Abstract
Melanoma is entering into an era of combinatorial approaches to build upon recent clinical breakthroughs achieved by novel single-agent therapies. One of the leading targets to emerge from the growing understanding of the molecular pathogenesis, heterogeneity, and resistance mechanisms of melanomas is the PI3K-AKT pathway. Multiple genetic and epigenetic aberrations that activate this pathway have been identified in melanomas de novo and in acquired resistance models. These developments have been paralleled by the establishment of models for preclinical testing, and the availability of compounds that target various effectors in the pathway. Thus, in addition to having a strong rationale for targeting, the PI3K-AKT pathway presents an immediate clinical opportunity. However, the development of effective strategies against this pathway must overcome several key challenges, including optimizing patient selection and overcoming feed-back loops and pathway cross-talk that can mediate resistance. This review will discuss the current understanding and ongoing research about the PI3K-AKT pathway in melanoma, and discuss emerging strategies to achieve clinical benefit in patients by targeting it.
Introduction
The PI3K-AKT cascade is one of the most studied pathways in cancer. The pathway is a critical regulator of many essential physiological processes that are critical to the aggressive nature and behavior of malignant cells. Previous studies have demonstrated that the pathway is among the most frequent targets of genetic aberrations across many types of cancer (1). These alterations include mutations and copy number changes within the core components of the pathway, as well as alterations in genes that utilize that pathway as a critical effector (i.e. receptor tyrosine kinases [RTKs]). For all of these reasons, the PI3K-AKT pathway has also been the focus of aggressive pharmacological development and testing (2, 3).
The high prevalence of activating mutations in BRAF and NRAS in cutaneous melanomas supports a critical role for activation of the RAS-RAF-MEK-ERK pathway in the pathogenesis of this disease (4). However, multiple lines of evidence have also demonstrated a significant role for the PI3K-AKT pathway. This review will highlight some of the key findings about the PI3K-AKT pathway in melanoma, and the rationale, approaches, and challenges to the development of effective therapeutic approaches against it.
Activation of the PI3K-AKT Pathway in Melanoma
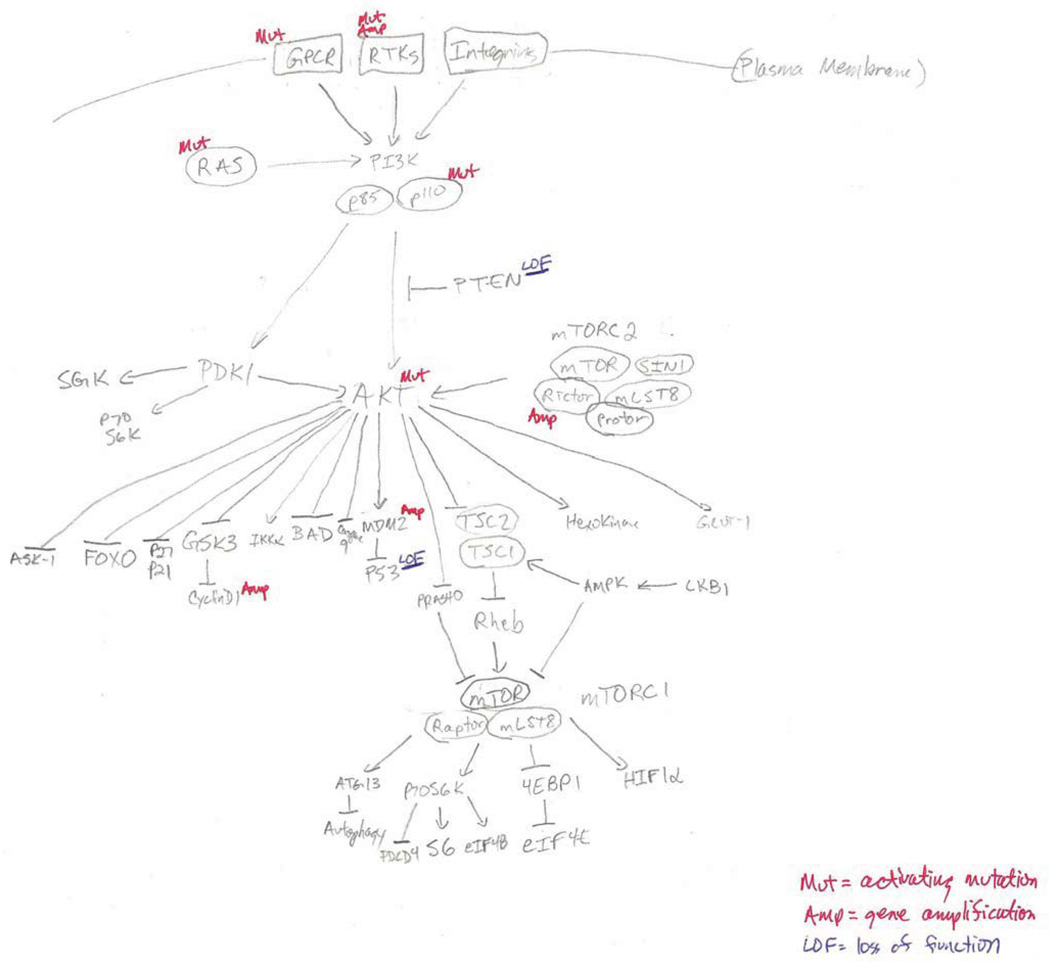
The physiological regulation of the PI3K-AKT cascade is shown in Figure 1 (5). PI3K, which consists of a dimer of catalytic (i.e. p110) and regulatory (i.e. p85) subunits, can be activated by multiple signals, including receptor tyrosine kinases (RTKs), RAS proteins, and cell-cell contacts, among others. Activated PI3K phosphorylates phosphatidylinositols in the plasma membrane at the 3’-OH group. These 3’-phospholipids attract proteins that contain a pleckstrin homology (PH) domain to the cell membrane, including AKT. AKT, which has 3 isoforms (AKT1/2/3), is phosphorylated at two critical and conserved residues, Thr308 (by PDK1) and Ser473 (by the mTORC2 complex), which fully activates its catalytic activity. Activated AKT then phosphorylates a number of effector proteins, thereby regulating multiple key cellular processes, including proliferation, survival, motility, metabolism, angiogenesis, and more. PTEN regulates the activity of the pathway by dephosphorylating phosphatidylinositols at the 3’-position, thereby antagonizing the activity of PI3K (6). Multiple other lipid and protein phosphatases also regulate various steps and effectors in the pathway (7).
Figure 1.
Regulators, effectors, and somatic alterations in the PI3K-AKT pathway in melanoma.
The PI3K-AKT pathway is activated multiple ways in melanoma. The two most common and studied events are activating mutations in the oncogene NRAS (15–20%) and loss of expression or function of the tumor suppressor PTEN (20–30%) (4). Similar to BRAF and NRAS mutations in the RAS-RAF-MEK-ERK signaling pathway, NRAS mutations and PTEN mutations/deletions are largely mutually exclusive. In contrast, PTEN loss commonly occurs in melanomas with activating BRAF mutations, resulting in concurrent activation of the RAS-RAF-MEK-ERK and PI3K-AKT pathways (8–10). The general mutual exclusivity of NRAS mutations and PTEN loss in melanoma is thought by many to be attributable to the fact that both events activate the PI3K-AKT pathway, thus rendering the presence of both alterations in the same tumor functionally redundant. However, similar to findings in other tumor types, quantitative analysis of melanoma cell lines and clinical specimens has demonstrated that melanomas with PTEN loss consistently have higher levels of AKT activation than those with NRAS mutations (11–13). Furthermore, experiments in an NRAS-mutant melanoma genetically engineered mouse model (GEMM) demonstrated that loss of PTEN increased invasiveness and metastatic potential (14). While rare, deletions and mutations of PTEN have been detected in some melanomas with activating NRAS mutations, including in two recent whole exome sequencing studies of >100 melanomas, which also detected PTEN alterations in melanomas with wild-type BRAF and NRAS (15, 16). However, this data should be interpreted with caution, as there is yet no standardized protocol for defining PTEN deletions. Preliminary analysis of TCGA data suggests that such a standard should take into account both copy number and focality, and would decrease the discrepancies between studies. Additional studies support that PTEN expression can be regulated epigenetically, including by miRNAs and the PTENP1 pseudogene (17–20). A more complete understanding of the prevalence, pattern, molecular causes, and clinical associations of PTEN loss will likely be possible with the completion of the ongoing melanoma TCGA effort, which will include DNA-, RNA-, and protein-based analyses of up to 500 clinically annotated melanoma specimens.
The functional significance of PTEN loss has been studied extensively in the setting of melanomas with activating BRAF mutations. To date, nearly all published patient-derived melanoma cell lines with complete loss of PTEN have concurrent BRAF mutations (11, 21–24). This strong association with BRAF mutations has also been demonstrated functionally in genetically engineered mouse models (GEMMs). While expression of the BRAF V600E protein in murine melanocytes results in increased proliferation of melanocytes, concurrent PTEN loss results in 100% penetrance of invasive, metastatic tumors, thus establishing the first such model for this disease (25). BRAF-mutant human melanoma cell lines with loss of PTEN are generally sensitive to growth inhibition by BRAF and MEK inhibitors, but they are significantly resistant to apoptosis induction by these treatments (26–29). Supporting the clinical relevance of these findings, two independent analyses of PTEN status, one genetic and one immunohistochemical, identified decreased clinical benefit with selective BRAF inhibitors (vemurafenib, dabrafenib) in patients with loss of PTEN in pre-treatment (including archival) tumor specimens (30, 31). While these studies support the potential value for evaluating PTEN function in BRAF-mutant melanomas in future studies, it is not yet clear what methodology of PTEN testing (i.e. DNA-, RNA- or protein-based) will prove most informative. Further, very little information is available at this time about the concordance of PTEN among different tumors in individual patients (32).
Both broad and focused sequencing studies have identified additional genetic events that can activate the PI3K-AKT pathway in melanoma. Point mutations in PIK3CA, which encodes the p110α catalytic subunit of PI3K, are detected in 2–6% of melanomas (15, 16, 33, 34). Notably, while some of these mutations are recurrent hotspots reported in other tumor types, others are novel and of unclear functional significance. Mutations producing the activating substitution E17K in AKT1, which are detected as rare events in several tumor types, have also been detected as rare events in melanoma (1–2%) (35, 36). However, melanoma is the only disease in which the analogous mutation in AKT3 has been detected (1–2%). The identification of AKT3 mutations builds upon previous studies reporting increased expression and activation of AKT3 in melanoma progression, potential implicating it as a novel therapeutic target (37, 38). Recently, amplification of a 5Mbp locus including RICTOR, which encodes a component of the multiprotein TORC2 complex that phosphorylates AKT at the Ser473 residue, has been reported in up to 5% of melanomas, particularly those that are relatively protected from ultraviolet radiation (UVR) (16). Temporally, PI3K activation appears to be a secondary event. In an immunohistochemical survey, PTEN protein loss was observed in melanoma but not nevi (39), in contrast to the uniformly high mutation rate of BRAF across all stages (40). Similarly, phosphorylated Akt was found to be high in melanoma, but not in nevi (41). The findings are also consistent with the lack of the melanocytic phenotype in the PTEN−/− mice in the absence of the mutant BRAF allele (25).
The PI3K-AKT pathway is also implicated as a critical effector of alterations that activate receptor tyrosine kinases (RTKs). Activating mutations in c-Kit are rare in cutaneous melanomas, but they are relatively common in acral and mucosal melanomas (42). While the low prevalence of BRAF and NRAS mutations in these subtypes, and their general mutual exclusivity with c-Kit mutations, suggested that signaling by mutant KIT proteins might activate the RAS-RAF-MEK-ERK pathway, functional studies in cell lines have demonstrated activation of, and in some studies dependence upon, the PI3K-AKT pathway (43–45). One study has also reported frequent (~20%) somatic mutations in the ERBB4 gene (46). Although these mutations do not cluster in any functional domain, preclinical studies suggested that multiple mutant forms of the encoded ERBB4 protein activated the PI3K-AKT pathway. However, recent whole exome sequencing studies did not identify ERBB4 as a significantly mutated gene by the algorithms used in those analyses (15, 16). In addition to genetic events, it appears that epigenetically mediated activation of RTKs plays a role in melanoma, specifically in resistance to BRAF inhibitors. Two different groups identified increased expression and activation of different RTKs (PDGFRβR and IGF1R, respectively) in progressing tumors and melanoma cell lines with acquired resistance to BRAF inhibitors (47–49). Both groups demonstrated that the activation of these RTKs did not rescue the activity of the MAPK pathway, but instead caused compensatory activation of the PI3K-AKT pathway. Importantly, no mutations or amplification in the genes encoding the RTKs were detected in the cell lines. Similar compensatory activation of this pathway via IGF1R was also reported in human melanoma cells with de novo resistance to killing by MEK inhibitors (29). More recently, two different groups demonstrated that secretion of hepatocyte growth factor (HGF) by non-transformed cells in the tumor microenvironment results in PI3K-AKT pathway activation in melanoma cells (50, 51). This interaction caused resistance to BRAF inhibitors in vitro, and correlated with inferior clinical outcomes in patients.
PI3K-AKT Pathway Inhibitors
The multiple ways in which the PI3K-AKT pathway is activated in melanoma, and existing evidence for a functional role in progression and resistance, support the rationale to target it therapeutically. Indeed, similar evidence in multiple tumor types has led to the development of multiple classes of inhibitors against this pathway. Classes of agents include inhibitors of PI3K (pan-isoform and isoform-specific), dual PI3K/mTOR, AKT, and mTOR (mTORC1 and dual mTORC1/2 inhibitors) [Table 1]. Multiple agents are available in each class, many of which are currently undergoing clinical evaluation in patients. While the availability of this spectrum of agents presents a tremendous opportunity, a key challenge for a relatively rare disease like metastatic melanoma is to rationally utilize and prioritize these agents in order to determine their clinical value effectively and efficiently.
Table 1.
PI3K pathway inhibitors currently in clinical trials for any cancer
| Target | Inhibitor | Alternative Name | Company |
|---|---|---|---|
| AKT | AZD5363 | AstraZeneca | |
| GDC-0068 | Genentech | ||
| GSK2110183 | GlaxoSmithKline | ||
| GSK2141795 | GlaxoSmithKline | ||
| GSK690693 | GlaxoSmithKline | ||
| KRX-0401 | Perifosine | Keryx | |
| MK2206 | Merck | ||
| SR13668 | SRI | ||
| mTORC1 | Rapamycin | Sirolimus | Pfizer |
| CCI779 | Temsirolimus | Pfizer | |
| MK-8669 | Ridaforolimus | Ariad | |
| RAD001 | Everolimus | Novartis | |
| Dual mTORC1/2 | AZD2014 | AstraZeneca | |
| AZD8055 | AstraZeneca | ||
| CC-223 | Celgene | ||
| MLN0128 | INK-128 | Millenium | |
| OSI-027 | Astellas | ||
| Palomid 529 | Paloma | ||
| PI3K p110α-selective | GDC-0032 | Genentech | |
| MLN1117 | INK-1117 | Millenium | |
| NVP-BYL719 | Novartis | ||
| PI3K p110β-selective | GSK2636771 | GlaxoSmithKline | |
| SAR260301 | Sanofi-Aventis | ||
| PI3K p110δ-selective | CAL101 | Gilead | |
| GSK2269557 | GlaxoSmithKline | ||
| Pan PI3K | BAY80-6946 | Bayer | |
| GDC-0941 | Genentech | ||
| NVP-BKM120 | Novartis | ||
| PX866 | Oncothyreon | ||
| SF1126 | Semafore | ||
| XL147 | SAR245408 | Exelixis | |
| ZSTK474 | Zenyaku Kogyo | ||
| Dual PI3K/mTOR | DS-7423 | Daiichi Sankyo | |
| GDC-0980 | Genentech | ||
| GSK2126458 | GlaxoSmithKline | ||
| NVP-BEZ235 | Novartis | ||
| NVP-BGT226 | Novartis | ||
| P7170 | Piramal | ||
| PF-05212384 | Pfizer | ||
| PF-4691502 | Pfizer | ||
| XL765 | SAR245409 | Exelixis | |
Experimental evidence from other tumor types supports that different ways of activating the PI3K-AKT pathway result in functional dependence upon different effectors, and thus sensitivity to different classes of therapeutic agents. Melanomas with loss of PTEN represent a high-priority opportunity, due to the high prevalence of this alteration de novo, the availability of models for functional testing, and the evidence for a role in resistance to MAPK pathway inhibitors. Previous studies in multiple tumor types demonstrated that loss of PTEN correlates with marked dependence on AKT, and sensitivity to AKT inhibition in gene knockdown experiments (13). However, recently reported experiments using the BRAF-mutant, PTEN-null melanoma GEMM suggests superior in vivo tumor growth inhibition with PI3K inhibitors than with AKT inhibitors (52, 53). While the results are interesting, it remains unclear how well this model will reflect results in BRAF-mutant, PTEN-null melanomas in patients, which will likely have significant heterogeneity and additional molecular alterations that cannot be modeled easily in GEMM systems. Testing of dual PI3K/mTOR inhibitors in melanoma cell lines has shown that these agents are broadly inhibitory, and superior to the inhibition achieved by PI3K or mTOR inhibition alone (54, 55). While improved anti-tumor activity is preferred, a key question is whether this will translate into an acceptable therapeutic index in patients due to the broad physiological functions of PI3K and mTOR. Recently reported experiments in other models have suggested that the efficacy of AKT inhibitors is relatively selective for tumors with PTEN loss (56). It is possible that this selectivity for cells with loss of PTEN will translate into selective killing of tumor cells in patients with AKT inhibitors at clinically tolerated doses, even if they are less potent. Rapamycin and its analogues, which inhibit mTORC1, are reasonably well-tolerated clinically, as demonstrated by longstanding use in patients who have undergone organ transplantation. However, mTORC1 inhibitors have not demonstrated significant clinical activity as single agents in metastatic melanoma patients, or in combination with RAF inhibitors (57–59). As will be discussed below, this lack of activity may be due to compensatory hyperactivation of AKT due to inhibition of an mTORC1-mediated negative feedback loop within the PI3K pathway. In contrast, dual mTORC1/2 inhibitors block this upregulation through the additional blockade of mTORC2-mediated phosphorylation/activation of AKT, and thus may represent a more effective strategy to test the effects of mTOR inhibition (29). A recent study has also demonstrated that genetic inhibition of PDK1 can induce melanoma regression (60), supporting the rationale for testing of PDK1 inhibitors in melanoma as they are developed clinically.
One strategy to achieve significant pathway inhibition clinically with an acceptable therapeutic index is the use of isoform-specific PI3K inhibitors. Genetic studies in mouse models have demonstrated that the PI3K catalytic subunit p110α is predominantly responsible for mediating growth factor signaling from RTKs, but it is largely dispensable for pathway activation in tumors with PTEN loss. Cells with PTEN loss instead appear to depend largely on p110β to activate the pathway, drive proliferation, and mediate tumorigenesis in vivo (61, 62). Testing of a p110β-selective inhibitor in a panel of >400 cancer cell lines demonstrated significantly greater activity in lines with loss of PTEN than in those with PTEN intact (63). However, despite the overall trend, some PTEN-intact cell lines were sensitive, and a number of PTEN-null cell lines were resistant. Clinical testing of two different p110β-selective inhibitors (GSK2636771, SAR260301) is currently ongoing, with planned analysis of PTEN built into both studies (www.clinicaltrials.gov). Other PI3K isoform-specific inhibitors, particularly BYL719 (p110α) and CAL-101 (p110δ), have been well-tolerated and demonstrated clinical efficacy in other cancer types (64). The use of a p110α-selective inhibitor may be a rational approach, in particular, for tumors with PI3K-AKT pathway activation mediated by RTKs, but to date there is no published experimental data testing this hypothesis in melanoma.
Another strategy to optimize the therapeutic index of PI3K-AKT pathway inhibitors is alternative dosing schedules. Multiple studies have demonstrated that induction of apoptosis by BRAF or MEK inhibitors in melanoma cell lines with activating BRAF mutations generally is not observed until the MAPK pathway has been suppressed for 48 to 72 hours (29, 65). In contrast, when PI3K pathway inhibitors are combined with those agents not only is apoptosis increased, but it is generally induced at much earlier timepoints (i.e. 24 hours or less) (29, 55). This suggests that relatively short-term exposure to PI3K-AKT pathway inhibitors may be effective clinically. This strategy is similar to that used conventionally with chemotherapy agents, in which dosing regimens have been developed to deliver the maximally tolerated doses of agents intermittently (i.e. every 7, 14, or 21 days). The clinical development of targeted therapies instead has generally utilized continuous dosing regimens. As one of the concerns about the clinical development of PI3K-AKT pathway inhibitors has been whether sufficient pathway inhibition is being achieved, the use of high, intermittent dosing may overcome this hurdle. Indeed, intermittent dosing of the combination of a MEK and a PI3K inhibitor exhibited marked anti-tumor activity in vivo in multiple xenograft models, including melanoma (66). While this strategy can be explored empirically in mouse models, one of the critical challenges to the rational development of this strategy is the identification of pharmacodynamic markers that correlate with the achievement of clinically effective pathway inhibition by PI3K-AKT inhibitors (67). Notably, PI3K inhibitors generally produce marked inhibition of AKT activation at doses that are much lower than those that correlate with anti-proliferative and/or pro-apoptotic effects. The identification of targets, and/or the degree of target modulation, that will correspond to clinical benefit, similar to what has been demonstrated for P-ERK and BRAF inhibitors (68), will facilitate the preclinical development and clinical evaluation of candidate agents and dosing regimens.
Feedback Loops and Cross-Talk
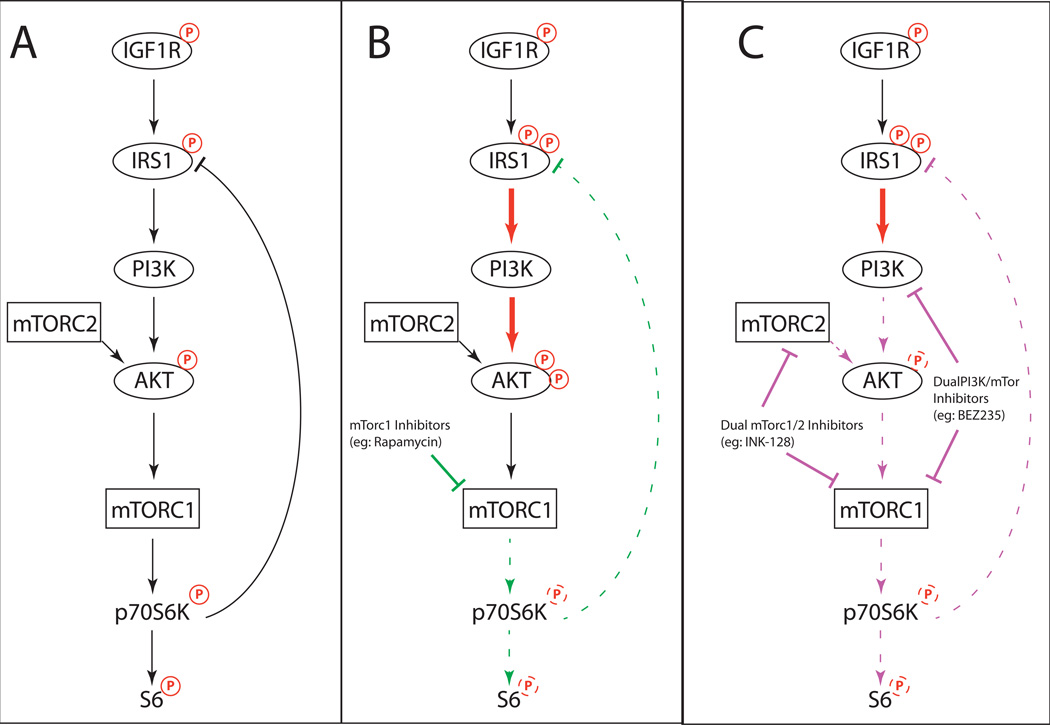
Growing experience with effective targeted therapies, particularly in melanoma with selective BRAF inhibitors, has demonstrated that compensatory signaling within and between signaling pathways can be critical to both clinical activity and the emergence of resistance (69–71). Consistent with this experience, effective clinical targeting of the PI3K-AKT pathway will likely also need to account for and overcome complex feedback loops that blunt the activity of single-target inhibitors against it. The seminal example is the feedback induction of AKT phosphorylation by mTORC1 inhibitors, such as rapamycin and RAD001 (Figure 2). In these studies (72, 73), the mTORC1 complexes were found to negatively regulate IRS1 at baseline, a critical second messenger from the IGF1R to PI3K. mTORC1 inhibitor-mediated relief of this negative loop activates PI3K, AKT, and sometimes ERK (74, 75), promoting cell survival. This particular feedback has been demonstrated in a variety of cancers (72–76) including melanoma (28, 77).
Figure 2.
Feedback signaling following mTORC1 inhibition. (A) The baseline status of the PI3K signaling cascade, indicating negative feedback from p70S6K to IRS1. (B) Inhibition of mTORC1 blocks the negative feedback loop, activating IRS1, and leading to PI3K and AKT activation. (C) Paradoxical activation of PI3K and AKT in the setting of mTORC1 inhibition can be overcome by dual PI3K/mTOR inhibitors, which also inhibit PI3K, or dual mTORC1/2 inhibitors, which block mTORC2-mediated AKT activation.
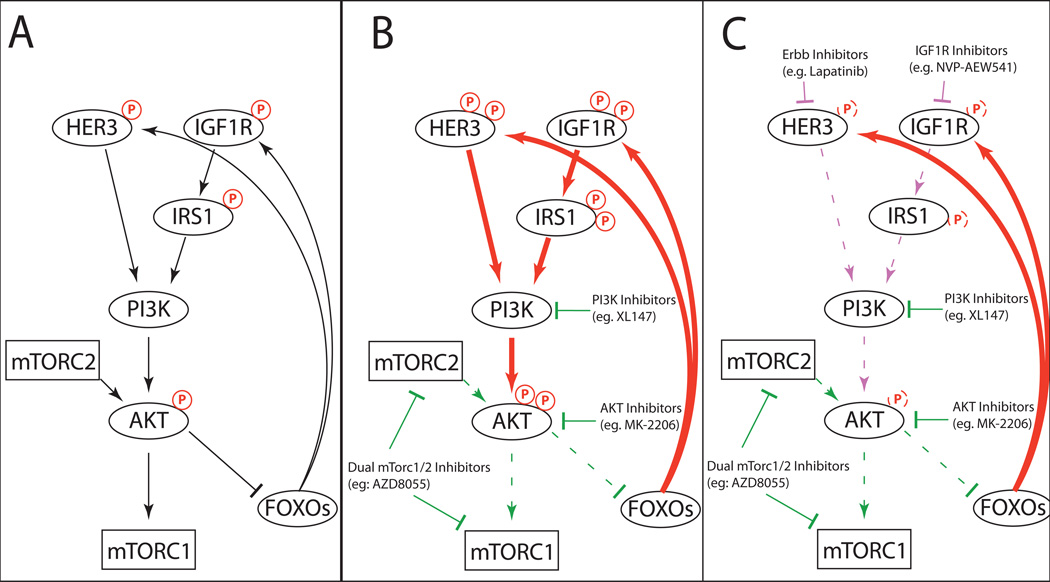
More complex feedback perturbations are generated by PI3K and AKT inhibitors (Fig. 3). In breast, lung, and prostate cancer cell lines, these inhibitors induced the FOXO-mediated transcription of multiple RTKs, most commonly HER3 and IGF1R (78–80). Independent studies showed that these RTKs are capable of transducing signals to both the PI3K-AKT (78) and the RAS-RAF-MEK-ERK pathways (80), potentially reinforcing mutual oncogenic crosstalk. Only when these RTKs were targeted by RNAi knockdown or by small-molecule inhibitors (lapatinib, NVP-AEW541) were these feedback activations extinguished. Indeed, combinations of the PI3K and RTK inhibitors displayed synergy in xenograft models (79–81), supporting their therapeutic value. However, one point of contention is whether dual mTORC1/2 inhibitors (also referred to as mTORC catalytic inhibitors) can induce RTKs, as some studies explicitly observe this (72, 80, 81) while others do not (78, 79), even in the same cell type. This may be due to differences in whether the readout is mRNA or protein, as post-translational modifications and/or protein turnover rates can result in discordant levels (82, 83), or whether or not the inhibitor hits both the mTORC1 and mTORC2 complexes. Regardless, these overall results serve as an important caution for the future development of PI3K inhibition in melanoma and reveals potential co-targets to suppress the feedback activity (Figures 2 and 3).
Figure 3.
Feedback signaling following PI3K, AKT, or dual mTORC1/2 inhibition. (A) The baseline status of the PI3K signaling cascade, indicating negative feedback to RTKs such as HER3 and IGF1R, via inactivation of the FOXO transcription factors by AKT. (B) PI3K, AKT, or dual mTORC1/2 inhibitor inactivate AKT, releasing the inhibition of FOXO transcription factors, leading to expression and activation of HER3, IGF1R, and other RTKs, leading to activation of PI3K and AKT activation, and potentially other pathways (i.e. RAS-RAF-MEK-ERK). This effect is delayed in vitro by 24–72 hours or more, and represents a reequilibration of the pathway over time. (C) The addition RTK inhibitors can block the compensatory signaling and induce synergy with PI3K, AKT, and/or dual mTORC1/2 inhibitors.
Dual PI3K-mTOR inhibitors (84) improve on these single-target agents by providing a built-in inhibition of the mTOR feedback loops. Characterization of multiple dual inhibitors in melanoma cell lines have demonstrated a potent and durable extinction of pAKT and its downstream targets, matching or even exceeding the effects of combining single PI3K and mTOR inhibitors (54, 77). Indeed, BEZ235 has shown preliminary success in various preclinical models, particularly in combination with MEK inhibitors (77, 85, 86). Relevantly, in a mouse model of melanoma, the combination of BEZ235 with the MEK inhibitor AZD6244 produced a 37% partial response rate (>30% decrease in tumor volume) (87). Various clinical trials are currently in progress with this class of drugs, though whether pharmacokinetic and toxicity issues can be optimized remains to be seen (88).
The existence of these and other feedback loops suggests that pharmacodynamic, mechanistic, and resistance tissue-based studies of PI3K-AKT pathway inhibitors in patients should optimally allow for the evaluation of multiple markers and/or pathways. Emerging proteomic technologies including phospho-RTK and reverse phase protein arrays (RPPA) facilitate such analyses by analyzing a large number of proteins in individual samples concurrently. Notably, while most experimental work to date examining markers and mechanisms of efficacy and resistance with PI3K-AKT pathway inhibitors have focused on the effects and changes observed in tumor cells, it becoming clear that anti-cancer treatments also have marked effects on the host, including the immune system and the tumor microenvironment. In melanoma, the demonstrated durable efficacy of immunotherapies (89), and a growing appreciation of the effects of MAPK-pathway inhibitors on the anti-tumor response (90–92)(93), mandates examination of the immunological effects of PI3K-AKT pathway inhibitors in this disease. The marked activity of p110δ-selective inhibitors in hematological malignancies, and the long-standing use of mTORC1 inhibitors (rapamycin) as immunosuppressants in transplant patients, raise the possibility that strategies that target the PI3K-AKT pathway could actually inhibit the anti-tumor immune response, and thus blunt long-term clinical benefit. However, an improved understanding of anti-tumor immunology and the differential effects of various PI3K-AKT pathway inhibitors on different immune cell populations (94), coupled with strategies (i.e. isoform-specific inhibitors) that are designed to achieve selective pathway inhibition in tumors, suggest that this challenge will not be insurmountable.
Summary
The PI3K-AKT pathway remains an attractive combinatorial target to improve clinical outcomes in patients with melanoma. As described, emerging understanding and models for this pathway are facilitating the development of rational strategies. However, critical challenges remain, including matching patients to the appropriate agents; developing appropriate markers to facilitate efficient and meaningful evaluation of doses that are achieved safely in patients; and ultimately identifying strategies that achieve acceptable therapeutic indices.
Acknowledgments
M.A. Davies receives research support from GlaxoSmithKline, Genentech, Astrazeneca, Merck, Oncothyreon, and Myriad. M.A. Davies discloses serving on advisory boards for GlaxoSmithKline, Genentech, and Novartis.
M.A. Davies is supported by NIH 1R01CA154710-01, a Melanoma Research Alliance Young Investigator Award, an American Society of Clinical Oncology Career Development Award, and Cancer Prevention Institute of Texas RP120505.
Footnotes
L.N. Kwong has no conflict of interest.
References
- 1.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 3.Courtney KD, Corcoran RB, Engelman JA. The PI3K Pathway As Drug Target in Human Cancer. J Clin Oncol. 2010;28:1075–10183. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hocker T, Tsao H. Ultraviolet radiation and melanoma: a systematic review and analysis of reported sequence variants. Hum Mutat. 2007;28:578–588. doi: 10.1002/humu.20481. [DOI] [PubMed] [Google Scholar]
- 5.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 6.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 7.Vasudevan KM, Garraway LA. AKT signaling in physiology and disease. Curr Top Microbiol Immunol. 2010;347:105–133. doi: 10.1007/82_2010_66. [DOI] [PubMed] [Google Scholar]
- 8.Goel VK, Lazar AJ, Warneke CL, Redston MS, Haluska FG. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol. 2006;126:154–160. doi: 10.1038/sj.jid.5700026. [DOI] [PubMed] [Google Scholar]
- 9.Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic Interaction Between NRAS and BRAF Mutations and PTEN//MMAC1 Inactivation in Melanoma. J Investig Dermatol. 2004;122:337–341. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsao H, Zhang X, Fowlkes K, Haluska FG. Relative Reciprocity of NRAS and PTEN/MMAC1 Alterations in Cutaneous Melanoma Cell Lines. Cancer Res. 2000;60:1800–1804. [PubMed] [Google Scholar]
- 11.Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, et al. Integrated Molecular and Clinical Analysis of AKT Activation in Metastatic Melanoma. Clin Cancer Res. 2009;15:7538–7546. doi: 10.1158/1078-0432.CCR-09-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park ES, Rabinovsky R, Carey M, Hennessy BT, Agarwal R, Liu W, et al. Integrative analysis of proteomic signatures, mutations, and drug responsiveness in the NCI 60 cancer cell line set. Mol Cancer Ther. 2010;9:257–267. doi: 10.1158/1535-7163.MCT-09-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16:21–32. doi: 10.1016/j.ccr.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogueira C, Kim KH, Sung H, Paraiso KHT, Dannenberg JH, Bosenberg M, et al. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010;29:6222–6232. doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodis E, Watson Ian R, Kryukov Gregory V, Arold Stefan T, Imielinski M, Theurillat J-P, et al. A Landscape of Driver Mutations in Melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nature genetics. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguissa-Toure AH, Li G. Genetic alterations of PTEN in human melanoma. Cell Mol Life Sci. 2012;69:1475–1491. doi: 10.1007/s00018-011-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X-P, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C. Epigenetic PTEN Silencing in Malignant Melanomas without PTEN Mutation. Am J Pathol. 2000;157:1123–1128. doi: 10.1016/S0002-9440(10)64627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karreth Florian A, Tay Y, Perna D, Ala U, Tan Shen M, Rust Alistair G, et al. In Vivo Identification of Tumor- Suppressive PTEN ceRNAs in an Oncogenic BRAF-Induced Mouse Model of Melanoma. Cell. 2011;147:382–395. doi: 10.1016/j.cell.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–6307. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stark M, Hayward N. Genome-Wide Loss of Heterozygosity and Copy Number Analysis in Melanoma Using High-Density Single-Nucleotide Polymorphism Arrays. Cancer Res. 2007;67:2632–2642. doi: 10.1158/0008-5472.CAN-06-4152. [DOI] [PubMed] [Google Scholar]
- 23.Haluska FG, Tsao H, Wu H, Haluska FS, Lazar A, Goel V. Genetic alterations in signaling pathways in melanoma. Clin Cancer Res. 2006;12:2301s–2307s. doi: 10.1158/1078-0432.CCR-05-2518. [DOI] [PubMed] [Google Scholar]
- 24.Daniotti M, Oggionni M, Ranzani T, Vallacchi V, Campi V, Di Stasi D, et al. BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene. 2004;23:5968–5977. doi: 10.1038/sj.onc.1207780. [DOI] [PubMed] [Google Scholar]
- 25.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. BRAF(V600E) cooperates with PTEN loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing F, Persaud Y, Pratilas CA, Taylor BS, Janakiraman M, She QB, et al. Concurrent loss of the PTEN and RB1 tumor suppressors attenuates RAF dependence in melanomas harboring (V600E)BRAF. Oncogene. 2012;31:248–258. doi: 10.1038/onc.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen A, Munko AC, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res. 2011;71:2750–2760. doi: 10.1158/0008-5472.CAN-10-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng W, Yennu-Nanda VG, Scott A, Chen G, Woodman SE, Davies MA. Role and Therapeutic Potential of PI3K-mTOR Signaling in De Novo Resistance to BRAF Inhibition. Pigment Cell & Melanoma Research. 2012;25:248–258. doi: 10.1111/j.1755-148X.2011.00950.x. [DOI] [PubMed] [Google Scholar]
- 29.Gopal YN, Deng W, Woodman SE, Komurov K, Ram P, Smith PD, et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in BRAF-mutant human cutaneous melanoma cells. Cancer Res. 2010;70:8736–8747. doi: 10.1158/0008-5472.CAN-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathanson K, Martin A, Letrero R, D/'Andrea K, O'Day S, Infante JR, et al. Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor GSK2118436 (GSK436) J Clin Oncol. 2011;29 doi: 10.1158/1078-0432.CCR-13-0827. abst 8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sosman JA, Pavlick AC, Schuchter LM, Lewis KD, McArthur GA, Cowey CL, et al. Analysis of molecular mechanisms of response and resistance to vemurafenib (vem) in BRAF V600E melanoma. J Clin Oncol. 2012;30:8503. [Google Scholar]
- 32.Niessner H, Forschner A, Klumpp B, Honegger JB, Witte M, Bornemann A, et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Medicine. 2013;2:76–85. doi: 10.1002/cam4.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curtin JA, Stark MS, Pinkel D, Hayward NK, Bastian BC. PI3-kinase subunits are infrequent somatic targets in melanoma. J Invest Dermatol. 2006;126:1660–1663. doi: 10.1038/sj.jid.5700311. [DOI] [PubMed] [Google Scholar]
- 34.Omholt K, Krockel D, Ringborg U, Hansson J. Mutations of PIK3CA are rare in cutaneous melanoma. Melanoma Res. 2006;16:197–200. doi: 10.1097/01.cmr.0000200488.77970.e3. [DOI] [PubMed] [Google Scholar]
- 35.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 36.Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, et al. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer. 2008;99:1265–1268. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–7010. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 38.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and Mutant V600EB-Raf Cooperate to Promote Early Melanoma Development. Cancer Research. 2008;68:3429–3439. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh RS, Diwan AH, Zhang PS, Prieto VG. Phosphoinositide 3-kinase is not overexpressed in melanocytic lesions. Journal of Cutaneous Pathology. 2007;34:220–225. doi: 10.1111/j.1600-0560.2006.00592.x. [DOI] [PubMed] [Google Scholar]
- 40.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 41.Kantrow SM, Boyd AS, Ellis DL, Nanney LB, Richmond A, Shyr Y, et al. Expression of activated Akt in benign nevi, Spitz nevi and melanomas. Journal of Cutaneous Pathology. 2007;34:593–596. doi: 10.1111/j.1600-0560.2006.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006;24:4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 43.Liang R, Wallace AR, Schadendorf D, Rubin BP. The phosphatidyl inositol 3-kinase pathway is central to the pathogenesis of Kit-activated melanoma. Pigment Cell Melanoma Res. 2011 doi: 10.1111/j.1755-148X.2011.00870.x. no-no. [DOI] [PubMed] [Google Scholar]
- 44.Monsel G, Ortonne N, Bagot M, Bensussan A, Dumaz N. c-Kit mutants require hypoxia-inducible factor 1[alpha] to transform melanocytes. Oncogene. 2009;29:227–236. doi: 10.1038/onc.2009.320. [DOI] [PubMed] [Google Scholar]
- 45.Todd JR, Becker TM, Kefford RF, Rizos H. Secondary c-Kit mutations confer acquired resistance to RTK inhibitors in c-Kit mutant melanoma cells. Pigment Cell & Melanoma Research. 2013 doi: 10.1111/pcmr.12107. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 46.Prickett TD, Agrawal NS, Wei X, Yates KE, Lin JC, Wunderlich JR, et al. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nat Genet. 2009;41:1127–1132. doi: 10.1038/ng.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to BRAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired Resistance to BRAF Inhibitors Mediated by a RAF Kinase Switch in Melanoma Can Be Overcome by Cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi H, Kong X, Ribas A, Lo RS. Combinatorial Treatments That Overcome PDGFRβ-Driven Resistance of Melanoma Cells to V600EB-RAF Inhibition. Cancer Research. 2011;71:5067–5074. doi: 10.1158/0008-5472.CAN-11-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marsh V, Silva J, Bosenberg M, Phillips W, McMahon M. Elucidating the role of the PI3-kinase pathway activation in melanoma progression in vivo. Pigment Cell & Melanoma Research. 2011;24:1000. [Google Scholar]
- 53.McMahon M, Thakur MD, Marsh V, Silva J, Landman AS, Deuker M, et al. Targeting BRAF and PI3'-kinase signaling for therapy of melanoma. 2013 AACR Annual Meeting; 2013; Washington, DC. 2013. p. SY17-03. [Google Scholar]
- 54.Aziz SA, Jilaveanu LB, Zito C, Camp RL, Rimm DL, Conrad P, et al. Vertical Targeting of the Phosphatidylinositol-3 Kinase Pathway as a Strategy for Treating Melanoma. Clinical Cancer Research. 2010;16:6029–6039. doi: 10.1158/1078-0432.CCR-10-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng W, Vashisht Gopal YN, Scott A, Chen G, Woodman SE, Davies MA. Role and therapeutic potential of PI3K-mTOR signaling in de novo resistance to BRAF inhibition. Pigment Cell & Melanoma Research. 2012;25:248–258. doi: 10.1111/j.1755-148X.2011.00950.x. [DOI] [PubMed] [Google Scholar]
- 56.Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, et al. Targeting Activated Akt with GDC-0068, a Novel Selective Akt Inhibitor That Is Efficacious in Multiple Tumor Models. Clinical Cancer Research. 2013;19:1760–1772. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- 57.Margolin K, Longmate J, Baratta T, Synold T, Christensen S, Weber J, et al. CCI-779 in metastatic melanoma: a phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 58.Davies MA, Fox PS, Papadopoulos NE, Bedikian AY, Hwu W-J, Lazar AJ, et al. Phase I Study of the Combination of Sorafenib and Temsirolimus in Patients with Metastatic Melanoma. Clinical Cancer Research. 2012;18:1120–1128. doi: 10.1158/1078-0432.CCR-11-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margolin KA, Moon J, Flaherty LE, Lao CD, Akerley WL, 3rd, Othus M, et al. Randomized phase II trial of sorafenib with temsirolimus or tipifarnib in untreated metastatic melanoma (S0438) Clin Cancer Res. 2012;18:1129–1137. doi: 10.1158/1078-0432.CCR-11-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 61.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110[bgr] in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wee S, Wiederschain D, Maira S-M, Loo A, Miller C, deBeaumont R, et al. PTEN-deficient cancers depend on PIK3CB. Proceedings of the National Academy of Sciences. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ni J, Liu Q, Xie S, Carlson C, Von T, Vogel K, et al. Functional Characterization of an Isoform-Selective Inhibitor of PI3K-p110β as a Potential Anticancer Agent. Cancer Discovery. 2012;2:425–433. doi: 10.1158/2159-8290.CD-12-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paraiso KHT, Fedorenko IV, Cantini LP, Munko AC, Hall M, Sondak VK, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer. 2010;102:1724–1730. doi: 10.1038/sj.bjc.6605714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoeflich KP, Merchant M, Orr C, Chan J, Den Otter D, Berry L, et al. Intermittent Administration of MEK Inhibitor GDC-0973 plus PI3K Inhibitor GDC-0941 Triggers Robust Apoptosis and Tumor Growth Inhibition. Cancer Research. 2012;72:210–219. doi: 10.1158/0008-5472.CAN-11-1515. [DOI] [PubMed] [Google Scholar]
- 67.Andersen JN, Sathyanarayanan S, Di Bacco A, Chi A, Zhang T, Chen AH, et al. Pathway-Based Identification of Biomarkers for Targeted Therapeutics: Personalized Oncology with PI3K Pathway Inhibitors. Science Translational Medicine. 2010;2:43ra55. doi: 10.1126/scitranslmed.3001065. [DOI] [PubMed] [Google Scholar]
- 68.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solit DB, Rosen N. Resistance to BRAF Inhibition in Melanomas. New England Journal of Medicine. 2011;364:772–774. doi: 10.1056/NEJMcibr1013704. [DOI] [PubMed] [Google Scholar]
- 70.Lu Y, Muller M, Smith D, Dutta B, Komurov K, Iadevaia S, et al. Kinome siRNA-phosphoproteomic screen identifies networks regulating AKT signaling. Oncogene. 2011;30:4567–4577. doi: 10.1038/onc.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–146. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]
- 72.Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Molecular Cancer Therapeutics. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 73.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carracedo A, Li M, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. Journal of Clinical Investigation: American Society for Clinical Investigation. 2008:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith J, Rozengurt E. Different Patterns of Akt and ERK Feedback Activation in Response to Rapamycin, Active-Site mTOR Inhibitors and Metformin in Pancreatic Cancer Cells. PLoS ONE. 2013;8:e57289. doi: 10.1371/journal.pone.0057289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2006;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 77.Marone R, Erhart D, Mertz AC, Bohnacker T, Schnell C, Cmiljanovic V, et al. Targeting Melanoma with Dual Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Inhibitors. Molecular Cancer Research. 2009;7:601–613. doi: 10.1158/1541-7786.MCR-08-0366. [DOI] [PubMed] [Google Scholar]
- 78.Chakrabarty A, Sánchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proceedings of the National Academy of Sciences. 2012;109:2718–2723. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT Inhibition Relieves Feedback Suppression of Receptor Tyrosine Kinase Expression and Activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serra V, Scaltriti M, Prudkin L, Eichhorn PJA, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR Kinase Inhibition Causes Feedback-Dependent Biphasic Regulation of AKT Signaling. Cancer Discovery. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shankavaram UT, Reinhold WC, Nishizuka S, Major S, Morita D, Chary KK, et al. Transcript and protein expression profiles of the NCI-60 cancer cell panel: an integromic microarray study. Mol Cancer Ther. 2007;6:820–832. doi: 10.1158/1535-7163.MCT-06-0650. [DOI] [PubMed] [Google Scholar]
- 83.Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8:393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 84.Maira S-M, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Molecular Cancer Therapeutics. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 85.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Migliardi G, Sassi F, Torti D, Galimi F, Zanella ER, Buscarino M, et al. Inhibition of MEK and PI3K/mTOR Suppresses Tumor Growth but Does Not Cause Tumor Regression in Patient-Derived Xenografts of RAS-Mutant Colorectal Carcinomas. Clinical Cancer Research. 2012;18:2515–2525. doi: 10.1158/1078-0432.CCR-11-2683. [DOI] [PubMed] [Google Scholar]
- 87.Roberts PJ, Usary JE, Darr DB, Dillon PM, Pfefferle AD, Whittle MC, et al. Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clin Cancer Res. 2012;18:5290–5303. doi: 10.1158/1078-0432.CCR-12-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS, et al. The Clinical Effect of the Dual-Targeting Strategy Involving PI3K/AKT/mTOR and RAS/MEK/ERK Pathways in Patients with Advanced Cancer. Clinical Cancer Research. 2012;18:2316–2325. doi: 10.1158/1078-0432.CCR-11-2381. [DOI] [PubMed] [Google Scholar]
- 89.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19 doi: 10.1158/1078-0432.CCR-13-0143. xx-xx. [DOI] [PubMed] [Google Scholar]
- 90.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw C-NJ, Sloss CM, et al. Selective BRAFV600E Inhibition Enhances T-Cell Recognition of Melanoma without Affecting Lymphocyte Function. Cancer Research. 2010 doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 91.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clinical Cancer Research. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 93.Kwong M, Neyns B, Yang J. Adoptive T-cell transfer therapy and oncogene targeted therapy for melanoma: the search for synergy. Clin Cancer Res. 2013;19 doi: 10.1158/1078-0432.CCR-13-0261. xx-xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, et al. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]