Abstract
Background
Studies suggest that prenatal vitamin D status may be inversely associated with lower respiratory tract infections (LRTIs) early in life. Studies of prenatal vitamin D status and development of asthma have inconsistent findings.
Methods
We examined associations of maternal mid-pregnancy 25-hydroxyvitamin D (25(OH)D) level with frequency of LRTIs by 36 months and with current asthma at 36 months using the Norwegian Mother and Child Cohort Study. Maternal plasma 25(OH)D level was measured using liquid chromatography-tandem mass spectrometry. Respiratory disorders were evaluated by maternal report through questionnaires. LRTIs were analyzed in a random sample of 1,248 children. Asthma was analyzed using a case-control design, including 489 cases and 1,183 controls. Multivariable generalized linear models calculated adjusted measures of association.
Results
The median gestational week of sample collection was 18 weeks (range 9, 35). The mean 25(OH)D level was 73.7 nmol/L (standard deviation 23.7). Higher maternal mid-pregnancy 25(OH)D level was associated with reduced risk of 3 or more LRTIs by 36 months versus none, adjusted risk ratio (RR) 0.74 [95% confidence interval (CI): 0.58, 0.93] per 20 nmol/L increase. Associations were similar when examining frequency of LRTIs by 18 months and frequency of LRTIs between 18 and 36 months. Maternal mid-pregnancy 25(OH)D level was not significantly associated with current asthma at 36 months, adjusted odds ratio (OR) 0.91 [95% CI: 0.81, 1.02] per 20 nmol/L increase.
Conclusions
Higher maternal mid-pregnancy 25(OH)D level was associated with a modestly reduced risk of recurrent LRTIs by 36 months, but was not associated with current asthma at 36 months.
Keywords: asthma, lower respiratory tract infection, pregnancy, vitamin D
Lower maternal vitamin D status during pregnancy is associated with increased risk of some adverse pregnancy outcomes and childhood diseases.1, 2 Controversy remains about whether maternal vitamin D status during pregnancy may influence lower respiratory tract infections (LRTIs) and/or asthma in the offspring. LRTIs are important health problems during early childhood, leading to substantial health related costs, and positively associated with asthma.3 Because asthma before school age is an uncertain diagnosis, and often reflects repeated wheezing episodes due to LRTIs, it is important to consider these different early childhood respiratory symptoms when evaluating early asthma phenotypes.4, 5
Vitamin D2 and D3 are the two forms of vitamin D. Vitamin D3 is primarily gained through skin exposure to UVB-light, while both forms are obtained through diet and supplements. 25-hydroxyvitamin D (25(OH)D) is the major circulating metabolite, and the most common measure of vitamin D status, while 1,25-dihydroxyvitamin D (1,25(OH)2D) is the biologically active form.6 1,25(OH)2D may influence innate and adaptive immune system responses.7 Maternal 25(OH)D level during pregnancy is an important determinant of the fetal 25(OH)D level, which subsequently influences the fetal 1,25(OH)2D level.2, 8
Five previous studies evaluated prenatal 25(OH)D levels, measured in either the mother during pregnancy or cord blood, and LRTIs in the offspring, four of which indicated an inverse association.9–13 Studies of prenatal 25(OH)D level and development of wheezing or asthma report conflicting findings.10–12, 14 Most previous studies evaluated cord blood 25(OH)D levels or maternal 25(OH)D late in pregnancy.9–11, 13, 14 Only one study examined maternal 25(OH)D early in pregnancy.12 Early pregnancy may be particularly important for both fetal immune system and lung development.15, 16 In addition, the previously conducted studies of prenatal 25(OH)D level and childhood LRTIs focused on disease development during the first 12 months of life, while one study examined disease development up to 24 months of age, but this study did not distinguish between LRTIs that occurred before and after 12 months of age.13
We examined associations of maternal mid-pregnancy 25(OH)D level with frequency of LRTIs during the first 36 months of life and with current asthma at 36 months.
Methods
Study subjects
Study subjects included participants in the Norwegian Mother and Child Cohort Study (MoBa), administered by the Norwegian Institute of Public Health (NIPH).17, 18 MoBa recruited pregnant women between 1999 and 2008, at approximately 18 weeks gestation. The participation rate was 38.5%, resulting in 90,680 mothers and 108,859 children. Participants gave a written informed consent. The current study used follow-up questionnaires at 18-, 22-, and 30- gestational weeks, and when the child was 6-, 18-, and 36- months.19 Data from MoBa was linked to the Medical Birth Registry of Norway using 11-digit person identification numbers. The Norwegian Data Inspectorate and the Regional Ethics Committee for Medical Research approved this study.
Study design
Among MoBa children born between July 1st 2002 and December 31st 2003 with information from questionnaires at 18 and 22 gestational weeks (n=17,005 eligible children) we measured maternal mid-pregnancy 25(OH)D level in a simple random sample of 2,473 MoBa children. This time period within the MoBa recruitment period was selected because of the large number of participants that were recruited during this period and because their biological specimens had been processed at the time the list of samples to be pulled from the MoBa biobank was generated. Table S1 shows characteristics of the eligible children based on these criteria and of the random sample with 25(OH)D measurements. Overall, the distribution of maternal characteristics was similar among the eligible and randomly sampled individuals, with the exception of a modest difference in season of delivery. Among the random sample with 25(OH)D measurements, a total of 1,248 singletons with information from all follow-up questionnaires up to 36 months were included in an evaluation of frequency of LRTIs by 36 months (Figure 1). From the random sample, mothers of 1,246 singletons with complete follow-up information at 36 months had responded to questions classifying current asthma at 36 months, of which 63 were asthmatic (Figure 1).
Figure 1. Illustration of sample size.
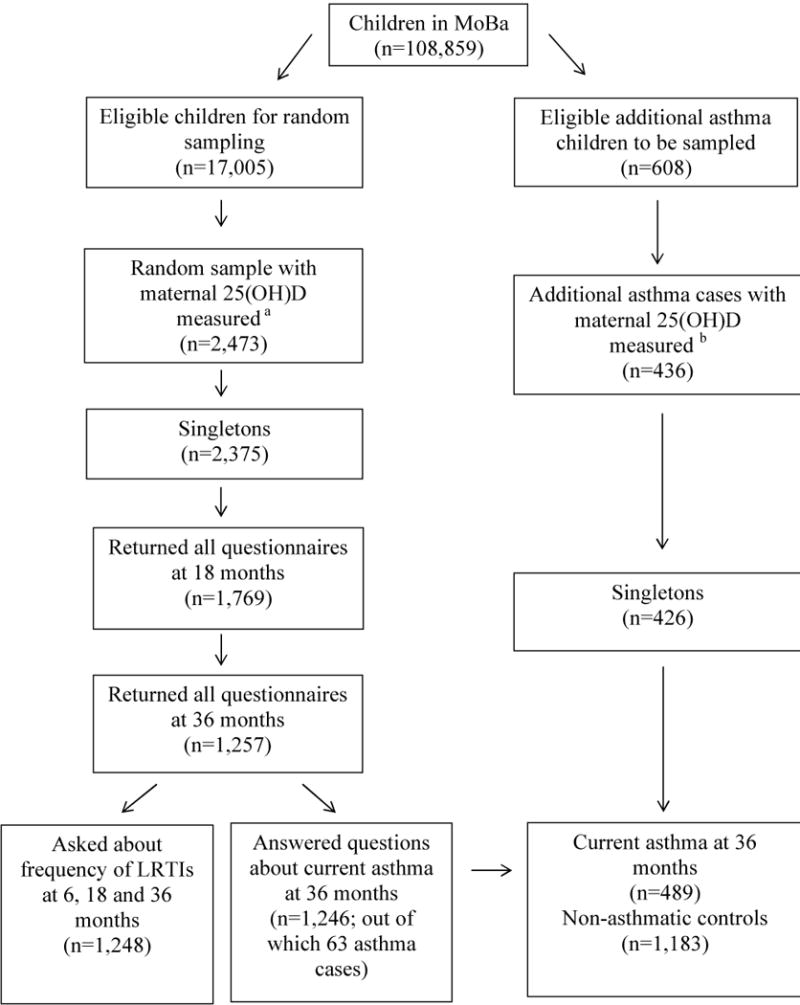
aA random sample of children born between July 2002 and December 2003 with information from questionnaires at 18-and 22-gestational weeks.
bChildren with current asthma at 36 months, born between July 2002 and June 2004, with information from all follow-up questionnaires at 36 months of age and not already selected into the random sample.
LRTIs: Lower Respiratory Tract Infections, MoBa: Norwegian Mother and Child Cohort Study
Furthermore, we measured maternal mid-pregnancy 25(OH)D level in an additional 426 singletons out of the 608 MoBa children with current asthma at 36 months, born between July 1st 2002 and June 30th 2004, with information from all follow-up questionnaires up to 36 months who had not already been selected into the random sample. Combining children from the random sample with the necessary follow-up information with these additional asthma cases, 489 children with current asthma at 36 months and 1,183 non-asthmatic controls were included in a case-control evaluation (Figure 1). The difference between the children from the random sample and the additional asthma cases included in this case-control evaluations is that the extra asthma cases were born over a 24 month period, instead of over the 18 month period that the random sample were born.
Exposure
MoBa collected venous blood samples from mothers during the routine ultrasound screening at approximately 18 gestational weeks. Plasma was mailed by regular mail to the biobank at the NIPH, and subsequently stored at −80ºC until analysis.20 The current study measured maternal plasma 25-hydroxyvitamin D2 and D3 levels using liquid chromatography-tandem mass spectrometry at BEVITAL laboratory. BEVITAL is approved by the Vitamin D External Quality Assurance Scheme. The within and between day coefficients of variance for 25-hydroxyvitamin D2 in this assay are 4.3%–4.5% and 4.6%–7.7%, respectively.21 The within and between day coefficients of variance for 25-hydroxyvitamin D3 are 4.4%–5.3% and 7.3%–8.2%, respectively.21 Only 2.7% of mothers in this study had 25-hydroxyvitamin D2 levels above the limit of detection. The exposure was therefore the sum of 25-hydroxyvitamin D2 and D3, subsequently referred to as 25(OH)D. 25(OH)D levels <=50 nmol/L has been considered deficient, 51–75 nmol/L insufficient and >75 nmol/L sufficient.22
Outcomes
The outcomes of interest included frequency of LRTIs by 36 months and current asthma at 36 months. LRTIs included the mother responding yes to whether the child had experienced “pneumonia/bronchitis, and/or respiratory syncytial virus” at 6 and 18 months, all queried in the same question, and the mother responding yes to whether the child had experienced “pneumonia” and/or “bronchitis” at 36 months, queried as two separate questions. The mother reported frequency of LRTIs during the following periods of the child’s life: up to 6 months on the 6 month questionnaire, between 6 and 18 months on the 18 month questionnaire, and between 18 and 36 months on the 36 month questionnaire. We categorized frequency of LRTIs by 36 months into none, 1–2 infections and 3 or more. Using 3 or more LRTIs as a cut off was selected a priori based on an association of 3 or more LRTIs with asthma exacerbations.23 To evaluate any indication of a dose response association of maternal 25-hydroxyvitamin D with frequency of LRTIs, children experiencing 1–2 LRTIs were included in a separate category, instead of being included in the comparison group. Current asthma at 36 months was classified based on meeting both criteria (i) the mother responding “yes, has now” to whether the child had asthma on the 36 month questionnaire; and (ii) the mother reporting names of inhaled asthma medications in response to which medications the child had taken the last 12 months on the 36 month questionnaire. Inhaled asthma medications included maternal report of inhaled glucocorticoids and/or beta-2 agonist.
Covariates
Potential confounders included maternal age at delivery (<25, 25–29, 30–34, ≥35), maternal parity (Primipara, 1, 2, 3 or more), maternal education (<12, 12, 13–16, ≥17 years), region of delivery (south/east, west, north, mid-country), maternal multivitamin intake during pregnancy (yes and no), maternal mid-pregnancy plasma folate level (≤ 6.18, 6.19–9.10, 9.11–15.36, ≥15.37 nmol/L), maternal pre-pregnancy body mass index (<18.5, 18.5–24.9, 25.0–29.9, ≥30), smoking during pregnancy (yes and no), maternal frequency of leisure time physical activity per week during pregnancy (None, 1–3, 4–5 and 6 or more), maternal history of asthma (yes and no), season of sample collection (winter, spring, summer and fall) and gestational week of sample collection (≤16, 17, 18, 19 and ≥20). Season of sample collection was adjusted for to accounted for natural variations in 25(OH)D level.24
Statistical analyses
In order to evaluate non-linear associations of maternal mid-pregnancy 25(OH)D level with frequency of LRTIs and/or asthma, the risk of LRTIs and log odds of asthma was plotted by maternal 25(OH)D level using fractional polynomial smoothed regression. As there were no indications of non-linear associations, the primary analyses evaluated maternal 25(OH)D level continuously, scaled to reflect the associations per 20 nmol/L increase in 25(OH)D level, approximately one standard deviation. We examined associations of maternal mid-pregnancy 25(OH)D level with frequency of LRTIs by 36 months using generalized linear models with a log-link function (i.e. log binomial regression), from which the risk ratio (RR) and 95% confidence interval (CI) were derived. Two separate models were run, first comparing children with 1–2 LRTIs against those who had experienced none, and second comparing children with 3 or more LRTIs against those who had experienced none. We did not examine the association of the clinical cut off values for 25(OH)D levels with frequency of LRTIs by 36 months because of the few number of cases and reduced statistical power. As a sensitivity analysis, frequency of LRTIs by 36 months was separated into frequency of LRTIs by 18 months (examined among 1,769 children with the necessary follow-up information at 18 months) and frequency of LRTIs between 18 and 36 months (examined among 1,248 children with the necessary follow-up information at 36 months) (Figure 1).
We examined the association of maternal mid-pregnancy 25(OH)D level with current asthma at 36 months using generalized linear models with a logit function (i.e. logistic regression), from which the odds ratios (OR) and 95% CI were derived. In addition, we evaluated the association of predefined clinical cut off values indicating maternal 25(OH)D deficiency, insufficiency and sufficiency with current asthma and 36 months. A sensitivity analysis was performed excluding the extra asthma cases born in 2004, because of the slight difference in the sampling strategy between the random sample and the extra asthma cases, meaning that we did not have any non-asthmatic controls born in 2004 with maternal 25(OH)D levels measured.
Multivariable models adjusted for all potential confounders identified, categorized as described and entered using dummy variables. Maternal age at delivery and pre-pregnancy BMI were adjusted for as continuous variables. Adjustment for season of sample collection is commonly done to evaluate the association of an “averaged” 25(OH)D level across the year.24 This might be less valuable if one is interested 25(OH)D levels during a specific exposure window such as a period during pregnancy. We therefore show adjusted associations with and without adjustment for season of sample collection. The amount of missing information on individual covariates was generally low (<3%), but approximately 8% of observations had missing information on one or more covariates in the multivariable analyses. We therefore imputed missing covariate information using chained equations, generating 10 imputed datasets.25 STATA version 12 was used for all analyses (Stata Corporation, College Station, Texas).
Results
The median gestational week of sample collection was 18 weeks (range 9, 35) and the mean 25(OH)D level was 73.7 nmol/L (standard deviation 23.7). A total of 16.8% of mothers had 25(OH)D levels <=50 nmol/L and 34.0% had 25(OH)D levels between 51–75 nmol/L. Maternal mid-pregnancy 25(OH)D level increased with age, education, folate level, frequency of physical activity, multivitamin use and gestational week of sample collection (Table 1). There was a non-linear variation in 25(OH)D level by pre-pregnancy body mass index. Furthermore, mothers who gave birth in the South/East region of Norway had the highest 25(OH)D levels compared to other regions (Table 1).
Table 1.
Distribution of maternal mid-pregnancy 25-hydroxyvitamin D by maternal and child characteristics (n=1,248)
| Covariate | Percent | Mean 25(OH)D (Std. Dev) | P-valuea |
|---|---|---|---|
|
| |||
| Maternal age at delivery, years | |||
| <25 | 9.9 | 67.8 (21.7) | 0.009 |
| 25–29 | 36.2 | 73.4 (23.8) | |
| 30–34 | 38.1 | 74.5 (23.5) | |
| ≥35 | 15.7 | 76.7 (24.5) | |
|
| |||
| Maternal parity | |||
| Primipara | 43.9 | 74.5 (23.0) | 0.610 |
| 1 | 36.1 | 72.6 (24.1) | |
| 2 | 14.5 | 74.4 (25.1) | |
| 3 or more | 5.5 | 74.2 (23.1) | |
|
| |||
| Maternal education, years | |||
| <12 | 6.4 | 67.4 (25.7) | 0.010 |
| 12 | 34.5 | 72.4 (23.5) | |
| 13–16 | 43.2 | 74.9 (23.1) | |
| ≥17 | 15.9 | 76.6 (24.0) | |
|
| |||
| Maternal pre-pregnancy BMI | |||
| <18.5 | 3.1 | 67.9 (19.8) | <0.001 |
| 18.5–24.9 | 66.1 | 76.1 (24.1) | |
| 25.0–29.9 | 20.5 | 71.9 (21.7) | |
| ≥30 | 10.2 | 67.4 (22.9) | |
|
| |||
| Maternal smoking during pregnancy | |||
| Yes | 12.3 | 71.3 (24.9) | 0.153 |
| No | 87.7 | 74.2 (23.4) | |
|
| |||
| Maternal multivitamin use during pregnancy | |||
| Yes | 590 | 75.2 (24.0) | 0.044 |
| No | 658 | 72.5 (23.3) | |
|
| |||
| Maternal plasma folate level, nmol/L | |||
| ≤6.18 | 25.0 | 69.1 (24.1) | <0.001 |
| 6.19–9.10 | 25.0 | 73.7 (23.7) | |
| 9.11–15.36 | 24.6 | 75.8 (22.6) | |
| ≥15.37 | 25.4 | 76.6 (23.6) | |
|
| |||
| Maternal average frequency of leisure time physical activity during pregnancy (per week) | |||
| None | 130 | 66.9 (24.4) | <0.001 |
| 1–3 | 661 | 71.6 (22.6) | |
| 4–5 | 287 | 79.0 (24.0) | |
| 6 or more | 170 | 78.7 (24.1) | |
|
| |||
| Maternal history of asthma | |||
| Yes | 6.4 | 73.2 (23.5) | 0.805 |
| No | 93.6 | 73.8 (23.7) | |
|
| |||
| Region of delivery | |||
| South/East | 43.9 | 76.4 (24.6) | 0.003 |
| West | 32.2 | 72.4 (21.4) | |
| North | 16.5 | 70.4 (23.5) | |
| Mid-country | 7.5 | 70.3 (25.0) | |
|
| |||
| Gestational week of sample collection | |||
| ≤16 | 7.4 | 70.2 (21.2) | 0.010 |
| 17 | 24.7 | 71.6 (23.8) | |
| 18 | 37.2 | 73.5 (23.2) | |
| 19 | 22.2 | 75.5 (24.8) | |
| ≥20 | 8.6 | 79.9 (23.1) | |
|
| |||
| Season of sample collection | |||
| Winter (December, January, February) | 6.6 | 60.3 (18.9) | < 0.001 |
| Spring (March, April, May) | 31.6 | 60.7 (21.8) | |
| Summer (June, July, August) | 40.2 | 82.6 (20.8) | |
| Autumn (September, October, November) | 21.6 | 80.5 (22.3) | |
25(OH)D:25-hydroxyvitamin D, BMI: body mass index
P-value from analysis of variance
A total of 19.5% of children experienced 1 or 2 LRTIs and 5.7% experienced 3 or more LRTIs by 36 months. Maternal mid-pregnancy 25(OH)D level was not associated with 1 or 2 LRTIs versus none the first 36 months of life, adjusted RR 0.98 [95% CI: 0.87, 1.12] (Table 2). In contrast, higher maternal mid-pregnancy 25(OH)D level was associated with a modest reduced risk of 3 or more LRTIs versus none by 36 months, adjusted RR 0.74 [95% CI: 0.58, 0.93] per 20 nmol/L increase (Table 2). Multivariable adjustment caused only modest changes of the crude associations. In the sensitivity analysis, the associations of maternal mid-pregnancy 25(OH)D level with risk of 3 or more LRTIs versus none by 18 months, adjusted RR 0.75 [95% CI: 0.51, 1.10] per 20 nmol/L increase, and with risk of 3 or more LRTIs versus none between 18 and 36 months, adjusted RR 0.73 [95% CI: 0.51, 1.05] per 20 nmol/L increase, were similar (Table 3).
Table 2.
Associations of a 20 nmol/L increase in maternal mid-pregnancy 25-hydroxyvitamin D level with frequency of lower respiratory tract infections the first 36 months of life
| Outcome | N | Mean 25(OH)D (Std. Dev) | Unadj. RR (95% CI) | Adj. RR (95% CI)a | Adj. RR (95% CI)b |
|---|---|---|---|---|---|
|
| |||||
| Frequency of LRTIs | |||||
| None | 883 | 74.7 (23.9) | 1 | 1 | 1 |
| 1 or 2 | 230 | 73.7 (23.4) | 0.97 (0.88, 1.07) | 0.98 (0.89, 1.08) | 0.98 (0.87, 1.12) |
| 3 or more | 67 | 66.4 (22.0) | 0.76 (0.62, 0.92) | 0.77 (0.63, 0.94) | 0.74 (0.58, 0.93) |
25(OH)D: 25-hydroxyvitamin D, LRTIs: lower respiratory tract infections
Maternal 25(OH)D level among children with 1 or 2 infections and children with 3 or more infections compared against children with none.
Adjusted for maternal age at delivery, maternal education, maternal parity, region of delivery, maternal pre-pregnancy BMI, maternal smoking during pregnancy, maternal mid-pregnancy plasma folate level, maternal history of asthma, maternal multivitamin use during pregnancy, maternal frequency of leisure time physical activity during pregnancy and gestational week of sample collection.
Adjustment for covariates specified in model a with additional adjustment for season of sample collection using three dummy variables.
Multiple imputation of missing covariate information using chained equations.
Table 3.
Associations of a 20 nmol/L increase in maternal mid-pregnancy 25-hydroxyvitamin D with frequency of lower respiratory tract infections by 18 months and between 18 and 36 months of age
| Outcome | n | Mean 25(OH)D (Std. Dev) | Unadj. RR (95% CI) | Adj. RR (95% CI)a | Adj. RR (95% CI)b |
|---|---|---|---|---|---|
|
| |||||
| Frequency of LRTIs by 18 months | |||||
| None | 1,392 | 74.5 (23.8) | 1 | 1 | 1 |
| 1 or 2 | 269 | 72.1 (23.5) | 0.93 (0.85, 1.02) | 0.95 (0.86, 1.04) | 0.97 (0.87, 1.07) |
| 3 or more | 27 | 67.8 (26.5) | 0.79 (0.58, 1.09) | 0.76 (0.55, 1.06) | 0.75 (0.51, 1.10) |
|
| |||||
| Frequency of LRTIs between 18 and 36 months | |||||
| None | 1,062 | 74.0 (23.9) | 1 | 1 | 1 |
| 1 or 2 | 145 | 74.0 (21.9) | 1.00 (0.88, 1.14) | 1.00 (0.88, 1.15) | 0.96 (0.83, 1.11) |
| 3 or more | 29 | 66.0 (22.6) | 0.76 (0.56, 1.03) | 0.73 (0.53, 1.00) | 0.73 (0.51, 1.05) |
25(OH)D - 25-hydroxyvitamin D; LRTIs- lower respiratory tract infections
Maternal 25(OH)D level among children with 1 or 2 infections and children with 3 or more infections compared against children with none.
Adjusted for maternal age at delivery, maternal education, maternal parity, region of delivery, maternal pre-pregnancy BMI, maternal smoking during pregnancy, maternal mid-pregnancy plasma folate level, maternal history of asthma, maternal multivitamin use during pregnancy, maternal frequency of leisure time physical activity during pregnancy and gestational week of sample collection.
Adjustment for covariates specified in modela with additional adjustment for season of sample collection using three dummy variables.
Multiple imputation of missing covariate information using chained equations.
Among children from the random sample, 5.1% had current asthma at 36 months, reflecting the distribution of asthma in MoBa. Maternal mid-pregnancy 25(OH)D level was not significantly associated with asthma, adjusted OR 0.91 [95% CI: 0.81, 1.02] per 20 nmol/L increase (Table 4). Adjusting for potential confounding factors did not change the observed association, while additional adjustment for season of sample collection attenuated the observed association towards the null. Examining the commonly used cut off values for 25(OH)D levels, children of mothers who had 25(OH)D levels >75 had a decreased odds of current asthma compared to children of mothers with 25(OH)D levels >=50, adjusted OR 0.67 [95% CI: 0.48, 0.95] (Table 4). Notably, this inverse association was not seen after excluding the extra asthma cases born in 2004.
Table 4.
Association of maternal mid-pregnancy 25-hydroxyvitamin D level with current asthma at 36 months
| Exposure | Cases (n=489) |
Controls (n=1,183) |
Crude OR (95%CI) |
Adj. OR (95% CI)a |
Adj. OR (95% CI)b |
Adj. OR (95% CI)c |
|---|---|---|---|---|---|---|
|
| ||||||
| 20 nmol/L increase in | Mean (std.dev.) | Mean (std.dev.) | ||||
| 25(OH)D | 68.6 (23.5) | 73.7 (23.8) | 0.83 (0.76, 0.91) | 0.84 (0.76, 0.92) | 0.91 (0.81, 1.02) | 0.95 (0.83, 1.08) |
|
| ||||||
| 25(OH)D level in nmol/L | % | % | ||||
| <=50 | 23.3 | 17.1 | 1 | 1 | 1 | 1 |
| 51–75 | 38.2 | 33.6 | 0.83 (0.62, 1.11) | 0.78 (0.58, 1.06) | 0.84 (0.61, 1.17) | 1.05 (0.72, 1.53) |
| >75 | 38.5 | 49.3 | 0.57 (0.43, 0.76) | 0.55 (0.41, 0.75) | 0.67 (0.48, 0.95) | 0.83 (0.56, 1.24) |
25(OH)D: 25-hydroxyvitamin D
Adjusted for maternal age at delivery, maternal education, maternal parity, region of delivery, maternal pre-pregnancy BMI, maternal smoking during pregnancy, maternal mid-pregnancy plasma folate level, maternal history of asthma, maternal multivitamin use during pregnancy, maternal frequency of leisure time physical activity during pregnancy and gestational week of sample collection.
Adjustment for covariates specified in model a with additional adjustment for season of sample collection using three dummy variables.
Sensitivity analysis excluded the asthma cases born in 2004 (172 cases). Adjustment for covariates specified in model a with additional adjustment for season of sample collection using three dummy variables.
Multiple imputation of missing covariate information using chained equations.
Comments
In this population based study, higher maternal mid-pregnancy 25(OH)D level was associated with a reduced risk of 3 or more LRTIs by 36 months. Similar associations of maternal mid-pregnancy 25(OH)D level with risk of 3 or more LRTIs by 18 months and with risk of 3 or more LRTIs between 18 and 36 months were observed, although they did not reach statistical significance, likely due to the fewer number of cases within these specific age periods. Maternal mid-pregnancy 25(OH)D level showed a borderline non-significant inverse association with current asthma at 36 months, which was further attenuated towards the null in sensitivity analyses.
A limited number of previous studies have examined associations of prenatal 25(OH)D levels with risk of LRTIs (Table S2).9–12 Inverse associations of cord blood 25(OH)D levels with 1: respiratory syncytial virus the first year of life, 2: experiencing a cold, cough, whooping cough, chest infections and/or ear infections by 3 months, and 3: bronchiolitis and/or pneumonia the first 24 months of life have been identified.9, 10, 13 Two previous studies evaluated maternal 25(OH)D levels during pregnancy. One study found that maternal 25(OH)D level around 33 gestational weeks was positively associated with LRTIs before 9 months.11 Another study reported an inverse association between maternal 25(OH)D level around 13 gestational weeks and LRTIs by 12 months.12
The current study supports the previous four studies indicating that higher prenatal 25(OH)D level is associated with reduced risk of LRTIs in the offspring.9, 10, 12 Notably, only two of the previous studies had a sample size of more than 500.10, 12 No previous study has to our knowledge examined frequency of LRTIs. We were therefore able to show that maternal mid-pregnancy 25(OH)D level was inversely associated with recurrent LRTIs. Furthermore, most of the previous studies focused on disease developed within the first 12 months, while one study examined 25(OH)D levels during pregnancy and development of LRTIs by 24 months, but this study did not distinguish between LRTIs occurring before 12 months and after.13 The current study therefore contributes information that the previously observed inverse association of prenatal 25(OH)D levels with LRTIs may extend beyond the first 12 months of life.
Four previous studies evaluated prenatal 25(OH)D level and development of wheezing and/or asthma (Table S3).10–12, 14 One study indicated that higher cord blood 25(OH)D level was associated with reduced risk of early childhood wheezing, another reported a positive association of maternal 25(OH)D level around 33 gestational weeks with asthma at age 9, while three other studies found no statistically significant associations of prenatal 25(OH)D levels with asthma at ages 4–6 years.10–12, 14 Furthermore, higher maternal vitamin D intake during pregnancy has been associated with reduced risk of wheezing and/or asthma in the offspring.26–29
The discrepancy between these previous studies may be due to differences in time point of 25(OH)D measurement and/or evaluating different wheezing/asthma phenotypes.4, 5 In contrast to the British study, our study provided no indication of a positive association of maternal mid-pregnancy 25(OH)D levels with current asthma at 36 months. With our study, there are now three studies indicating no association of prenatal 25(OH)D levels with early asthma phenotypes.12, 14 The current study had twice the number of asthma cases compared to the previous studies, providing better statistical power to examine the association. Furthermore, only one study has previously examined maternal 25-hydroxyvitamin D levels early in pregnancy, around 13 gestational weeks, and development of asthma, while the other studies measured 25(OH)D levels in cord blood or in the mother during late pregnancy.12 Finally, the differences in associations identified in studies evaluating vitamin D intake during pregnancy versus prenatal 25(OH)D levels and it’s association with asthma phenotypes might be due to differences in confounding factors that were accounted for.
An inverse association of prenatal 25(OH)D levels with childhood respiratory disorders has at least three potential explanations. First, maternal 25(OH)D levels during pregnancy may directly influence the fetal 1,25(OH)2D level.2, 8 1,25(OH)2D binds to vitamin D response elements (VDRE) on DNA, and several immune regulatory genes contain VDRE.7, 30 Higher 1,25(OH)2D increases antimicrobial peptides, inhibits dendritic cell maturation and increases Treg cells, while its influence on Th1/Th2 balance remains unclear. 7, 31 Pregnancy trimester specific differences in these mechanisms cannot be excluded. We measured 25(OH)D around 18 gestational weeks, the canalicular stage of lung development, characterized by formation of the capillary network and differentiation of alveolar cells.15, 32
Secondly, the association of higher prenatal 25(OH)D level with reduced risk of recurrent LRTIs the first 36 months of life may be due to lasting epigenetic changes, such as DNA methylation, histone modifications, and/or expression of micro RNAs.33 An animal study identified that vitamin D deficiency in utero resulted in changes in invariant natural killer T cells in the offspring, hypothesized to be due to epigenetic mechanisms.34 To our knowledge, there are no published studies of maternal 25(OH)D level during pregnancy and epigenetic changes in the offspring among humans.
Third, maternal 25(OH)D during pregnancy may simply be an imperfect marker of the child’s own vitamin D status the first years of life. Maternal and cord blood 25(OH)D have a strong correlation at delivery, with a correlation coefficient of 0.79.35 This strong correlation at delivery is expected to decrease over time. Case-control studies found lower 25(OH)D levels among children suffering from LRTIs and among children suffering from asthma.36–39 No prospective study identified has measured both maternal 25(OH)D level during pregnancy and the child’s 25(OH)D during the first years of life, and it therefore remains to properly distinguish prenatal and postnatal influences of 25(OH)D on respiratory health.
Strengths of this study include the large population based sample, prospective data collection, and adjustment for a wide range of potential confounders. This study also has limitations. We only measured maternal 25(OH)D level at one point during pregnancy. 25(OH)D has a half-life of about 20 days, indicating the time period that our one measurement might reflect.40 Mothers reported childhood respiratory disorders through questionnaires, which may have caused misclassification. Due to the prospective data collection, any outcome misclassification is unlikely to be influenced by the exposure of interest. Furthermore, asthma at 36 months likely contains a large proportion of wheezing due to LRTIs.4 Furthermore, using maternal report of LRTIs has a degree of uncertainty. As only a portion of children with LRTIs are admitted to a hospital, using hospital discharge registries would only cover a small portion of cases, and using maternal report through questionnaires allowed us to cover a larger spectrum of disease severity. The child’s vitamin D status the first 36 months of life was also not available. However, adjustment for child characteristics that may influence their vitamin D status the first 36 months of life, such as gender, duration of breast feeding and vitamin D supplement intake when the child was 6 and 18 months of age, did not change the associations. A selection bias may also be present due to low participation rate of invited pregnant women or because mothers of children with respiratory disorders might have lower response rate. Mothers of children with complete follow-up information at 36 months were more educated and less likely to smoke (Table S4). Notably, there was no difference in maternal history of asthma or maternal mid-pregnancy 25(OH)D level by follow-up information available (Table S4).
In conclusion, higher maternal mid-pregnancy 25(OH)D level was associated with a modest reduced risk of recurrent LRTIs by 36 months, but was not significantly associated with current asthma at 36 months. The current study provides further evidence that the previously observed inverse association of prenatal 25(OH)D levels with risk of LRTIs may extend beyond the first year of life. Future studies would benefit from obtaining both repeat measurements of maternal 25(OH)D levels during pregnancy and child 25(OH)D levels early in life, in order to properly distinguish between prenatal and postnatal influences.
Supplementary Material
Acknowledgments
The Norwegian Mother and Child Cohort Study is supported by NIH (NIH/NIEHS project number ZIAES049019, NIH/NINDS grant no.1 UO1 NS 047537-01 and grant no.2 UO1 NS 047537-06A1), in addition to a grant from the Norwegian Research Council/FUGE (grant number 151918/S10). The first author was further supported by a grant from the Norwegian Extra-Foundation for Health and Rehabilitation (grant number 2011.2.0218). The authors are grateful to all the families participating in the Norwegian Mother and Child Cohort Study.
References
- 1.Dror DK. Vitamin D status during pregnancy: maternal, fetal, and postnatal outcomes. Currrent Opinion in Obstetrics and Gynecology. 2011;23:422–426. doi: 10.1097/GCO.0b013e32834cb791. [DOI] [PubMed] [Google Scholar]
- 2.Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients. 2012;4:208–230. doi: 10.3390/nu4030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt PG, Rowe J, Kusel M, Parsons F, Hollams EM, Bosco A, et al. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. Journal of Allergy and Clinical Immunology. 2010;125:653–659. doi: 10.1016/j.jaci.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Bisgaard H, Bonnelykke K. Long-term studies of the natural history of asthma in childhood. Journal of Allergy and Clinical Immunology. 2010;126:187–197. doi: 10.1016/j.jaci.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Boehmer AL. Paediatric asthma: everything that seemed to be certain no longer is. Paediatric Respiratory Reviews. 2010;11:185–190. doi: 10.1016/j.prrv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Jones G. Metabolism and biomarkers of vitamin D. Scandinavian Journal of Clinical and Laboratory Investigation Supplementum. 2012;243:7–13. doi: 10.3109/00365513.2012.681892. [DOI] [PubMed] [Google Scholar]
- 7.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Current Opinion in Pharmacology. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Novakovic B, Galati JC, Chen A, Morley R, Craig JM, Saffery R. Maternal vitamin D predominates over genetic factors in determining neonatal circulating vitamin D concentrations. American Journal of Clinical Nutrition. 2012;96:188–195. doi: 10.3945/ajcn.112.035683. [DOI] [PubMed] [Google Scholar]
- 9.Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JL, et al. Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics. 2011;127:e1513–e1520. doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 10.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 11.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. European Journal of Clinical Nutrition. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, Vioque J, et al. Maternal Vitamin D Status in Pregnancy and Risk of Lower Respiratory Tract Infections, Wheezing, and Asthma in Offspring. Epidemiology. 2012;23:64–71. doi: 10.1097/EDE.0b013e31823a44d3. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed WA, Al-Shehri MA. Cord blood 25-hydroxyvitamin D levels and the risk of acute lower respiratory tract infection in early childhood. Journal of Tropical Pediatrics. 2013;59:29–35. doi: 10.1093/tropej/fms042. [DOI] [PubMed] [Google Scholar]
- 14.Pike KC, Inskip HM, Robinson S, Lucas JS, Cooper C, Harvey NC, et al. Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax. 2012;67:950–956. doi: 10.1136/thoraxjnl-2012-201888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bizzarro MJ, Gross I. Effects of hormones on fetal lung development. Obstetrics & Gynecology Clinics of North America. 2004;31:949–961. doi: 10.1016/j.ogc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Ygberg S, Nilsson A. The developing immune system - from foetus to toddler. Acta Paediatrica. 2012;101:120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 17.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) International Journal of Epidemiology. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 18.Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatric and Perinatal Epidemiology. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 19.Norwegian Institute of Public Health. The Norwegian Mother and Child Cohort Study Questionnaires. ( http://www.fhi.no/eway/default.aspx?pid=240&trg=MainContent_6894&Main_6664=6894:0:25,7372:1:0:0:::0:0&MainContent_6894=6706:0:25,7375:1:0:0:::0:0) (Accessed December 1st2012)
- 20.Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, et al. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. European Journal of Epidemiology. 2006;21:619–625. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Midttun O, Ueland PM. Determination of vitamins A, D and E in a small volume of human plasma by a high-throughput method based on liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2011;25:1942–1948. doi: 10.1002/rcm.5073. [DOI] [PubMed] [Google Scholar]
- 22.Dietary Reference Intakes for Vitamin D and Calcium. Washington DC, USA: National Academy Press; 2010. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 23.Ten BA, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26:812–818. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Jacobs EJ, McCullough ML, Rodriguez C, Thun MJ, Calle EE, et al. Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25-hydroxyvitamin d. American Journal of Epidemiology. 2009;170:88–94. doi: 10.1093/aje/kwp086. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Jacobs EJ, McCullough ML, Rodriguez C, Thun MJ, Calle EE, et al. Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25-hydroxyvitamin d. American Journal of Epidemiology. 2009;170:88–94. doi: 10.1093/aje/kwp086. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. The American Journal of Clinical Nutrition. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. The American Journal of Clinical Nutrition. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 29.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clinical & Experimental Allergy. 2009;39:875–882. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 30.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. European Respiratory Journal. 2010;35:1228–1234. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 31.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. Journal of Molecular Medicine. 2010;88:441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatric Research. 2009;65:106R–113R. doi: 10.1203/PDR.0b013e31819dba91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest. 2007;132:651–656. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- 34.Gabory A, Attig L, Junien C. Developmental programming and epigenetics. American Journal of Clinical Nutrition. 2011;94:1943S–1952S. doi: 10.3945/ajcn.110.000927. [DOI] [PubMed] [Google Scholar]
- 35.Yu S, Cantorna MT. Epigenetic reduction in invariant NKT cells following in utero vitamin D deficiency in mice. The Journal of Immunology. 2011;186:1384–1390. doi: 10.4049/jimmunol.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouillon R, Van BH, De MP. 25-hydroxyvitamin D and its binding protein in maternal and cord serum. The Journal of Clininical Endocrinology & Metabolism. 1977;45:679–684. doi: 10.1210/jcem-45-4-679. [DOI] [PubMed] [Google Scholar]
- 37.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. European Journal of Clinical Nutrition. 2009;63:473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 38.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. European Journal of Clinical Nutrition. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 39.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. Journal of Allergy and Clinical Immunology. 2010;126:52–58. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, et al. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. European Respiratory Journal. 2011;38:1320–1327. doi: 10.1183/09031936.00029011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


