years old) and adults. They binned significantly correlated voxels from each seed map as within 5 mm Euclidean distance, 5– 10 mm distance, 10–15 mm distance, and so forth. For the cingulate region purported to be involved in motor control there was essentially no difference between the groups. For the regions purportedly related to cognitive control and conflict monitoring, there were significant age-related decreases in the number of nearby voxels (0–20 mm) showing correlations with those regions. There were also significant age-related decreases in the number of nearby voxels showing functional connections to the regions thought to be involved in social processing and emotional regulation. However, these regions also showed age related increases in the number of correlated voxels far from the original region location (101–140 mm). Thus it seems that regions related to functions that reach maturity relatively early (i.e., motor control) have already developed a mature pattern of relationships at the age range studied, while regions involved in later developing functions (i.e., emotional regulation) show continued decreases in their local rs-fcMRI relationships (segregation) and increases in their distant relationships (integration).
Fig. 6.
Development of rs-fcMRI correlations via functional segregation and integration occurs differentially in functionally distinct regions. a Location of anterior cingulate regions. S1, S3, S5, S7, and I9 are used in the developmental analysis. b Plots reflect the number of voxels significantly correlated with the seed region (S1, S3, S5, S7, and I9) in bins of 20 mm Euclidean distance from the original seed. Significant differences between age groups are denoted with an asterisk. While the S1 region, related to motor control, shows no developmental effects, the S3 region related to attentional control shows decreased correlations with nearby voxels and the S5, S7 and I9 regions related to conflict monitoring, social processing, and emotional regulation, repectively, show both decreased correlations with nearby voxels and increased correlations with distant voxels. (Figure adapted from Kelly et al. 2009)
Children and Adults Show Similar Small World Properties
Interestingly, these developmental differences do not appear to cause or reflect large changes in small world network properties. Fair et al. (2009) determined the path length and clustering coefficient for the task control and default mode network regions in children and adults and found no qualitative differences between the two groups. When Supekar et al. (2009) calculated path length and clustering coefficient on a brain wide scale, children and adults did not differ significantly in small world metrics tested at a single matrix threshold. Fransson and colleagues qualitatively observed small-world networks in infants and in adults, but could not perform direct comparisons (Fransson et al. 2010). Taken together, these findings from the handful of available studies suggest that across development, the brains of infants, children, adolescents, and young adults possess a functional network architecture that has demonstrable small world features. This implies that despite substantial organizational differences within the functional network architecture across development, there does not appear to be a gross deficit in efficiency of network organization in the pediatric age range. Rather, the mature organization appears to emerge largely from a reconfiguration of an already efficient scheme. That said, it will be necessary to re-evaluate the developmental trajectory of network features quantitatively using larger node sets and direct comparisons across age groups.
Synaptic and Anatomical Changes May Underlie Developmental Changes in Resting State Functional Connectivity
It is likely that progressive events (e.g., myelination, axon terminal arborization, synapse formation and elaboration) and regressive events (e.g., axon collateral pruning, removal of axon terminal branching, synaptic pruning) in neurogenesis (Cowan et al. 1984; Luo and O'Leary 2005), play some role in the functional connectivity changes observed here. It is possible that developmental segregation of regions in local networks may be partly related to synaptic pruning. Synaptic density increases early in development, but by the age range studied in the papers presented here, the major effect is synaptic pruning (Huttenlocher 1979). Synaptic pruning is also thought to result in decreased gray matter, which is seen throughout this age range in structural MRI scans (Sowell et al. 2004). In contrast, integration of anatomically disparate regions into an adult network may be assisted by myelination of long distance cortical axon tracts. Structural MRI measurements of white matter (Giedd et al. 1999), diffusion tensor imaging (DTI, Snook et al. 2005) and post-mortem myelin staining (Yakovlev and Lecours 1967) have demonstrated increased cortical myelination in the age ranges found in the studies described in this review. However, despite these demonstrable changes, a note of caution is needed regarding directly linking processes such as myelination and synaptic pruning to the phenomenology observed in the development of the brain's functional architecture. One should avoid assuming that there is an isomorphic relationship between myelination and integration on the one hand, and pruning and segregation on the other.
Changes in synaptic density and myelination cannot be fully responsible for the observed developmental changes. Primarily, not all regions showing functional “connections” show monosynaptic anatomical connections. For example, non-human primates show robust rs-fcMRI connections between eccentric visual field representations in V1 (Vincent et al. 2007); this location lacks direct homotopic callosal connections (Newsome and Allman 1980). While an anatomic study of synaptic pruning shows an adult-like synaptic density in frontal cortex by age 16 (Huttenlocher 1990), there seems to be continued changes in rs-fcMRI connectivity in frontal cortex through the early part of the third decade (Fair et al. 2009; Dosenbach et al. 2010). Additionally, gross changes in myelination cannot explain the specific increases in functional connectivity between regions of adult networks, or decreases in connectivity between regions that continue to show increased myelination with development (i.e., prefrontal cortex). In fact, Supekar and colleagues found developmental increases in functional connectivity in the absence of increased fractional anisotropy, a DTI marker of myelination, although not myelination specifically (Supekar et al. 2010). Moreover, a modest amount of training in adults, which is unlikely to induce myelination or synaptic pruning, can increase functional connectivity between regions (Lewis et al. 2009; Stevens et al. 2010; Tambini et al. 2010).
Rather, increased rs-fcMRI connectivity with training supports the hypothesis that at least some of the developmental changes in resting state networks are due to an increased history of co-activation, such as might be found with Hebbian processes (Hebb 1949). Lewis et al. (2009) demonstrated modulations in connectivity between a section of retinotopic visual cortex and frontal eye fields and retinotopic visual cortex and default mode brain regions when subjects repeatedly made eye movements to the same location in the visual field. Similarly, Stevens et al. (2010) showed increased connectivity between the right inferior frontal gyrus and fusiform face area or a fusiform area responsive to scenes when subjects performed a repetition priming task with face and scene stimuli respectively. Finally, a similar increase in resting state correlations between the medial temporal lobes and lateral occipital cortex was observed by Tambini et al. (2010) following an associative object learning task. Taken together, these studies suggest that rs-fcMRI connectivity is likely related to a history of co-activity, a process that may be especially prominent in child and adolescent development.
Understanding the Typical Network Developmental Pattern of Segregation and Integration Will Aid in Our Understanding of Disordered Development
Many common neurologic and psychiatric illnesses, such as autism, attention deficit hyperactivity disorder (ADHD), and Tourette syndrome (among others) have their origin in infancy or childhood. Without knowing the typical developmental network trajectories, we cannot know whether those with developmental disorders differ from that typical trajectory. Without knowledge of the typical developmental track, we also cannot know whether therapeutic interventions help place children back on the typical track or achieve their benefits via an alternate route. Understanding where in the brain and in what ways the rs-fcMRI connectivity deviates from typical development may also inform what types of therapeutic interventions might be useful in treating specific developmental disorders.
The finding of network development via segregation and integration has already increased our understanding of network dysfunction in Tourette syndrome (Church et al. 2009a). Church and colleagues demonstrated that children with Tourette syndrome showed immature as well as atypical functional connectivity in the task control networks. Specifically, for functional connections between task control regions that had a known typical developmental profile, the authors determined that for adolescents with Tourette syndrome, several functional connections within both the cingulo-opercular and fronto-parietal task control systems were immature (lower than expected for integrating connections, higher for segregating connections) in comparison to typically developing cohorts of the same age. Additionally, multiple atypical functional connections, with values lying well outside the typical developmental trajectory were observed in the fronto-parietal, but not the cingulo-opercular systems, suggesting that in addition to a global immaturity, there was also aberrant functional connectivity selectively involving the system most involved in rapid, adaptive online control. In a subsequent fMRI study, frank functional abnormality of the fronto-parietal network and immaturity of the cingulo-opercular task control network were confirmed in adolescents with Tourette syndrome (Church et al. 2009b). It remains unclear whether these results are specific to Tourette Syndrome; a topic for future investigation.
Likewise, an understanding of the developmental trajectory utilizing segregation and integration in other region sets may help inform our understanding of developmental disorders such as autism and ADHD. rs-fcMRI connectivity studies of the default mode network in autism show reduced connectivity within the network in adults (Cherkassky et al. 2006; Kennedy and Courchesne 2008) and adolescents (Jones et al. 2010). Similarly, Uddin et al. (2008) show decreased homogeneity in default mode network connectivity in adults with ADHD. Since correlations between long distance regions in the default mode and control networks increase with age, these findings are consistent with a hypothesis of an “immature” default network underlying these clinical states. Moreover, Fair et al. (2010) have shown immaturity in the default mode network via both reduced correlation values for rs-fcMRI connections that increase with age and an increased correlation value for an rs-fcMRI connection that decrease with age. This atypical default mode network functional connectivity in ADHD is consistent with the hypothesis that inattention and impulsivity are consequences of intrusions of the default mode network during intended maintenance of the task state (Castellanos et al. 2008). However, while disorders as different as autism and ADHD may both display “immaturity” of the default network, they likely arise from disparate pathophysiological mechanisms, and subsequent studies should investigate the possible causes of “immaturity” in these systems.
Advantages and Caveats in Using rs-fcMRI Connectivity to Study Development
The use of resting state data to study developmental differences has many advantages (Fair et al. 2007). Data acquisition requires minimal task demands, so task-related differences between children and adults that confound many developmental functional imaging studies are not an issue (see Church et al. 2010 for a discussion). Moreover, rs-fcMRI analyses require relatively little time to acquire: 5–10 min of data are often adequate to perform these analyses. rs-fcMRI analyses can also be performed on a brain-wide scale, in contrast with connectivity measured based on functional data such as effective connectivity (Friston et al. 2003) and Granger causality (Granger 1969; Eichler 2005). It may also allow network definition that considers a broad history of co-activity across many tasks, rather than relying on relationships defined in a single task, like those used in dynamic causal modeling (Friston et al. 2003) or Granger causality (Granger 1969; Eichler 2005). Note that these comments are by no means intended to denigrate the role of task-based connectivity measures. Such measures accomplish what rs-fcMRI cannot—a measure of the relationship between regions activated in a specific task. But this approach addresses a different question. In rs-fcMRI studies the question is “what is the general functional network structure?”, while in task-based connectivity the question is “what are the functional relationships between regions during the performance of a specific task?”.
Yet there are many considerations of which one should be mindful when using rs-fcMRI in developmental studies. While there is no “task” per se during rest, these analyses do require the subjects to be still, which may be harder for some subject groups than others. The results discussed in this review used a variety of motion correction approaches. In some, groups of adults and children were matched for movement (Fair et al. 2007, 2009; Church et al. 2009a), while in others data was corrected for movement frame by frame (Fair et al. 2007, 2009; Church et al. 2009a) or with a six parameter movement regression (Kelly et al. 2009). Some authors also did a “visual inspection” of movement effects (Kelly et al. 2009). Efforts to remove the effect of movement are particularly important given the principles of segregation and integration. Movement increases the noise in the signal, making long distance correlations more difficult to detect (Fair et al. 2007).
Additionally, there are continued methodological debates regarding rs-fcMRI data acquisition and processing. Some groups define rest as lying quietly with eyes closed (e.g., Supekar et al. 2009, 2010), some with eyes open, some using fixation blocks extracted from block design studies (e.g., Dosenbach et al. 2006; Fair et al. 2007, 2009), and some using data acquired during task with the task regressed out (Fair et al. 2007; Andrews-Hanna et al. 2007). These various acquisition conditions may affect rs-fcMRI relationships as there are small changes in rs-fcMRI correlations during sleep (Larson-Prior et al. 2009), and certainly some changes even with task regressed (Fair et al. 2007; Jones et al. 2010). There is also continued debate about what steps should be taken to remove nuisance variable such as heart rate and breathing (Birn et al. 2006; Chang et al. 2009) and whether using a whole brain signal regression to account for these noise effects is appropriate (Fox et al. 2009) or induces spurious negative correlations (Murphy et al. 2009).
Finally, there is considerable debate over the various analysis regimes used to evaluate rs-fcMRI data. The field is in the process of evaluating the relative merits of various node and edge definitions in network analyses (Rubinov and Sporns 2009; Power et al. 2010b), and comparisons of graph theoretic networks to component analyses and seed maps are generally lacking. It is also important to note that graph theoretic network metrics are best performed using a large set of nodes or regions (e.g., >100), since in small networks the addition or removal of a single edge can impact findings. However, with a large enough collection of nodes, small world features are essentially assured (Zalesky et al. 2010). These methodological debates are beyond the scope of this review, though hopefully many of them will be resolved as the field matures.
Conclusions
rs-fcMRI is increasingly used to study the development of functional brain networks. Thus far, developmental studies of a number of brain networks including task control networks (Fair et al. 2007, 2009), the default mode network (Fair et al. 2008, 2009), and even whole brain anatomical networks (Supekar et al. 2009) indicate a general principle of anatomical segregation and functional integration whereby child networks are organized primarily by anatomical proximity, while adult networks are organized in a distributed manner across the brain.
Acknowledgments
Portions of this work were funded by NIH NS61144, NS4624, K02 NS0534425, and R01HD057076.
Contributor Information
Alecia C. Vogel, Email: vogela@wustl.edu, Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA.
Jonathan D. Power, Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA
Steven E. Petersen, Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA, Department of Radiology, Washington University School of Medicine, St. Louis, MO, USADepartment of Anatomy and Neurobiology, Washington University School of Medicine, St. Louis, MO, USADepartment of Psychology, Washington University in St. Louis, St. Louis, MO, USA
Bradley L. Schlaggar, Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA, Department of Radiology, Washington University School of Medicine, St. Louis, MO, USADepartment of Anatomy and Neurobiology, Washington University School of Medicine, St. Louis, MO, USADepartment of Pediatrics, Washington University School of Medicine, St. Louis, MO, USA
References
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: a DTI study. Cerebral Cortex. 2010;20(9):2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;13(4):1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam MM, et al. Developmental changes in activation and effective connectivity in phonological processing. Neuroimage. 2007;38(3):564–575. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Current Opinion in Neurobiology. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly AMC, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44:857–869. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. NeuroReport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex. 2008;18(9):2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Fair DA, Dosenbach NU, Cohen AL, Miezin FM, Petersen SE, et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain: A Journal of Neurology. 2009a;132(Pt 1):225–238. doi: 10.1093/brain/awn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Wenger KK, Dosenbach NU, Miezin FM, Petersen SE, Schlaggar BL. Task control signals in pediatric Tourette syndrome show evidence of immature and anomalous functional activity. Frontiers in Human Neuroscience. 2009b;3(38) doi: 10.3389/neuro.09.038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. The “Task B Problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping. 2010;31(6):852–862. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland PS, Sejnowski TJ. Perspectives on cognitive neuroscience. In: Lister RG, Weingartner HJ, editors. Perspectives on cognitive neuroscience. Oxford; Oxford University Press; 1991. [Google Scholar]
- Cowan WM, Fawcett J, O'Leary DDM, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225(468):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvers-horn L, Mueller BA, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Nardos B, Cohen AL, Fair DA, Church JA, Nelson SM, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler M. A graphical approach for evaluating effective connectivity in neural systems. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:953–967. doi: 10.1098/rstb.2005.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, et al. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Costa Dias TG, Mills KL, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry, epub. 2010 doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex, epub. 2010 doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, et al. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37(3):424–438. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. The Journal of Neuroscience. 2006;26(51):13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, King RA, Constable RT, Leckman JF. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biological Psychiatry. 2009;65:594–599. doi: 10.1016/j.biopsych.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic Resonance Imaging, epub. 2010 doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53(6):905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- He Y, Wang J, Wang L, Chen ZJ, Yan C, Yang H, et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS ONE. 2009;4(4):e5226. doi: 10.1371/journal.pone.0005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior: A neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex— developmental changes and effects of aging. Brain Research. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in infants: elements of an interactive specialization framework. Child Development. 2000;71(1):75–81. doi: 10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- Jones TB, Bandettini PA, Kenworthy L, Case LK, Milleville SC, Martin A, et al. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49(1):401–414. doi: 10.1016/j.neuroimage.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Kelly C, Shehzad Z, Penesetti D, Castellanos FX, Milham MP. Reading networks at rest. Cerebral Cortex, epub. 2010 doi: 10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small world networks. Physical Review Letters. 2001;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(41):17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7(2):119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luo L, O'Leary D. Axon retraction and degeneration in development and disease. Annual Review of Neuroscience. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, et al. Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage. 2010;51:1445–1452. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ME. Modularity and community structure in networks. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(23):8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. Networks: An introduction. Oxford University Press; 2010. [Google Scholar]
- Newman MEJ, Girvan M. Finding and evaluating community structure in networks. Physical Review E: Statistical, Nonlinear, and Soft Matter Physics. 2004;69(2 Pt 2):026113. doi: 10.1103/PhysRevE.69.026113. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Allman JM. Interhemispheric connections of visual cortex in the owl monkey, Aotus trivergatus, and the bushbaby, Galago senegalensis. The Journal of Comparative Neurology. 1980;194(1):209–233. doi: 10.1002/cne.901940111. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460(3):425–429. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Interpreting developmental changes in neuroimaging signals. Human Brain Mapping. 2010;31:872–878. doi: 10.1002/hbm.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Miezin FM, Schlaggar BL, Petersen SE. rs-fcMRI networks preferentially link auditory and sensorimotor mouth regions across development from 8-26 years of age Paper presented at the. Society for Neuroscience; Chicago, IL: 2009. [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Miezin FM, Vogel AC, et al. The network architecture of functionally defined regions spanning the brain reorganize from a predominantly local architecture in children to a distributed, functional architecture in adults. Paper presented at the Cognitive Neuroscience Society; Mntreal, QC, Canada: 2010a. [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010b;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom CT. Maps of random walks on complex networks reveal community structure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(4):1118–1123. doi: 10.1073/pnas.0706851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Saur D, Schelter B, Schnell S, Kratochvil D, Kupper H, Kellmeyer P, et al. Combining functional and anatomical connectivity reveals brain networks for auditor language comprehension. Neuroimage. 2010;49:3187–3197. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulcre J, Liu H, Talukdar T, Martincorena I, Yeo BTT, Buckner RL. The organization of local and distant functional connectivity in the human brain. PLOS Computational Biology. 2010;6(6):e1000808. doi: 10.1371/journal.pcbi.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Korshidi G, Webster M, Beckmann CF, Nichols TE, et al. Network modeling methods for fMRI. NeuroImage. doi: 10.1016/j.neuroimage.2010.08.063. in press. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex, epub. 2010 doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Honey CJ. Small worlds inside big brains. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(51):19219–19220. doi: 10.1073/pnas.0609523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Human Brain Mapping. 2009;30(8):2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cerebral Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J. The fundamentals of brain development: Integrating nature and nurture. Harvard University Press; 2008. [Google Scholar]
- Stiles J, Moses P, Passarotti A, Dick FK, Buxton R. Exploring developmental change in the neural bases of higher cognitive functions: the promise of functional magnetic resonance imaging. Developmental Neuropsychology. 2003;24(2–3):641–668. doi: 10.1080/87565641.2003.9651914. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biology. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52(1):290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. Enhanced brain correlations furing rest are related to memory for recent experiences. Neuron. 2010;65(2):280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomic parcellation of the MNI MRI single subject brain. NeuroImage. 2002;15(1):293–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. Journal of Neuroscience Methods. 2008;169(1):249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anesthetized monkey brain. Nature. 2007;447(7140):46–47. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Church JA, Power JD, Cohen AL, Miezin FM, Schlaggar BL, et al. Development of network structure in reading related regions Paper presented at the. Society for Neuroscience; Chicago, IL: 2009. [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford: Blackwell Scientific; 1967. pp. 3–70. [Google Scholar]
- Zalesky A, Fornito A, Harding IA, Cocchi L, Yucel M, Pantelis C, et al. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage. 2010;50:970–983. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]