Abstract
The posteromedial cortex (PMC) is strongly linked to episodic memory and age-related memory deficits. The PMC shows deactivations during a variety of demanding cognitive tasks as compared to passive baseline conditions and has been associated with the default-mode of the brain. Interestingly, the PMC exhibits opposite levels of functional MRI activity during encoding (learning) and retrieval (remembering), a pattern dubbed the encoding/retrieval flip (E/R-flip). Yet, the exact role of the PMC in memory function has remained unclear. This review discusses the possible neurofunctional and clinical significance of the E/R-flip pattern. Regarding neurofunctional relevance, we will review four hypotheses on PMC function: (1) the internal orienting account (2) the self-referential processing account (3) the reallocation account and (4) the bottom-up attention account. None of these accounts seem to provide a complete explanation for the E/R-flip pattern in PMC. Regarding clinical relevance, we review work on aging and Alzheimer’s disease, indicating that amyloid deposits within PMC, years before clinical memory deficits become apparent. High amyloid burden within PMC is associated with detrimental influences on memory encoding, in particular, the attenuation of beneficial PMC deactivations. Finally, we discuss functional subdivisions within PMC that help to provide a more precise picture of the variety of signals observed within PMC. Collective data from anatomical, task-related fMRI and resting-state studies all indicate that the PMC is composed of three main regions, the precuneus, retrosplenial, and posterior cingulate cortex, each with a distinct function. We will conclude with a summary of the findings and provide directions for future research.
Introduction
The posteromedial cortex (PMC) is strongly associated with episodic memory and considered a central node of the default-mode (Buckner, Andrews-Hanna, & Schacter, 2008; Raichle, et al., 2001). The default-mode network (DMN) involves a set of strongly connected regions that in functional neuroimaging studies tends to be activated during rest but deactivated during demanding cognitive tasks (Mazoyer, et al., 2001; McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003; Shulman, et al., 1997). According to the default-mode hypothesis, these deactivations arise, because PMC and other DMN regions support cognitive processes that normally occur during rest, but must be temporarily shut down when available resources are needed for active task performance (Raichle, et al., 2001). Interestingly, successful learning of events (episodic encoding) has been associated with reduced activity in the PMC, whereas successful retrieval of events (episodic retrieval) has been associated with increased activity in the same region (e.g. Buckner, Raichle, Miezin, & Petersen, 1996; Daselaar, Prince, & Cabeza, 2004; Hayama, Vilberg, & Rugg, 2012; Kim, 2011; Otten & Rugg, 2001; Shrager, Kirwan, & Squire, 2008; Wagner, et al., 1998; Wagner, Shannon, Kahn, & Buckner, 2005). These opposing effects, which have been dubbed the encoding/retrieval flip (E/R-flip), were originally reported by Daselaar, Cabeza, and colleagues who observed this pattern not across participants in separate encoding and retrieval studies, but within the same study and within the same participants for a variety of stimuli and memory paradigms (Daselaar, et al., 2009). Since then, the E/R-flip pattern has been replicated in several other studies (Gilbert, Armbruster, & Panagiotidi, 2011; Huijbers, Pennartz, Cabeza, & Daselaar, 2009, 2011; Kim, Daselaar, & Cabeza, 2010; Vannini, O'Brien, et al., 2011). Yet, despite the robustness of the E/R-flip, the functional significance of this pattern and the role of the PMC in memory still remain unclear.
This review aims to clarify the relation between the function of the PMC and the E/R-flip pattern, and includes four different sections. The first section reviews studies that found the E/R-flip pattern and discusses how the E/R-flip may lead to competition between encoding and retrieval processes. The second section discusses the relevance of the E/R-flip for clinical studies of aging and Alzheimer’s disease and provides a direct link between PMC deactivations during encoding and memory-decline. The third section focuses on four different hypotheses that could potentially explain the E/R-flip pattern in the PMC. The last section of our review discusses anatomical, functional, and connectivity findings indicating three functionally distinct subregions within PMC; the precuneus (Pcun), posterior cingulate cortex (PCC), and retrosplenial cortex (RsC). Distinguishing between these subregions should help to further clarify the role of PMC in memory function. The review ends with a concluding section and directions for future research.
The Encoding / Retrieval Flip
Converging evidence for the encoding/retrieval flip
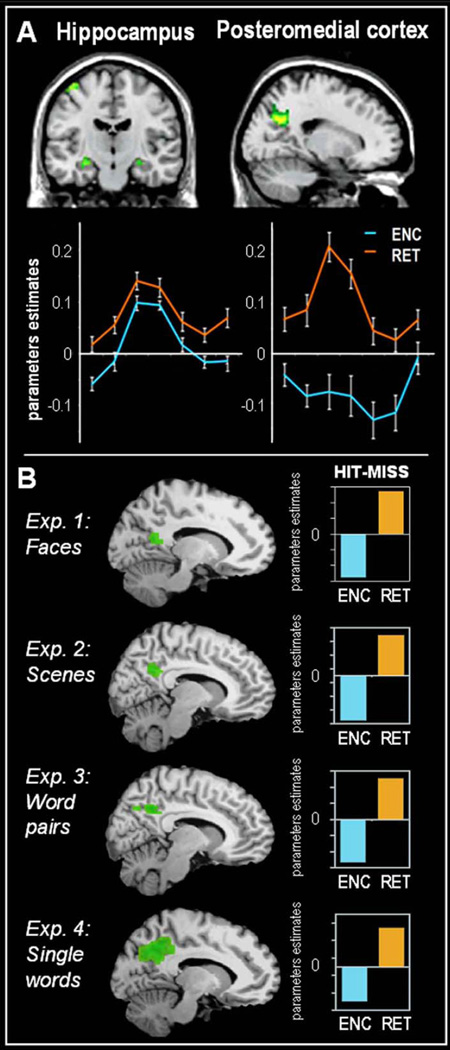
The most powerful method for identifying brain regions associated with successful memory encoding processes using fMRI is known as the subsequent memory paradigm. In this paradigm, encoding trials are back-sorted based on whether they are subsequently remembered (hit) or forgotten (miss). There have been numerous fMRI studies using this paradigm, which have generally found greater activity for encoding hits than misses, or a positive encoding success effect, in the medial temporal lobe (MTL), a pivotal region for episodic memory function (Kim, 2011; Paller & Wagner, 2002; Uncapher & Wagner, 2009). In contrast, several studies have also found less activity for hits than misses, or a negative encoding success effect in the PMC (Figure 1; e.g. Daselaar, Prince, & Cabeza, 2004; Otten & Rugg, 2001). These positive and negative encoding effects have led to the idea that the MTL and PMC support distinct cognitive processes, which are both important for successful memory encoding (Daselaar, et al., 2009; Vannini, O'Brien, et al., 2011). In contrast to the negative encoding success effect in PMC during encoding, most retrieval studies report a positive retrieval success effect in this region during memory retrieval, reflecting greater rather than less activity for retrieval hits than misses (Hayama, Vilberg, & Rugg, 2012; Spaniol, et al., 2009). Similar to the effects of memory encoding, a positive retrieval success effect is assumed to reflect neural mechanisms contributing to the successful remembering of past events.
Figure 1.
Panel A In green, overlapping activity in the medial temporal lobe and posteromedial cortex during encoding and retrieval. The lines shows the average time course of activity during successful encoding (ENC) in blue and successful retrieval (RET) in orange for both regions. Adapted from Vannini et. al. (2011). Panel B shows the encoding/retrieval flip in four different experiments using (1) faces, (2) spatial scenes (3) word pairs and (4) single words. Bars demonstrate the negative encoding success effect in PMC during encoding (ENC: MISS -HIT) in blue and the positive retrieval success effect (RET: HIT - MISS) in orange. Adapted from Daselaar et. al. (2009).
The E/R-flip pattern appears to be robust. First, it has been shown that this pattern occurs regardless of the type of information (words, faces, spatial scenes), stimulus modality (auditory or visual), and memory test (item or relational memory) (Figure 1B, Daselaar, et al., 2009; Huijbers, Pennartz, Cabeza, & Daselaar, 2011). Initially, the E/R-flip was defined using contrast of hits versus misses (encoding: hits < miss retrieval: hit > miss), but recently a similar pattern has been demonstrated using hits as compared to fixation (Vannini, Hedden, et al., 2012; Vannini, O'Brien, et al., 2011). Furthermore, the pattern is not restricted to fMRI studies. Recent evidence suggests that the E/R-flip can also be observed when using electroencephalography measurements during encoding and retrieval stages, from cortical sources in PMC (Jaiswal, Ray, & Slobounov, 2010). Taken together, these findings indicate that the E/R-flip pattern represents a robust neural activity pattern that occurs independently of the specific memory task, stimulus characteristics, and neuroimaging method being used.
Competition between encoding and retrieval
Influential models of memory assume that encoding and retrieval cannot occur at the same time and that the two processes compete for neural resources (Hasselmo, Bodelon, & Wyble, 2002; Norman & O'Reilly, 2003; Yassa & Stark, 2011). In line with these models, we have recently hypothesized that the E/R-flip could also lead to a competition between encoding and retrieval states (Huijbers, Pennartz, Cabeza, & Daselaar, 2009). Given that global activity in a particular brain region cannot increase and decrease at the same time, we hypothesized that the negative encoding, and positive retrieval, success effects in PMC cannot occur simultaneously and will interact.
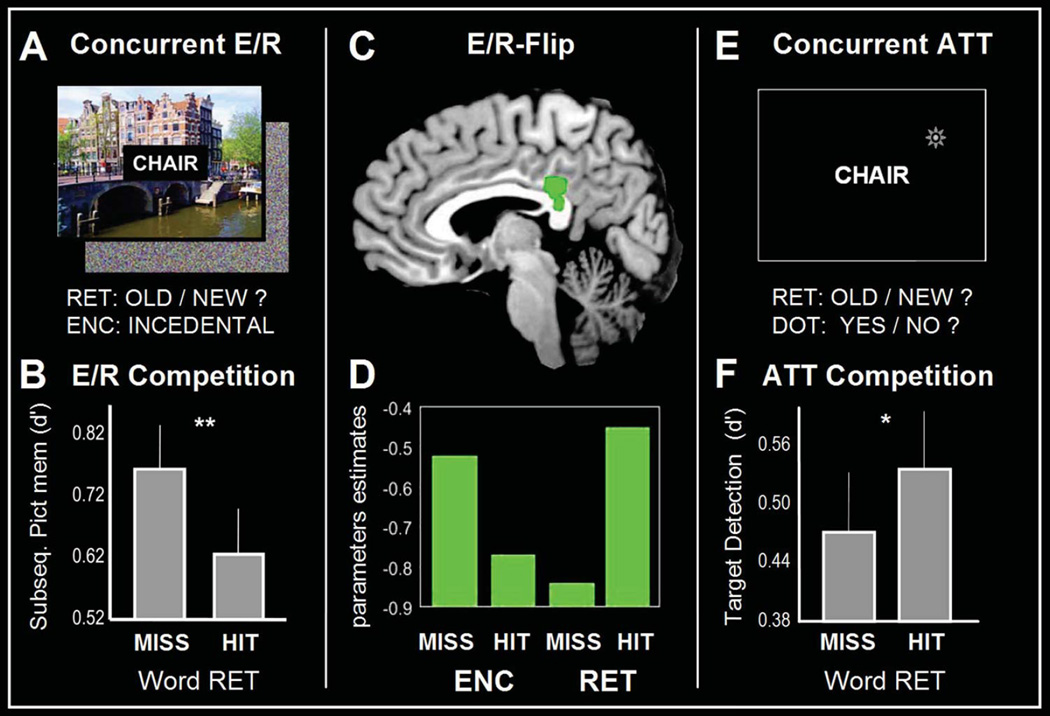
We investigated the hypothesis that the E/R-flip can lead to a competition using an fMRI experiment in which participants encoded and retrieved information within a brief period of time. Participants rapidly encoded words by processing their meaning (living/nonliving decisions) and then performed an old/new word recognition task including words presented at the word encoding phase intermixed with new words (Figure 2A). The key difference with a standard old/new word recognition test is that, while recognizing the words, participants also encoded spatial scenes that were presented in the background. The paradigm was not simply measuring potential interference between viewing scenes and making recognition responses, but specifically measured interference between successful encoding and successful retrieval. Potential interference from perceptual or motor processes was subtracted out, because all trials had scenes in the background and all involved recognition responses. In line with a memory competition, we found that during successful word retrieval the scenes were less likely to be successfully encoded, and vice versa (Figure 2B). Moreover, whereas previous studies found the E/R-flip pattern across encoding and retrieval sessions, this study showed that the E/R-flip pattern could also be found within the same session and within the same trials (Figure 2C). Thus, PMC showed greatest activity when retrieval of words was successful and encoding of spatial scenes was unsuccessful, and least activity when encoding of spatial scenes was successful and retrieval of words unsuccessful (Figure 2D). In order to assess whether the apparent competition between encoding and retrieval was the result of divided attention between word and scene processing, we conducted a follow-up behavioral experiment. For this experiment, we replaced the encoding task with an attention-task involving the detection of a small dot that was flashed on the screen during memory retrieval (Figure 2E). In this case, we actually found the opposite pattern: target-detection performance was worse, rather than better, during unsuccessful retrieval (Figure 2F). This fits with the idea that unsuccessful retrieval tends to coincide with a more demanding and extended search process (Rugg & Wilding, 2000), and thus less attention is available for concurrent target-detection. Together, these findings indicate that a mere attentional account can not easily explain the competition between encoding and retrieval. Thus, similar to the findings regarding competition between encoding and retrieval mediated by the hippocampus (Hasselmo, et al., 2002), our results suggest that the E/R-flip pattern also reflects a processing bottleneck between encoding and retrieval states. However, even though trail-by-trial fluctuations in theta or gamma oscillations have been associated with differences in fMRI signal (Scheeringa, et al., 2011; Scheeringa, et al., 2009) and theta power predicts encoding-related deactivations of the PMC (White, et al., 2012), there is currently no direct evidence linking competitive neuronal processes to the E/R-flip.
Figure 2.
Panel A. shows the experimental design of the concurrent scene encoding/word retrieval task. Panel B. shows the behavioral results, the accuracy of memory encoding (d-prime) is lower for successful (HIT) as compared to unsuccessful (MISS) retrieval. Panel C shows brain activity within PMC when encoding and retrieval occur concurrently. Panel D. brain activity: the green bars at the bottom represent average PMC activity within the PMC for unsuccessful (MISS) / successful (HIT) encoding (ENC) and retrieval (RET). Panel E. shows the experimental design of the attentional control experiment: target-detection of visual dots compared to word retrieval. Panel F. shows the behavioral results, an opposite pattern as compared concurrent encoding/retrieval. The accuracy of target detection (d-prime) is higher for successful (HIT) as compared to unsuccessful (MISS) retrieval. Adapted from Huijbers et. al. (2009).
Clinical relevance of the encoding/retrieval flip
Recently, clinical interest in PMC function has intensified following the development of new neuroimaging tools that allow in-vivo visualization of amyloid-β deposition-one of the hallmark pathologies of Alzheimer’s disease (AD). The accumulation of amyloid-β in fibrillar plaques in conjunction with neurofibrillary tangles are the histopathological features required for the post-mortem confirmation of AD (Braak & Braak, 1992). The recent development of a molecular marker - Pittsburgh Compound-B (PiB) and other tracers has made it possible to visualize fibrillar forms of amyloid-β in vivo using PET imaging (Klunk, et al., 2004). Using PiB, it has been shown that in older adults PMC is particularly vulnerable to early amyloid-β deposition (Buckner, et al., 2008). Although, tau-pathology and hippocampal atrophy- are linked more closely to the clinical syndrome of memory impairment, amyloid-β accumulation is one of the earliest preclinical markers of AD (Frisoni, Fox, Jack, Scheltens, & Thompson, 2010; Jack, et al., 2010), and about one third of clinically normal older adults already harbor amyloid-β within PMC (Sperling, et al., 2011). Therefore, it is believed that amyloid-β accumulation begins many years - perhaps a decade or more – prior to the emergence of the clinical syndrome of AD (Rowe, et al., 2010)
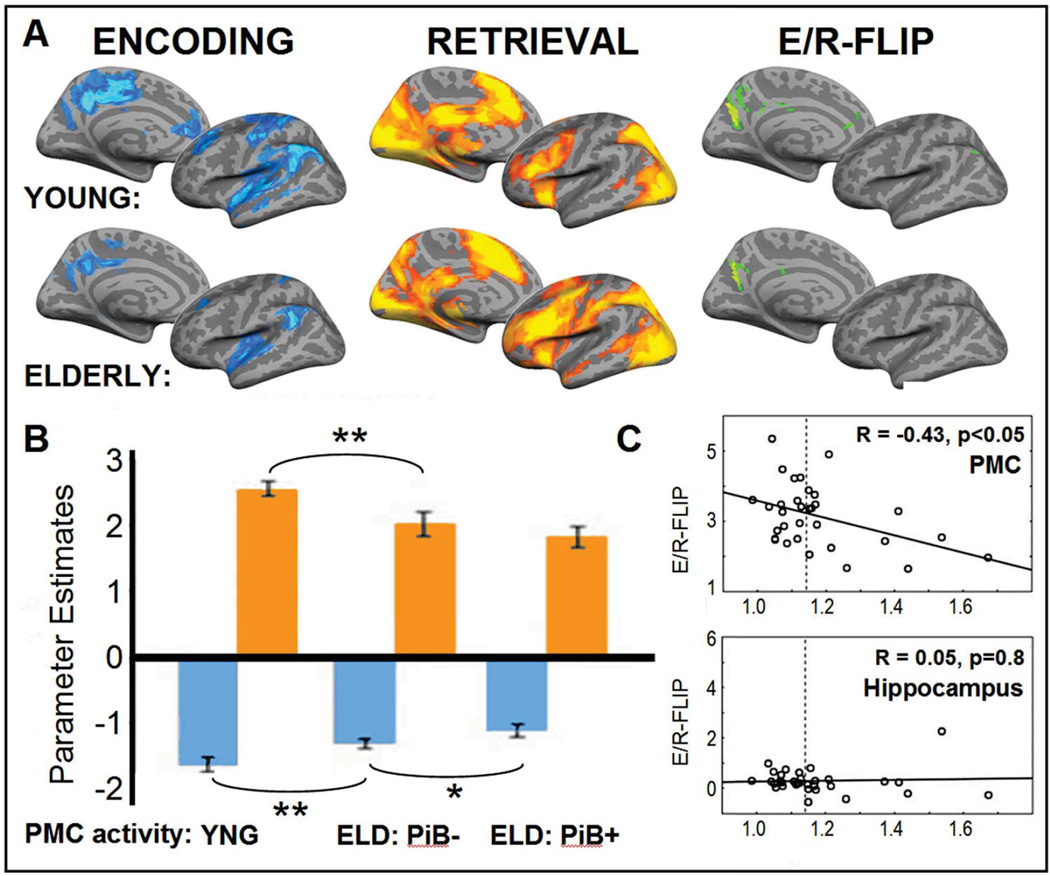
Functional MRI studies have found evidence for disrupted PMC activity in older adults who diagnosed with early stages of AD (Lustig, et al., 2003). Furthermore, healthy older adults who are at high-risk of developing AD, for example those with relatively poor memory or who carry the APEO-4 allele, already show a reduced negative encoding success effect in the PMC, akin to AD patients (Miller, et al., 2008; Pihlajamaki, et al., 2010). Interestingly, several fMRI studies have shown that many normal older adults who have high amounts of amyloid-β also exhibit aberrant brain activity in the PMC during memory encoding (Kennedy, et al., 2012; Mormino, et al., 2012; Sperling, et al., 2009). Recently, Vannini et. al. (2012) specifically investigated the effects of amyloid-β in relation to the E/R-flip, by directly contrasting the difference between the deactivations during successful encoding with activations during successful retrieval (Figure 3A). Older adults showed a reduced E/R-flip within PMC, and this reduction was more pronounced in the older adults with high-amounts of amyloid-β (Figure 3B). Although, it should be noted that this pattern was mostly driven by a reduction of the negative encoding success effect. The reduced ability to modulate the activation between encoding and retrieval was also related to decreased performance in the memory task. In contrast, activity in hippocampus was not correlated with levels of amyloid-β (Figure 3C) or performance. This finding provides a link between aberrant amyloid-β levels, and the E/R-flip in the PMC. Yet, its remains debated if the amyloid burden especially affects memory-related activity or leads to a more general failure to modulate activity in the PMC (Nestor, Scheltens, & Hodges, 2004; Park, Polk, Hebrank, & Jenkins, 2010).
Figure 3.
Panel A: In blue, brain regions along the left midline and hemisphere that show deactivations during memory encoding (Baseline – HIT, P < 0.01) for young and elderly In orange, brain regions that show activations during memory retrieval (HIT - Baseline, P < 0.01). In green, E/R-flip chance: overlapping activity between encoding (Baseline – HIT, P < 0.01) and retrieval (HIT – Baseline, P < 0.01). Panel B: Bars demonstrate the average activity in PMC during encoding in blue and retrieval in orange, separately for young (YNG), elderly (ELD) with low (PiB-) and high (PiB+) amounts of amyloid-β deposition. Panel C: The PMC shows a correlation between the fMRI signal and amyloid-β (R = −0.43). The hippocampus, a control regions, shows no correlation between the fMRI signal and amyloid-beta (R = 0.05). Adapted from Vannini, et. al. 2012.
Although amyloid accumulation has been strongly linked to synaptic activity (Selkoe, 2001), the exact reason why amyloid starts to aggregate within the PMC is not entirely clear. Several studies have shown that amyloid-β -under normal circumstances- serves as a negative feedback signal that maintains neuronal activity within a normal dynamic range (Cirrito, et al., 2005; Ting, Kelley, Lambert, Cook, & Sullivan, 2007). Thus, it has been suggested that the vulnerability of the PMC to amyloid-β might be a consequence of the high-levels of synaptic activity in default-mode regions (Bero, et al., 2011; D. Zhang & Raichle, 2010). This view is consistent with PET studies that found spatial overlap between hypo-metabolism, disruption of connectivity and the accumulation of amyloid-β (Drzezga, et al., 2011). Thus, the reason why the PMC might be particularly vulnerable to amyloid-β, might be a consequence of it metabolic demands. These metabolic demands, in turn, might reflect the PMC’s dynamic function, with both up-and down regulated activity in response to cognitive demands as reflected by the E/R-flip pattern.
Theoretical accounts
The clinical research reviewed in the previous section suggests a link between PMC integrity, the E/R-flip pattern, and episodic memory. However, these findings do not explain the E/R-flip in terms of underlying cognitive processes. Four prevailing theories could potentially explain the E/R-flip: (1) the internal orienting account, (2) the self-referential processing account, (3) the reallocation account and (4) the bottom-up attention account. Below, we discuss evidence in favor of, and opposition to, each account.
The internal orienting account
The internal orienting account states that activity within the PMC and other DMN regions is dependent on a competition between two modes of attention: attention towards information coming from the external environment, and attention to internal processes and thoughts (Nakao, Ohira, & Northoff, 2012; Wagner, et al., 2005). When attention is oriented to the external environment the PMC is deactivated, but when attention is oriented internally the PMC is activated. Evidence in support of the internal orienting account is provided by the observation that a wide variety of studies found increased activity in the PMC using task conditions that require processing of internally generated information. These tasks include autobiographical memory retrieval, thinking about the future, mental imagery and complex moral judgments (Greene, Sommerville, Nystrom, Darley, & Cohen, 2001; Hassabis, Kumaran, & Maguire, 2007; Schacter, Addis, & Buckner, 2007; Szpunar, Watson, & McDermott, 2007). In contrast, most studies that observed deactivations in PMC used task conditions that require attention to external information (Mazoyer, et al., 2001; Raichle, et al., 2001; Shulman, et al., 1997). The internal orienting account could explain the E/R-flip when we simply assume that retrieval benefits from internally-oriented attention to memory representations and drives up activity in PMC, while encoding benefits from externally-oriented attention to study items and drives down activity in PMC (Huijbers, et al., 2011).
Evidence in opposition to the internal orienting account is provided by several fMRI studies that used experimental paradigms involving internally-oriented attention, including mental rotation, mental calculation and working memory (Habeck, et al., 2005; Stanescu-Cosson, et al., 2000; Vingerhoets, de Lange, Vandemaele, Deblaere, & Achten, 2002). Here, we give three examples. First, Vingerhoets et. al. (2002) showed that mental rotation of a complex object is associated with less activity within the PMC, as compared to a control object that required no rotation. Second, Stancescu-Cosson et. al. (2000) showed that mental calculation with a small set of numbers evokes more activity within PMC versus calculations with a large set. Finally, Habeck et. al (2005) showed that the maintenance of a large set of items in working memory results in deactivations of the PMC as compared to maintenance of a small set. All these findings are inconsistent with the idea that conditions that require more internal attention evoke greater PMC activity.
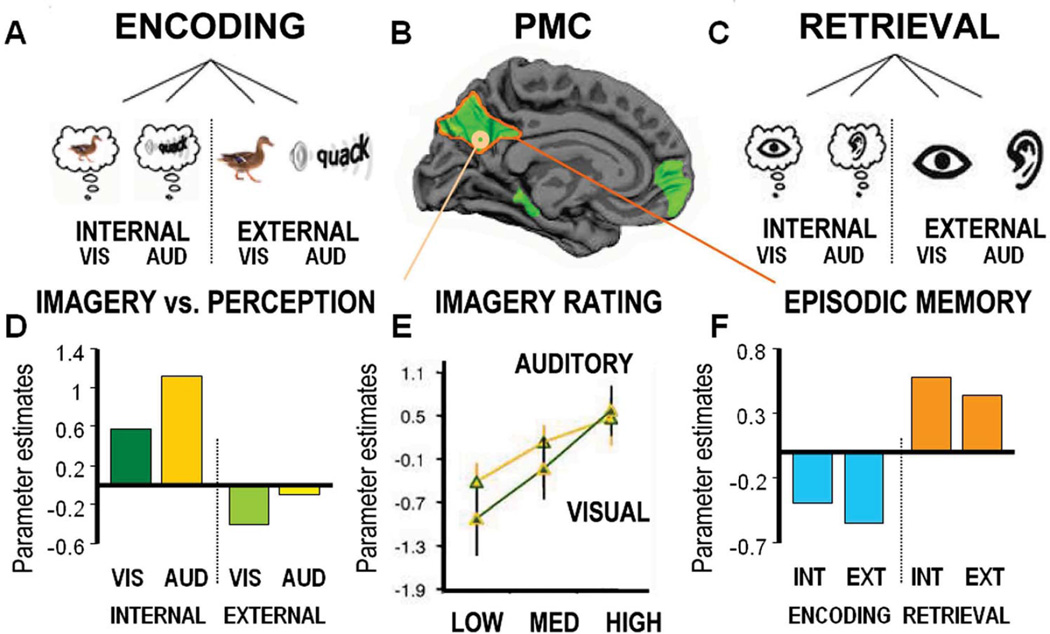
Recently, the internal orienting account was directly tested by Huijbers et al. (2011a) by using a task that scanned encoding and retrieval of both externally-presented and internally-generated sounds and images (Figure 4A–C). In the external condition, participants viewed images or sounds associated with words (e.g., “duck”), whereas in the internal condition they imagined similar images or sounds. Although the internal condition also included external, visual cues, behavioral results verified that vivid mental imagery enhanced memory encoding (Figure 4E). In other words, orienting of attention toward imagined experiences resulted in improved memory encoding. If orienting of attention is the main driver of PMC activity, one would expect a reversal of the E/R-flip when imagery benefits encoding (Figure 4F). In other words, a positive, rather than, a negative encoding success effect in the internal condition. Compatible with the internal orienting account, we found more PMC activity during mental imagery and this activity correlated with the mental experience (Figure 4D). Also in line with the internal orienting account, the conventional E/R-flip in PMC was observed in the external-condition. However, counter to the internal orienting account, PMC also showed the E/R-flip pattern for the internal condition
Figure 4.
Top left and right corners show the experimental design. At the top left, on day 1 individuals encoded cues using visual (VIS) and auditory (AUD) imagery (internal) or visual and auditory perception (external). In the bottom left corner, the bars represent activity within a sub regions of the PMC (MNI(x,y,z) = 9,−57,18), associate with visual imagery (dark green), auditory imagery (dark yellow), visual perception (light green) and auditory perception (light yellow). Bottom middle, lines indicate level of activity for visual imagery (green), auditory imagery (yellow) isolated according to the “richness” of the mental experience. Adapted from Daselaar et. al. (2010). Top middle shows the PMC - as identified using resting-state fMRI - in order to specify the default-mode network regions as regions of interest. Top right corner shows the experimental design at day 2, individuals retrieved the experience from day 1 by indicating whether they used visual imagery, auditory imagery, visual perception or auditory perception to encode the cue. Bottom right corner, the bars represent overall activity within the PMC, as identified by functional connectivity during resting-state fMRI. In blue, negative encoding success effect. separately for internal (INT) and external (EXT) conditions, In orange, positive retrieval success effect. Adapted from Huijbers et. al. (2011).
In sum, the differences in fMRI signal observed within the PMC during encoding and retrieval are not adequately described by the internal orienting account (Huijbers, et al., 2011; Nakao, Ohira, & Northoff, 2012). Another account is that the ER-flip pattern does not reflect internal processing per se, but that it is linked to the self-referential nature of the cognitive processes involved in episodic memory tasks.
The self-referential processing account
The self-referential processing account states that activity within the PMC, together with other DMN regions, including medial prefrontal cortex (mPFC), reflects attention to self-referential thoughts (Buckner & Carroll, 2007; Northoff, et al., 2006). For example, PMC shows increased activity when a personality profile is judged as self-descriptive, or when judging photos made by oneself versus someone else (St Jacques, Conway, Lowder, & Cabeza, 2011). Self-referential thought can also incorporate spatial features. For instance, when navigating a virtual maze, individuals who use an egocentric spatial strategy - based on internal proprioceptive information from ones own body – show more PMC activity than those who use an allocentric strategy - based on external perceptual information from the environment (e.g. Jordan, Schadow, Wuestenberg, Heinze, & Jäncke, 2004). Further support for the self-referential processing account is provided by studies using a variety of tasks that require self-referential information, such as autobiographical memory retrieval, imaging one’s self in the future, and theory of mind (Daselaar, et al., 2008; Dodell-Feder, Koster-Hale, Bedny, & Saxe, 2011; Schacter, et al., 2007; Szpunar, et al., 2007). The self-referential processing account could potentially explain the E/R-flip when one assumes that orienting to self-referential information benefits episodic retrieval and is associated with an increase in PMC activity. At the same time, encoding may benefit from externally-oriented attention and therefore is associated with decreased PMC activity. The self-referential processing account could explain why encoding internally-generated information is associated with a negative encoding success effect in PMC (Figure 4), even though these mental images are internally generated, they are not necessarily self-referential.
Evidence at odds with the self-referential processing account is provided by studies that examined the influence of self-referential processes on memory encoding (Gutchess, Kensinger, & Schacter, 2010; Macrae, Moran, Heatherton, Banfield, & Kelley, 2004). Information that is regarded as self-referential is often remembered better, consistent with the levels of processing model (Craik & Lockhart, 1972). The levels of processing model states that information processed at deeper levels, which includes self-referential information, is encoded better, and therefore, more likely to be remembered. Analogous to our experimental test of the internal orienting account (Huijbers, et al., 2011), the self-referential processing account would predict that self-referential encoding should be accompanied by a positive encoding success effect rather than a negative encoding success effect. Yet, available evidence does not seem to support this hypothesis. For example, Macrae et. al. (2004) only reported a positive encoding success effect for self-referential information in the mPFC. Furthermore, Gutchess et. al 2010 actually found the conventional negative encoding success effect in PMC for self-referential information in healthy young individuals, but not in older adults. Further evidence seemingly at odds is provided by fMRI studies in humans investigating pain. The sensation of pain causes reorienting of attention toward one’s own body, thus an egocentric orienting of attention. However, unlike self-referential navigation, the sensation of pain typically reduces activity in the PMC (Kong, et al., 2010; B. A. Vogt, Derbyshire, & Jones, 1996). Thus, for the self-referential processing account to hold, it seems that self-referential processing requires both a representation of self in context to other information. In sum, circumstantial evidence does not clearly support the self-referential processing account. However, as an explanation for the E/R-flip, the self-referential processing account has not yet been explicitly tested. A third account frames PMC function in terms of reallocation of available resources.
The reallocation account
The reallocation account states that activity within the PMC reflects spontaneous cognitive memory processes that occur during wakeful rest (Gusnard & Raichle, 2001; McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003). These resting state processes are disrupted whenever cognitive resources are required for the performance of active tasks, resulting in decreased activity within regions of the default-mode network, including PMC (Raichle, et al., 2001) In contrast, when task-related resources are not required, they revert back to the default-mode processes and activity within the PMC increases again. In line with the reallocation account, task-induced deactivations in PMC have been shown to be proportional to task demands (e.g. McKiernan, et al., 2003; Park, et al., 2010). Thus, when cognitive demands increase and more resources are reallocated, PMC activity is reduced. At the same time, when cognitive demands are low, spontaneous task-irrelevant processes come to the fore, and activity within the PMC increases. Note that the reallocation account makes no specific claim about whether these processes are linked to memory per se (Mason, et al., 2007; Weissman, Roberts, Visscher, & Woldorff, 2006). The reallocation account could explain the E/R-flip. When we assume that encoding and retrieval processes put different demands on cortical resources. Specifically, encoding draws additional resources when it is successful, while retrieval – as a more automatic process -- draws more resources when it is unsuccessful leading to additional, demanding, retrieval attempts (McClelland, McNaughton, & O'Reilly, 1995).
We recently tested the reallocation account in relation to memory retrieval, using a manipulation of retrieval lags within the context of a continuous recognition paradigm (Huijbers, Pennartz, & Daselaar, 2010). Using shorter and longer lags between encoding and retrieval, we varied the task-demands on episodic memory. If the PMC and the other DMN regions are associated with task demands, one would expect that activity would be greater for the longer than the shorter lags. At the same time, regions associated with longer lags should be more reliant on episodic memory regions such as the MTL (Ranganath, Heller, Cohen, Brozinsky, & Rissman, 2005) Behavioral results confirmed that retrieval for long lags was more difficult than for short lags. The fMRI results indicated three distinct activity patterns within the PMC. First, RsC - together with the MTL - showed a pattern consistent with retrieval from long-term episodic memory, showing a relatively flat response for short lags, but a sharp increase for longer lags (Figure 5). In contrast, regions overlapping with the dorsal attention system (Corbetta, Patel, & Shulman, 2008) - including Pcun and dorsal parietal cortex - showed the opposite pattern, decreased activity with longer lags. However, counter to the reallocation account, PCC and the other DMN regions showed a V-shaped pattern as a function of retrieval lag. Specifically, between short to medium repetition lags activity decreased, but for medium to long lags, these regions showed an increase in activity (Figure 5). These findings suggest a complex interaction between task demands and the recruitment of memory. For example, at long delays the PMC activates as a consequence of task-relevant memory processes. Yet, at short delays, cognitive demands are so low they might leave room for mind-wandering again resulting in PMC activations. Regardless of exact interpretation, these results indicate that the reallocation account alone also cannot explain the E/R-flip within PMC. A fourth account explains the E/R-flip pattern by distinguishing between bottom-up and top-down attention systems.
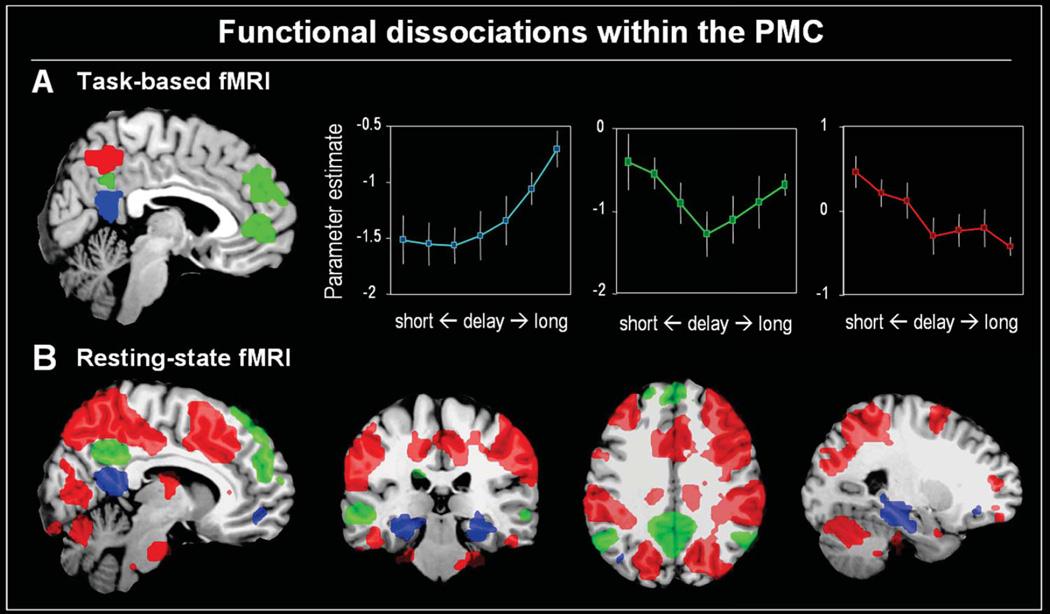
Figure 5.
Panel A. shows brain regions significantly activated during memory retrieval and modulated by a task-difficulty (easy/short-delay vs. difficult/long-delay). In blue the retrosplenial cortex (RsC), which shows more activity for longer delays. Note that RsC connectivity extends into the ventral PCC and does not exactly match anatomical boundaries. In green the posterior cingulate cortex (PCC), which shows an interaction between long and short delays. In red, the precuneus (Pcun), which shows less activity for longer delays, a pattern consistent with the reallocation account. Adapted from Huijbers et al. (2010). Panel B shows the relative functional connectivity of each these of regions with a distributed cortical network. In blue the RsC which is part of the hippocampal-memory network. In green the PCC which is part of the default-mode network and in red, the Pcun which is part of the dorsal attention network.
The bottom-up attention account
The bottom-up attention account, which is a component of the Attention-to-Memory model (AtoM) (Cabeza,Ciaramelli, Olson, & Moscovitch, 2008; Ciaramelli, Grady, Levine, Ween, & Moscovitch, 2010; Ciaramelli, Grady, & Moscovitch, 2008), has previously been used to explain the E/R-flip in ventral parietal cortex, but it could be extended to explain the E/R-flip in PMC (Cabeza, et al., 2011). Top-down attention typically refers to attention that is guided by goals, while bottom-up attention refers to the capture of attention by incoming sensory information (Corbetta & Shulman, 2002; Knudsen, 2007). According to the AtoM model, bottom-up attention is not only captured by sensory information, but also by information coming from the memory system. According to AtoM, the E/R-flip reflects a different relationship between bottom-up attention and memory success during typical retrieval tasks and typical encoding tasks (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Cabeza, et al., 2011; Huijbers, et al., 2011). During typical retrieval tasks, bottom-up attention is often captured by recovered memories, and hence bottom-up attention tends to be associated with retrieval success. During typical encoding tasks, in contrast, to-be-encoded stimuli are at the focus of top-down attention, and bottom-up attention is often captured by irrelevant thoughts or environmental stimuli, thereby establishing a link between bottom-up attention and encoding failure (Cabeza, 2008). Consistent with the bottom-up attention account, fMRI studies have found that ventral parietal cortex, a region associated with bottom-up attention, overlaps between bottom-up attention and retrieval success (Cabeza, et al., 2011) and between bottom-up attention and encoding failure (Uncapher, Hutchinson, & Wagner, 2011).
As noted above, the bottom-up attention account was originally used to explain the E/R-flip in ventral parietal cortex (Cabeza, Ciaramelli, & Moscovitch, 2012; Cabeza, et al., 2008), but one could extend it to explain the E/R-flip in PMC. This extension is supported by the fact that in many conditions, PMC behaves similarly as the ventral parietal cortex. For example, both regions tend to deactivate during demanding cognitive conditions and are assumed to be core components of the DMN (Buckner, et al., 2008; McKiernan, et al., 2003). However, whereas the ventral parietal cortex has been linked to bottom-up attention by functional neuroimaging and patient data in attention and episodic memory domains (Cabeza, et al., 2008; Corbetta & Shulman, 2002), the link in the literature between PMC and bottom-up attention is not as strong.
In sum, none of the four accounts adequately explain the E/R-flip pattern within the PMC. These cognitive accounts are also not mutually exclusive. For example, internal orienting might engage more self-referential processing. Likewise, bottom-up orienting to salient information might lead to greater reallocation of resources (Raichle, et al., 2001). At present, there is no overarching account that can fully explain PMC’s behavior in various cognitive conditions. The lack of a uniform theory of PMC function might also reflect the fact that the PMC is not a single uniform brain region, and aforementioned accounts might actually be more-or-less applicable to distinct regions within PMC. Thus, in order to obtain a more complete picture of the functional role of PMC, it is critical to consider the existence of different subregions within PMC more carefully.
Functional subdivisions of PMC
One critical issue when interpreting fMRI results regarding PMC is that there is considerable evidence indicating functional subdivisions within PMC. Thus, simply considering these different areas as a single PMC region with one unitary function can hinder our understanding of the PMC. As noted at the beginning of this review, three major regions can be roughly discriminated within PMC: RsC, Pcun, and PCC.
The E/R-flip has been reported most consistently within PCC and Pcun (e.g. Daselaar, et al., 2009; Vannini, et al., 2011). Regarding RsC, a small number of fMRI studies that generally involved memory for contextual information have even reported positive encoding success effects in this region (Davachi, Mitchell, & Wagner, 2003; Hayes, Nadel, & Ryan, 2007; Kim & Cabeza, 2007; Staresina & Davachi, 2006; Uncapher, Otten, & Rugg, 2006). In other words, RsC has been found to show increased activity during both encoding and retrieval, particularly for context-dependent information. As discussed below, these findings might relate to the strong coupling between RsC and the hippocampal memory system. In the next section, we describe the evidence derived from anatomical data, task-related fMRI, and resting state fMRI studies for functional subregions in PMC, involving RsC, Pcun and PCC.
Anatomical data from macaque monkey and humans, indicate that the RsC, Pcun and PCC each have distinct cortical connections and consist of different anatomical Brodmann Areas (BA). RsC is a relatively small region located at the ventral and posterior end of the cingulate gyrus and includes BA 29 and 30. RsC has dense anatomical connections to regions within the MTL - including the hippocampus and entorhinal cortex - as well as the thalamus (Kobayashi & Amaral, 2007; B. A. Vogt, Vogt, & Laureys, 2006; B.A. Vogt, Vogt, Perl, & Hof, 2001). In humans, damage to RsC can result in memory deficits that mimic problems observed after hippocampal insult, providing a direct link between episodic memory and RsC function (Milner, 1966; Valenstein, et al., 1987). Pcun is a relatively large region located dorsal and posterior to the cingulate gyrus, anterior to the parieto-occipital sulcus and includes Brodmann areas 7a and 7b. Pcun has dense anatomical connections to prefrontal and other parietal regions, as well as the striatum and thalamus (Margulies, et al., 2009). Finally, PCC is located at the center of PMC between RsC and Pcun, and includes Brodmann areas 23 and 31. Monkey and human data have shown that PCC has the strongest reciprocal connections with the other DMN regions, VPC and mPFC (Kobayashi & Amaral, 2007; Margulies, et al., 2009). Thus, although RsC, Pcun and PCC are closely adjacent regions with strong interconnectivity, human and animal data also indicate that these regions show clear anatomical differences.
Further evidence for different sub-regions within PMC is provided by data from task-related fMRI studies in humans. RsC is typically associated with cognitive processes also ascribed to MTL, including recollection of past events and spatial navigation (Vann, Aggleton, & Maguire, 2009). Pcun is mostly associated with mental imagery and is often referred to as the “mind’s eye” (Daselaar, Porat, Huijbers, & Pennartz, 2010; Fletcher, et al., 1995). Besides mental imagery, the Pcun has also been implicated in top-down attention, working memory and self-referential processes (Cavanna & Trimble, 2006; Corbetta, et al., 2008). The PCC is activated in a variety of task-conditions closely related to episodic memory (Buckner, et al., 2008; Spreng, Mar, & Kim, 2009) and deactivated in a variety of task-conditions that have no direct episodic component (McKiernan, et al., 2003). It should be noted that due to the lower spatial resolution of functional MRI, activity in RsC often extends into the ventral PCC (Vann, et al., 2009). Yet, several fMRI studies on memory retrieval have reported functional dissociations between the RsC, Pcun and PCC within the same task, again indicating that these sub-regions have distinct functions in episodic memory (Daselaar, Fleck, & Cabeza, 2006; Huijbers, Pennartz, & Daselaar, 2010; Yonelinas, Otten, Shaw, & Rugg, 2005). For instance, using recognition confidence functions both Yonelinas et. al. (2005) and Daselaar et. al. (2006) found distinct patterns in RsC, Pcun and PCC relating differentially to recollection and familiarity-based recognition. Also, Summerfield et. al. (2009) found that the RsC has a more general role in autobiographical retrieval, while the PCC distinguishes “real” from “imagined” events. In sum, fMRI-task data clearly indicate that the RsC, Pcun and PCC are functionally distinct regions within PMC.
In line with both anatomical and task-data, resting-state fMRI data provide further evidence that PMC is composed of at least three distinct sub-regions, each connected to a distinct cortical network (Damoiseaux, et al., 2006; Yeo, et al., 2011; S. Zhang & Li, 2012). Using a standard seed-based connectivity analysis (Chao-Gan & Yu-Feng, 2010) on previously published resting-state data (Huijbers, et al., 2011), we visualized the relative connectivity of the RsC, PCC and Pcun. Resting-state scans were collected in two 8 minutes sessions with a Phillips Intera 3.0T (TR 2000 ms, TE 30 ms, 34 slices, voxel size 2.3 × 2.3 × 3-mm, see Huijbers et. al. 2011 for details). Seeds, with a radius of 6mm, were defined by the local maxima from the triple dissociation between the PMC subregions taken from the memory study that manipulated retrieval lags (Huijbers et. al. 2010, MNI coordinates: RsC(x,y,z) = [-6,-51,15], Pcun(x,y,z) = [6,-60,51] and PCC(x,y,z) = [0,-51,33]). Connectivity difference maps were created by subtracting each seed-map from the other seed-maps (i.e., RsC – Pcun; Pcun - PCC; PCC – Pcun). Next, a random effects analysis was conducted using a one-sample t-test (P < 0.001, cluster size = 10), resulting in six group-maps. Finally, using a conjunction approach, we extracted the relative connectivity of each PMC seed ([RsC > Pun RsC > PCC], [Pcun > PCC Pcun > RsC], [PCC > RsC PCC > Pcun]). The resulting seed-maps again confirmed that the RsC, Pcun and PCC are neuroanatomically distinct regions, each preferentially connected to a different cortical network (Figure 5B). Specifically, the RsC is associated more with the hippocampal-memory system (Vann, et al., 2009), the Pcun is associated more with the dorsal-attention system (Corbetta, et al., 2008), and the PCC is associated more with the default-mode network (Buckner, et al., 2008). Note again, that due to the relatively low spatial resolution of functional MRI, connectivity of the RsC extends somewhat into the most ventral part of PCC. In sum, the anatomical, fMRI-task, and resting-state connectivity data clearly indicate that the RsC, Pcun and PCC form functionally distinct regions within the PMC.
Conclusions
The PMC reliably shows opposing levels of activation during encoding and retrieval, the E/R-flip pattern, and this can lead to a competition between encoding and retrieval states. In terms of clinical relevance, age-related pathology, specifically amyloid deposition within the PMC, has detrimental effects on the E/R-flip. Thus, E/R-flip is an interesting candidate for tracking longitudinal changes in episodic memory during pre-clinical stages of Alzheimer’s disease (Sperling, et al., 2011). We also reviewed four hypotheses that may explain the E/R-flip pattern, the internal orienting account, the self-referential processing account, the reallocation account, and the bottom-up attention account. The internal orienting account asserts that PMC involvement in encoding and retrieval is dependent on the internal vs. external orientation of attentional. The self-referential processing account explains PMC activity in terms of orienting toward self-relevant thoughts versus the external environment. The reallocation account states that the activation differences in PMC depend on task-demands and follows response times. Finally, the bottom-up attention account asserts that activity within default-mode regions reflects bottom-up orienting of attention towards information retrieved from memory. Finally, we addressed the issue that hinders understanding of the PMC. Specifically, anatomical studies, task-based fMRI studies, and resting state fMRI studies all indicate that PMC is not a single, homogeneous, region but consists of different sub regions, the retrosplenial cortex, the posterior cingulate cortex and the precuneus, each making separate contributions to memory encoding and retrieval. Future research should take these distinctions into account in order to clarify the E/R-flip pattern in PMC.
On a final note, the underlying neuronal mechanisms that give rise to the E/R-flip are still unknown. The fMRI signal is known to correlate with local field potentials and is believed to reflect a combination of neuronal input and output (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001). Recently, it has been shown that electrical stimulation can induce BOLD deactivations in highly connected brain regions through inhibitory neuronal input (Logothetis, et al., 2010). One possible mechanism is that the E/R-flip in the PCC reflects a combination of fMRI signal from inhibitory input in layers II and III and excitatory output in layers V and VI. For example, inhibitory input from sensory regions could drive encoding-related deactivations in the PCC, while excitatory output to the parahippocampus could drive retrieval-related activations. However, human imaging studies currently lack the temporal and spatial resolution to investigate such mechanisms. Studies in monkeys and epilepsy patients, that involve intracranial recording techniques with high spatial and temporal resolution should help to elucidate the neural mechanisms that underlie the E/R-flip.
Figure 6.
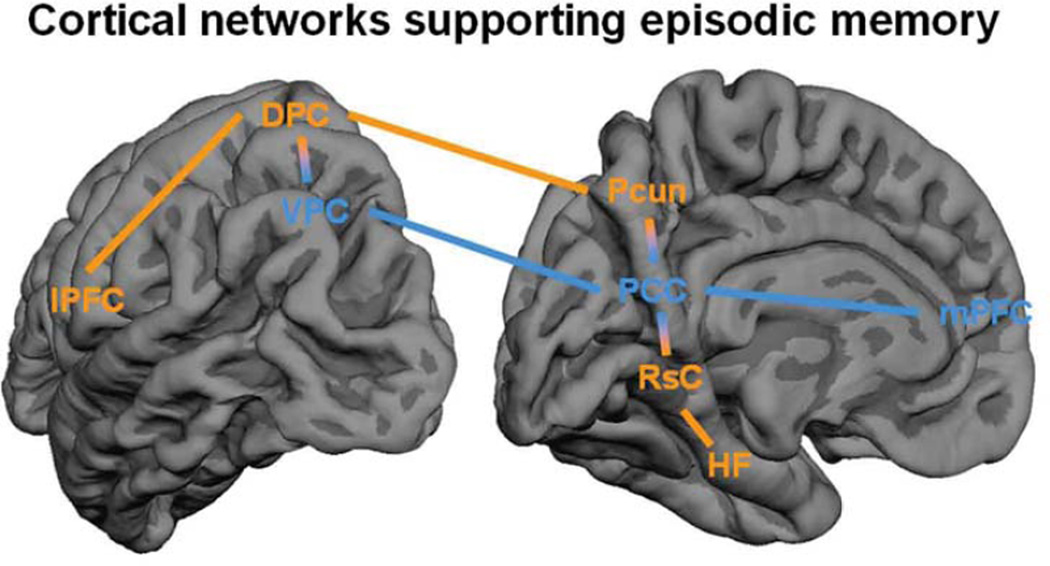
Simplified representation of the cortical networks involved in episodic memory. On the left the lateral surface and right the medial surface of the left-hemisphere. In orange, regions that tend to activate during both encoding and retrieval, which include the hippocampal-memory network, consisting of the hippocampal formation (HF) and the retrosplenial cortex (RsC) and the dorsal-attention network consisting of the precuneus (Pcun), the dorsal parietal cortex (DPC) and the lateral prefrontal cortex (lPFC). In blue regions that tend to show the encoding/retrieval flip, including default-mode network regions: posterior cingulated cortex (PCC), the ventral parietal cortex (VPC) and medial prefrontal cortex (mPFC).
Highlights.
Posteromedial cortex (PMC) deactivates when Encoding and activates when Retrieving
Pre-clinical markers of Alzheimer's are linked to PMC changes and the E/R-flip
Four neurocognitive accounts are discussed that can possibly explain the E/R-flip
Future research on PMC function requires consideration of its functional subregions
Acknowledgements
This work was supported by the European Molecular Biology Organization: ALTF 318-2011 [W.H] the Amsterdam Brain Imaging Platform [W.H, S.D], the Marie Curie Fellowship: FP7-PEOPLE-2007-4-1-IOF from the European Union [P.V], the Swedish Brain Foundation and Swedish Society for Medicine [P.V], the Institutes of Health: K24 AG035007 [R.S.], R01 AG027435-S1 [R.S], P01AG036694 [R.S], P50AG00513421 [R.S], and the Alzheimer’s Association: IIRG-06-27374 [R.S] and the Netherlands Organization for Scientific Research, VICI 918.46.609 [C.P] and VENI 916.66.022 [S.D].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-[beta] deposition.[10.1038/nn.2801] Nature Neuroscience. 2011;14(6):750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neuroscience Research. 1992;15(1–2):6–31. doi: 10.1016/0168-0102(92)90014-4. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, Petersen SE. Functional anatomic studies of memory retrieval for auditory words and visual pictures. Journal of Neuroscience. 1996;16(19):6219–6235. doi: 10.1523/JNEUROSCI.16-19-06219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012 doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nature Reviews Neuroscience. 2008;9(8):613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Mazuz YS, Stokes J, Kragel JE, Woldorff MG, Ciaramelli E, et al. Overlapping parietal activity in memory and perception: evidence for the attention to memory model. Journal of Cognitive Neuroscience. 2011;23(11):3209–3217. doi: 10.1162/jocn_a_00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. (Pt3) [DOI] [PubMed] [Google Scholar]
- Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in Systems Neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Behavior. 1972;11:671–684. [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96(4):1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Porat Y, Huijbers W, Pennartz CM. Modality-specific and modality-independent components of the human imagery system. Neuroimage. 2010;52(2):677–685. doi: 10.1016/j.neuroimage.2010.04.239. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23(3):921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100(4):2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Becker JA, Van Dijk KRA, Sreenivasan A, Talukdar T, Sullivan C, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain. 2011;134(6):1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RJ, Dolan RJ. The minds eye-precuneus activation in memory-related imagery. Neuroimage. 1995;2(3):195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293(5537):2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48(1):211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27(52):14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14(4):793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Hayama HR, Vilberg KL, Rugg MD. Overlap between the Neural Correlates of Cued Recall and Source Memory: Evidence for a Generic Recollection Network? J Cogn Neurosci. 2012 doi: 10.1162/jocn_a_00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17(9):873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Cabeza R, Daselaar SM. When learning and remembering compete: a functional MRI study. PLoS Biol. 2009;7(1):e11. doi: 10.1371/journal.pbio.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Cabeza R, Daselaar SM. The Hippocampus Is Coupled with the Default Network during Memory Retrieval but Not during Memory Encoding. PLoS One. 2011;6(4):e17463. doi: 10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Daselaar SM. Dissociating the “retrieval success” regions of the brain: effects of retrieval delay. Neuropsychologia. 2010;48(2):491–497. doi: 10.1016/j.neuropsychologia.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Ray W, Slobounov S. Encoding of visual-spatial information in working memory requires more cerebral efforts than retrieval: Evidence from an EEG and virtual reality study. [doi: 10.1016/j.brainres.2010.05.086] Brain Research. 2010;1347(0):80–89. doi: 10.1016/j.brainres.2010.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K, Schadow J, Wuestenberg T, Heinze H-J, Jäncke L. Different cortical activations for subjects using allocentric or egocentric strategies in a virtual navigation task. Neuroreport. 2004;15(1):135–140. doi: 10.1097/00001756-200401190-00026. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Rodrigue KM, Devous MD, Sr, Hebrank AC, Bischof GN, Park DC. Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage. 2012;62(1):1–8. doi: 10.1016/j.neuroimage.2012.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. Neuroimage. 2011;54(3):2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Differential contributions of prefrontal, medial temporal, and sensory-perceptual regions to true and false memory formation. Cereb Cortex. 2007;17(9):2143–2150. doi: 10.1093/cercor/bhl122. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental Components of Attention. Annual Review of Neuroscience. 2007;30(1):57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502(5):810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, LaViolette P, Gollub RL. Exploring the brain in pain: Activations, deactivations and their relation. [doi: 10.1016/j.pain.2009.11.008] Pain. 2010;148(2):257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Augath M, Murayama Y, Rauch A, Sultan F, Goense J, et al. The effects of electrical microstimulation on cortical signal propagation. [10.1038/nn.2631] Nat Neurosci. 2010;13(10):1283–1291. doi: 10.1038/nn.2631. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences. 2009;106(47):20069–20074. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315(5810):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McClelland J, McNaughton B, O'Reilly R. Why There Are Complementary Learning Systems in the Hippocampus and Neocortex: Insights From the Successes and Failures of Connectionist Models of Learning and Memory. Psychological Review. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Amnesia following operations on the medial temporal lobes. In C. W. M. Whitty & O. L. Zangwill (Eds.), Amnesia: Butterworth, London. 1966 [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Marks S, Baker SL, Jagust WJ. Abeta Deposition in Aging Is Associated with Increases in Brain Activation during Successful Memory Encoding. Cereb Cortex. 2012;22(8):1813–1823. doi: 10.1093/cercor/bhr255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Ohira H, Northoff G. Distinction between externally vs. internally guided decisionmaking: Operational differences, meta-analytical comparisons and their theoretical implications. [Review] Frontiers in Neuroscience, 6. 2012 doi: 10.3389/fnins.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer's disease. Nat Med. 2004;(10 Suppl):S34–41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110(4):611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11(19):1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins L. Age differences in default mode activity on easy and difficult spatial judgment tasks. [Original Research] Frontiers in Human Neuroscience, 3. 2010 doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki M, K OK, Bertram L, Tanzi RE, Dickerson BC, Blacker D, et al. Evidence of altered posteromedial cortical FMRI activity in subjects at risk for Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24(1):28–36. doi: 10.1097/WAD.0b013e3181a785c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15(8):997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. Retrieval processing and episodic memory. Trends in Cognitive Sciences. 2000;4(3):108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69(3):572–583. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Oostenveld R, Norris DG, Hagoort P, Bastiaansen MC. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. Neuroimage. 2009;44(3):1224–1238. doi: 10.1016/j.neuroimage.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's Disease: Genes, Proteins, and Therapy. Physiological Reviews. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR. Activity in both hippocampus and perirhinal cortex predicts the memory strength of subsequently remembered information. Neuron. 2008;59(4):547–553. doi: 10.1016/j.neuron.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks 2. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Conway MA, Lowder MW, Cabeza R. Watching my mind unfold versus yours: an fMRI study using a novel camera technology to examine neural differences in self-projection of self versus other perspectives. J Cogn Neurosci. 2011;23(6):1275–1284. doi: 10.1162/jocn.2010.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. J Neurosci. 2006;26(36):9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. Neuroimage. 2009;44(3):1188–1200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA. 2007;104(2):642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Hutchinson JB, Wagner AD. Dissociable effects of top-down and bottom-up attention during episodic encoding. J Neurosci. 2011;31(35):12613–12628. doi: 10.1523/JNEUROSCI.0152-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52(3):547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110:1631–1646. doi: 10.1093/brain/110.6.1631. ( Pt 6) [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? [10.1038/nrn2733] Nat Rev Neurosci. 2009;10(11):792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Becker JA, Sullivan C, Putcha D, Rentz D, et al. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Huijbers W, Ward AM, Johnson KA, Sperling RA. The Ups and Downs of the Posteromedial Cortex: Age- and Amyloid-Related Functional Alterations of the Encoding/Retrieval Flip in Cognitively Normal Older Adults. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur J Neurosci. 1996;8(7):1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Perl DP, Hof PR. Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. Journal of Comparative Neurology. 2001;438(3):353–376. doi: 10.1002/cne.1320. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281(5380):1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- White TP, Jansen M, Doege K, Mullinger KJ, Park SB, Liddle EB, et al. Theta power during encoding predicts subsequent-memory performance and default mode network deactivation. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25(11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li C-s. R. Functional connectivity mapping of the human precuneus by resting state fMRI. [doi: 10.1016/j.neuroimage.2011.11.023] Neuroimage(0) 2012 doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]