Abstract
The rubber oxygenase (RoxA) of Xanthomonas sp. strain 35Y (RoxAXsp) is so far the only known extracellular c-type diheme cytochrome that is able to cleave poly(cis-1,4-isoprene). All other rubber-degrading bacteria described are Gram positive and employ a nonheme protein (latex-clearing protein [Lcp]) for the postulated primary attack of polyisoprene. Here, we identified RoxA orthologs in the genomes of Haliangium ochraceum, Myxococcus fulvus, Corallococcus coralloides, and Chondromyces apiculatus. The roxA orthologs of H. ochraceum (RoxAHoc), C. coralloides BO35 (RoxACco), and M. fulvus (RoxAMfu) were functionally expressed in a ΔroxA Xanthomonas sp. 35Y background. All RoxA orthologs oxidatively cleaved polyisoprene, as revealed by restoration of clearing-zone formation and detection of 12-oxo-4,8-dimethyltrideca-4,8-diene-1-al (ODTD) as a cleavage product. RoxAXsp, RoxAMfu, and RoxACco were purified and biochemically characterized. The optimal temperature of RoxACco and RoxAMfu was between 22 and 30°C. All RoxA orthologs as isolated showed an oxidized UV-visible spectrum. Chemical reduction of RoxACco and RoxAMfu indicated the presence of two slightly different heme centers with absorption maxima between 549 and 553 nm, similar to RoxAXsp. Sequence analysis and modeling of the three-dimensional structures of the RoxA orthologs revealed a high degree of similarity to the recently solved RoxAXsp structure and included several conserved residues, notably, W302, F317, and a MauG motif at about H517. Lcp-like sequences were not detected in the genomes of the Xanthomonas sp. 35Y, H. ochraceum, M. fulvus, and C. coralloides. No RoxA orthologs were found in Gram-positive bacteria, and this first description of functional RoxA in Gram-negative bacteria other than Xanthomonas proves that RoxA is more common among rubber degraders than was previously assumed.
INTRODUCTION
Natural rubber [NR; poly(cis-1,4-isoprene)] is a hydrocarbon polymer that has been produced by cultivating the rubber tree (Hevea brasiliensis) on a large scale for over 150 years. Innumerable products based on NR are known in industry and households, but the majority of the NR produced goes into the production of tires. Abrasion from tires generates a constant supply of small rubber particles that go into roadside ditches. The fact that no enrichment of rubber particles in the environment has been observed already indicates that rubber is effectively degraded in the environment. However, the fate of rubber materials in soil and the biochemical mechanism of its biodegradation are poorly understood.
Due to the high stability of carbon-carbon bonds, biodegradation of NR is a slow process compared to biodegradation of other biopolymers, such as proteins or polysaccharides, in which the polymer backbone contains heteroatoms, such as nitrogen and/or oxygen. Heteroatoms are absent in polyisoprene, apart from sulfur atoms in vulcanized rubber. The double bonds in polyisoprene are the only functional groups in NR that allow an attack by oxidizing enzymes. In fact, rubber-degrading organisms are widespread in nature (1, 2). Some rubber-degrading bacteria, such as Xanthomonas sp. strain 35Y (3) and many actinomycetes, produce clearing zones on opaque latex agar, while others grow adhesively on rubber without clearing zone formation. Gordonia polyisoprenivorans and Gordonia westfalica belong to the latter group (see reference 4 and references cited therein).
Rubber oxygenase A (RoxA) and latex-clearing protein (Lcp) represent two types of enzymes that share no detectable amino acid similarities but which both catalyze the primary attack of NR and cleave the hydrocarbon polymer to low-molecular-weight degradation products. Lcp was first described in Streptomyces sp. strain K30 and is widely distributed in rubber-degrading bacteria (5, 6), including clearing zone formers (e.g., streptomycetes) and in adhesively growing species, such as G. polyisoprenivorans (7). Lcp is apparently secreted via a TAT-dependent pathway (8). No cofactors are known for Lcp, and the biochemical mechanism by which Lcp catalyzes the cleavage of polyisoprene is unknown. RoxA consists of 678 amino acids and is a c-type cytochrome that features two binding sites for covalent attachment of heme as well as a MauG motif (9). In contrast, the Lcp amino acid sequence (397 amino acids) does not indicate the presence of metals. Both Lcp and RoxA are responsible for the cleavage of rubber to low-molecular-weight degradation products with aldehyde and keto end groups and therefore apparently catalyze a similar or even the same reaction. RoxA has been purified and studied in vitro (10–12). Isolated RoxA is active in aqueous environments if the enzyme substrates, rubber and dioxygen, are present and the physical conditions (pH, temperature) are appropriate. Notably, no separate cofactors are required for activity. 12-Oxo-4,8-dimethyltrideca-4,8-diene-1-al (ODTD) was identified to be the major degradation product, and a dioxygenase cleavage mechanism was shown (13).
Xanthomonas sp. 35Y is the only known Gram-negative rubber degrader. Attempts to isolate other rubber-degrading Gram-negative bacteria were not successful (1; unpublished data). When we screened the database for RoxA orthologs after the first cloning of the Xanthomonas sp. 35Y roxA gene (roxAXsp) about 10 years ago (9), no other RoxA-related sequence could be identified. However, a recently repeated BLAST search revealed several potential RoxA orthologs. Remarkably, all identified RoxA-related sequences were from members of the myxobacteria, namely, Haliangium ochraceum, Corallococcus coralloides, Myxococcus fulvus, and Chondromyces apiculatus. Rubber-degrading activities have not been described for myxobacteria or any other Gram-negative bacteria, except for Xanthomonas sp. 35Y. This prompted us to investigate the potential of the putative RoxA orthologs from myxobacteria for oxidative cleavage of polyisoprene.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
All strains and plasmids used in this study are given in Table 1. Recombinant Xanthomonas sp. 35Y ΔroxA-attB harboring roxA under the control of a rhamnose promoter was grown in modified LB medium with a reduced concentration of yeast extract (10 g/liter tryptone, 5 g/liter NaCl, 0.25 g/liter yeast extract, 1 g/liter l-rhamnose) for 3 to 4 days. The growth temperature was 30°C for expression of RoxAXsp and 22°C for expression of RoxA orthologs. H. ochraceum was grown on DSMZ medium 246 (pH 7.3) that included beef extract (1%), peptone (1%), and artificial seawater (750 ml per liter medium). The beef extract and peptone were dissolved in 250 ml of tap water and combined with 750 ml of artificial seawater. M. fulvus HW1 and C. coralloides BO35 were grown on solid nutrient broth agar.
Table 1.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or oligonucleotide | Relevant characteristic or sequencea | Reference or source |
|---|---|---|
| Strains | ||
| Escherichia coli S17-1 | Conjugation strain | 28 |
| E. coli XL1-Blue | Transformation strain | |
| Corallococcus coralloides | Strain BO35, source of RoxACco | T. Schäberle, University of Bonn |
| Myxococcus fulvus HW1 | Source of RoxAMfu | Y. Li, Shandong University |
| Haliangium ochraceum | Source of RoxAHoc | DSMZ 14365 |
| Xanthomonas sp. 35Y (SN5065) | Growth on poly(cis-1,4-isoprene) latex, clearing zone formation | 3 |
| Xanthomonas sp. 35Y-CM | Chloramphenicol-resistant mutant of strain 35Y | 15 |
| Xanthomonas sp. 35Y-CM ΔroxA-attB (SN4114) | Chromosomal deletion of roxA, attB at former roxA site, no clearing zone formation on latex agar | 12 |
| Xanthomonas sp. 35Y-CM ΔroxA-attB/pNH1-roxA-attP (in chromosome; SN4230) | Expression of RoxA from rhamnose promoter, Kmr Cmr, clearing zone formation in the presence of rhamnose | 12 |
| Xanthomonas sp. 35Y-CM ΔroxA-attB/pNH1-roxAHoc-attP (in chromosome; SN5127) | Expression of RoxAHoc from rhamnose promoter, Kmr Cmr | This study |
| Xanthomonas sp. 35Y-CM ΔroxA-attB/pNH1-roxACco-attP (in chromosome; SN5129) | Expression of RoxACco from rhamnose promoter, Kmr Cmr | This study |
| Xanthomonas sp. 35Y-CM ΔroxA-attB/pNH1-roxAMfu-attP (in chromosome; SN5132) | Expression of RoxAMfu from rhamnose promoter, Kmr Cmr | This study |
| Plasmids | ||
| pJOE6787.1 | pBR322 ori, aphII (Kmr) mob int (phiC31 integrase) attP | J. Altenbuchner |
| pNH1 | pJOE6787.1 with rhamnose regulation genes | 12 |
| pNH1-roxAXsp | Coding sequence of roxAXsp under rhamnose promoter | 12 |
| pNH1-roxAXsp-attP (SN4230) | Coding sequence of roxAXsp under rhamnose promoter and attP site | 12 |
| pNH1-roxAHoc-attP (SN4126) | Coding sequence of roxAHoc under rhamnose promoter and attP site | This study |
| pNH1-roxAMfu-attP (SN5021) | Coding sequence of roxAMfu under rhamnose promoter and attP site | This study |
| pNH1-roxACco-attP (SN5084) | Coding sequence of roxACco under rhamnose promoter and attP site | This study |
| Oligonucleotides | ||
| NdeI-roxHoc_f | GGAATTCCATATGACGACCCGAGCGAACTTAC | |
| roxHoc-HindIII_r | CCCAAGCTTCTACAGGGTCTTGAGGTACTC | |
| NdeI-roxCco_f | GGAATTCCATATGAGACTGCGATGGAGCC | |
| roxCco-HindIII_r | CCCAAGCTTCTACAGCGTCTTCATGTACT | |
| NdeI-roxMfu_f | GGAATTCCATATGCGGCTACACTGGAGTC | |
| roxMfu-HindIII_r | CCCAAGCTTTCAGAGCGTCTTCACGTATT |
Underlining indicates the restriction enzyme sites.
Cloning of roxA from myxobacteria.
The coding sequences of the roxA genes of H. ochraceum (roxAHoc), C. coralloides BO35 (roxACco), and M. fulvus HW1 (roxAMfu) were PCR amplified from the respective genomic DNAs (for primer sequences, see Table 1) and cloned into pUC9. In the case of roxACco, the chromosomal template DNA was isolated from C. coralloides strain BO35 and the roxA region was sequenced de novo. Finally, the roxAXsp-coding sequence of the pNH1-roxA-attP plasmid was replaced by the roxA orthologs (roxAHoc, roxAMfu, or roxACco), resulting in plasmids pNH1-roxAHoc-attP, pNH1-roxAMfu-attP, and pNH1-roxACco-attP, respectively (Table 1). The three plasmids were conjugatively transferred from Escherichia coli S17-1 to Xanthomonas sp. 35Y ΔroxA-attB and were chromosomally integrated via attP/attB recombination, as previously described in detail (12). The correct integration of roxA orthologs and DNA sequences was confirmed by PCR and subsequent DNA sequencing of the roxA genes for each Xanthomonas sp. 35Y clone.
Purification of recombinant RoxA.
Construction of tagged variants of RoxA did not result in a simplified purification process, regardless of whether a C-terminal, N-terminal, or internal tag (a His6 or Strep tag) was chosen (unpublished result). Therefore, conventional chromatography methods were applied for purification of RoxA. Xanthomonas sp. 35Y ΔroxA-attB harboring the roxA ortholog of interest (Table 1) was grown in 10 to 20 individual 600-ml cultures of modified LB medium (each in a 3-liter Erlenmeyer flask) supplemented with 0.1% (wt/vol) l-rhamnose for 92 h at 22°C by continuous shaking. Cells were harvested (4°C) by centrifugation, and RoxA was purified from the culture supernatant. The cell-free supernatant was concentrated by ultrafiltration (10-kDa cutoff) and was applied to a Q-Sepharose fast-flow column (Q-FF 50/11) that had been equilibrated with 20 mM Tris-HCl (pH 8.5; flow rate, 8 ml/min). RoxA was eluted in a subsequent step gradient at ≈50 mM NaCl in equilibration buffer. Combined RoxA-containing fractions were concentrated ≈20-fold, and Tris-HCl buffer was exchanged against potassium phosphate buffer (10 mM, pH 6.8) by gel filtration on a HiPrep 26/10 column. The RoxA pool was then applied to a hydroxyapatite column (CHT5-I) that had been equilibrated with the same buffer. RoxA was eluted with a linear gradient of 10 to 200 mM potassium phosphate, pH 6.8, at ≈40 mM. RoxA fractions were pooled and concentrated via ultrafiltration (30-kDa cutoff) in the presence of 300 mM NaCl at 0 to 4°C (on ice). High ionic strength prevented precipitation of RoxA. Purity was tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by determination of the quotient of the absorption at 406 nm and that at 280 nm, which was ≈1.35 for pure RoxA, 1.48 for RoxACco, and 1.27 for RoxAMfu. Purified RoxA was frozen in liquid nitrogen and stored at −70°C.
Determination of RoxA activity.
RoxA was assayed by determination of the polyisoprene cleavage product ODTD via high-pressure liquid chromatography (HPLC). The assay mixture contained 5 μg of the respective purified RoxA ortholog, rubber latex (0.2% [wt/vol] emulsion), and potassium phosphate buffer (100 mM; pH 4.5 to 9.0) in a total volume of 1 ml. In some experiments, artificial seawater consisting of 28.1 g NaCl, 0.77 g KCl, 1.6 g CaCl2·2H2O, 4.8 g MgCl2·6H2O, 0.11 g NaHCO3, and 3.5 g MgSO4·7H2O (all components were dissolved in 1 liter water) was additionally present in the assay buffer. The reaction was performed at the indicated temperatures for 18 h in a 15-ml Falcon tube. The mixture was extracted with 1.5 ml ethyl acetate, and 1.0 ml of the extract was dried, dissolved in 100 μl methanol, and then subjected to HPLC analysis (20 μl per sample). An RP8 HPLC column (12 by 4 mm; particle size, 5 μm) was used with water (solution A) and methanol (solution B) as the mobile phases. The concentration of solution B was increased starting from 50% (vol/vol) to 100% (vol/vol) at 20 min at a flow rate of 0.7 ml/min; products were detected at 210 nm. ODTD eluted after 15.3 ± 0.4 min. Purified ODTD was used as the standard. Mixtures without RoxA served as controls.
Clearing zone assay.
RoxA activity was semiquantitatively estimated by streaking recombinant Xanthomonas sp. 35Y ΔroxA::attB that contained the different roxA orthologs (Table 1) onto previously described solid mineral salts agar plates (3) supplemented with 0.05% (wt/vol) yeast extract and 0.1% (wt/vol) l-rhamnose and with an opaque overlay agar (≈8 ml) of purified polyisoprene latex (0.25% rubber [dry wt/vol in agar]). Polyisoprene latex was obtained from Weber and Schaer, Hamburg, Germany, and was purified by washing 3 times with an aqueous solution of 0.1% (wt/vol) Nonidet P-40. Xanthomonas sp. 35Y ΔroxA::attB harboring chromosomally anchored wild-type roxA under rhamnose control (pNH1-roxA-attP) and Xanthomonas sp. 35Y ΔroxA served as positive and negative controls, respectively. Xanthomonas sp. 35Y wild type was used as a supplementary control. The plates were incubated at room temperature (≈22°C) or at 30°C. The intensity of clearing zone formation semiquantitatively indicated RoxA activity and consumption of the ODTD produced by the strain.
Other methods.
Heme staining was performed after separation of RoxA-containing fractions by SDS-PAGE and subsequent assay for pseudoperoxidase activity of the RoxA heme groups, as described previously (11). The concentrations of the purified RoxA proteins were determined using the molar absorption coefficient of RoxA at 406 nm (ε406; 2.07 × 105 M−1 cm−1). RoxA structures were visualized and modeled using the SWISS MODEL server (14). The genomic DNA of H. ochraceum and M. fulvus was prepared by standard methods.
Nucleotide sequence accession numbers.
Annotations of the RoxA sequences from Xanthomonas sp. 35Y, H. ochraceum, C. coralloides, and C. apiculatus have been submitted to GenBank under accession numbers KC980911 to KC980914.
RESULTS
Identification, cloning, and expression of RoxA orthologs in Xanthomonas sp. 35Y.
A BLAST search (January 2013) using the Xanthomonas sp. 35Y RoxA-coding sequence (GenBank accession number AAP41848.1) as the template revealed the presence of several putative RoxA orthologs in the database: The amino acid sequences of COCOR_02166 from C. coralloides DSM 2259, of LILAB_11505 from M. fulvus HW1, and of A176_5502 from C. apiculatus DSM 436 had 65% amino acid sequence identity (75 to 78% similarity) to the RoxAXsp amino acid sequence, and the amino acid sequence of Hoch_1661 from H. ochraceum DSM 14365 was 63% identical (75% similar) to that of RoxAXsp (Table 2). All RoxA-like sequences were characterized by a high fraction of aromatic residues (Table 3). Apparently, roxA-like genes are present in the genomes of several species of myxobacteria.
Table 2.
Identities and similarities between RoxA amino acid sequences
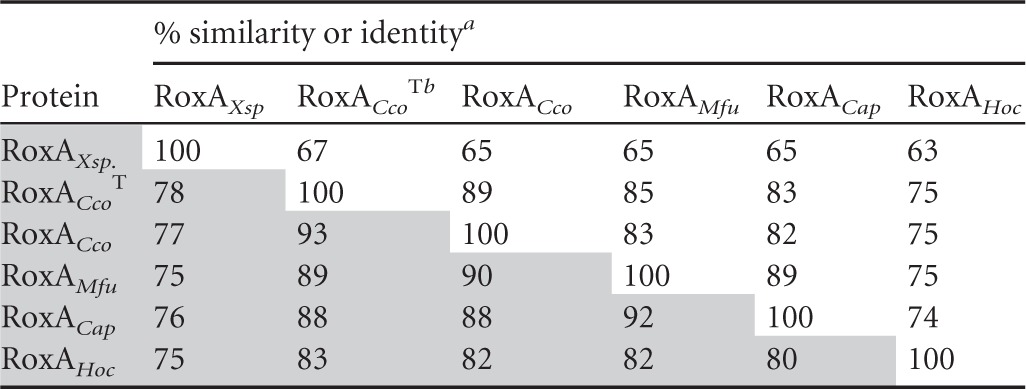
Percent similarity is identified by shading.
RoxACcoT, RoxA from Corallococcus coralloides type strain (DSMZ 2259).
Table 3.
Properties of RoxA orthologsa
| Attribute | RoxAXsp | RoxACcoTb | RoxACco | RoxAMfu | RoxAHoc | RoxACap |
|---|---|---|---|---|---|---|
| Gene length (bp) | 2,037 | 2,022 | 2,022 | 2,025 | 2,031 | 2,016 |
| Molecular mass (kDa) | ||||||
| Mature protein | 71.5 | 71.0 | 71.0 | 71.9 | 72.1 | 71.9 |
| Mature protein + 2 hemes | 73.0 | 72.5 | 72.5 | 73.3 | 73.7 | 73.4 |
| Pi (theoretical) | 7.3 | 6.1 | 6.7 | 7.0 | 4.9 | 6.6 |
| % aromatic amino acids (no. of F, W, and Y amino acids) | 11.4 (24, 20, 30) | 11.3 (31, 16, 26) | 11.3 (34, 16, 23) | 11.6 (32, 16, 27) | 12.7 (35, 17, 30) | 11.1 (36, 15, 21) |
| Heme attachment (N terminus/C terminus | CSACH195/CASCH394 | CSACH181/CASCH379 | CSACH181/CASCH379 | CSACH181/CASCH379 | CSACH181/CASCH379 | CSACH181/CASCH379 |
| Axial heme ligands (N terminus/C terminus) | H195/H394 H641 | H181/H379 H626 | H181/H379 H626 | H181/H379 H626 | H181/H379 H626 | H181/H379 H626 |
| MauG motif | PYFH517 NGSVP | PFFH502 NGSVP | PFFH502 NGSVP | PYFH502 NSSVP | PYFH502 NGSVP | PYFH502 NSSVP |
| F317 equivalent | F317 | F302 | F302 | F302 | F302 | F302 |
| W302 equivalent | W302 | W287 | W287 | W287 | W287 | W287 |
| pH optimum at 22°C | 6–7 | 6–7 | 7 | |||
| Optimal temp range at pH 7 (°C) | 30–37 | 22–30 | 22–30 | ≈22c | ||
| Sp act (ODTD area/μg RoxA [%]) | 6,700 (100) | 660 (9.9) | 590 (8.7) | |||
| Soret maximum (nm) | ||||||
| Oxidized | 407 | 407 | 408 | 409d | ||
| Reduced | 418 | 418 | 418 | 418d | ||
| α band (reduced) (nm) | 549, 553 | 549, 553 | 551–552 | 550, 553d | ||
| β band (reduced) (nm) | 521 | 522 | 522 | 523d | ||
| Main cleavage product | ODTD | ODTD | ODTD | ODTDd | ||
| Salt tolerance | + | + | + | |||
| Activity with the following activators/inhibitors (%): | ||||||
| Ethanol solvent (1%/5%/10%) | 110/140/140 | 120/190/140 | ||||
| Squalene | 75 | 85 | ||||
| α-Tocopherol | 25 | 35 | ||||
| Carotenee | 55 | 10 | ||||
| Pyridine | <3 | <3 |
Inhibitor compounds were dissolved in ethanol (final concentration, 1%, vol/vol). Blank cells indicate that the value was not determined.
RoxACcoT, RoxA from Corallococcus coralloides type strain (DSMZ 2259).
From Fig. 1.
The experiment was performed with a cell-free culture supernatant of the Xanthomonas sp. ΔroxA strain with chromosomally integrated pNH1-roxAHoc-attP plasmid. The red shift in UV-vis signals of the RoxAHoc protein by 1 nm is probably explained by impurities of medium components.
Mixture of carotenes (Roth; catalog no. 7864.1).
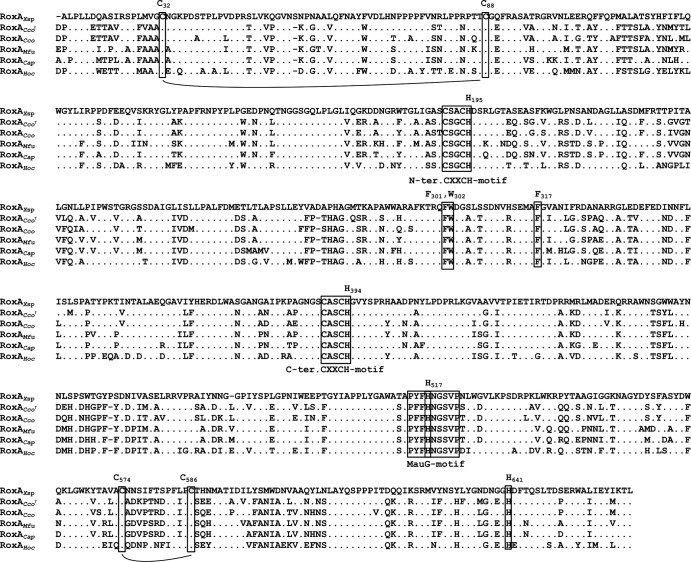
The deduced amino acid sequences of all putative RoxA orthologs have potential signal peptides for secretion; the apparent molecular masses of the putative mature proteins were highly similar and were 73.7 kDa for RoxAHoc, 72.5 kDa for RoxACco, 73.3 kDa for RoxAMfu, 73.4 kDa for RoxACap, and 73.0 kDa for RoxAXsp (Table 3). All RoxA orthologs (i) harbored two heme motifs (CXXCH) for covalent attachment of heme, (ii) had conserved histidine residues as axial heme ligands, (iii) contained a conserved MauG motif, and (iv) had conserved W302 and F317 residues (Fig. 1; Table 3). W302 in RoxAXsp is located between the two hemes, and F317 is essential for optimal binding of dioxygen (12). In conclusion, the high similarity of the identified putative proteins suggests a structure similar to that of RoxAXsp and, presumably, a biochemical function similar to that of RoxAXsp. To test whether the identified myxobacteria can degrade polyisoprene, H. ochraceum was cultivated on opaque latex agar (with 0.05% yeast extract and 0.1% polyisoprene latex supplemented with artificial seawater). Although the growth of H. ochraceum was poor, clearing zone formation became visible within 2 weeks and suggested that the putative roxA gene could have the same function for the cleavage of polyisoprene as it does in Xanthomonas sp. 35Y. To find evidence for this assumption, we functionally analyzed RoxAHoc, RoxACco, and RoxAMfu.
Fig 1.
Alignment of amino acid sequences of RoxA orthologs. Amino acid residues of RoxA orthologs that were identical to those of RoxAXsp are indicated by dots. Residues different from those in RoxAXsp are given by the 1-letter amino acid code. Heme binding and MauG motifs as well as important residues in RoxAXsp are emboxed. Conserved cysteine residues that contribute to disulfide bridges are connected with solid lines. Numbering of residues refers to that of RoxAXsp, as described in reference 9. RoxACcoT, RoxA from Corallococcus coralloides type strain (DSMZ 2259).
Cloning and functional expression of RoxAHoc, RoxACco, and RoxAMfu.
Substantial expression of RoxAXsp in E. coli, other Gram-negative bacteria, or Bacillus subtilis was not successful, as observed earlier for RoxA and MauG (12, 15, 16). When we attempted expression of RoxAHoc in an E. coli strain that harbored the cytochrome c maturation (ccm) genes on pEC86 (17), growth of the recombinant E. coli strain stopped shortly after induction and no substantial expression was detected. We concluded that experiments aimed at the expression of RoxACco or RoxAMfu in E. coli also might not be successful. Therefore, we decided to express roxA orthologs in Xanthomonas sp. 35Y ΔroxA via attP/attB recombination using the phiC31 integrase system that had successfully been developed in our lab for RoxAXsp expression; this system is so far the only way to obtain reproducible expression of recombinant RoxA (12).
The RoxAHoc-, RoxACco-, and RoxAMfu-encoding genes were PCR amplified, cloned, and finally, integrated into the chromosome of Xanthomonas sp. 35Y ΔroxA under rhamnose promoter control, as described in detail in Materials and Methods. Successful expression of the chromosomally anchored roxA orthologs was tested by growth of the recombinant strains in modified latex medium supplemented with rhamnose. Expression of active RoxA proteins was indicated (i) by the formation of clearing zones around the colonies on opaque latex-rhamnose agar (Fig. 2) and (ii) by the appearance of bands with pseudoperoxidase activity and with relative mobility in SDS-polyacrylamide gels similar to that for RoxAXsp for all three cloned roxA orthologs (Fig. 3A). The clearing zone diameter of the RoxAMfu strain was similar to that of the RoxAXsp strain (control), while the clearing zones for the RoxACco and RoxAHoc strains were considerably smaller. Those strains formed small clearing zones at 22°C but no or only very small clearing zones at 30°C. Therefore, we assume a considerable temperature sensitivity of the RoxAHoc and RoxACco orthologs. In no case were any clearing zones formed on latex agar in the absence of rhamnose. This confirmed that RoxA expression by recombinant Xanthomonas sp. 35Y depends on the presence of the inducer rhamnose.
Fig 2.
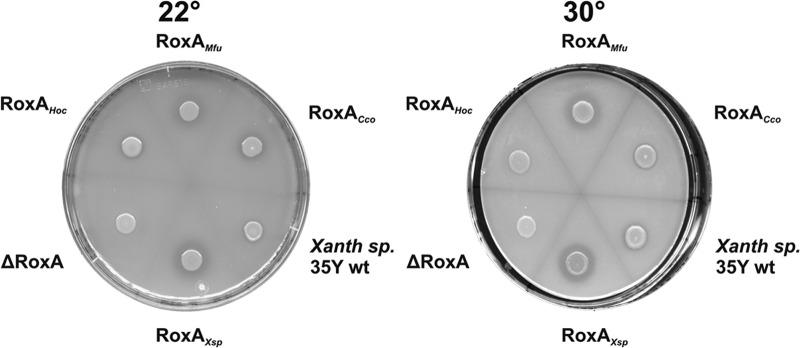
Functional expression of RoxA orthologs in Xanthomonas sp. 35Y ΔroxA. Xanthomonas sp. 35Y wild type (Xanth sp. 35Y wt) and Xanthomonas sp. 35Y ΔroxA (ΔRoxA) with chromosomally integrated roxA orthologs from M. fulvus (RoxAMfu), C. coralloides BO35 (RoxACco), Xanthomonas sp. 35Y (RoxAXsp), and H. ochraceum (RoxAHoc) under rhamnose control were grown on opaque latex medium that had been supplemented with l-rhamnose (0.1%) at 22°C and at 30°C. The formation and the diameter of the clearing zones semiquantitatively indicate expression of the RoxA protein and consumption of polyisoprene cleavage products. Note the reduced clearing zone formation (RoxAHoc) at 30°C compared to that at 22°C.
Fig 3.
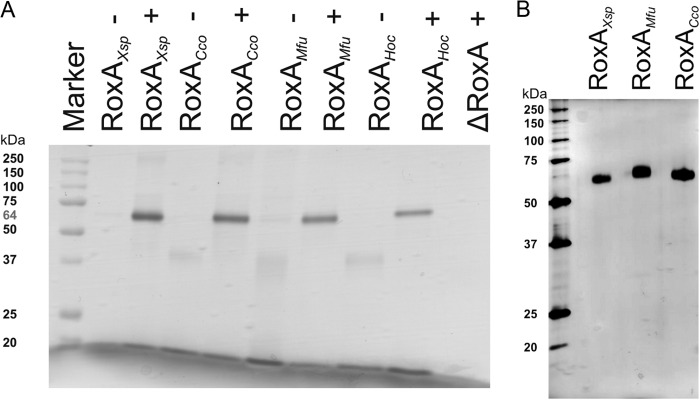
(A) SDS-PAGE and subsequent pseudoperoxidase staining (heme staining) of culture supernatants of Xanthomonas sp. 35Y ΔroxA harboring chromosomally integrated roxAXsp orthologs. Each strain was grown in the absence (−) or presence (+) of the inducer l-rhamnose (0.1%). Xanthomonas sp. 35Y ΔroxA and Xanthomonas sp. 35Y ΔroxA with pNH1-roxAXsp (RoxAXsp) were used as negative and positive controls, respectively. (B) Purification of recombinant RoxA orthologs. RoxAXsp, RoxACco, and RoxAMfu were purified from rhamnose-grown cultures by chromatography of concentrated cell-free culture fluid on Q-Sepharose and hydroxyapatite, as described in Materials and Methods. Aliquots of the respective RoxA pools after the hydroxyapatite step were separated by SDS-PAGE and stained with silver. Overloading of the lanes indicates the absence of significant amounts of contaminating proteins.
Purification and biochemical properties of RoxAMfu and RoxACco.
Xanthomonas sp. 35Y RoxA and two RoxA orthologs (RoxAMfu and RoxACco) were purified from 10, 11, or 5 liters of cell-free culture supernatants by subsequent chromatography on Q-Sepharose and hydroxyapatite (for details, see Materials and Methods). A 32-mg quantity of RoxAXsp, 1.1 mg of RoxAMfu, and 7.7 mg of RoxACco were obtained as yields after the final chromatography and concentration step. All purified RoxA orthologs were highly pure (>98%), as revealed by SDS-PAGE analysis (Fig. 3B) and by spectral analysis (not shown).
ODTD was identified as the major in vitro product of oxidative cleavage of natural polyisoprene latex using the three purified RoxA orthologs (RoxAXsp, RoxACco, and RoxAMfu) (Table 3). RoxAHoc was not purified, but the polyisoprene-cleaving activity of RoxAHoc was verified using a 10-fold-concentrated culture supernatant of a RoxAHoc-expressing culture, and ODTD was identified to be the main cleavage product as well. These results confirmed that RoxAMfu, RoxACco, and RoxAHoc are true rubber oxygenases (RoxAs). Purified RoxAXsp had an approximately 10-fold higher specific activity than purified RoxACco and RoxAMfu. Determination of the pH and temperature profiles showed that the purified RoxA orthologs were active in a range from pH 5 to 9 and at 20 to 37°C. The highest activities were determined at between pH 6 and 7 and 30°C (Table 3). The thermal stability of RoxAHoc was slightly lower at 30°C than at room temperature, as revealed by the reduced clearing zone formation of the RoxAHoc-expressing Xanthomonas sp. 35Y (Fig. 2) and by quantitative determination of ODTD peak areas in RoxA activity assays.
RoxAHoc and RoxAMfu originate from marine species, suggesting that salts could influence their activity. However, repetition of enzyme assays in the presence of different salt concentrations (0, 1, and 3% NaCl tested with or without artificial seawater) had no or only a minor influence on the formation of ODTD. RoxA orthologs of soil bacteria or of bacteria from marine habitats apparently do not differ significantly in salt tolerance. They do not depend on the presence of high salt concentrations but are not negatively affected if salts are present at considerable concentrations. Substrate analogs, such as squalene, carotene, or α-tocopherol, partially inhibited RoxACco to variable extents (only RoxAXsp and RoxACco were tested with substrate analogs; Table 3). Notably, ethanol, which was used as the solvent for the inhibitor compounds, moderately increased the ODTD formation activity of RoxA in a dose-dependent manner, possibly by changing the buoyant force in the emulsion. This may simply lead to a faster concentration of latex aggregated to RoxA at the surface of the test tube, resulting in a more efficient enzyme/substrate ratio. The highest inhibitory effect on activity was, however, found for pyridine, which can act as a heme ligand. A 1 mM concentration of this compound completely inhibited the RoxA activity of all RoxA orthologs, consistent with previous investigations with RoxAXsp (11).
Spectral properties of RoxA orthologs.
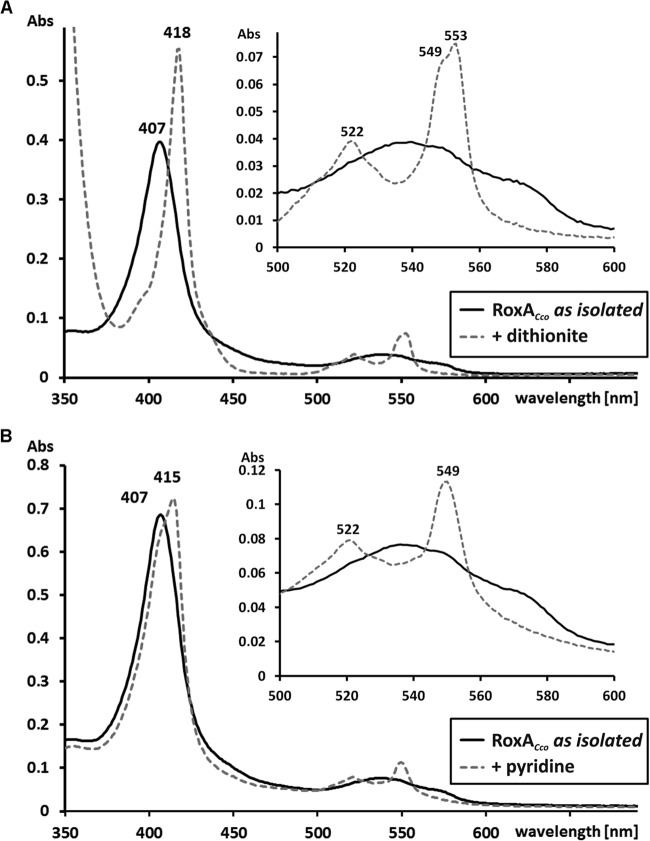
UV-visible (UV-vis) spectroscopy of RoxAXsp as isolated indicated an oxidized spectrum of the heme groups. Both heme groups could be differentiated by separate absorption maxima of the α bands (549 nm and 553 nm) after chemical reduction with dithionite (11). The newly isolated RoxA preparations (RoxAMfu, RoxACco, as well as the 10-fold-concentrated supernatant of the RoxAHoc-expressing culture) in the state as isolated also showed an oxidized spectrum (Soret band at 408 ± 1 nm). In the chemically reduced state, a comparison of the UV-vis properties of RoxAXsp with those of RoxA of myxobacteria revealed almost identical Soret bands (at 418 nm; shown for RoxACco as an example in Fig. 4) and maxima of β bands (522 ± 1 nm) and α bands (549 ± 1 and 553 ± 1 nm) (Table 3; Fig. 4A). Only the α bands of RoxAMfu were not well separated and composed a broad maximum from 551 to 552 nm, indicating different but very closely located maxima (not shown). In conclusion, the two hemes of RoxAXsp and the hemes of the orthologs RoxAMfu, RoxACco, and RoxAHoc have very similar optical properties with uncommon features (11, 12) which can be seen to be characteristic for RoxA. This suggests very similar chemical environments in the immediate vicinities of the heme centers of all RoxA orthologs.
Fig 4.
UV-vis spectra of purified RoxACco. (A) UV-vis spectra of RoxACco as isolated (solid line) and of dithionite-reduced RoxACco (dashed line); (B) UV-vis spectrum of RoxACco as isolated (solid line) compared with the spectrum obtained after incubation of RoxA with 2 mM pyridine. The Soret band region is enlarged in the insets in both panels, and the absorption maxima of RoxA are indicated. Note that the presence of pyridine resulted in an increase of only the 549-nm α band, while in chemically reduced RoxACco, both α bands (549 to 553 nm) are visible. Abs, absorbance.
Structures of RoxA orthologs.
Determination of the recently solved three-dimensional structure of the RoxAXsp protein showed that the N-terminal heme has only one axial amino acid ligand (H195) on the proximal side and that dioxygen represents the distal heme ligand (18, 19). The N-terminal heme constitutes the active site of RoxA. The other (C-terminal) heme is coordinated by two axial histidine ligands (for residue numbers, see Table 3). UV-vis and electron paramagnetic resonance (EPR) spectroscopy data suggested that a considerable part of the isolated RoxA molecules is in an Fe2+-O2 ↔ Fe3+-O2− equilibrium at the N-terminal heme comparable to that of oxyhemoglobin (20, 21). Incubation of RoxAXsp with amino acid-like molecules, such as imidazole or pyridine, that can function as axial ligands to heme resulted in a selective increase of the 549-nm α band and in inactivation of the enzyme. This can be explained by the displacement of dioxygen and binding of the ligand to Fe2+. A selective increase of the 549-nm α band was also observed in the UV-vis spectrum upon incubation of RoxACco with pyridine (Fig. 4B) and indicated that the N-terminal heme of RoxACco apparently has no amino acid ligand and is present in an oxygenated state, as in RoxAXsp. This assumption was supported by comparing the modeled structures of RoxA orthologs shown in Fig. 5. It is evident that all structures are almost identical (root mean square deviation [RMSD] values, <1 Å). Only a minor difference was noticed for the RoxAMfu structure: the major α helix (the top helix in Fig. 5) was reduced in length, and a small α helix in the enzyme center was present as a random coil. In conclusion, the structures of all RoxA orthologs, including the positions and relative orientations of the two heme centers, are highly similar and support the same biochemical function, i.e., oxidative cleavage of polyisoprene.
Fig 5.
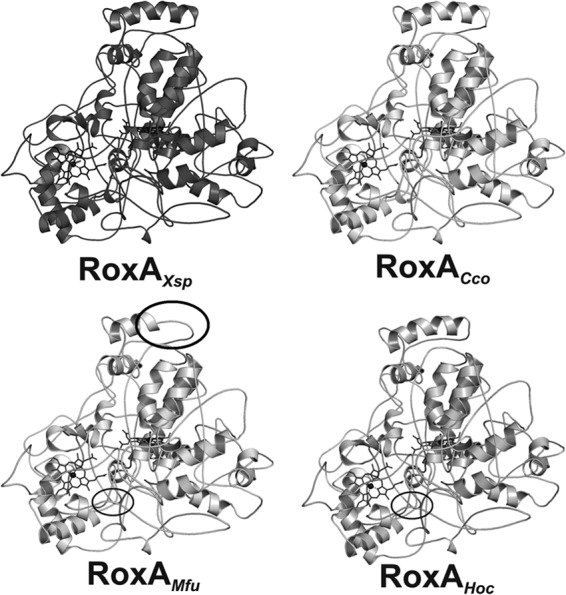
Similarity of the RoxAXsp structure to the structures of RoxAMfu, RoxACco, and RoxAHoc. Cartoons of the modeled structures of RoxAMfu, RoxACco, and RoxAHoc are shown and are compared with the known RoxAXsp structure (Protein Data Bank accession number 4B2N). Areas with differences in secondary structures are indicated (circles). All modeled structures showed RMSD values of <1 Å (0.62 to 0.71 Å) compared to the RoxAXsp structure.
DISCUSSION
About 12 million tons of poly(cis-1,4-isoprene) are produced every year by cultivating the rubber tree (Hevea brasiliensis; natural rubber), and a comparable amount is produced by chemical synthesis (synthetic rubber). Polyisoprene is also produced by many plants and some fungal species (4), although the concentration of polyisoprene in the latex is much lower than that in the latex from H. brasiliensis, where the rubber solid content of the laticifers can be as high as 35% (wt/vol). For example, the common dandelion (Taraxacum) and lactiferous mushrooms contain substantial amounts of polyisoprene. The portion of natural rubber that is produced by non-Hevea species is difficult to estimate, but it is reasonable to assume that polyisoprene compounds are ubiquitous.
Most of the artificially produced polyisoprene is released to the environment after use and, together with polyisoprene-containing biomass, constitutes an abundantly available carbon source for microorganisms. Streptomycetes and related actinobacteria are well-known for their potential to degrade biopolymers, such as proteins, polysaccharides, or polyesters. Previous analysis of the distribution of rubber-degrading bacteria showed that, indeed, members of the actinobacteria are frequently able to utilize polyisoprene as a source of carbon and energy (1, 6, 22–24). As far as it has been investigated, most—if not all—of the rubber-degrading actinobacteria employ latex-clearing protein (Lcp) as the catalyst for the primary attack of polyisoprene (6, 7). Xanthomonas sp. 35Y is so far the only known rubber-degrading Gram-negative bacterium, and this strain does not have Lcp but produces rubber oxygenase (RoxA). Reports on the isolation of other Gram-negative bacteria that can utilize natural rubber as a sole source of carbon and energy have not been published since the description of latex degradation by Xanthomonas sp. 35Y, and even a directed search among related strains was not successful (1). One could assume that Xanthomonas sp. 35Y represents an exception and that most Gram-negative bacteria are not able to utilize polyisoprene as a carbon source. Few gene products with high similarity to RoxA that are described in this study have been deposited in databases, and none were deposited before 2009, suggesting that RoxA, at least, is not widespread in nature. We verified the function of RoxA as a polyisoprene-cleaving enzyme in at least 3 species (H. ochraceum, M. fulvus, and C. coralloides) by functional expression. On the basis of the high amino acid sequence similarities (76 to 92%; Table 2), it is very likely that C. apiculatus has a functional RoxA ortholog as well.
Myxobacteria are known to prefer a variety of insoluble biopolymers, such as cellulose, chitin, and starch, for growth. They are also able to grow bacteriotrophically by lysing intact bacterial cells and proteins (25). However, their ability to degrade poly(cis-1,4-isoprene) has not been described prior to this study but seems plausible for myxobacteria, which are decomposers of recalcitrant substrates. The finding of RoxA orthologues only in myxobacteria, besides Xanthomonas sp. 35Y, is indeed conspicuous and suggests that RoxA is overrepresented in this group (compared to other Gram-negative bacteria). The rubber degradation ability may generally be restricted to certain groups of bacteria, like the Gram-positive actinomycetes or the Gram-negative myxobacteria, which both share the capacity to decompose hardly accessible molecules. Although the rubber degradation ability among Gram-negative bacteria was not discovered first in myxobacteria, this group may represent the counterpart to the actinomycetes, which combine the majority of Gram-positive bacteria that are rubber degraders. Our results suggest that rubber-degrading Gram-negative bacteria use RoxA instead of Lcp as the rubber-cleaving enzyme. Inspection of the genome sequences of Xanthomonas sp. 35Y and that of the myxobacteria indicated that none of them harbor any Lcp orthologs. In conclusion, nature has evolved polyisoprene-cleaving enzymes at least two times: the Lcp system is widespread in and apparently specific for Gram-positive bacteria (Streptomyces, Gordonia, and other actinomycetes), and the RoxA system is present only in Gram-negative bacteria. It will be interesting to see whether exceptions to this rule can be found in future.
Nevertheless, a commonality of all rubber degraders seems to be the metabolization of polyisoprene cleavage products like ODTD. β-Oxidation has been proposed to be a further metabolic route for rubber latex cleavage products by Lcp in Gram-positive bacteria that are rubber degraders (26, 27). Moreover, genes necessary for β-oxidation are also found in all the myxobacteria investigated in the genomic context surrounding the respective roxA genes. Only a small region of 7.6 kb around roxA of Xanthomonas sp. 35Y has been sequenced. However, genes coding for a putative aminoglycoside-phosphotransferase, enoyl coenzyme A (enoyl-CoA) hydratase, and acetyl-CoA acetyltransferase are located within this region, suggesting that a β-oxidation cluster is also present around roxAXsp and that there is a metabolic connection between the function of RoxA and β-oxidation. One could speculate that the localization of roxA in this metabolic gene cluster is indicative of the potential of the rubber oxygenase to use other hydrocarbons, in addition to poly(cis-1,4-isoprene), as substrates which are metabolized by β-oxidation.
Expression of active RoxA in E. coli or other heterologous hosts was not successful, regardless of the absence or presence of cytochrome c maturation genes (ccm genes). We assume that the maturation machinery for the covalent attachment of two hemes and/or for the formation of two disulfide bridges proceeds via different mechanisms in Xanthomonas sp. 35Y than in E. coli or B. subtilis. Since expression of RoxA from three species of myxobacteria was possible in recombinant Xanthomonas cells, we assume that the RoxA maturation systems for H. ochraceum, C. coralloides, and M. fulvus are related and are interchangeable. We postulate the existence of a yet unknown (or at least modified) cytochrome c maturation system in Xanthomonas sp. 35Y and RoxA-expressing myxobacteria, and we assume that RoxA of C. apiculatus and other not yet described RoxA orthologs from other Gram-negative bacteria can be functionally expressed in recombinant Xanthomonas sp. 35Y as well.
ACKNOWLEDGMENTS
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to D.J. The support of the Weber and Schaer Company for providing polyisoprene latex is also acknowledged.
We thank Y. Li (Shandong University, Shandong, China) and T. Schäberle (University of Bonn, Bonn, Germany) for providing M. fulvus HW1 and C. coralloides BO35, including BO35 genomic DNA.
Footnotes
Published ahead of print 9 August 2013
REFERENCES
- 1.Jendrossek D, Tomasi G, Kroppenstedt RM. 1997. Bacterial degradation of natural rubber: a privilege of actinomycetes? FEMS Microbiol. Lett. 150:179–188 [DOI] [PubMed] [Google Scholar]
- 2.Rose K, Steinbüchel A. 2005. Biodegradation of natural rubber and related compounds: recent insights into a hardly understood catabolic capability of microorganisms. Appl. Environ. Microbiol. 71:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuchii A, Takeda K. 1990. Rubber-degrading enzyme from a bacterial culture. Appl. Environ. Microbiol. 56:269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yikmis M, Steinbüchel A. 2012. Historical and recent achievements in the field of microbial degradation of natural and synthetic rubber. Appl. Environ. Microbiol. 78:4543–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose K, Tenberge KB, Steinbüchel A. 2005. Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 6:180–188 [DOI] [PubMed] [Google Scholar]
- 6.Yikmis M, Steinbüchel A. 2012. Importance of the latex-clearing protein (Lcp) for poly(cis-1,4-isoprene) rubber cleavage in Streptomyces sp. K30. Microbiologyopen 1:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiessl S, Schuldes J, Thürmer A, Halbsguth T, Broker D, Angelov A, Liebl W, Daniel R, Steinbüchel A. 2012. Involvement of two latex-clearing proteins during rubber degradation and insights into the further degradation pathway revealed by the genome sequence of Gordonia polyisoprenivorans strain VH2. Appl. Environ. Microbiol. 78:2874–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yikmis M, Arenskötter M, Rose K, Lange N, Wernsmann H, Wiefel L, Steinbüchel A. 2008. Secretion and transcriptional regulation of the latex-clearing protein, Lcp, by the rubber-degrading bacterium Streptomyces sp. strain K30. Appl. Environ. Microbiol. 74:5373–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jendrossek D, Reinhardt S. 2003. Sequence analysis of a gene product synthesized by Xanthomonas sp. during growth on natural rubber latex. FEMS Microbiol. Lett. 224:61–65 [DOI] [PubMed] [Google Scholar]
- 10.Braaz R, Fischer P, Jendrossek D. 2004. Novel type of heme-dependent oxygenase catalyzes oxidative cleavage of rubber (poly-cis-1,4-isoprene). Appl. Environ. Microbiol. 70:7388–7395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt G, Seiffert G, Kroneck PMH, Braaz R, Jendrossek D. 2010. Spectroscopic properties of rubber oxygenase RoxA from Xanthomonas sp., a new type of dihaem dioxygenase. Microbiology 156:2537–2548 [DOI] [PubMed] [Google Scholar]
- 12.Birke J, Hambsch N, Schmitt G, Altenbuchner J, Jendrossek D. 2012. Phe317 is essential for rubber oxygenase RoxA activity. Appl. Environ. Microbiol. 78:7876–7883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braaz R, Armbruster W, Jendrossek D. 2005. Heme-dependent rubber oxygenase RoxA of Xanthomonas sp. cleaves the carbon backbone of poly(cis-1,4-isoprene) by a dioxygenase mechanism. Appl. Environ. Microbiol. 71:2473–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwede T, Kopp J, Guex N, Peitsch MC. 2003. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31:3381–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hambsch N, Schmitt G, Jendrossek D. 2010. Development of a homologous expression system for rubber oxygenase RoxA from Xanthomonas sp. J. Appl. Microbiol. 109:1067–1075 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Graichen ME, Liu A, Pearson AR, Wilmot CM, Davidson VL. 2003. MauG, a novel diheme protein required for tryptophan tryptophylquinone biogenesis. Biochemistry 42:7318–7325 [DOI] [PubMed] [Google Scholar]
- 17.Arslan E, Schulz H, Zufferey R, Künzler P, Thöny-Meyer L. 1998. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251:744–747 [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann M, Braaz R, Jendrossek D, Einsle O. 2008. Crystallization of the extracellular rubber oxygenase RoxA from Xanthomonas sp. strain 35Y. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 64:123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seidel J, Schmitt G, Hoffmann M, Jendrossek D, Einsle O. 2013. Structure of the processive rubber oxygenase RoxA from Xanthomonas sp. Proc. Natl. Acad. Sci. U. S. A. 110:13833–13838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss JJ. 1964. Nature of the iron-oxygen bond in oxyhaemoglobin. Nature 202:83–84 [DOI] [PubMed] [Google Scholar]
- 21.Wittenberg JB, Wittenberg BA, Peisach J, Blumberg WE. 1970. On the state of the iron and the nature of the ligand in oxyhemoglobin. Proc. Natl. Acad. Sci. U. S. A. 67:1846–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heisey RM, Papadatos S. 1995. Isolation of microorganisms able to metabolize purified natural rubber. Appl. Environ. Microbiol. 61:3092–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai S, Ichikawa K, Muramatsu Y, Kasai D, Masai E, Fukuda M. 2011. Isolation and characterization of Streptomyces, Actinoplanes, and Methylibium strains that are involved in degradation of natural rubber and synthetic poly(cis-1,4-isoprene). Enzyme Microb. Technol. 49:526–531 [DOI] [PubMed] [Google Scholar]
- 24.Warneke S, Arenskötter Q, Tenberge KB, Steinbüchel A. 2007. Bacterial degradation of poly(trans-1,4-isoprene) (Gutta percha). Microbiology 153:347–356 [DOI] [PubMed] [Google Scholar]
- 25.Dworkin M. 1966. Biology of the myxobacteria. Annu. Rev. Microbiol. 20:75–106 [DOI] [PubMed] [Google Scholar]
- 26.Bode HB, Kerkhoff K, Jendrossek D. 2001. Bacterial degradation of natural and synthetic rubber. Biomacromolecules 2:295–303 [DOI] [PubMed] [Google Scholar]
- 27.Banh Q, Arenskötter M, Steinbüchel A. 2005. Establishment of Tn5096-based transposon mutagenesis in Gordonia polyisoprenivorans. Appl. Environ. Microbiol. 71:5077–5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon R, Priefer U, Pühler A. 1983. A broad host-range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Nat. Biotechnol. 1:784–791 [Google Scholar]