Abstract
A gene encoding a homolog to the cation diffusion facilitator protein DmeF from Cupriavidus metallidurans has been identified in the genome of Rhizobium leguminosarum UPM791. The R. leguminosarum dmeF gene is located downstream of an open reading frame (designated dmeR) encoding a protein homologous to the nickel- and cobalt-responsive transcriptional regulator RcnR from Escherichia coli. Analysis of gene expression showed that the R. leguminosarum dmeRF genes are organized as a transcriptional unit whose expression is strongly induced by nickel and cobalt ions, likely by alleviating the repressor activity of DmeR on dmeRF transcription. An R. leguminosarum dmeRF mutant strain displayed increased sensitivity to Co(II) and Ni(II), whereas no alterations of its resistance to Cd(II), Cu(II), or Zn(II) were observed. A decrease of symbiotic performance was observed when pea plants inoculated with an R. leguminosarum dmeRF deletion mutant strain were grown in the presence of high concentrations of nickel and cobalt. The same mutant induced significantly lower activity levels of NiFe hydrogenase in microaerobic cultures. These results indicate that the R. leguminosarum DmeRF system is a metal-responsive efflux mechanism acting as a key element for metal homeostasis in R. leguminosarum under free-living and symbiotic conditions. The presence of similar dmeRF gene clusters in other Rhizobiaceae suggests that the dmeRF system is a conserved mechanism for metal tolerance in legume endosymbiotic bacteria.
INTRODUCTION
Nickel and cobalt are essential microelements for microbial nutrition that participate in a variety of cellular processes. In particular, nickel participates as a cofactor in at least nine enzymes, including urease and hydrogenase (1), whereas cobalt is required for activity of corrinoid-containing enzymes such as isomerases and methyl transferases (2). These two elements are usually present at low concentrations in soils, and bacteria have developed high-affinity metal uptake systems for the cations (3). In contrast, moderate concentrations of the same elements can become toxic by displacing other metals from the active site of metalloenzymes, by catalyzing the production of free radicals, or by interfering with the assembly of FeS clusters (4, 5). Nickel homeostasis requires the balance of import and export pathways to control metal concentration inside the bacterial cell (6). Active transport efflux pumps represent the largest category of metal resistance systems (7). Most studies on bacterial metal resistance have been carried out with the heavy metal-resistant organism C. metallidurans CH34 (8). In this organism, three main groups of efflux systems have been characterized: RND (resistance, nodulation, and cell division) proteins, cation diffusion facilitators (CDFs), and P-type ATPases (9). Bacteria utilize primarily the first two groups for dealing with Ni(II) and Co(II) (2).
Members of the RND group are membrane proteins that participate in trimeric complexes along with outer membrane factors and bridging periplasmic proteins (10). Such complexes are able to export toxic substances, including heavy metals, acting as a kind of peristaltic pump driven by proton motive force to pump out metals from the periplasm across the outer membrane (11).
CDF proteins use a Me2+/H+ proton-antiport mechanism to drive the translocation of heavy metals across membranes (12). CDF substrates are divalent cations such as Zn(II), Mn(II), Cd(II), Fe(II), Zn(II), and Co(II). As a general rule, CDF proteins contain six putative transmembrane domains (TMD) with a C terminus protruding into the cytoplasm and carrying metal binding sites (12). Many CDF transporters also contain a histidine-rich domain to allow more efficient metal binding. At least some of the members of the family function as homo-oligomeric complexes. CDFs are present in organisms from the three kingdoms of life (13). The model example of bacterial CDF is Escherichia coli YiiP, a homodimeric protein involved in the efflux of Fe, Zn, and Cd (14). In the metal-resistant bacterium Cupriavidus metallidurans, a CDF protein (DmeF, for Divalent metal efflux) is essential for cobalt export to the periplasm (15).
A key aspect of metal homeostasis is the regulation of expression of transporter proteins. Bacteria contain metalloregulatory proteins to fine-tune the expression of genes involved in uptake and efflux of metals (3). Two E. coli proteins, NikR and RcnR, are involved in metal sensing and regulation of gene expression in response to nickel or cobalt ions. NikR is a member of the bacteriophage P22 Arc repressor superfamily. This protein controls the expression of Ni uptake genes (E. coli nikABCDE) and other nickel-related genes such as urease genes in Helicobacter pylori (16). RcnR is a metal-responsive repressor that constitutes, along with the copper regulator CsoR, the most recent addition to the list of major groups of metalloregulators (17, 18). E. coli RcnR represses the expression of the Ni- and Co-specific efflux system RcnAB by binding to a specific sequence with G/C tracts flanked by AT-rich inverted repeats in the operator region (19). This repression is released upon binding of Ni or Co ions to a histidine-rich motif (H-C-H-H) critical for RcnR function (20).
The symbiotic interaction between Rhizobium and legume plants is a key component of sustainable agricultural systems, due to the ability of these endosymbiotic bacteria to fix atmospheric nitrogen into ammonia provided to the plant (21). In this symbiosis, the bacteria infect the legume roots and induce the formation of nodules in which bacteria proliferate and fix nitrogen. A plant-derived peribacteroid membrane surrounds bacteroids, the symbiotic form of the bacteria, thus controlling nutrient exchange between both symbionts (22). Although the Rhizobium-legume symbiosis has been proposed as a tool for bioremediation of heavy metal-polluted soils (23, 24), the information on determinants involved in metal resistance in Rhizobiaceae is scarce, restricted to a few reports on the levels of metal tolerance by members of this relevant group of endosymbiotic bacteria (25, 26). However, this bacterial group might be a relevant reservoir of genetic determinants mediating survival under high-metal conditions, as deduced from the large number of metal resistance genes identified in the genome of a Mesorhizobium amorphae isolate obtained from a Zn/Pb mine tailing (27). In this paper, we describe the functional characterization and expression analysis of an Ni(II)- and Co(II)-inducible system (DmeRF) involved in resistance to these metals in both free-living and symbiotic states of Rhizobium leguminosarum bv. viciae.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The R. leguminosarum bv. viciae strains used in this work were routinely grown at 28°C in tryptone-yeast extract (TY) (28), Rhizobium minimal (Rmin) (29), or yeast-mannitol (30) media. Strain SPF25 is derived from UPM791 by replacement of the native NifA-dependent hupSL promoter by the FnrN-dependent fixN promoter to allow expression of hydrogenase in free-living cells (31). E. coli strains DH5α (Bethesda Research Laboratories, United Kingdom) and S17.1 (32) were used for cloning and conjugation purposes, respectively. Antibiotics were added at the following concentrations (in μg · ml−1): tetracycline, 5; kanamycin, 50; and spectinomycin, 50.
MICs for nickel, cobalt, zinc, and copper were estimated by the ability of the Rhizobium strains to grow on TY plates containing increasing concentrations of NiCl2 (0.1, 0.2, 0.3, 0.5, 1.0, 1.5, 2.0, and 2.5 mM), CoCl2 (0.1, 0.2, 0.3, 0.4, 0.5, 0.75, and 1.0 mM), ZnSO4 (0.5, 1.0, 1.5, and 2.0 mM), or CuSO4 (0.5, 1.0, 1.5, and 2.0 mM). For disk diffusion susceptibility tests (33), bacterial cultures grown to the exponential phase were mixed with warm TY agar medium and poured into petri plates. Disks soaked with different amounts of NiCl2 (100, 200, or 500 mM), CoCl2 (20, 50, or 100 mM), CuSO4 (100, 200, or 500 mM), ZnSO4 (100, 200, or 500 mM), and MnCl2 (100, 200, or 500 μM) were placed on the surface. The zone of inhibition was measured after 48 h of incubation at 28°C.
DNA manipulation techniques and plasmid constructions.
Plasmid DNA preparations, restriction enzyme digestions, and DNA transformations into E. coli were carried out by standard protocols (34). For analysis of promoter expression, transcriptional gene fusions were generated with the promoterless lacZ gene present in plasmid pMP220 (35). DNA fragments containing the 5′ end of dmeR and dmeF genes along with upstream regions were amplified using primer pairs dmeR1_F/dmeR1_R (5′-AGAGCGGCACGAGAATGG-3′/5′-GGACGGAGGCGAGCAGTT-3′) and dmeR2_F/dmeRF_R(5′-TTGAAGGGGCAGATGGAG-3′/5′-GGCACGGGATTGGAAAGG-3′), respectively, cloned in plasmid pCR2.1-TOPO, and subcloned in pMP220 as KpnI/XbaI fragments, thus generating plasmids pDL13 (dmeR′-lacZ) and pDL43 (dmeF′-lacZ). An additional fusion plasmid containing the region upstream dmeR, the whole dmeR gene, and the 5′ end of dmeF was constructed by a similar strategy using primers dmeR1_F and dmeRF_R (fusion plasmid pDL10, dmeRF′-lacZ). These plasmids were introduced into R. leguminosarum by mating, and transconjugants were selected in Rhizobium minimal medium supplemented with tetracycline.
Generation of the dmeRF-deficient mutant strain D15 was carried out by a marker exchange approach. Two DNA regions of ca. 1 kb corresponding to the dmeR upstream and dmeF downstream regions were amplified by PCR using primer pairs dmeR1_F/dmeR1_R and dmeF_F (5′-TTGTTGCCGTCCTTACCT-3′)/dmeF2_R (5′-CCGCTCCTTGCCTGTCGT-3′), respectively. Both regions were cloned in plasmid pK18mobsacB with an intervening DNA fragment containing a spectinomycin resistance cassette. This construction was introduced into R. leguminosarum SPF25 by conjugation, and single and double recombination events were selected in Rhizobium minimal medium as described previously (36). The dmeRF deletion was confirmed through Southern blot analysis of EcoRI-digested genomic DNA from the mutants, using a digoxigenin (DIG)-labeled DNA fragment containing dmeRF genes as a probe. Hybridizing bands were visualized using a chemiluminescent DIG detection substrate as described by the manufacturer (Roche Diagnostics GmbH, Mannheim, Germany).
qRT-PCR analysis.
For RNA preparation, R. leguminosarum SPF25 cells were grown in standard TY medium or in TY medium supplemented with metals (200 μM NiCl2 or 10 μM CoCl2) and incubated for 24 h at 28°C. Cells were harvested from 5 ml of culture by centrifugation and resuspended in 500 μl of Tris-EDTA (TE) buffer, and RNA was stabilized with RNA Protect Bacteria reagent (Qiagen, Hilden, Germany) and purified with an RNeasy minikit (Qiagen). Contaminating DNA was removed with Turbo DNA-free (Ambion, Life Technologies Ltd., Paisley, United Kingdom). cDNAs were obtained using SuperScript III reverse transcriptase (Invitrogen Life Technologies Ltd., Paisley, United Kingdom) according to the manufacturer's instructions. Quantitative reverse transcription-PCR (qRT-PCR) was carried out with a Power SYBR green master mix (Applied Biosystems Life Technologies Ltd., Paisley, United Kingdom) in a final volume of 25 μl.
For transcript analysis of dmeF, orf03473, and orf03476, cDNA was used as the template for qRT-PCR using primer pairs qdmeF(5′-AGGACGCTGCCGATACAA-3′)/qdmeR(5′-TCCTGCCGTTGTTAACGC-3′), orf03476F(5′-GACACGCTCGGCAATCTGAC-3′)/orf03476R(5′-GCACGGTCGTCTCGCTGATA-3′), and orf03473F(5′-CCATTCTCGTGCCGCTCTAC-3′)/orf03473R(5′-GGGTGAAATCCAGCTGTTCG-3′), respectively. Primers rpoD_F (5′-GATGAAGTCGATCGGCAATCTG-3′) and rpoD_R (5′-GCTTCGACCATTTCCTTCTTGG-3′) were used to estimate expression of rpoD as an internal reference. The qRT-PCR program consisted of 10 min of incubation at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C and a final cycle of 15 s at 95°C, 60 s at 60°C, 15 s at 95°C, and 15 s at 60°C. Determinations were carried out with RNA extracted from three independent biological samples, with the threshold cycle (CT) determined in triplicate for each biological replicate. The relative levels of transcription were calculated by using the 2−ΔΔCT method (37). To control DNA contamination, PCRs were performed on RNA preparations prior to reverse transcription using the same primer pairs.
Plant tests and enzymatic activity.
Pea (Pisum sativum L. cv. Frisson) and lentil (Lens culinaris L. cv. Magda) seeds were surface sterilized and planted in Leonard jar-type assemblies under bacteriologically controlled conditions (38). Plants were grown in a greenhouse with 16-h/8-h light-dark cycle and 25/23°C day-night temperature. When required, the nitrogen-free plant nutrient solution was supplemented with 85 μM NiCl2 or 42.5 μM CoCl2 10 days after seedling inoculation (39).
Plant shoot dry weight was determined after drying at 60°C for 24 h. Nitrogen content of the shoot was determined by a Kjeldhal method (40) with 0.5 g of ground plant material per sample.
β-Galactosidase activities in R. leguminosarum free-living cultures and in bacteroid suspensions obtained from 21-day-old plants were determined as described by Miller (41). For this purpose, free-living cells were grown in TY liquid medium overnight, diluted in fresh TY medium supplemented with increasing concentrations of NiCl2 or CoCl2, and incubated for 24 h at 28°C. The assays were conducted using ortho-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate. Values for β-galactosidase activity were calculated as Miller units. The protein contents of bacteroid suspensions and free-living cultures were measured by the bicinchoninic acid method (42) with the modifications described by Brito et al. (43).
RESULTS
Identification of dmeRF genes in R. leguminosarum bv. viciae.
The cation diffusion facilitator (CDF) encoded by dmeF has a key role in resistance to nickel and cobalt in the metal-resistant strain C. metallidurans CH34 (15). In order to identify potential genetic systems involved in nickel and cobalt resistance in R. leguminosarum bv. viciae, the genome of this bacterium (JGI-128C53, Gi08894) was analyzed for homologs to this gene. An open reading frame (ORF; orf03475) encoding a protein with 39% amino acid identity to dmeF was identified. This ORF encodes a 343-amino-acid protein with a predicted structure including six transmembrane (TM) domains (see Fig. S1 in the supplemental material). In this protein we also identified the two motifs characteristic of CDF proteins identified by Montanini et al. (13) and a histidine-rich stretch located between TM4 and TM5, so we designated the R. leguminosarum gene dmeF. A phylogenetic tree (see Fig. S2 in the supplemental material) built with proteobacterial CDF protein sequences grouped R. leguminosarum dmeF within the Zn-CDF group previously defined (13).
Upstream of dmeF, the R. leguminosarum UPM791 genome presents an ORF encoding a 90-amino-acid protein showing high similarity (39% identical residues) to E. coli RcnR, one of the founding members of the RcnR/CsoR structural class of metal-responsive transcriptional regulators (17, 44). Alignment of the two proteins revealed that the R. leguminosarum gene product contained a conserved cysteine residue (Cys-35) and three out of the four conserved histidine residues (His-3, His-60, and His-64) involved in response to Ni(II) and Co(II) (20, 45); furthermore, structural prediction based on I-TASSER software (46) indicated the presence of three alpha helices similar to those present in CsoR/RcnR (see Fig. S1). Based on the regulatory role of this protein in R. leguminosarum (see below), the corresponding gene was designated dmeR.
Functional analysis of dmeRF in R. leguminosarum free-living and symbiotic cells.
In order to carry out the functional analysis of dmeRF genes, a ΔdmeRF::Spcr mutant of R. leguminosarum SPF25 was constructed by replacement of these genes with a spectinomycin resistance cassette, thus resulting in strain D15.
We first analyzed the effect of the elimination of dmeRF genes on the levels of nickel and cobalt resistance. As shown in Table 1, inactivation of dmeRF genes led to decreased levels of tolerance to nickel and cobalt both in rich and minimal media (TY and Rmin). Similarly, assays with disks soaked with Ni(II) or Co(II) solutions revealed significant increases in the size of inhibition halos for these two metal ions, but not in the case of Zn(II) or Cu(II). All these data indicate that the dmeRF system is involved in resistance to nickel and cobalt in R. leguminosarum.
Table 1.
Effect of deletion of dmeRF genes on nickel and cobalt resistance in R. leguminosarum bv. viciae SPF25
| Strain | Genotype | Medium | MIC (mM)a |
Inhibition zone (mm)b |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NiCl2 | CoCl2 | CuSO4 | ZnSO4 | NiCl2 | CoCl2 | CuSO4 | ZnSO4 | |||
| SPF25 | Wild type | TY | 1 | 0.75 | 2 | 2 | 23 | 21 | 17 | 20 |
| D15 | ΔdmeRF | TY | 0.75 | 0.3 | 2 | 2 | 27 | 30 | 18 | 20 |
| SPF25 | Wild type | Rmin | 0.1 | 0.2 | ND | ND | ND | ND | ND | ND |
| D15 | ΔdmeRF | Rmin | 0.05 | 0.05 | ND | ND | ND | ND | ND | ND |
Values represent the results of three separate experiments.
Values are the averages of three replicates. Standard errors were below 5%. ND, not determined.
We tested the potential relevance of dmeRF genes to the symbiosis with host legumes grown under low and high levels of metals. To this aim, pea plants inoculated with wild-type R. leguminosarum SPF25 or with its dmeRF-deficient derivative D15 were grown for 3 weeks under greenhouse conditions either with a standard nutrient solution or with the same solution supplemented with nickel or cobalt. The data obtained (Table 2) indicate that the mutation had no significant effects on symbiotic performance when plants were grown with standard nutrient solutions. In the case of plants supplemented with cobalt, however, deletion of the dmeRF system resulted in a statistically significant decrease (26%) of the average dry-weight values in the mutant compared to the wild type. Similar reductions in the average values were observed in the case of nickel, although in this case differences were not statistically significant, likely due to the high heterogeneity of the plants. These results indicate that the dmeRF system has a relevant role in the symbiotic performance of the pea-R. leguminosarum association when pea plants were grown under high-metal conditions. We also tested the effect of the dmeRF system on the symbiotic performance of lentil plants. This alternative host is known to provide lower levels of nickel to the bacteroids, thus resulting in reduced levels of hydrogenase activity (39). In the case of lentils, symbiotic performance was not affected by the deletion of the dmeRF system under either low- or high-metal conditions (Table 2). We interpret this result as a consequence of the lower level of metals available to the bacteroids in this plant species, thus avoiding noxious metal buildup in the absence of the efflux system.
Table 2.
Effect of deletion of dmeRF genes on symbiotic performance of R. leguminosaum bv. viciae with pea and lentil as plant hosts
| Straina | Concn of metal added (μM) |
Pea |
Lentil |
|||
|---|---|---|---|---|---|---|
| Ni(II) | Co(II) | Shoot DWb | N fixedc | Shoot DWb | N fixedc | |
| SPF25 | 0 | 0 | 269.6 ± 25.3 | 10.8 ± 1.5 | 196.2 ± 23.3 | 5.2 ± 0.7 |
| D15 | 0 | 0 | 249.9 ± 32.0 | 9.9 ± 1.3 | 177.9 ± 38.6 | 4.8 ± 1.2 |
| Controld | 0 | 0 | 155.7 ± 24.8* | 1.8 ± 0.2* | 120.1 ± 21.1* | 1.4 ± 0.1* |
| SPF25 | 85 | 0 | 220.1 ± 46.9 | 8.4 ± 1.6 | 190.7 ± 54.4 | 4.9 ± 1.9 |
| D15 | 85 | 0 | 141.7 ± 47.6 | 5.4 ± 2.0 | 195.2 ± 40.2 | 5.2 ± 1.5 |
| SPF25 | 0 | 42.5 | 293.8 ± 45.6 | 11.3 ± 1.7 | 182.9 ± 38.0 | 4.7 ± 1.0 |
| D15 | 0 | 42.5 | 218.3 ± 40.8* | 8.8 ± 1.9* | 174.6 ± 12.3 | 4.6 ± 0.6 |
R. leguminosarum strains used were SPF25 (wild type) and D15 (dmeRF deletion derivative).
Values of shoot dry weight (DW) (mg · plant−1) correspond to the averages of at least three replicates ± standard errors. *, statistically significant difference (analysis of variance, P < 0.05) from the wild type grown under the same conditions.
Values (mg of N · plant−1) correspond to the averages of at least three replicates ± standard errors.*, statistically significant difference (analysis of variance, P < 0.05) from the wild type grown under the same conditions.
As a control, noninoculated plants were used to verify the absence of cross-contamination.
Expression of dmeRF genes is induced by nickel and cobalt in R. leguminosarum.
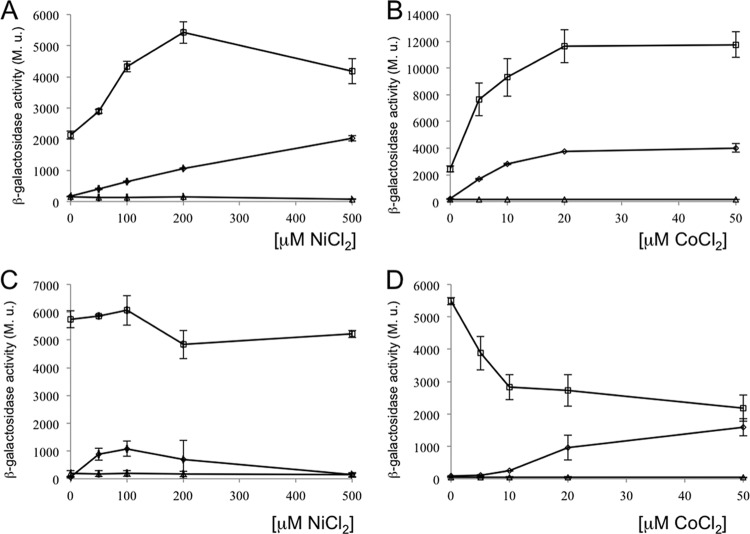
Since metal efflux systems from E. coli and other bacteria are regulated by the presence of the corresponding metal cation (2), we decided to study the effect of the addition of metal ions on the expression of dmeRF genes. Expression analysis of dmeRF genes was performed first by using fusions to the lacZ reporter gene. The DNA region containing the dmeR upstream region along with the dmeR gene and the 5′ end of dmeF was cloned into the pMP220 vector to obtain the dmeRF′-lacZ fusion plasmid pDL10 (Fig. 1). Also, the dmeR and dmeF upstream regions were cloned independently in vector pMP220 to generate transcriptional fusion plasmids pDL13 (dmeR′-lacZ) and pDL43 (dmeF′-lacZ). These plasmids were introduced into R. leguminosarum strains SPF25 and D15, and the reporter activity was determined in cell cultures grown in media supplemented with increasing nickel and cobalt concentrations.
Fig 1.
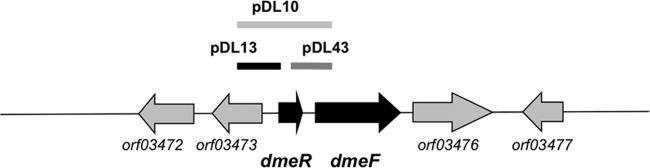
Genetic map of dmeRF genes in the R. leguminosarum bv. viciae UPM791 genome. Thick lines above the genetic map indicate the DNA regions cloned in pMP220 in the indicated fusion constructs. ORF designation corresponds to those used in the Joint Genome Institute database (Gi08894).
Expression of the dmeRF genes was analyzed first in free-living cells from R. leguminosarum strain SPF25 (Fig. 2). In this background, the dmeRF′-lacZ fusion pDL10 was associated with basal levels of β-galactosidase activity (below 50 Miller units [MU]) in media with no added metals (Fig. 2A). Interestingly, reporter activity mediated by this fusion gradually increased to more than 2,000 MU when the level of nickel added was increased until 500 μM NiCl2, close to the level of toxicity for this metal. In the case of cobalt, metal-dependent induction of reporter activity was even higher and reached a maximum (ca. 4,000 MU) at a cobalt concentration of 20 μM (Fig. 2B). No significant induction of reporter activity over basal levels was observed when other metals [Zn(II), Cu(II), Mn(II), or Cd(II)] were added at concentrations up to 100 μM (data not shown). When similar experiments were carried out with plasmid pDL43 (dmeF′-lacZ), only basal levels of reporter activity were observed, irrespective of the Ni(II) or Co(II) levels present in the medium. We conclude from these results that expression of dmeRF is induced in response to the presence of nickel and cobalt metal ions from a promoter region located upstream of dmeR gene. The existence of a transcriptional unit including both dmeR and dmeF was confirmed by RT-PCR experiments carried out with cDNA from R. leguminosarum cultures in media either supplemented or not supplemented with Ni(II) (see Fig. S3 in the supplemental material).
Fig 2.
Expression analysis of R. leguminosarum dmeRF genes as a function of nickel or cobalt concentration in the culture medium. R. leguminosarum wild-type SPF25 (A and B) and dmeRF mutant D15 (C and D) containing reporter fusion plasmids pDL10 (dmeRF′-lacZ) (diamonds), pDL13 (dmeR′-lacZ) (squares), or pDL43 (dmeF′-lacZ) (triangles) were grown in TY medium supplemented with the indicated amounts of NiCl2 (A and C) and CoCl2 (B and D). β-Galactosidase activities are expressed in Miller units. Values are averages of three independent experiments. Error bars indicate standard errors.
The reporter expression associated with plasmid pDL13 (dmeR′-lacZ) was also analyzed. In this case, significantly higher levels of β-galactosidase activity were detected under all conditions tested, even in the absence of added metals in the medium. For this fusion, the addition of increasing concentrations of nickel or cobalt resulted in higher values of reporter activity, with maxima at ca. 200 μM Ni(II) and 20 μM Co(II) (5,000 and 10,000 MU, respectively), likely due to repressor titration by the higher number of copies of the dmeRF promoter region.
When the reporter fusions were introduced into the dmeRF mutant D15, activity profiles associated with fusion plasmid pDL10, containing a whole copy of the dmeR gene, again showed a Ni(II)- and Co(II)-dependent regulation, whereas those associated with pDL13 were quite different (Fig. 2C and D). In this genetic background, the dmeR′-lacZ fusion induced very high β-galactosidase activities even in the absence of added nickel or cobalt in the medium. These data strongly suggest that DmeR acts as a repressor of dmeRF expression and that the DmeR protein synthesized from the dmeR gene cloned in plasmid pDL10 restores the metal-dependent control of expression of the dmeRF promoter. Titration of DmeR would also explain the high values of reporter activity associated with fusion pDL13 in the wild-type strain.
The metal-responsive induction of dmeRF genes deduced from the lacZ fusion assays was confirmed by qRT-PCR experiments. In this analysis (Fig. 3), we found that the presence of Ni(II) (200 μM) or Co(II) (10 μM) in the medium induced 6-fold and 8-fold increases, respectively, in the level of transcription of the dmeF gene, whereas the level of expression of flanking genes orf03473 and orf03476 was not modified by the addition of the cations. The lack of metal-induced expression of orf03476, along with the basal levels of expression associated with the dmeF-lacZ fusion, and the results of RT-PCR experiments (see Fig. S3 in the supplemental material) indicate that the dmeRF genes constitute an operon whose expression is induced in response to the presence of Ni(II) and Co(II) ions. Our data also indicate that the expression of dmeRF is negatively controlled by the product of dmeR and that this repression is likely alleviated by the presence of these cations.
Fig 3.
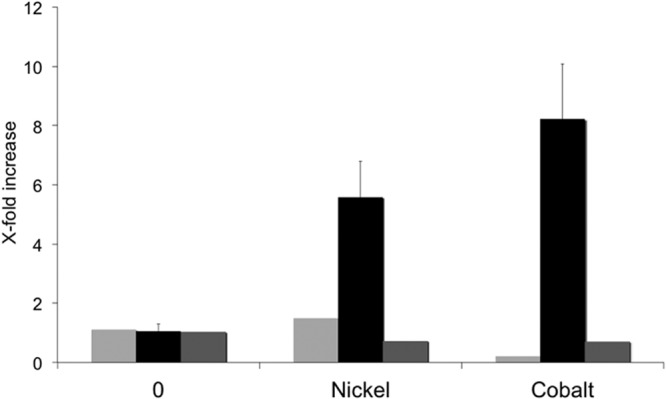
qRT-PCR analysis of expression of R. leguminosarum dmeF and flanking genes. Histograms correspond to qRT-PCR expression analysis of orf03473 (light gray), dmeF (black), and orf03476 (dark gray) genes in cells grown in standard TY culture medium (0) or in the same medium supplemented with nickel (200 μM NiCl2) or cobalt (10 μM CoCl2). Bars indicate standard errors from three experimental replicates.
Expression of dmeRF genes was also studied in pea bacteroids from SPF25 and dmeRF-deficient mutant D15 derivatives carrying each of the reporter gene fusions (Table 3). In this analysis, the wild-type strain R. leguminosarum SPF25 exhibited low levels of reporter activity in the case of fusion plasmid pDL10 (dmeRF-lacZ), with values similar to those associated with plasmid pDL43 (30 to 40 Miller units), considered the basal level. In the same experiments, the presence of dmeR-lacZ fusion plasmid pDL13 was associated with slightly higher reporter activities, irrespective of whether Ni(II) or Co(II) was added to the plants. Our interpretation of these results is that the product of the genomic copy of dmeR is able to repress the dmeRF promoter under symbiotic conditions. Analysis of the same fusions in bacteroids of the dmeRF mutant revealed that expression of these genes was significantly enhanced by cobalt. Under these conditions, the dmeR-lacZ fusion was associated with higher levels of unregulated expression in this genetic background (ca. 350 MU). Again, the high reporter activities associated with this fusion are likely the result of the absence of an active copy of dmeR in this genetic background.
Table 3.
Symbiotic expression of R. leguminosarum dmeRF genes
| lacZ fusion | β-Galactosidase activity (Miller units)a |
|||||
|---|---|---|---|---|---|---|
| SPF25 |
D15 |
|||||
| Control | Ni(II) | Co(II) | Control | Ni(II) | Co(II) | |
| pDL43 | 33 ± 12 | 39 ± 11 | 33 ± 6 | 33 ± 5 | 43 ± 15 | 46 ± 13 |
| pDL10 | 37 ± 12 | 35 ± 8 | 46 ± 7 | 40 ± 17 | 41 ± 13 | 113 ± 24 |
| pDL13 | 56 ± 17 | 60 ± 17 | 63 ± 17 | 333 ± 114 | 367 ± 141 | 367 ± 74 |
Values are β-galactosidase activities of pea bacteroids obtained from plants inoculated with R. leguminosarum SPF25 (wild type) and D15 (dmeRF deletion mutant) harboring dmeF′-lacZ (pDL43), dmeRF′-lacZ (pDL10), or dmeR′-lacZ (pDL13) fusions. Plants were grown in standard nutrient solutions (control) or in nutrient solutions supplemented with Ni(II) (85 μM NiCl2) or Co(II) (42.5 μM CoCl2). Values are the averages of four replicates ± standard errors.
Effect of dmeRF system on NiFe hydrogenase activity.
Since it was previously shown that an Ni/Co metal efflux system, RcnRA, has an effect on NiFe hydrogenase in E. coli, the effect of inactivation of dmeFR genes on induction of hydrogenase activity in microaerobic free-living cells of R. leguminosarum was determined. In these assays, microaerobic cultures of the wild-type strain SPF25 induced normal levels of O2-dependent H2 uptake, whereas microaerobic cultures of D15 mutant exhibited significantly lower levels (ca. 50% reduction) (Table 4). This reduction in activity was not reverted by the addition of nickel at a concentration (1 μM) able to revert the low hydrogenase activity in mutant SPF22, devoid of both nickel transporter genes hupE and hupE2 (47).
Table 4.
Effect of dmeRF genes on hydrogenase activity of R. leguminosarum bv. viciae SPF25
| Strain | Relevant genotype | Hydrogenase activity with metal additiona |
|
|---|---|---|---|
| No metal | 1 μM NiCl2a | ||
| SPF25 | Wild type | 970 ± 170 | 1,000 ± 160 |
| D15 | SPF25 ΔdmeRF | 510 ± 140 | 470 ± 190 |
| SPF22 | SPF25 ΔhupE hupE2 | 280 ± 50 | 1,060 ± 110 |
Microaerobic cultures were assayed for hydrogenase activity after incubation under 1% oxygen for 16 h. Values are given in nmol of H2 · h−1 · mg of protein−1 and represent the averages of four experiments ± standard errors.
The effect of the deletion of the dmeRF system on the level of hydrogenase activity was also tested under symbiotic conditions. To this aim, the levels of hydrogenase activity in bacteroids from nodules induced in pea and lentil plants were determined (Table 5). In the case of pea, both wild-type and mutant strains induced normal levels of hydrogenase activity when plants were grown under standard nutrient conditions. Such levels were greatly enhanced by the addition of nickel, irrespective of the presence of the mutation in the dmeRF genes. The addition of cobalt resulted in partial inhibition (40% reduction) of hydrogenase activity in both wild-type and mutant strains. We also measured hydrogenase activity in bacteroids induced in lentil. In this host, the level of hydrogenase activity in plants grown under standard conditions was ca. 10 times lower than in pea, as we had previously observed (47). Again, the addition of nickel to lentil plants resulted in a 5-fold increase of hydrogenase activity, but interestingly, lentil bacteroids from the dmeRF-deficient strain induced significantly lower levels of hydrogenase activity under both standard and Ni-enriched conditions. The addition of cobalt resulted in a decrease of the activity, irrespective of the presence of the dmeRF deletion. These results suggest that bacteroids induced in lentil plants, but not in pea plants, require the dmeRF system to achieve an appropriate balance of intracellular nickel for expression of optimal levels of hydrogenase.
Table 5.
Effect of deletion or R. leguminosarum dmeRF gene on hydrogenase activity in symbiosis with different hosts
| Strain | Relevant genotype | Hydrogenase activity in bacteroids froma: |
|||||
|---|---|---|---|---|---|---|---|
| Pea |
Lentil |
||||||
| Control | Ni(II) | Co(II) | Control | Ni(II) | Co(II) | ||
| SPF25 | Wild type | 2,400 ± 140 | 6,650 ± 1080 | 1,470 ± 230 | 210 ± 50 | 1,190 ± 110 | 90 ± 10 |
| D15 | ΔdmeRF | 2,870 ± 890 | 7,700 ± 890 | 1,020 ± 10 | 135 ± 20 | 580 ± 60 | 100 ± 10 |
Values are given in nmol of H2 · h−1 · mg of protein−1. Plants were grown with normal nutrient solutions (control) or with nutrient solutions supplemented with NiCl2 (85 μM) or CoCl2 (42.5 μM). Values are the averages of at least three replicates ± standard errors.
DISCUSSION
Active transport by efflux pumps is one of the most relevant mechanisms for metal resistance (48). Analysis of R. leguminosarum bv. viciae genome led to the identification of a dmeF-like gene. This gene encodes a member of the cation diffusion facilitator family. This family of metal-proton antiporters is involved in resistance to Zn(II) and other metals (12). R. leguminosarum DmeF presents a predicted topology of 6 TM domains, with two characteristic motifs (HX3H at the beginning of TM2 and HX3D at the beginning of TM5), and a histidine-rich stretch characteristic of the group of CDFs having Co(II) and Zn(II) as substrates (13) (see Fig. S1 in the supplemental material). Also, phylogenetic analysis placed the R. leguminosarum protein within the previously defined Zn-CDF group (13). Analysis of the R. leguminosarum dmeRF-deficient mutant indicates a major role for this protein in cobalt detoxification, whereas no effect on the tolerance to Zn(II) was observed in this mutant. This lack of effect on resistance to Zn(II) could be the result of the Co(II)- and Ni(II)-responsive regulation of dmeF. However, assays carried out in the presence of cobalt levels leading to full induction of the system (10 μM) did not result in significant effects on resistance to zinc in a disk susceptibility assay (data not shown), suggesting either that R. leguminosarum DmeF has no relevant role in tolerance to zinc in this bacterium or that other systems providing resistance are present.
Analysis of data obtained by using lacZ fusions and by qRT-PCR determinations indicates that expression of dmeRF operon is strongly induced by nickel and cobalt in free-living cells. This is consistent with the presence of a gene (dmeR) encoding a protein homologous to RcnR, an E. coli nickel- and cobalt-responsive transcriptional regulator that, in the absence of nickel, represses synthesis of the efflux system RcnAB in this bacterium (17, 49). The unregulated high levels of expression of the system in the dmeRF-deficient mutant are corrected when the introduced fusion plasmid contains an active dmeR copy, suggesting that DmeR is actually a repressor whose effect is alleviated by the presence of these metals. Such a mode of regulation represents an alternative model to that described for C. metallidurans. In this bacterium, dmeF expression is constitutive and not inducible by metals (15). Conversely, the Ni and Co resistance cnr system described for the same organism shows an Ni-responsive regulation dependent on an alternative sigma factor (50).
It has been previously shown that nickel and cobalt binding to E. coli RcnR inhibits interaction of this protein with the rcnAB promoter region, thus removing transcriptional repression (45, 51). Sites critical for metal binding were mapped to residues His-3, Cys-35, His-60, and His-64 (45). All these residues are fully conserved in R. leguminosarum DmeR. However, the relative response to Ni(II)/Co(II) cations is different in rcnR versus dmeR. In the case of the E. coli system, RcnAB expression is induced to similar levels by Ni(II) and by Co(II) (45, 51), whereas in the case of R. leguminosarum, the level of induction of dmeF expression by Co(II) is higher than that by Ni(II). This difference might be due to the effect of sequence variations affecting residues other than those listed above. For instance, an E34Q mutation in RcnR yielded a higher response to cobalt (45). Also, recent evidence indicates that RcnR His-67 is involved in the interaction of RcnR with cobalt (20). These two residues are not conserved in the case of R. leguminosarum dmeR.
Our expression studies using lacZ fusions have shown that the relevant sequences for metal-induced expression of dmeF genes are located upstream of dmeR. Sequence analysis of this region in different Rhizobium species reveals the presence of a conserved palindromic sequence (ATA-X2-ATA-C6-TAT-X2-TAT) (see Fig. S4 in the supplemental material). This sequence corresponds to the type I site (a single G/C tract flanked by an AT-rich palindromic sequence) proposed by Iwig and Chivers (19) for DNA binding of E. coli RcnR. Based on the constitutive expression associated with the absence of DmeR and on the existence of this potential binding site, a similar repression mechanism can be hypothesized for the control of dmeF expression in the absence of metals. In the case of endosymbiotic bacteria, genes for transcriptional repressor and efflux protein form a single operon, with the two genes transcribed in the same direction. In this case, the amount of repressor synthesized increases with the derepression of the system, thus allowing a tighter control of the regulation process than with the divergent promoter situation described to occur in E. coli for rcnR-rcnA genes. In that case, other modes of regulation might be also present that affect the expression of regulator and the regulated genes differently, as was exemplified by the differential regulation of rcnR and rcnA by iron (52).
Our results with lacZ fusions indicate a low level of expression of dmeRF genes in SPF25 pea bacteroids, even in the presence of added metals. This level of expression of the system was significantly induced in response to the presence of cobalt ions but only in the case of the dmeRF mutant. These data are consistent with the deleterious effect of high cobalt levels in the case of the mutant strain, suggesting the existence of a buildup of cytoplasmic metal concentrations in the absence of this efflux system. Our interpretation of these data is that the amount of metals actually available to the bacteroids is very low compared to the free-living situation. Even in this situation, a low level of expression of the DmeRF system is apparently required to maintain an adequate level of metals inside the bacteroids, since a significant decrease of plant growth was observed when pea plants inoculated with the dmeRF-deficient mutant were exposed to an excess of cobalt. The lower symbiotic performance of this mutant under high-metal conditions is consistent with a previous report on metal-susceptible mutants generated from Bradyrhizobium japonicum strains isolated from Ni-rich soils (53) and suggests that the mechanisms for metal resistance in the microsymbiont are relevant for the development of the symbiosis under high-metal conditions, at least in the case of pea. The situation is likely different in the case of lentil, where no impairment of plant symbiotic performance was associated with the deletion of the dmeRF system. This might reflect a lower exposure of lentil bacteroids to metals, as was concluded after analysis of the differential nickel-dependent limitations of NiFe hydrogenase in these two legume hosts (39).
The marked reduction in hydrogenase activity associated with the deletion of dmeRF system in free-living cells and lentil bacteroids is an unexpected result that might reflect an additional layer of complexity in the control of nickel homeostasis in this bacterium. Nickel is a key element for hydrogenase synthesis (43), and we had previously demonstrated that the deletion of nickel uptake transporter genes hupE and hupE2 results in significant decreases in hydrogenase activity in free-living cells and in lentil bacteroids (47). A different situation was observed in the case of pea bacteroids. In this particular symbiosis there was no effect of HupE/HupE2 nickel transporters on the level of hydrogenase activity, suggesting the induction of a different mechanism for nickel provision in this symbiosis (47). Interestingly, we observe here a parallel pattern of results regarding the effect of the deletion of the dmeRF system on hydrogenase activity. It is tempting to speculate on the existence of an interaction between efflux and uptake systems, so both systems could be connected to maintain an optimal intracellular nickel level; such an interaction would not occur with the alternative uptake system proposed for pea bacteroids. The effect of a nickel efflux system in modulating the activity of other nickel enzymes, such as urease, has been previously documented for Helicobacter pylori (54), stressing the relevance of efflux systems for the maintenance of nickel homeostasis.
Analysis of the genome of other strains of R. leguminosarum bv. viciae, Sinorhizobium meliloti, Rhizobium etli, and Agrobacterium tumefaciens revealed that the metal efflux system presented in this work is likely conserved within the Rhizobiaceae (see Fig. S4 in the supplemental material). These data suggest that this model of a metal-inducible, RcnR-regulated CDF system has been selected by this group of bacteria as a general strategy for metal detoxification. Further studies are required to ascertain the actual role of this system in the maintenance of metal homeostasis and its relationship with metal availability for metalloenzyme biosynthesis in endosymbiotic bacteria.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by projects from Spain's MICINN (BIO2010-15301), Comunidad Autónoma de Madrid (S-505/AMB/0321 MICROAMBIENTE-CM), and Universidad Politécnica de Madrid [AL09-P(I+D)-06].
We thank Tomás Ruiz-Argüeso for critical reading of the manuscript.
Footnotes
Published ahead of print 9 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01954-13.
REFERENCES
- 1.Mulrooney SB, Hausinger RP. 2003. Nickel uptake and utilization by microorganisms. FEMS Microbiol. Rev. 27:239–261 [DOI] [PubMed] [Google Scholar]
- 2.Hausinger RP, Zamble DB. 2007. Microbial physiology of nickel and cobalt, p 287–320 In Nies DH, Silver S. (ed), Molecular microbiology of heavy metals. Springer, Berlin, Germany [Google Scholar]
- 3.Ma Z, Jacobsen FE, Giedroc DP. 2009. Metal transporters and metal sensors: how coordination chemistry controls bacterial homeostasis. Chem. Rev. 109:4644–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macomber L, Hausinger RP. 2011. Mechanisms of nickel toxicity in microorganisms. Metallomics 3:1153–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. 2007. Cobalt stress in Escherichia coli. The effect on the iron-sulfur proteins. J. Biol. Chem. 282:30442–30451 [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Zamble DB. 2009. Nickel homeostasis and nickel regulation: an overview. Chem. Rev. 109:4617–4643 [DOI] [PubMed] [Google Scholar]
- 7.Bruins MR, Kapil S, Oehme FW. 2000. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf. 45:198–207 [DOI] [PubMed] [Google Scholar]
- 8.Mergeay M, Monchy S, Vallaeys T, Auquier V, Benotmane A, Bertin P, Taghavi S, Dunn J, van der Lelie D, Wattiez R. 2003. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 27:385–410 [DOI] [PubMed] [Google Scholar]
- 9.Nies DH. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313–339 [DOI] [PubMed] [Google Scholar]
- 10.Blair JM, Piddock LJ. 2009. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Curr. Opin. Microbiol. 12:512–519 [DOI] [PubMed] [Google Scholar]
- 11.Goldberg M, Pribyl T, Juhnke S, Nies DH. 1999. Energetics and topology of CzcA, a cation/proton antiporter of the resistance-nodulation-cell division protein family. J. Biol. Chem. 274:26065–26070 [DOI] [PubMed] [Google Scholar]
- 12.Haney CJ, Grass G, Franke S, Rensing C. 2005. New developments in the understanding of the cation diffusion facilitator family. J. Ind. Microbiol. Biotechnol. 32:215–226 [DOI] [PubMed] [Google Scholar]
- 13.Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. 2007. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 8:107. 10.1186/1471-2164-8-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu M, Chai J, Fu D. 2009. Structural basis for autoregulation of the zinc transporter YiiP. Nat. Struct. Mol. Biol. 16:1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munkelt D, Grass G, Nies DH. 2004. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 186:8036–8043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Vliet AH, Ernst FD, Kusters JG. 2004. NikR-mediated regulation of Helicobacter pylori acid adaptation. Trends Microbiol. 12:489–494 [DOI] [PubMed] [Google Scholar]
- 17.Iwig JS, Rowe JL, Chivers PT. 2006. Nickel homeostasis in Escherichia coli—the rcnR-rcnA efflux pathway and its linkage to NikR function. Mol. Microbiol. 62:252–262 [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. 2007. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat. Chem. Biol. 3:60–68 [DOI] [PubMed] [Google Scholar]
- 19.Iwig JS, Chivers PT. 2009. DNA recognition and wrapping by Escherichia coli RcnR. J. Mol. Biol. 393:514–526 [DOI] [PubMed] [Google Scholar]
- 20.Higgins KA, Hu HQ, Chivers PT, Maroney MJ. 2013. Effects of select histidine to cysteine mutations on transcriptional regulation by Escherichia coli RcnR. Biochemistry 52:84–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canfield DE, Glazer AN, Falkowski PG. 2010. The evolution and future of Earth's nitrogen cycle. Science 330:192–196 [DOI] [PubMed] [Google Scholar]
- 22.Kereszt A, Mergaert P, Kondorosi E. 2011. Bacteroid development in legume nodules: evolution of mutual benefit or sacrificial victims? Mol. Plant Microbe Interact. 24:1300–1319 [DOI] [PubMed] [Google Scholar]
- 23.Mandal SM, Bhattacharyya R. 2012. In Zaidi A, Wani PA, Khan MS. (ed), Toxicity of heavy metals to legumes and bioremediation. Springer, Vienna, Austria [Google Scholar]
- 24.Pajuelo E, Rodiguez-Llorente ID, Lafuente A, Caviedes MA. 2011. Legume-Rhizobium symbioses as a tool for bioremediation of heavy metal polluted soils, p 95–123 In Khan MS, Zaidi A, Goel R, Musarrat J. (ed), Biomanagement of metal-contaminated soils, vol 20 Springer, Berlin, Germany [Google Scholar]
- 25.El-Aziz R, Angle JS, Chaney RL. 1991. Metal tolerance of Rhizobium meliloti isolated from heavy-metal contaminated soils. Soil Biol. Biochem. 23:795–798 [Google Scholar]
- 26.Pereira SI, Lima AI, Figueira EM. 2006. Screening possible mechanisms mediating cadmium resistance in Rhizobium leguminosarum bv. viciae isolated from contaminated Portuguese soils. Microb. Ecol. 52:176–186 [DOI] [PubMed] [Google Scholar]
- 27.Hao X, Lin Y, Johnstone L, Baltrus DA, Miller SJ, Wei G, Rensing C. 2012. Draft genome sequence of plant growth-promoting rhizobium Mesorhizobium amorphae, isolated from zinc-lead mine tailings. J. Bacteriol. 194:736–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beringer J. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188–198 [DOI] [PubMed] [Google Scholar]
- 29.O'Gara F, Shanmugam KT. 1976. Regulation of nitrogen fixation by rhizobia: export of fixed nitrogen as NH4+. Biochim. Biophys. Acta 437:313–321 [DOI] [PubMed] [Google Scholar]
- 30.Vincent JM. 1970. A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom [Google Scholar]
- 31.Brito B, Palacios JM, Imperial J, Ruiz-Argüeso T. 2002. Engineering the Rhizobium leguminosarum bv. viciae hydrogenase system for expression in free-living microaerobic cells and increased symbiotic hydrogenase activity. Appl. Environ. Microbiol. 68:2461–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon R, Priefer U, Pühler A. 1983. Vector plasmids for in-vivo and in-vitro manipulations of Gram-negative bacteria, p 98–106 In Pühler A. (ed), Molecular genetics of the bacteria-plant interaction. Springer-Verlag, Berlin, Germany [Google Scholar]
- 33.Bauer AW, Kirby WM, Sherris JC, Turck M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45:493–496 [PubMed] [Google Scholar]
- 34.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 35.Spaink HP, Okker RJH, Wijffelman CA, Pees E, Lugtenberg BJJ. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 9:27–39 [DOI] [PubMed] [Google Scholar]
- 36.Schäfer A, Tauch A, Jäger Kalinowski WJ, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73 [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Argüeso T, Hanus FJ, Evans HJ. 1978. Hydrogen production and uptake by pea nodules as affected by strains of Rhizobium leguminosarum. Arch. Microbiol. 116:113–118 [Google Scholar]
- 39.Brito B, Toffanin A, Prieto RI, Imperial J, Ruiz-Argüeso T, Palacios JM. 2008. Host-dependent expression of Rhizobium leguminosarum bv. viciae hydrogenase is controlled at transcriptional and post-transcriptional levels in legume nodules. Mol. Plant Microbe Interact. 21:597–604 [DOI] [PubMed] [Google Scholar]
- 40.Bremmer JM. 1996. Nitrogen—total. In Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME. (ed), Methods of soils analysis. Part 3. Chemical methods. SSSA and ASA, Madison, WI [Google Scholar]
- 41.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 42.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85 [DOI] [PubMed] [Google Scholar]
- 43.Brito B, Palacios JM, Hidalgo E, Imperial J, Ruiz-Argüeso T. 1994. Nickel availability to pea (Pisum sativum L.) plants limits hydrogenase activity of Rhizobium leguminosarum bv. viciae bacteroids by affecting the processing of the hydrogenase structural subunits. J. Bacteriol. 176:5297–5303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giedroc DP, Arunkumar AI. 2007. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 29:3107–3120 [DOI] [PubMed] [Google Scholar]
- 45.Iwig JS, Leitch S, Herbst RW, Maroney MJ, Chivers PT. 2008. Ni(II) and Co(II) sensing by Escherichia coli RcnR. J. Am. Chem. Soc. 130:7592–7606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy A, Kucukural A, Zhang Y. 2010. I-TASSER: a unified platform for automated protein structure and function prediction. Nat. Protoc. 5:725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brito B, Prieto RI, Cabrera E, Mandrand-Berthelot MA, Imperial J, Ruiz-Argueso T, Palacios JM. 2010. Rhizobium leguminosarum hupE encodes a nickel transporter required for hydrogenase activity. J. Bacteriol. 192:925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirete S, de Figueras CG, Gonzalez-Pastor JE. 2007. Novel nickel resistance genes from the rhizosphere metagenome of plants adapted to acid mine drainage. Appl. Environ. Microbiol. 73:6001–6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blériot C, Effantin G, Lagarde F, Mandrand-Berthelot MA, Rodrigue A. 2011. RcnB is a periplasmic protein essential for maintaining intracellular Ni and Co concentrations in Escherichia coli. J. Bacteriol. 193:3785–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grass G, Grosse C, Nies DH. 2000. Regulation of the cnr cobalt and nickel resistance determinant from Ralstonia sp. strain CH34. J. Bacteriol. 182:1390–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaha D, Arous S, Bleriot C, Dorel C, Mandrand-Berthelot MA, Rodrigue A. 2011. The Escherichia coli metallo-regulator RcnR represses rcnA and rcnR transcription through binding on a shared operator site: insights into regulatory specificity towards nickel and cobalt. Biochimie 93:434–439 [DOI] [PubMed] [Google Scholar]
- 52.Koch D, Nies DH, Grass G. 2007. The RcnRA (YohLM) system of Escherichia coli: a connection between nickel, cobalt and iron homeostasis. Biometals 20:759–771 [DOI] [PubMed] [Google Scholar]
- 53.Chaintreuil C, Rigault F, Moulin L, Jaffre T, Fardoux J, Giraud E, Dreyfus B, Bailly X. 2007. Nickel resistance determinants in Bradyrhizobium strains from nodules of the endemic New Caledonia legume Serianthes calycina. Appl. Environ. Microbiol. 73:8018–8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stähler FN, Odenbreit S, Haas R, Wilrich J, Van Vliet AH, Kusters JG, Kist M, Bereswill S. 2006. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infect. Immun. 74:3845–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.