Key Points
Molecular profiling was used to optimize an ex vivo modulation protocol with dmPGE2 for UCB transplantation.
Pulse treatment of UCB with dmPGE2 is safe and may lead to accelerated UCB engraftment and preferential cord chimerism.
Abstract
Umbilical cord blood (UCB) is a valuable source of hematopoietic stem cells (HSCs) for use in allogeneic transplantation. Key advantages of UCB are rapid availability and less stringent requirements for HLA matching. However, UCB contains an inherently limited HSC count, which is associated with delayed time to engraftment, high graft failure rates, and early mortality. 16,16-Dimethyl prostaglandin E2 (dmPGE2) was previously identified to be a critical regulator of HSC homeostasis, and we hypothesized that brief ex vivo modulation with dmPGE2 could improve patient outcomes by increasing the “effective dose” of HSCs. Molecular profiling approaches were used to determine the optimal ex vivo modulation conditions (temperature, time, concentration, and media) for use in the clinical setting. A phase 1 trial was performed to evaluate the safety and therapeutic potential of ex vivo modulation of a single UCB unit using dmPGE2 before reduced-intensity, double UCB transplantation. Results from this study demonstrated clear safety with durable, multilineage engraftment of dmPGE2-treated UCB units. We observed encouraging trends in efficacy, with accelerated neutrophil recovery (17.5 vs 21 days, P = .045), coupled with preferential, long-term engraftment of the dmPGE2-treated UCB unit in 10 of 12 treated participants. This study was registered at www.clinicaltrials.gov as #NCT00890500.
Introduction
Umbilical cord blood (UCB) is a valuable source of hematopoietic stem cells (HSCs) for use in allogeneic transplantation when a suitable adult donor is unavailable.1,2 However, many of the publically accessible UCB units are currently unfit for use in adult transplantation because of a low HSC content, which is associated with delayed time to engraftment, high graft failure rates, and early mortality.3,4 Several approaches are being evaluated to address this limiting stem cell dose problem, including cotransplantation of multiple UCB units,5,6 cotransplantation with progenitor cells from an alternative source,7,8 direct intramarrow injection of UCB units,9 or expanding HSCs in culture.10-12 Although each strategy has its benefits, significant limitations are associated with these approaches, including induction of differentiation of long-term HSCs during extended culture, high manufacturing cost, and the risk of introducing infectious agents.
An alternative strategy to improve outcomes for patients undergoing UCB transplantation is to use a brief pulse treatment with a small molecule modulator to enhance the homing and engraftment potential of HSCs. One potential ex vivo-enhancing agent, the stable prostaglandin E2 (PGE2) derivative 16,16-dimethyl PGE2 (dmPGE2), was previously identified in a chemical screen using zebra fish embryos to be a potent regulator of vertebrate HSC homeostasis.13 dmPGE2 modulates HSCs primarily through the G-protein–coupled prostaglandin receptors PTGER2 (EP2) and PTGER4 (EP4), which use cyclic adenosine monophosphate (cAMP) as a secondary messenger to upregulate the expression of genes involved in homing (eg, CXCR4), proliferation (eg, cyclinD1), and cell survival (eg, survivin).14,15 dmPGE2 also increases Wnt signaling in HSCs and enhances self-renewal, which is important for the long-term maintenance of HSCs.16 An extensive set of characterization studies in zebra fish, murine, and nonhuman primate models demonstrated the ability of dmPGE2 to enhance hematopoietic engraftment of HSCs.13-15,17
On the basis of promising preclinical evidence and prior human safety data,18,19 a phase 1 clinical trial was initiated to evaluate the safety and efficacy of using an ex vivo treatment with dmPGE2 to improve HSC engraftment following reduced-intensity double UCB transplantation. Double UCB transplantation provides a unique platform to examine the potential of enhanced hematopoietic engraftment of dmPGE2-modulated UCB compared with unmanipulated UCB by virtue of the competitive engraftment between UCB units. In addition, the second unmanipulated UCB unit provides a safety mechanism in the event of damage to the treated UCB unit during the ex vivo manipulation. Herein, we describe a point-of-care, ex vivo pulse treatment strategy designed to accelerate engraftment in UCB transplantation, describe the molecular studies that lead to the optimization of this ex vivo modulation process, and report outcomes treated on this phase 1 clinical trial.
Materials and methods
Patients and treatment program
The primary objective of this phase 1 trial was to evaluate the safety of dmPGE2-treated UCB (dmPGE2-UCB) cotransplantation with an unmanipulated UCB unit in patients with hematologic malignancies. Secondary objectives were to determine the kinetics of engraftment and the fractional chimerism of dmPGE2-UCB after transplantation. Participants with hematologic malignancies for whom no HLA-matched donor was available received conditioning with fludarabine (180 mg/m2), melphalan (100 mg/m2), and antithymocyte globulin (4 mg/kg) and received graft-versus-host disease (GVHD) prophylaxis with sirolimus (target trough concentration, 3-12 ng/mL) and tacrolimus (target trough concentration 5-10 ng/mL), as described previously.20 UCB units were required to be ≥4/6 HLA-allele matched with the recipient and each other. Each UCB unit was required to be ≥1.5 × 107 total nucleated cells (TNCs)/kg before cryopreservation, and the combined cell dose was required to be ≥3.7 × 107 TNC/kg. UCB units were hierarchically selected from international cord blood banks based on TNC count, HLA match, and unit age. Units against which participants had preformed anti-HLA antibodies were excluded.21
On the day of transplantation, 2 cryopreserved UCB units were thawed and resuspended in a saline solution (0.9% NaCl) containing 5% human serum albumin (Baxter or Talecris) and 8% Dextran 40 (Hospira) (LMD/HSA). A total of 2 cohorts of patients were enrolled. In cohort 1, one of the 2 UCB units was incubated with 10 µM of dmPGE2 (Fate Therapeutics) for 60 minutes at 4°C in LMD/HSA (9 patients). In the initial 6 patients, the smaller unit (by TNC before cryopreservation) was treated with dmPGE2, and in subsequent 3 patients, the larger unit was treated. Cohort 2 consisted of 12 patients in whom the larger of the 2 UCB units was incubated with 10 µM of dmPGE2 for 120 minutes at 37°C in LMD/HSA. After incubation, excess dmPGE2 was removed using a second centrifugation procedure and was resuspended in LMD/HSA for infusion. All patients received both UCB units within 4 hours of each other, with the larger UCB (whether dmPGE2-treated or not) always administered first. Standard posttransplantation care was delivered to all participants. In accordance with the Declaration of Helsinki, all participants provided informed consent to participate, and this trial was prospectively registered at www.clinicaltrials.gov (#NCT00890500). The study was approved by the Office for Protection of Research Subjects at the Dana-Farber/Harvard Cancer Center.
Patient baseline characteristics were reported descriptively. Neutrophil engraftment was defined as the first of 3 consecutive days with neutrophil recovery to at least 0.5 × 109 cells/L. Platelet engraftment was defined as the first day of a platelet count of at least 20 × 109 cells/L, without supporting transfusion in the prior 3 days. Donor chimerism was determined from peripheral blood mononuclear cells by analyses of informative short tandem repeat loci using the ABI Profiler-Plus Kit (Applied Biosystems) and the ABI 310 GeneticAnalyzer. Overall survival (OS) was defined as the time from transplant to death from any cause, whereas progression-free survival (PFS) was defined as the time from transplant to malignant disease progression or death from any cause. Surviving patients were censored at their date of last known follow-up. OS and PFS estimates were calculated using the method of Kaplan and Meier.22 A comparative control cohort of 53 participants treated at our institution using the same UCB unit selection criteria, conditioning, and GVHD prophylaxis regimen was used for comparison (supplemental Table 1, available on the Blood Web site). All P values are based on 2-sided tests and were computed using SAS v9.2 (SAS Institute, Cary, NC).
Primary cells
Cryopreserved human UCB CD34+ primary cells were purchased from AllCells or Stem Cell Technologies (Vancouver, BC, Canada).
RNA preparation
RNA was extracted using the PicoPure RNA Isolation kit (Life Technologies) using the manufacturer’s recommended protocol. Total RNA was quantified using the Nanodrop 2000 Spectrophotometer (Thermo Scientific). RNA integrity was confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies).
Genome-wide microarray analysis
Biotinylated antisense RNA was prepared from 10 to 100 ng of total RNA involving the Message Amp II kit (Life Technologies) following the standard 2-round amplification protocols and hybridized to Affymetrix U133-plus-2.0 GeneChips according to the manufacturer’s instructions. Arrays were processed in the GeneChip Fluidics Station 450 and were scanned on the 3000 7G Scanner (Affymetrix). Probe intensities were normalized according to a log-scale robust multiarray analysis (RMA) method (Affymetrix) and were visualized in Spotfire for Genomics 4.5 (Tibco). Raw expression data files are available on Gene Expression Omnibus (GSE46569).
Parametric paired t tests (Benjamini-Hochberg false discovery rate < .05, adjusted P value/q-value < .05, and fold change > or < threefold) detected probes with significant changes due specifically to the dmPGE2 treatment conditions. Biological pathway enrichment analysis of the upregulated probes was performed against the Gene Ontology (GO) database (Singular Enrichment to GO Biological Process and false discovery rate < .01).
Microfluidic quantitative PCR using the Fluidigm platform
The messenger RNA sequences of the dmPGE2 signature genes were taken from the National Center for Biotechnology Information Gene database, and amplification primers were designed using Primer3. (supplemental Table 2) For real-time polymerase chain reaction (PCR) transcript quantitation, we used the BioMark Dynamic Array microfluidics system and GE Dynamic Array 96.96 chips (Fluidigm) using the manufacturer’s protocol. Amplification results were analyzed using BioMark Real-Time PCR Analysis software. Samples with Cycle Thresholds above 28 or amplified products with inappropriate melting curve properties were excluded from the calculations. Log2 fold change results are displayed in Spotfire for Genomics 4.0 in a heat-map format.
Human CD34+ HSC murine homing
Human CD34+ cells isolated from UCB were treated with 10 µM of dmPGE2 or vehicle (dimethylsulfoxide) for 2 hours at 37°C in StemSpan-SFEM (Stem Cell Technologies) or a minimal media containing LMD/HSA. For the last 15 minutes of the incubation, Vybrant Dye DiI (Life Technologies) was added to label the cells. After incubation, 1 × 105 cells from each condition were washed and injected in sublethally irradiated NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice in groups of 5 per condition. At 20 hours after the injections, hind limb long bones were harvested and the bone marrow (BM) extracted. Flow cytometry was performed on the BM using LSRII (Becton Dickinson) and gating criteria based on remaining DiI-stained CD34+ cells and control mouse BM. This homing experiment was performed in duplicate using CD34+ cells from 2 separate UCB units. Data were plotted as the increase in homed events relative to the vehicle-treated cells. Results were averaged for both donor groups in each of the conditions tested. All protocols using animals in this study were approved by the Institutional Animal Care and Use Committee at Indiana University School of Medicine (supplemental Methods).
Results
Cohort 1
Nine participants with high risk hematological malignancies were treated using ex vivo modulation conditions based on preclinical studies (10 µM of dmPGE2, exposure for 60 minutes at 4°C in LMD/HSA media) between May 2009 and June 2010. Patient and UCB unit characteristics are described in Table 1. No safety concerns were identified in this cohort; however, neither an improvement in rates of neutrophil recovery nor preferential engraftment of the dmPGE2-UCB over the unmanipulated UCB units was observed (Figure 1A). The median times to neutrophil and platelet engraftment were 24 and 72.5 days, respectively. Two of 7 patients undergoing engraftment demonstrated prolonged hematopoiesis from the dmPGE2-UCB units, and 2 patients had primary or late graft failure (Figure 1B-C).
Table 1.
Patient and UCB characteristics
| Patient characteristics | Cohort 1 | Cohort 2 | ||||
|---|---|---|---|---|---|---|
| Sample size (n) | 9 | 12 | ||||
| Median age, y (range) | 43.0 (29-64) | 57.5 (19-66) | ||||
| Male gender, n (%) | 4 (44.4) | 8 (66.7) | ||||
| Median weight (kg, range) | 73.8 (44.7-126) | 78.7 (48.7-149.6) | ||||
| Primary malignancy, n (%) | ||||||
| AML | 3 (33.3) | 5 (41.7) | ||||
| MDS | 2 (22.2) | 4 (33.3) | ||||
| NHL/CLL | 2 (22.2) | 3 (25.0) | ||||
| ALL | 2 (22.2) | 0 | ||||
| Prior autologous transplant, n (%) | 2 (22.2) | 2 (16.7) | ||||
| CMV seropositive, n (%) |
7 (77.8) |
7 (58.3) |
||||
|
UCB unit characteristics |
dmPGE2-UCB |
Untreated UCB |
P |
dmPGE2-UCB |
Untreated UCB |
P |
| HLA match | ||||||
| 4/6 | 8 | 8 | NS | 10 | 8 | .64 |
| 5/6 | 1 | 1 | 2 | 4 | ||
| Precryopreservation | ||||||
| TNC (×107/kg) | 3.03 | 2.53 | .43 | 2.64 | 1.95 | .02 |
| CD34+ (×105/kg) | 1.58 | 1.54 | .79 | 1.21 | 1.01 | .69 |
| Postthaw | ||||||
| TNC (×107/kg) | 2.17 | 1.8 | .45 | 1.8 | 1.7 | .43 |
| CD34+ (×105/kg) | 0.7 | 0.68 | .94 | 0.74 | 0.56 | .71 |
| CFU-GM (×103/kg) | 6.76 | 2.65 | .19 | 4.5 | 6.89 | .77 |
| First infused unit |
3 |
6 |
12 |
0 |
||
|
dmPGE2 processing |
||||||
| CD34+ cell count (%) | −20.92% | −7.87% | ||||
| TNC viability (7-AAD) | −0.27% | 1.81% | ||||
| CD34+ viability (7-AAD) | −1.40% | −1.28% | ||||
ALL, acute lymphoblastic leukemia; AML, acute myeloblastic leukemia; CMV, cytomegalovirus; GM, granulocyte-macrophages (in CFUs); MDS, myelodysplastic syndrome; NHL/CLL, non-Hodgkin lymphoma/chronic lymphocytic leukemia.
Figure 1.
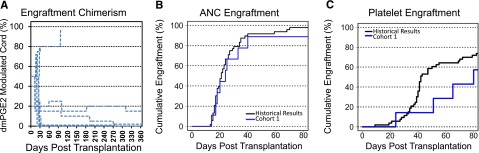
Cohort 1 clinical results. (A) Donor chimerism of the dmPGE2-treated UCB unit during 360 days posttransplantation (percentage of total) as determined from peripheral blood mononuclear cells by analyses of informative short tandem repeat loci. (B) Cumulative incidence of neutrophil engraftment of >500 cells/mm3, in cohort 1 (n = 9, blue) compared with historical institutional control participants (n = 53, black). (C) Cumulative incidence of platelet engraftment in cohort 1 (n = 9, blue) compared with historical institutional control participants (n = 53, black).
Optimization of the ex vivo modulation
Given the lack of accelerated engraftment in the initial cohort of patients, we sought to determine if the ex vivo incubation conditions were optimal in the clinical setting. To evaluate the effects of temperature, CD34+ cells were incubated at 4°C, 25°C, or 37°C for 2 hours in the presence of dmPGE2 or vehicle. After the incubation, genome-wide expression arrays were used to identify genes upregulated by dmPGE2. Only 2 probe sets across the entire human transcriptome were significantly upregulated (>fourfold) when the CD34+ cells were incubated at 4°C (Figure 2A). Performing the ex vivo incubation at 25°C also resulted in a modest biological response, with 19 probe sets being significantly upregulated. In contrast, increasing the incubation temperature to 37°C resulted in a robust biological response with 192 probe sets being significantly upregulated, suggesting that physiological temperatures are required for cryopreserved human CD34+ cells to effectively activate the prostaglandin signaling pathway. The gene ontology pathway analysis for the dmPGE2 signature showing genes involved in proliferation, migration, and receptor signaling is shown in Figure 2B.
Figure 2.
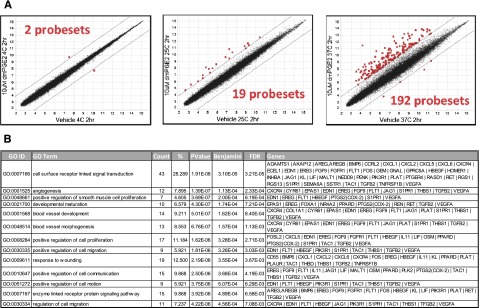
Evaluation of ex vivo modulation temperature with cryopreserved human UCB CD34+ cells with dmPGE2. (A) Genome-wide expression analysis on Affymetrix U133 plus 2.0 GeneChips of human UCB CD34+ cells treated with 10 µM of dmPGE2 for 2 hours at 4°C, 25°C, and 37°C. RMA log2 normalized expression levels for cells treated with dmPGE2 (y-axis) in comparison with vehicle-treated cells (x-axis). There were 2, 19, and 192 probe sets (red) with expression levels changing greater (or less) than fourfold due to dmPGE2 treatment at 4°C (left), 25°C (center), and 37°C (right), respectively. (B) GO enrichment analysis showing biological processes enriched in the upregulated probes induced by treatment with dmPGE2 at 37°C. Genes upregulated by dmPGE2 treatment at 37°C in CD34+ are listed for each GO category.
To determine the optimal incubation time, CD34+ cells were treated with 10 µM of dmPGE2 or vehicle (dimethylsulfoxide) for increasing amounts of time at 37°C. After the incubation, dmPGE2-induced changes in gene expression were measured using a high-throughput microfluidic quantitative PCR-based system. Amplification primers were designed for 6 reference genes and 90 of the top dmPGE2 upregulated genes, selected based on the genome-wide expression analysis and biological significance for homing, proliferation, and survival (supplemental Table 1). Figure 3A shows that 2-hour incubation at 37°C is required to induce the maximal activation of the prostaglandin pathway. We also demonstrated that the prostaglandin must be present during the entire 2-hour incubation to achieve a full biological response (supplemental Figure 1). This eliminates the possibility of using a brief pulse treatment (<60 minutes) to initiate a signaling event that could mature once the enhanced HSCs are infused into the patient.
Figure 3.
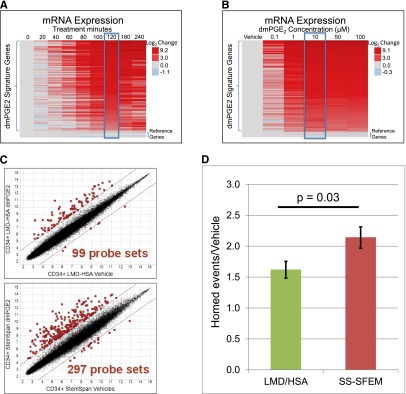
Optimization of incubation time, dmPGE2 concentration, and modulation media in cryopreserved UCB CD34+ cells. (A) Microfluidic RT-PCR gene expression analysis of the top 90 signature genes after 10 μM of dmPGE2 treatment at 37°C incubated for 0 to 240 minutes. The heat map shows log2 fold changes in expression levels relative to vehicle control treatments of the same time. (B) Microfluidic RT-PCR gene expression analysis of the top 90 signature genes after 120 minutes at 37°C with varying dmPGE2 concentrations. The heat map shows log2 fold changes in expression levels relative to vehicle control treatments. (C) Genome-wide expression analysis on Affymetrix U133 plus 2.0 GeneChips of human UCB CD34+ cells treated with 10 µM of dmPGE2 for 2 hours at 37°C in 8% LMD and 5% HSA or StemSpan-SFEM. RMA log2 normalized expression levels of expression for cells treated with dmPGE2 (y-axis) in comparison with vehicle-treated cells (x-axis). There were 99 and 297 probe sets (red) with expression levels changing greater (or less) than fourfold due to dmPGE2 treatment in LMD/HSA and StemSpan-SFEM, respectively. (D) Fold increase of homed CD34+ cells over control (vehicle-treated) after incubation with 10 µM of dmPGE2 for 2 hours at 37°C in LMD/HSA (green) and StemSpan-SFEM (red) (t test, P = .03).
To determine the optimal concentration of dmPGE2 for ex vivo modulation, cryopreserved human CD34+ cells were treated with increasing concentrations of dmPGE2 (0.1 µM, 1 µM, 10 µM, 50 µM, and 100 µM) for 2 hours at 37°C. Supraphysiological concentrations of dmPGE2 are permissible because the molecule is removed with the supernatant during washing prior to infusion. Expression changes in the prostaglandin signature genes were monitored using a microfluidic quantitative reverse transcription (RT)-PCR platform. The amount able to induce a maximal pathway response was 10 µM (Figure 3B). Higher concentrations of dmPGE2 were not able to increase pathway activation at 4°C (supplemental Figure 2).
The majority of dmPGE2 preclinical homing, engraftment, and optimization studies were performed in “complete” media such as StemSpan-SFEM (Stem Cell Technologies), which contains glucose, amino acids, and other nutrients designed to support long-term culturing of HSCs. Unfortunately, this type of complete media cannot be used in a clinical setting because differentiated cell types (eg, granulocytes) that do not survive the cryopreservation process lyse upon thawing and incubation in this media, which causes a significant reduction in the TNC counts (supplemental Figure 3). Typically, the media used in a clinical setting for thawing and washing cryopreserved UCB contain LMD/HSA to minimize cell loss. We sought to determine whether the ex vivo modulation could be performed in these clinically compatible, nutrient-free media. To characterize the effects of media on the level of pathway activation, CD34+ cells were treated with 10 µM of dmPGE2 for 2 hours at 37°C in either StemSpan-SFEM or LMD/HSA. After the incubations, prostaglandin-induced expression changes were analyzed using genome-wide expression arrays. Figure 3C demonstrates that both media formulations were able to support activation of the prostaglandin pathway by dmPGE2; however, threefold more probe sets were upregulated in StemSpan-SFEM (297 vs 99 probe sets). We used a mouse homing model to determine whether the incubation media affect the functional properties of the enhanced HSCs. The homing properties of human CD34+ cells were significantly improved in both types of media with a 2.2-fold increase in the number of human cells that had migrated to the BM of mice when the CD34+ cells were pulse treated in StemSpan-SFEM (P < .001) compared with a 1.6-fold increase with LMD/HSA (P = .002) (Figure 3D). The difference in homing properties between the 2 types of media (1.6-fold vs 2.2-fold) is also statistically significant (P = .03). A subtle decrease in viable cell recovery (7-aminoactinomycin D [7-AAD] and colony-forming units [CFU] in culture) was observed in the LMD/HSA relative to nutrient-rich media. On the basis of these results, we decided to move forward with the clinically proven LMD/HSA media for the initial clinical studies.
To characterize the engraftment properties of HSCs treated using the optimized conditions, lethally irradiated mice were injected with decreasing numbers of mouse BM cells (200K, 100K, 50K, or 10K) treated with 10 µM of dmPGE2 or vehicle for 2 hours at 37°C. Results from this experiment demonstrate that ex vivo treatment with dmPGE2 significantly increased the survival rates of mice transplanted with limiting numbers of HSCs (supplemental Figure 4) We also observed accelerated recovery of neutrophils and platelets using this murine transplantation model (supplemental Figure 5A-B).
Cohort 2
A total of 12 additional participants were accrued between August 2010 and August 2011 and were treated according to the optimized ex vivo dmPGE2 modulation protocol(10 µM of dmPGE2 and 2-hour exposure at 37°C in LMD/HSA media). Clinical and UCB unit characteristics are described in Table 1. The patient median age was 57.5 years (age range, 19-66 years) and the median weight was 78.7 kg (range, 48.7-149.6 kg). The majority of UCB units were 4/6 matched to each other and the recipient, and the postthaw cell doses between units were very similar. Ex vivo incubation with dmPGE2 did not result in significant cell loss, with a mean viable CD34+ cell recovery of 90%. Adverse events attributed to dmPGE2-UCB infusion included grade 1 to 2 infusion-related events in 4 participants, consisting of chills, flushing, abdominal pain, and cough. One additional participant with known coronary artery disease experienced transient grade 4 ST-elevation after infusion and evidence of myocardial ischemia by cardiac troponin assay.
The median time to neutrophil engraftment was 17.5 days (range, 14-31 days), which was shorter than a historical control regimen of similarly treated patients at our institution (21 days, n = 53, P = .045, Figure 4B), as well as shorter than the time to engraftment of patients in cohort 1 (P = .09). No patient experienced primary graft failure. The median time to platelet engraftment was 43 days (range, 26-60 days), and 11 of 12 patients had engrafted platelets by day 60 (Figure 4C). Chimerism assessment demonstrated that 10 of 12 patients had early and sustained engraftment of the dmPGE2-UCB unit, and that this unit contributed 100% to hematopoiesis (Figure 4A). Using a binomial distribution and historical engraftment rates of the first administered cord, the probability of this occurring is only 2.4% (P = .03). Sustained dmPGE2-UCB hematopoiesis has been demonstrated for up to 27 months from transplantation. Chimerism in CD33+ myeloid and CD3+ lymphoid subsets mirrored total chimerism assessments.
Figure 4.
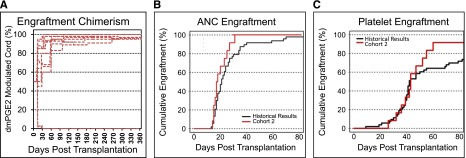
Cohort 2 clinical results. (A) Donor chimerism of the dmPGE2-treated UCB unit during 360 days posttransplantation (percentage of total) as determined from peripheral blood mononuclear cells by analyses of informative short tandem repeat loci. (B) Cumulative incidence of neutrophil engraftment of >500 cells/mm3, in cohort 2 (n = 12, red) compared with historical institutional control participants (n = 53, black). (C) Cumulative incidence of platelet engraftment in cohort 2 (n = 12, red) compared with historical institutional control participants (n = 53, black).
Three participants experienced grade 1 GVHD, and 2 participants experienced skin-limited grade 2 GVHD. One patient had skin-limited chronic GVHD. Relapse occurred in 3 participants, and 8 participants have died. Causes of death include relapse (n = 3), treatment-related complications (n = 4), and suicide (n = 1). With a median follow-up among survivors of 24.6 months (range, 21.9-27.4 months), the 1- and 2-year PFS rates were 61.7% and 31.3%, respectively. The corresponding 1- and 2-year OS rates were 75% and 38.9%.
Discussion
The development of HSCs during embryogenesis is regulated by pathways that regulate HSC homing and engraftment after transplantation in adulthood. dmPGE2 was originally identified as a small molecule that could increase HSC formation in the developing aorta of the zebra fish embryo, and enhanced HSC engraftment in murine HSC transplantation after brief ex vivo exposure.13 Further studies established that dmPGE2 improved human UCB engraftment in immunodeficient mice, and demonstrated safety in long-term primate transplantation studies.14 Given that the limiting number of HSCs in UCB units results in delayed hematopoietic engraftment, graft failure, and incomplete immunologic reconstitution, we sought to determine if modulation of UCB with dmPGE2 before transplantation could improve these outcomes. Of note, our study is the first in which the function of a small molecule discovered in the zebra fish system has reached a human clinical trial. UCB units from the initial cohort of 9 participants were treated under ex vivo modulation conditions designed to maximize viability of the HSCs. After demonstrating safety, but lack of clear efficacy, we enrolled a second cohort of patients using ex vivo modulation conditions designed to increase the prostaglandin pathway stimulation. Results from this second group of patients demonstrated a correlation between the enhanced biologic activity, accelerated engraftment, and preferential hematopoiesis from the modulated unit in a double UCB transplantation model.
In translating this academic discovery into the clinic, one of the challenges that we faced was determining the optimal incubation conditions to use in the clinical setting. The incubation conditions used for the preclinical studies varied significantly with respect to the temperature, duration, dose, and media tested. Molecular profiling approaches were used to identify a set of ex vivo modulation conditions, which enable rapid and robust activation of the prostaglandin pathway in cryopreserved human cord blood HSCs. The key changes to the incubation protocol were increasing the temperature from 4°C to 37°C and extending the incubation from 1 hour to 2 hours. In making these changes, we had to consider the potential impacts of the optimized conditions on cell viability, especially in the clinically established nutrient-free media. Before human use, we confirmed that these optimized conditions were not only safe but resulted in enhanced engraftment and hematopoiesis in murine models.
There were limitations to the conclusions that can be made from this phase 1 clinical trial. In the second cohort of patients, it was always the larger unit before cryopreservation that was modulated with dmPGE2, and it was this unit that dominated hematopoiesis in 10 of 12 treated patients. Although firm conclusions cannot be drawn based on the small sample sizes tested, 2 important factors should be noted. First, in the latter patients enrolled in cohort 1, the same protocol of treating and infusing the larger unit first was used as in cohort 2, and no signal hinting at early engraftment or chimerism dominance was noted; this factor led to the early closure of the trial and the redesign of the ex vivo expansion process. In addition, in cohort 2, although the larger unit (based on TNC before cryopreservation) was to be infused first, the UCB units, in fact, were extraordinarily comparable with respect to TNC (1.8 vs 1.7 × 107 TNCs/kg, P = .43), CD34+ cells (0.74 vs 0.56 × 105 CD34+/kg, P = .71), and CFU growth (4.5 vs 6.9, P = .77) (Table 1). In dual UCB transplantation, 1 unit dominates in the vast majority of patients on the basis of incompletely understood biological factors. Factors such as TNC dose,23,24 CD34+ cell dose,23,24 CD3+ T-cell dose,25,26 HLA match,26,27 CXCR4 expression,28 and CFU assay24 have been suggested as relevant contributors to this phenomenon. To determine if order of infusion or cell size was relevant in the outcomes we reported in this manuscript, we compared the UCB dominance patterns with those in the historical control cohort, and noted that UCB dominance was nearly random in the control group, with ∼50% of participants undergoing engraftment with each of the transplanted units, using varying thresholds for the determination of UCB dominance. The dominance seen with the dmPGE2-treated patients in the active cohort suggests that enhanced homing may be relevant to this process. We have previously demonstrated that CXCR4 expression is enhanced on human UCB cells treated with dmPGE2.14,15 As a chemokine receptor to SDF1, increased CXCR4 expression may enhance engraftment.29,30 We also demonstrated that dmPGE2 functions as a modifier pathway for Wnt signaling via cAMP, which could lead to enhanced self-renewal of the HSCs.16 Rather than increasing the number of HSCs, this pharmacologic ex vivo modulation increases the “effective stem cell dose” for use in transplantation. Many additional cell types (eg, regulatory T cells, CD4+ T cells, CD8+ T cells, monocytes, dendritic cells, and natural killer cells) in UCB units express the EP2/4 receptors and have been shown to be affected by dmPGE2.31 It is likely that effects on differentiated cell types in cord blood are involved in stimulating engraftment and driving hematopoiesis. We are currently in the process of characterizing the molecular responses across these cell types and the potential mechanism(s) by which a pulse treatment with dmPGE2 may affect UCB unit dominance, GVHD, rates of infections, and rates of viral reactivation.
Other groups have attempted to expand HSCs to improve engraftment and clinical outcomes after UCB transplantation. A recent trial established that the notch ligand, Delta1ext-IgG could be used to expand UCB in culture for 14 days, and these cells could be infused with a second UCB unit.11 Although the expanded UCB unit did engraft early, these cells did not contribute to long-term hematopoiesis and likely acquired a committed myeloid progenitor phenotype during ex vivo culture. Similarly, a mesenchymal stem cell coculture UCB expansion trial demonstrated a 40-fold expansion of CD34+ cells; however, transplantation of these cells with a second unexpanded UCB unit similarly resulted in the loss of the expanded cells with time.12 We have demonstrated sustained multilineage dmPGE2-UCB–derived hematopoiesis for 27 months after transplantation, but further optimization of these and other ex vivo protocols may prove that each approach has usefulness in the transplantation setting.
Despite the limited conclusions that can be drawn from the clinical data, the potential implications of this prospective competitive engraftment clinical trial are broad. First, by generating more effective HSCs, the minimal nucleated cell dose required for adult UCB transplantation might be lowered, increasing the number of acceptable UCB units currently available in public UCB banks. Currently, fewer than 5% of UCB units in the National Marrow Donor Program inventory are of adequate size for use in single UCB transplantation for average-weight American adults. By increasing the effective stem cell dose by as little as fourfold, 98% of these units would be accessible for this use (Michael Boo, J.D., National Marrow Donor Program, email communication with D. Shoemaker). In addition, by increasing the number of accessible units, the likelihood of identifying better HLA-matched units would be increased, potentially leading to improved UCB transplantation outcomes.32,33 From a resource point of view, reducing the need for costly second UCB units and reducing the length and complexity of hospital stays represent a substantial potential cost savings for health care payers. In addition, unlike the significant time requirements associated with HSC expansion approaches, this simple ex vivo manipulation procedure with dmPGE2 is inherently exportable to all stem cell–processing facilities.
In summary, in this preliminary human experience, we have demonstrated that ex vivo modulation of UCB stem cells by using dmPGE2 results in enhanced and more rapid engraftment in human UCB transplantation. On the basis of these positive results, we are now expanding the use of dmPGE2 in randomized phase 2 trials, in addition to initiating new phase 1 studies in single UCB transplantation and autologous peripheral blood stem cell transplantation.
Acknowledgments
The authors wish to thank John Thomas (National Institutes of Health, National Heart, Lung, and Blood Institute) for his support of this project.
This study was supported by the National Heart, Lung, and Blood Institute (U54HL081030, U24HL074355), T32HL087735 (J.H.), R03DK085445 and R01DK090311 (W.G.), K01DK080226 (T.E.N.), R01HL096305 (L.M.P.), and R01HL048801 (L.I.Z.); the Howard Hughes Medical Institute (L.I.Z.); and the Stem Cell Cyclists of the Pan-Mass Challenge and the Patrick Carney Foundation (C.C.)
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.C. designed research, cared for patients, collected data, drafted the manuscript, and approved the manuscript; P.M. designed research, collected data, and approved the manuscript; D.R. performed statistical analyses, performed preclinical laboratory studies, drafted the manuscript, and approved the manuscript; H.T.K. designed research, collected data, performed statistical analyses, approved the manuscript; T.L., J.H., L.M.P., C.D., B.R., and P.H. performed preclinical laboratory studies and approved the manuscript; Y.-B.C., P.A., J.K., B.G., V.T.H., E.A., K.B., and R.J.S. cared for patients and approved the manuscript; M.I. collected data and approved the manuscript; G.K., M.A., and L.S. performed clinical laboratory studies and approved the manuscript; J.R. designed research, performed clinical laboratory studies, and approved the manuscript; D.T.S. designed research and approved the manuscript; W.G. and T.E.N. performed preclinical laboratory studies and approved the manuscript; J.M. approved the manuscript; L.I.Z. designed research, performed preclinical laboratory studies, and approved the manuscript; J.H.A. designed research, cared for patients, and approved the manuscript; and D.D.S. designed research, performed preclinical laboratory studies, drafted, and approved the manuscript.
Conflict-of-interest disclosures: P.M., D.R., T.L., C.D., B.R., J.M., and D.S. are employees of and own stock in Fate Therapeutics. C.C., H.T.K., J.H., and L.P. have received consulting fees from Fate Therapeutics. W.G. and T.E.N. receive patent royalties and consulting fees from Fate Therapeutics. D.S. and L.I.Z. are founders of, own stock in, and serve as members of the Scientific Advisory Board for Fate Therapeutics.
Correspondence: Corey Cutler, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: corey_cutler@dfci.harvard.edu.
References
- 1.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321(17):1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Pasquini R, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2011. http://www.cibmtr.org. 2011.
- 3.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344(24):1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 4.Rocha V, Labopin M, Sanz G, et al. Acute Leukemia Working Party of European Blood and Marrow Transplant Group; Eurocord-Netcord Registry. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22):2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 5.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344(24):1870–1871. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 6.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 7.Kim DW, Chung YJ, Kim TG, Kim YL, Oh IH. Cotransplantation of third-party mesenchymal stromal cells can alleviate single-donor predominance and increase engraftment from double cord transplantation. Blood. 2004;103(5):1941–1948. doi: 10.1182/blood-2003-05-1601. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118(24):6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frassoni F, Gualandi F, Podestà M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9(9):831–839. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 10.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329(5997):1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.North TE, Goessling W, Walkley CR, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447(7147):1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goessling W, Allen RS, Guan X, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8(4):445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goessling W, North TE, Loewer S, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelus LM, Hoggatt J, Singh P. Pulse exposure of haematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif. 2011;44(Suppl 1):22–29. doi: 10.1111/j.1365-2184.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson DE, Quertermus J, Raiser M, Curran J, Robért A. Inhibition of stimulated gastric secretion by an orally administered prostaglandin capsule. A study in normal men. Ann Intern Med. 1976;84(6):688–691. doi: 10.7326/0003-4819-84-6-688. [DOI] [PubMed] [Google Scholar]
- 19.Ippoliti AF, Isenberg JI, Hagie L. Effect of oral and intravenous 16,16-dimethyl prostaglandin E2 in duodenal ulcer and Zollinger-Ellison syndrome patients. Gastroenterology. 1981;80(1):55–59. [PubMed] [Google Scholar]
- 20.Cutler C, Stevenson K, Kim HT, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46(5):659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cutler C, Kim HT, Sun L, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118(25):6691–6697. doi: 10.1182/blood-2011-05-355263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 23.Haspel RL, Kao G, Yeap BY, et al. Preinfusion variables predict the predominant unit in the setting of reduced-intensity double cord blood transplantation. Bone Marrow Transplant. 2008;41(6):523–529. doi: 10.1038/sj.bmt.1705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avery S, Shi W, Lubin M, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117(12):3277–3285, quiz 3478. doi: 10.1182/blood-2010-08-300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milano F, Heimfeld S, Gooley T, Jinneman J, Nicoud I, Delaney C. Correlation of infused CD3+CD8+ cells with single-donor dominance after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(1):156–160. doi: 10.1016/j.bbmt.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez P, Wagner JE, DeFor TE, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant. 2012;47(6):799–803. doi: 10.1038/bmt.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delaney M, Cutler CS, Haspel RL, et al. High-resolution HLA matching in double-umbilical-cord-blood reduced-intensity transplantation in adults. Transfusion. 2009;49(5):995–1002. doi: 10.1111/j.1537-2995.2008.02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez P, Wagner JE, Defor TE, et al. CXCR4 expression in CD34+ cells and unit predominance after double umbilical cord blood transplantation. Leukemia. doi: 10.1038/leu.2012.261. 2013;27(5):1181-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner S, Whiting-Theobald N, Kawai T, et al. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22(7):1128–1133. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Liao C, Chen J, Gu S, Wu S, Xu Z. Significance of increasing adhesion of cord blood hematopoietic cells and a new method: platelet microparticles. Am J Hematol. 2003;74(3):216–217. doi: 10.1002/ajh.10412. [DOI] [PubMed] [Google Scholar]
- 31.Foudi N, Gomez I, Benyahia C, Longrois D, Norel X. Prostaglandin E2 receptor subtypes in human blood and vascular cells. Eur J Pharmacol. 2012;695(1-3):1–6. doi: 10.1016/j.ejphar.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115(9):1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]


