Abstract
Coal consumption is one important contributor to energy production, and is regarded as one of the most important sources of air pollutants that have considerable impacts on human health and climate change. Emissions of polycyclic aromatic hydrocarbons (PAHs) from coal combustion were studied in a typical stove. Emission factors (EFs) of 16 EPA priority PAHs from tested coals ranged from 6.25 ± 1.16 mg kg−1 (anthracite) to 253 ± 170 mg kg−1 (bituminous), with NAP and PHE dominated in gaseous and particulate phases, respectively. Size distributions of particulate phase PAHs from tested coals showed that they were mostly associated with particulate matter (PM) with size either between 0.7 and 2.1 μm or less than 0.4 μm (PM0.4). In the latter category, not only were more PAHs present in PM0.4, but also contained higher fractions of high molecular weight PAHs. Generally, there were more than 89% of total particulate phase PAHs associated with PM2.5. Gas-particle partitioning of freshly emitted PAHs from residential coal combustions were thought to be mainly controlled by absorption rather than adsorption, which is similar to those from other sources. Besides, the influence of fuel properties and combustion conditions was further investigated by using stepwise regression analysis, which indicated that almost 57 ± 10% of total variations in PAH EFs can be accounted for by moisture and volatile matter content of coal in residential combustion.
Keywords: PAHs, Residential coal combustion, Emission factors, Size distribution
1. Introduction
Coal consumption contributed about one fourth of the world total primary energy (IEA, 2009), and about 69–76% in China (NBSC, 2009). Total consumed quantities of coal in industrial and residential sectors in 2007 were 2.5 × 109 and 8.1 × 107 tons, respectively (NBSC, 2009). However, emissions of pollutants, like polycyclic aromatic hydrocarbons (PAHs), were much higher from the latter primarily due to relatively higher emission factors (EFs) of residential coal combustion (Zhang et al., 2008a,b). Amount of coal used in residential sector in China increased continuously till 1990, and then decreased slowly, and reached a plateau since 2000 (NBSC, 2009). Currently in China, though coal combustion in large cities is limited and well controlled, extensive and dispersive use without effective control technologies still exists in rural area, and one common use is consumption in extensive residential stoves for cooking and heating in Northern China, especially in winter (Chen et al., 2005; Zhang et al., 2008a; Liu et al., 2009).
A large amount of coal consumption has arisen high concerns due to considerable adverse impacts on both regional and global air quality, and also on human health (Zhang and Smith, 2007; Smith et al., 2009). Coal combustion produces groups of gaseous pollutants and particulate matter (PM), which often contains toxic components like heavy metals, PAHs and their derivatives (Borm, 1997; Zhang et al., 2008a; Liu et al., 2009). Moreover, PM and its toxic constituents from coal combustions are often formed and emitted in small diameter size (Bond et al., 2002; Chen et al., 2004), and can penetrate deeper into the lung region which may result in more harmful effect (Dockey et al., 1993). Exposure to household smoke is believed to be responsible for the diseases, like respiratory infections, chronic obstructive pulmonary disease, lung cancer, etc. (Chapman et al., 2005). It ranked the eighth and the sixth largest risk factor for global and Chinese disease, respectively, and caused the more than 1.6 million deaths in world (Ezzati et al., 2002; Zhang and Smith, 2007).
Among various pollutants from coal combustion, PAHs, which are ubiquitous and often emitted from the incomplete combustion, are one of the most toxic organic pollutants of concern in China. Exposure to high level of PAHs is believed to be related to increased cancer risk (Boström et al., 2002; Zhang et al., 2009). For example, inhalation exposure to ambient PAHs was estimated to cause the overall population attributable fraction (PAF) for lung cancer at 0.91–2.6% in China (Zhang et al., 2009). Global emissions of total PAHs in 2004 were 520 Gg year−1, of which about 29% from China (Zhang and Tao, 2009). Due to the rapid growth of the economy and population in China, total emissions of PAHs increased continuously in the last several decades with the increase of energy consumption. It was estimated that approximately 10.7% of the total PAHs emissions in China in 2004 were from residential coal combustion, which contributed 22.4% of total BaPeq (BaP toxic equivalent quality) emissions (Zhang and Tao, 2009). In one extensive survey with passive air sampler (PAS) in Northern China, Liu et al. (2007) reported that PAHs concentrations during winter time were about 514 ng m−3 and 610 ng m−3 in urban and rural areas, respectively. Due to the predominant sources of residential biomass and coal combustion, rural areas often have higher PAHs pollution (Wu et al., 2006; Liu et al., 2007; Wang et al., 2009), which could have more threat to human health when considering that more toxic high molecular weight PAHs often tend to be associated fine and can penetrate deeper into the lung region (Dockey et al., 1993; Chen et al., 2004; Wang et al., 2009).
With the purpose of diagnosing the relative contributions of various sources and making effective controlling strategies, accurate and updated inventories are often conducted based on the strengths of emission activities and EFs. EFs regarded as the key parameters when developing emission inventories often vary in orders of magnitude and are often combustion process dependent due to different fuel types, stove/coal combination, combustion conditions and operation technologies (Zhang et al., 2008a,b; Liu et al., 2009), and this often introduces high uncertainties in emission estimation (Gullett et al., 2003; Oanh et al., 2005; Xu et al., 2006). Measurement of PAH EFs from coal combustion had been done in the past, and there is more attention on the residential coal stove emissions (Chen et al., 2004, 2005; Liu et al., 2009). In residential combustion, relatively low combustion temperature and limited oxygen supply often yield higher PAHs emissions (Chen et al., 2005). There were several studies focusing on the PAH emissions from different coal/stove combinations (Chen et al., 2004, 2005; Zhang et al., 2008a; Liu et al., 2009). However, EF data are still limited, and most of them were conducted in laboratory, rather than real household combustions. The difference in PM EFs and composition profiles between field and laboratory chamber combustion of crop and wood had been documented in some studies (Dhammapala et al., 2007; Jimenez et al., 2007; Roden et al., 2006, 2009). It was found that both PM and CO emissions from actual cooking practice were significantly higher than those measured in laboratory simulated combustions (Roden et al., 2009). Hence, there was a desire to get both EFs and distributions of PAHs from coal combustions under actual residential conditions.
In this study, emissions of PAHs from selected coals burnt in a commonly used stove in rural China were studied. In addition to get more realistic EFs under actual residential conditions, the influence of fuel properties and combustion conditions, size distributions of particulate phase PAHs, and gas-particle partitioning of freshly emitted PAHs were also investigated.
2. Materials and methods
2.1. Coals and combustion experiments
Information about the coal, stove and combustion processes was described elsewhere in detail (Shen et al., in press), and summarized here in brief. Two honeycomb briquettes (a 16 hole column with diameter at 15 cm and 11 cm thick), and three raw chunk coals from Beijing, Taiyuan and Yulin, where coal productions contributed large percentage of the total in China, were tested in this study. Elemental and proximate analysis results of tested coals are provided in the supplementary material (S1). Honeycomb briquettes from Beijing were the only anthracite tested with 4% volatile matter (VM). The other honeycomb coal was low volatile bituminous (LVB) with 14% volatile matter, and three raw chunk coals were all median volatile bituminous (MVB) with volatile matter content at 23–29%.
Coal combustion experiments were conducted in duplicate in one rural kitchen according to the real practice as rural residents do. A commonly used coal stove was purchased from the local market. This type of coal stove is widely used in rural China, especially after the 1980s when the National Improved Stove Program was conducted (Smith et al., 1993). It is estimated that these so-called improved stoves had been introduced into about 349 million homes until 2006 (Ministry of Agriculture, 2007). Coals were ignited outdoor using small wood chips, and then, the stove was moved into the kitchen and placed under a stainless hood, which was connected to the mixing chamber (4.5 m3) for sampling. Combustion conditions, including relative humidity, smoke temperature, and exact duration were recorded.
EFs were measured based on the carbon mass balance method (Zhang et al., 2000). It assumed the total carbon burnt was emitted in the form of gaseous CO2, CO, total hydrocarbon carbon (THC) and total carbon in PM. The advantage of the method is that it is not necessary to collect all the species, and it was widely used in many EF measurements, especially in field studies (Zhang et al., 2000; Roden et al., 2006; Dhammapala et al., 2007). CO2 and CO concentrations were measured with online NDIR detectors and automatically recorded every 2 s. Background concentrations of CO, CO2, PM and PAHs in the mixing chamber before each combustion experiment were measured and corrected with the results. Statistica was used for data comparison (the non-parametric Wilcoxon test for paired samples) and Spearman correlation analysis, and a significant level of 0.05 was adopted.
2.2. Sampling and PAHs analysis
Gaseous and particulate phases PAHs were respectively sampled on polyurethane foam (PUF) plug (Supelco, 22 mm diameter × 7.6 cm) and quartz fiber filters (QFFs, 22 mm in diameter), using an active sampler (Jiangsu Eltong Electric Corp. Co., Ltd., China) at a flow rate of 1.5 l min−1. To investigate the size distribution of freshly emitted particulate phase PAHs, a nine stage cascade impactor at a flow rate of 28.3 l min−1 with cutoff diameters of <0.4, 0.4–0.7, 0.7–1.1, 1.1–2.1, 2.1–3.3, 3.3–4.7, 4.7–5.8, 5.8–9.0, and 9.0–10.0 μm (FA-3, Kangjie, China) was used.
After sampling, both PUF and QFF were packed in aluminum foil and stored at −20 °C. In laboratory, PAHs analysis, including extraction, cleanup and detection, followed the procedure in our previous study (Wang et al., 2009). Briefly, PUF was Soxhlet extracted with 150 ml dichloromethane (DCM) for 8 h. Particulate loaded QFF was extracted by 25 ml hexane/acetone mixture (1:1) using a microwave accelerated reaction system (CEM Corporation, Matthews, NC, USA). Microwave power was set at 1200 W and the temperature program was: ramp to 110 °C in 10 min and held at 110 °C for another 10 min. Both PUF and QFF extracts were concentrated to 1 ml and then transferred to a silica/alumina chromatography for cleanup. The elution solution (70 ml hexane/DCM mixture, 1:1) was collected, concentrated, conversed to hexane solution, and then added with 250 ng internal standards (2-fluoro-1, 1′-biphenyl and p-terphenyl-d14, J&W Chemical Ltd., USA).
A gas chromatography (GC, Agilent 6890) connected to a mass spectrometry (MS, Agilent 5973), equipped with an HP-5MS capillary column (30 m × 0.25 mm × 0.25 μm) was used for PAHs analysis. The oven temperature was kept at 50 °C for 1 min, increased to 150 °C at a rate of 10 °C min−1, to 240 °C at 3 °C min−1, and then to 280 °C held for 20 min. Target PAHs were identified based on retention time and qualified ions of standards. The following compounds were measured: naphthalene (NAP), acenaphthylene (ACY), acenaphthene (ACE), fluorene (FLO), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLA), pyrene (PYR), benzo(a)anthracene (BaA), chrysene (CHR), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenz(a,h)anthracene (DahA), indeno(l,2,3-cd)pyrene (IcdP), and benzo(g,h,i)perylene (BghiP).
2.3. Quality control and assurance
Before sampling, PUF used for collecting gaseous PAHs, was pre-extracted by acetone, followed by dichloromethane and then hexane. Each of them lasted for about 8 h. QFFs were baked at 450 °C for about 6 h, and stored in desiccators before use. All solvents (Beijing Reagent, China) were re-distillated and checked for blank of target before use. There were no target PAHs detected in re-distillated solvent. The silica gel and alumina (100–200 mesh) were baked at 450 °C for 6 h, activated at 300 °C for 12 h, and deactivated with deionized water (3%, w/w) prior to use. The anhydrous sodium sulfate was baked at 450 °C for 8 h. All glassware was cleaned in an ultrasonic cleaner and baked at 500 °C for at least 10 h. Field transport and laboratory procedure blank of PAHs were also measured and subtracted from the results.
Instrument detection limits (IDLs) of targets ranged from 0.13 ng (ACY) to 0.92 ng(BghiP), and method detection limits (MDLs) were in the range of 0.23 ng mL−1 (NAP)−1.42 ng mL−1 (BghiP) for gaseous samples, and from 0.53 ng mL−1 (PHE) to 1.32 ng mL−1 (BghiP) for particulate-bound PAHs, respectively. Recoveries of spiked standard PAHs were in the range of 70–121% for gaseous, and 68–120% for particulate phase compounds (five duplicates). Surrogate recoveries of spiked deuterated PAHs (Nap-d8, Ant-d10, Ane-d10, Chr-d12, and Perylene-d12) during analysis were 88–125% and 59–128% for gaseous and particulate phases PAHs, respectively.
3. Results and discussion
3.1. Emission factors
EFs of 16 EPA priority PAHs from residential coal combustion ranged from 4.78 mg kg−1 (anthracite) to 373 mg kg−1 (bituminous), averaged at 82.9 mg kg−1. Means and standard deviations of PAH EFs for each coal are listed in the supplementary material (Table S2) in detail. Gaseous PAH EFs were in the range of 3.83–174 mg kg−1 with most abundance of low molecular weight PAHs (NAP–PHE), while average PAH EFs in particulate phase ranged from 0.851 to 214 mg kg−1 dominated by high molecular weight PAHs (BaA–BghiP).
Large variations of PAH EFs within different coal types (honeycomb/chunk, and anthracite/bituminous) had already be documented in literature (Levendis et al., 1996; Oanh et al., 1999; Chen et al., 2005; Zhang et al., 2008a; Liu et al., 2009). It is accepted that chunk coals generally emit more PAHs than honeycomb ones, and PAH EFs are higher for bituminous than anthracite (Mitra et al., 1987; Chen et al., 2004; Liu et al., 2009). The same trend was also shown in this study for both gaseous and particulate phases PAHs. EFs of total PAHs for two honeycomb briquettes were 6.25 ± 1.16 mg kg−1 (Beijing) and 14.3 ± 0.3 mg kg−1 (Taiyuan), respectively. Of three tested raw chunk, coals from Yulin had the highest EFs at 253 ± 170 mg kg−1, followed by those from Taiyuan with PAH EFs at 140 ± 35 mg kg−1. Calculated BaPeq for tested coals showed the similar decrease trend, with the highest BaPeq EF for bituminous chunk coals from Yulin (15.8 ± 11.6 mg kg−1), and lower EFs in two honeycomb briquettes (Table S2).
Chen et al. (2004, 2005) measured PAH EFs for residential coals in laboratory equipped with combustion hood and dilution sampling system, and reported EFs of thirteen parent PAHs (without NAP, ACY and ACE) for honeycomb anthracite with 7% VM were 0.12 mg kg−1, which was lower than those (3.72 ± 1.80 mg kg−1) from our honeycomb anthracite coals with VM content at 4%. They also reported bituminous coals emitted thirteen PAHs at 66–151 mg kg−1. In another laboratory study, Liu et al. (2009) measured 15 PAH EFs (except NAP) from Beijing and Shanxi coals ranging in 53–405 mg kg−1 and 78–1435 mg kg−1 in high heat (mainly used for cooking and heating in daytime) and low heat (mainly in night with decreased oxygen supply) modes, respectively. Due to large difference in fuel properties and combustion conditions, reported EFs from coals burnt in stoves often varied several orders of magnitude. Generally, our EFs were more or less comparable to those reported data (Levendis et al., 1996; Oanh et al., 1999; Chen et al., 2004, 2005; Liu et al., 2009).
3.2. Influencing factors
As expected, EFs of PAHs were significantly correlated with other co-emitted pollutants, including CO, PM, black carbon (BC) and organic carbon (OC) (p < 0.05) (see supplementary material, S3).
In coal combustion, fuel properties including VM, ash content and heat value are expected to be among the most important factors influencing PAH emissions (Mitra et al., 1987; Chen et al., 2004; Liu et al., 2009). Coals with higher VM are often more difficult to achieve complete combustion, and hence produce more PAHs (Chen et al., 2005; Liu et al., 2009). Significantly positive correlation between VM content and PAH EFs was found (p = 0.001). Coals from Beijing, which had the lowest VM (4%) and were the only anthracite coal tested, emitted the least PAHs when comparing to the others. Heat values of coals were also positively correlated to EFs of PAHs (p = 0.004), which might be related to the suitable PAHs formation temperature under residential conditions.
Not only the origin and type of the fuel, but also a group of factors, like oxygen supply, combustion temperature and operation technologies, can influence PAHs formation and emissions (Mitra et al., 1987; Mastral and Callen, 2000; Chen et al., 2005). Higher combustion efficiency or modified combustion efficiency (MCE, defined as CO2 (CO2 + CO)) often associated with lower emissions. Combustion efficiency can be influenced by many factors, like oxygen supply and moisture. On the other hand, the influence of moisture was not only associated with different combustion temperature and efficiency, but also related to the steam stripping or volatilization of organic pollutants in combustion processes (Simoneit, 2002).
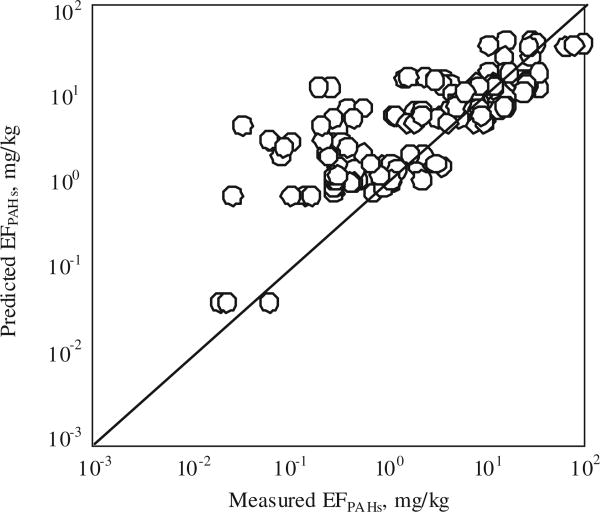
Stepwise regression models were applied with factors including element content (C, H, N), moisture, VM content (ash content and heat value were both significantly correlated with VM content of the coals (p < 0.05)), and MCE, and PAH EFs as independent and dependent variables, respectively, to quantify the influence of these factors on EFs. It was found that moisture (M) and VM were two most important factors, and they can explain about 57 ± 10% of total variation in EFs of PAH individuals (Fig.1), and total PAH EFs (EFPAHs) can be predicted from these two factors based on the equation:
Fig. 1.
Comparison between the measured and predicted EFs of PAHs from coal combustion. The prediction was based on the regression models for each individual PAHs using moisture and volatile matter as independent variables. A 1:1 line is also shown.
3.3. Composition profile
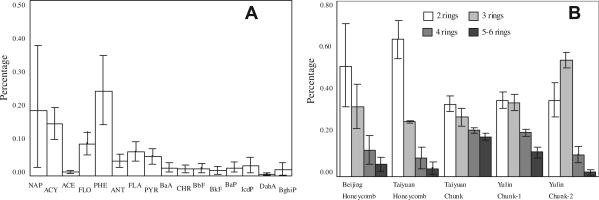
Of 16 PAHs, PHE and NAP were two most abundant compounds, contributing about 24% and 19%, respectively (Fig. 2A), while NAP, ACY and PHE were dominated gaseous PAHs, and in particulate phase, PHE made up to 16 ± 9% of total, followed by FLA (13 ± 4%) and PYR (11 ± 4%). The profile was similar to the reported results for coal combustions, and also comparable to that for other sources, like crop residue and wood combustion (Chen et al., 2004; Bignal et al., 2008; Liu et al., 2009).
Fig. 2.
Composition profile of PAHs from residential coal combustion (A) and relative distribution of 4 groups with different PAHs rings for each coal (B).
In comparison to lower molecular weight PAHs, compounds with higher molecular weight often show considerable tendency to accumulate on PM (Bond et al., 2002; Liu et al., 2007, 2009). Taking coals with higher VM emit more PM into considering, it is believed that VM content might be related to the EFs of PAHs with different molecular weight (Liu et al., 2009). As shown in Fig. 2B, one chunk coal from Taiyuan with VM content at 23%, and another chunk one from Yulin (chunk-1 with 28% VM) emitted more 4 rings (21 ± 1% and 20 ± 2%, respectively) and 5–6 rings PAHs (18 ± 2% and 11 ± 2%, respectively) when comparing to other coals, from which 4 and 5–6 rings PAHs made up to only about 9–12% and 2–6%, respectively.
Several PAH isomer ratios are often used for source apportionment by comparing them between sources and receptors (Watson, 1984; Yunker et al., 2002). For example, ratios larger than 0.50 and 0.56 were often adopted for FLA/(FLA + PYR) and IcdP/(IcdP + BghiP) from coal combustion (Yunker et al., 2002; Zhang et al., 2008a). In this study, six often used isomer ratios were calculated. Means and standard deviations of all five coals were 0.17 ± 0.07, 0.56 ± 0.02, 0.48 ± 0.13, 0.58 ± 0.16, 0.54 ± 0.06, and 0.58 ± 0.16 for ANT/(ANT + PHE), FLA/(FLA + PYR), BaA/(BaA + CHR), IcdP/(IcdP + BghiP), BbF/(BbF + BkF), and BaP/(BaP + BghiP), respectively. These values were generally comparable to those reported in literature for coal combustion (Chen et al., 2004, 2005; Oanh et al., 2005; Yunker et al., 2002; Zhang et al., 2008a). The ratios were also calculated for each tested coal and listed in S4. In general, the difference in the ratios among the five coals were significant (one-way ANOVA, p < 0.05), except FLA/(FLA + PYR). It appears that the application of parent PAHs isomer ratios in source apportionment should be used with caution (Zhang et al., 2005).
3.4. Size distributions
Fine PM is believed to be more harmful as it can penetrate deeper into the lung region, and the dominance of fine PM from coal combustion was often reported (Bond et al., 2002; Chen et al., 2004). In our five tested coals, more than 77% of total was fine PM fraction with size less than 2.5 μm (PM2.5) (Shen et al., in press). It would be interesting to look into the size distribution of particulate phase PAHs because they, especially high molecular weight PAHs, are often associated with PM, and hence, can produce more threat to human health (Chen et al., 2004).
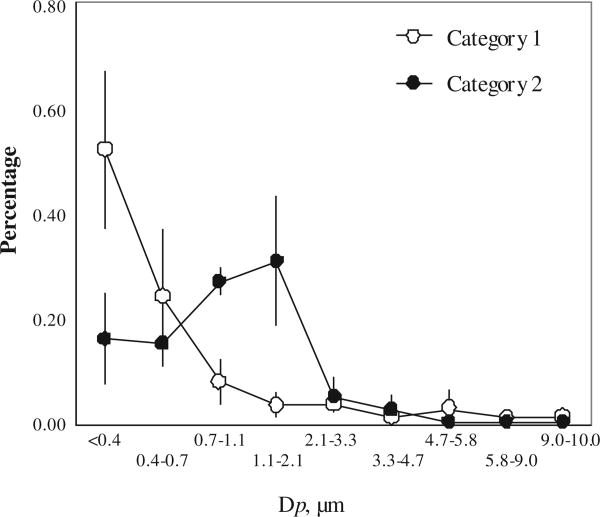
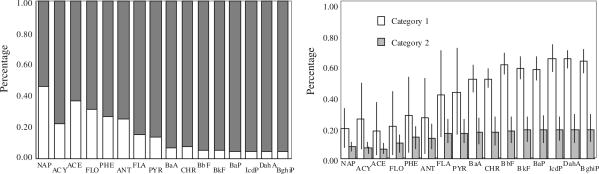
More than 89 ± 6% particulate phase PAHs were found in PM2.5 and the size distributions fell into two categories (Fig. 3). Two honeycomb coals and one chunk coal from Yulin (chunk-2) produced about 52 ± 15% PAHs of the total in fine PM with size less than 0.4 μm (PM0.4). For the other two coals, most of PAHs (58 ± 13%) were found in PM with size of 0.7–2.1 μm. In a laboratory study, Chen et al. (2004) reported that the most mass percentage of all PAHs (56–76%) were emitted in the finest PM on the backup filter (<0.49 μm), followed by the second fraction associated with PM with size between 0.49 and 0.95 μm. The distribution was similar to our results for coals in category 1. Freshly emitted PAHs that were mainly in submicron PM were also found in biofuel combustion in Indian stoves (Venkataraman et al., 2002). PAHs distributions were similar to those of co-emitted PM (Shen et al., in press), and of all coal properties and combustion status factors identified, only one parameter (char residue characteristic, CRC) was found to be able to distinguish the two different distributions. CRC, ranging from 1 to 8, is often used to quantify the caking properties, and the higher CRC the stronger caking and swelling properties of the coal. CRC values of three coals in the first category 1 were 1 or 2, while the other two coals had a higher CRC values at 5 and 6, respectively. In our previous study, coals with lower CRC tended to produce more PM with small diameter, like PM0.4 (Shen et al., in press). It appeared that not only finer PM, but also more PAHs associated with fine PM from the weakly or non-caking coals.
Fig. 3.
Size distributions of total particulate phase PAHs from tested residential coal combustion. Two distribution categories are shown with peak values less than 0.4 μm (category 1, n = 6) and between 1.1 and 2.1 μm (category 2, n = 4).
Because of the difference in diffusivities and vapor pressures of PAHs with different molecular weight, and different organic matter content in PM of different diameter size, it was reported that mass percentage of PAHs in fine PM from either combustion sources or in ambient atmosphere, increased with the increase of MW (Venkataraman et al., 2002; Hays et al., 2003; Keshtkar and Ashbaugh, 2007; Wang et al., 2009). The phenomenon was shown in Fig. 4 (left panel). It reveals that 55 ± 18% of NAP was bound in PM2.1; while about 87–96% of PAHs more than 4 rings (from PYR to BghiP) were in PM2.1.
Fig. 4.
Distribution of PAHs in fine (<2.1 μm, filled symbol) and coarse (2.1–10 μm, open symbol) particulate matter (left panel), and mass percentage of PM0.4 bound PAHs in two categories (right panel).
As discussed above, PM from coals in category 1 was smaller than that from other coals. These coals might also produce more PAHs in fine PM, especially in PM0.4. For each individual PAH from coals in category 1, there was about 18–63% of the total in PM0.4, while for those in category 2, PAHs bound in PM0.4 only made up to 6–19% of the total (Fig. 4, right panel). The difference was significant for 4- to 6-ring PAHs from PYR to BghiP (p < 0.05).
3.5. Gas-particulate partitioning
PAHs in particulate phase made up to 26 ± 10%, 9 ± 4%, 59 ± 10%, 52 ± 8% and 12 ± 5% of the total for Beijing honeycomb, Taiyuan honeycomb, Taiyuan chunk and two Yulin chunk coals, respectively. Higher particulate-bound PAHs emissions for coals from Taiyuan and Yulin (chunk-1) were related to the higher PM emissions. Simultaneously measured PM EFs for these two coals were 4.55 ± 1.66 and 10.8 ± 0.6 g kg−1, while EFPM of the other three coals were only at 0.0652–0.258 g kg−1, which might result from different coal properties and combustion conditions (Shen et al., in press).
A partition coefficient (Kp) is often used to describe gas-particle partitioning of organic pollutants (Pankow, 1987), and the partitioning is believed to be controlled by absorption which is associated with the organic matter in PM, or adsorption that is more related to the particulate surface area and BC content (Goss and Schwarzenbach, 1998; Lohmann and Lammel, 2004). To evaluate the partition mechanism(s), Kp is often plotted versus subcooled liquid-vapor pressure , and it is suggested that a steeper slope than −1 indicates adsorption dominance, while a shallower slope than −0.6 is responsible for absorption governance (Goss and Schwarzenbach, 1998). In addition, octanol–air partition coefficient (KOA) was thought to be a good parameter for absorption dominated partition (Lohmann and Lammel, 2004).
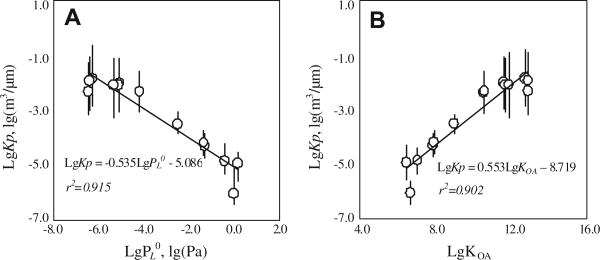
In this study, though two chunk coals produced more particulate-bound PAHs than the others, there was no significant difference in Kp of PAHs from different coals, which implied that partitioning of freshly emitted PAHs from residential coal combustion might be controlled by the similar mechanism(s). One shallow slope of −0.54 (p = 1.3 × 10−6) when plotting Kp versus , was derived (Fig. 5A), and Kp significantly positively correlated (p = 7.3 × 10−6) to KOA (Fig. 5B), both of which indicated that absorption rather than adsorption might govern partitioning of freshly emitted PAHs from residential coal combustions. Besides, correlation between PAHs and OC (r = 0.830, p = 2.3 × 10−4) were also more significant than that between PAHs and BC (r = 0.657, p = 7.4 × 10−3). A similar organic absorption was also found in other combustion sources, like wood and diesel combustions (Roden et al., 2006; Spezzano et al., 2009).
Fig. 5.
Dependence of lg(Kp) on (A) and lg(KOA) (B). and KOA were both calculated based on the measured temperatures and equations supplied by Odabasi et al. (2006).
4. Conclusions
Among 5 coals studied, one anthracite coal with 4% VM had the lowest EFs of PAHs at about 6.25 ± 1.16 mg kg−1, while PAH EFs for the other bituminous coals were in the range of 14.3–253 mg kg−1. Of five tested coals, combustion of two honeycomb briquettes produced lower PAHs, as well as BaPeq, than the other three chunk coals.
Fuel properties and combustion conditions were further analyzed to address the influence on the emissions of PAHs from residential coal combustion. It was found that moisture and volatile matter content were the two most important factors, and about 57% of the total variations can be explained by them.
In residential coal combustion, more than 89% of particulate phase PAHs were abundant in fine PM2.5. By plotting partitioning coefficient (Kp) versus and KOA, it was thought that gas-particle partitioning of freshly emitted PAHs was mainly controlled by absorption into the organic matter.
Supplementary Material
Acknowledgments
This study was funded by the National Natural Science Foundation of China (40730737 and 140710019001), the National Basic Research Program (2007CB407301), the Ministry of Environmental Protection (200809101), and NIEHS (P42 ES016465). We thank Marcus Trail and Eva Land for proof reading of the manuscript.
Footnotes
Appendix. supplementary material: Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.atmosenv.2010.08.042.
References
- Bignal KL, Langridge S, Zhou JL. Release of polycyclic aromatic hydrocarbons, carbon monoxide and particulate matter form biomass combustion in a wood-fired boiler under varying boiler conditions. Atmospheric Environment. 2008;42:8863–8871. [Google Scholar]
- Bond TC, Covert DS, Kramlich JC, Larson TV, Charlson RJ. Primary particle emissions from residential coal burning: optical properties and size distributions. Journal of Geophysical Research. 2002;107(D21):8347. doi: 10.1029/2001JD000571. [DOI] [Google Scholar]
- Borm PJA. Toxicity and occupational health hazards of coal fly ash (CFA). A review of data and comparison to coal mine dust. Annals of Occupational Hygiene. 1997;41:659–676. doi: 10.1016/S0003-4878(97)00026-4. [DOI] [PubMed] [Google Scholar]
- Boström C, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Tönqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environmental Health Perspectives. 2002;110:451–488. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RS, He X, Blair AE, Lan Q. Improvement in household stoves and risk of chronic obstructive pulmonary disease in Xuanwei, China: retrospective cohort study. British Medical Journal. 2005;331:1050–1052A. doi: 10.1136/bmj.38628.676088.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Bi XH, Mai BX, Sheng GY, Fu JM. Emission characterization of particulate/gaseous phases and size association for polycyclic aromatic hydrocarbons from residential coal combustion. Fuel. 2004;83:781–790. [Google Scholar]
- Chen Y, Sheng G, Bi X, Feng Y, Mai B, Fu J. Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environmental Science & Technology. 2005;39:1861–1867. doi: 10.1021/es0493650. [DOI] [PubMed] [Google Scholar]
- Dhammapala R, Claiborn C, Simpson C, Jimenez J. Emission factor from wheat and Kentucky bluegrass stubble burning: comparison of field and simulated burn experiments. Atmospheric Environment. 2007;41:1512–1520. doi: 10.1021/es062039v. [DOI] [PubMed] [Google Scholar]
- Dockey DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An association between air pollution and mortality in 6 United States cities. The New England Journal of Medicine. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJL. Comparative risk assessment collaborating group, 2002. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Goss K, Schwarzenbach RP. Gas/solid and gas/liquid partitioning of organic compounds: critical evaluation of the interpretation of equilibrium constants. Environmental Science & Technology. 1998;32:2025–2032. [Google Scholar]
- Gullett BK, Touati A, Hays MD. PCDD/F, PCD, HxCBz, PAH and PM emission factors for fireplace and woodstove combustion in the San Francisco Bay Region. Environmental Science & Technology. 2003;37:1758–1765. doi: 10.1021/es026373c. [DOI] [PubMed] [Google Scholar]
- Hays MD, Smith ND, Kinsey J, Dong Y, Kariher P. Polycyclic aromatic hydrocarbon size distributions in aerosols from appliances of residential wood combustion as determined by direct thermal desorption-GC/MS. Journal of Aerosol Science. 2003;34:1061–1084. [Google Scholar]
- International Energy Agency (IEA) Key World Energy Statistics. 2009 http://www.iea.org.
- Jimenez JR, Claiborn CS, Dhammapala RS, Simpson CD. Methoxyphenols and levoglucosan ratios in PM2.5 from wheat and Kentucky bluegrass stubble burning in eastern Washington and northern Idaho. Environmental Science & Technology. 2007;41:7824–7829. doi: 10.1021/es062039v. [DOI] [PubMed] [Google Scholar]
- Keshtkar H, Ashbaugh LL. Size distribution of polycyclic aromatic hydrocarbon particulate emission factors form agricultural burning. Atmospheric Environment. 2007;41:2729–2739. [Google Scholar]
- Levendis YA, Atal A, Carlson J, Dunayevskiy Y, Vouros P. Comparative study on the combustion and emissions of waste tire crumb and pulverized coal. Environmental Science & Technology. 1996;30:2742–2754. [Google Scholar]
- Liu S, Tao S, Liu W, Liu Y, Dou H, Zhao J, Wang L, Wang J, Tian Z, Gao Y. Atmospheric polycyclic aromatic hydrocarbons in north China: a winter-time study. Environmental Science & Technology. 2007;41:8256–8261. doi: 10.1021/es0716249. [DOI] [PubMed] [Google Scholar]
- Liu WX, Dou H, Wei ZC, Chang B, Qiu WX, Liu Y, Tao S. Emission characteristics of polycyclic aromatic hydrocarbons from combustion of different residential coals in North China. Science of the Total Environment. 2009;407:1436–1446. doi: 10.1016/j.scitotenv.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Lohmann R, Lammel G. Adsorptive and absorptive contributions to the gas-particle partitioning of polycyclic aromatic hydrocarbons: state of knowledge and recommended parametrization for modeling. Environmental Science & Technology. 2004;38:3793–3803. doi: 10.1021/es035337q. [DOI] [PubMed] [Google Scholar]
- Mastral AM, Callen MS. A review on polycyclic aromatic hydrocarbon (PAH) emissions from energy generation. Environmental Science & Technology. 2000;34:3051–3057. [Google Scholar]
- Mitra A, Sarofim AF, Bar-Ziv E. The influence of coal type on the evolution of polycyclic aromatic hydrocarbons during coal devolatilization. Aerosol Science and Technology. 1987;6:261–271. [Google Scholar]
- Ministry of Agriculture, PRC. China Agriculture Statistical Report 2006. China Agriculture Press; 2007. [Google Scholar]
- NBSC. China Statistical Yearbook. China Statistics Press; Beijing, China: 2009. http://www.stats.gov.cn/tjsj/ndsj/2009/indexch.htm. [Google Scholar]
- Oanh NTK, Reutergårdh LB, Dung NT. Emission of polycyclic aromatic hydrocarbons and particulate matter from domestic combustion of selected fuels. Environmental Science & Technology. 1999;33:2703–2709. [Google Scholar]
- Oanh NTK, Albina DO, Ping L, Wang X. Emission of particulate matter and polycyclic aromatic hydrocarbons from select cookstove-fuel systems in Asia. Biomass & Bioenergy. 2005;28:579–590. [Google Scholar]
- Odabasi M, Cetin E, Sofuoglu A. Determination of octanol-air partition coefficients and supercooled liquid vapor pressures of PAHs as a function of temperature: application to gas-particle partitioning in an urban atmosphere. Atmospheric Environment. 2006;40:6615–6625. [Google Scholar]
- Pankow JF. Review and comparative analysis of the theories on partitioning between the gas and aerosol particulate phases in the atmosphere. Atmospheric Environment. 1987;21:2275–2283. [Google Scholar]
- Roden CA, Bond TC, Conway S, Pinel ABO. Emission factors and real-time optical properties of particles emitted from traditional wood burning cookstoves. Environmental Science & Technology. 2006;40:6750–6757. doi: 10.1021/es052080i. [DOI] [PubMed] [Google Scholar]
- Roden CA, Bond TC, Conway S, Pinel ABO, MacCarty N, Still D. Laboratory and field investigations of particulate and carbon monoxide emissions from traditional and improved cookstoves. Atmospheric Environment. 2009;43:1170–1181. [Google Scholar]
- Shen GF, Yang YF, Wang W, Tao S, Zhu C, Min Y, Xue M, Ding J, Wang B, Wang R, Shen H, Li W, Wang X, Russell AG. Emission factors of particulate matter and elemental carbon for crop residues and coals burned in typical household stoves in China. Environmental Science & Technology. doi: 10.1021/es101313y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneit BRT. Biomass burning – a review of organic tracers for smoke from incomplete combustion. Applied Geochemistry. 2002;17:129–162. [Google Scholar]
- Smith KR, Gu S, Huang K, Qiu D. One hundred million improved cook-stoves in China: how was it done? World Development. 1993;21:941–961. [Google Scholar]
- Smith KR, Jerrett M, Anderson HR, Burnett RT, Stone V, Derwent R, Atkinson RW, Cohen A, Shonkoff SB, Krewski D, Pope CA, III, Thun MJ, Thurston G. Public health benefits of strategies to reduce greenhouse-gas emissions: health implications of short-lived greenhouse pollutants. Lancet. 2009;374:2091–2103. doi: 10.1016/S0140-6736(09)61716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spezzano P, Picini P, Cataldi D. Gas- and particle-phase distribution of polycyclic aromatic hydrocarbons in two-stroke, 50-cm3 moped emission. Atmospheric Environment. 2009;43:539–545. [Google Scholar]
- Venkataraman C, Negi G, Sardar SB, Rastogi R. Size distributions of polycyclic aromatic hydrocarbons in aerosol emissions from biofuel combustion. Journal of Aerosol Science. 2002;33:503–518. [Google Scholar]
- Watson JG. Overview of receptor model principles. Journal of the Air Pollution Control Association. 1984;34:619–623. [Google Scholar]
- Wang W, Tao S, Wang W, Shen G, Zhao J, Lam K. Airborne particulates and polycyclic aromatic hydrocarbons (PAHs) in ambient air in Donghe, Northern China. Journal of Environmental Science and Health, Part A Toxic/Hazardous Substances and Environmental Engineering. 2009;44:854–860. doi: 10.1080/10934520902958526. [DOI] [PubMed] [Google Scholar]
- Wu SP, Tao S, Liu WX. Particle size distributions of polycyclic aromatic hydrocarbons in rural and urban atmosphere of Tianjin, China. Chemosphere. 2006;62:357–367. doi: 10.1016/j.chemosphere.2005.04.101. [DOI] [PubMed] [Google Scholar]
- Xu SS, Liu WX, Tao S. Emission of polycyclic aromatic hydrocarbons in China. Environmental Science & Technology. 2006;40:702–708. doi: 10.1021/es0517062. [DOI] [PubMed] [Google Scholar]
- Yunker MB, Macdonald RW, Vingarzan R, Mitchell HR, Goyette D, Sylvestre S. PAHs in the Fraser River basin: a critical appraisal PAH ratios as indicators of PAH source and composition. Organic Geochemistry. 2002;33:489–515. [Google Scholar]
- Zhang J, Smith KR, Ma Y, Ye S, Jiang F, Qi W, Liu P, Khalil MAK, Rasmussen RA, Thorneloe SA. Greenhouse gases and other airborne pollutants from household stoves in China: a database for emission factors. Atmospheric Environment. 2000;34:4537–4549. [Google Scholar]
- Zhang XL, Tao S, Liu WX, Yang Y, Zuo Q, Liu SZ. Source diagnostics of polycyclic aromatic hydrocarbons based on species ratios: a multimedia approach. Environmental Science & Technology. 2005;39:9109–9114. doi: 10.1021/es0513741. [DOI] [PubMed] [Google Scholar]
- Zhang J, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environmental Health Perspectives. 2007;115:848–855. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schauer JJ, Zhang Y, Zeng L, Wei Y, Liu Y, Shao M. Characteristics of particulate carbon emissions from real-world Chinese coal combustion. Environmental Science & Technology. 2008a;42:5068–5073. doi: 10.1021/es7022576. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dou H, Chang B, Wei Z, Qiu W, Liu S, Liu W, Tao S. Emission of polycyclic aromatic hydrocarbons form indoor straw burning and emission inventory updating in China. Annals of the New York Academy of Sciences. 2008b;1140:218–227. doi: 10.1196/annals.1454.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tao S, Shen H, Ma J. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21063–21067. doi: 10.1073/pnas.0905756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tao S. Global atmospheric emission inventory of polycyclic aromatic hydrocarbons (PAHs) for 2004. Atmospheric Environment. 2009;43:812–819. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.