Abstract
“Candidatus Cardinium hertigii” (Bacteroidetes) is a maternally inherited endosymbiont known from several arthropods. Its mechanisms for persistence in host populations are mostly reproductive manipulation, though it has been occasionally reported to improve fitness parameters in several hosts. In Culicoides (Diptera: Ceratopogonidae) biting midges, the prevalence of “Candidatus Cardinium” infection was documented as moderate, with no detectable sex bias. We therefore investigated whether “Candidatus Cardinium” affects important fitness parameters, such as survival and body size, in Culicoides imicola, a dominant vector species. Field-collected midges were trapped and analyzed for survival under different environmental conditions and antibiotic treatment, taking into account “Candidatus Cardinium” infection status and parity status (i.e., parous or nulliparous). Additionally, wing lengths were measured as a proxy parameter for body size and analyzed together with “Candidatus Cardinium” infection data. The findings revealed no difference in survival of Culicoides infected with “Candidatus Cardinium” and that of uninfected midges in both parity states and under all tested conditions: optimal, starvation, heat, and antibiotic treatment. Beyond survival, no wing length difference was found for “Candidatus Cardinium”-infected versus uninfected midges. In aggregate, these findings support our conclusion that “Candidatus Cardinium” does not have an overt effect on the survival and size of adult C. imicola midges. “Candidatus Cardinium” may affect immature stages or may alter adult reproductive performance.
INTRODUCTION
The spread and persistence of facultative symbionts in host populations are assumed to be facilitated through reproductive manipulation and/or by improving host fitness (1). “Candidatus Cardinium hertigii” (Bacteroidetes) is a maternally inherited reproductive manipulator symbiont known from several arthropods, and it may cause feminization, parthenogenesis, and cytoplasmic incompatibility (CI) (2–5). In biting midges in Japan, “Candidatus Cardinium” presented infection rates ranging from 20 to 100% (6), yet its role in its Culicoides (Diptera: Ceratopogonidae) host, if any, remains obscure.
Recently, we documented average “Candidatus Cardinium” prevalences of 50.7% and 31.4% in adult Culicoides imicola and Culicoides oxystoma, respectively, with no difference between sexes within each species (7). Such intermediate prevalence, with no sex bias, has been speculated to be associated with either a weak CI effect or some facultative benefit of infection under particular environmental conditions (8). Indeed, environmental conditions can strongly affect interactions between an arthropod host and its microbial tenants (9). Interestingly, our previous data revealed that “Candidatus Cardinium” prevalence in Culicoides vector species in Israel is associated with land surface temperature (LST) (7), suggesting that the symbiont may interact with the host in early developmental stages.
Although best known as a reproductive manipulator, “Candidatus Cardinium” also has been reported to have beneficial influences in some hosts. Elevated fecundity was previously shown in the mite Metaseiulus occidentalis when infected with “Candidatus Cardinium” (10), and removal of “Candidatus Cardinium” showed negative effects on the survival of immature stages, as well as reduced fecundity, in Liposcelis bostrychophila (Psocoptera) (11). In addition, “Candidatus Cardinium” increased survival and male production when singly infecting the parasitoid wasp Encarsia inaron. However, the fitness effect responsible for persistence of “Candidatus Cardinium” in the Encarsia population remained unclear (1).
Culicoides spp. are minute hematophagous flies (order Diptera) of the family Ceratopogonidae and are important vectors of several major veterinary disease agents, notably arboviruses. In the family Reoviridae, key Culicoides-transmitted pathogens include bluetongue virus, epizootic hemorrhagic disease virus, and African horse sickness virus, and Culicoides spp. recently were discovered as vectors of Schmallenberg virus (family Bunyaviridae) (12–14). The diseases caused by these viruses have major impacts on animal welfare, international trade, and the economics of agricultural production (15, 16), so reducing the vectorial capacity of Culicoides is quite an important goal.
As a first step toward investigating the role of “Candidatus Cardinium” in Culicoides vector species, we focused here on two significant fitness parameters: adult survival and body size. Survival is known to be influenced by symbionts in multiple hosts (1, 17–19) and is a principal parameter in vectorial capacity (20, 21). Adult body size is known to be linked with fitness characteristics, such as fecundity, survival, reproductive success, and blood feeding, in mosquitoes (22, 23) and is an important fitness indicator in fruit flies (24). We chose to compare wing lengths as a proxy parameter for Culicoides body size, and it reflects fecundity in at least some species, such as Culicoides sonorensis and Culicoides melleus (25, 26). Traditionally, body size is also well correlated with wing length in mosquitoes (23).
Presently, very few Culicoides species have been cultured in the laboratory (27), so the alternative is to study Culicoides midges taken from the field. Therefore, in this study, we initially focused on comparisons of life span and wing length in individuals from field populations of C. imicola in which the “Candidatus Cardinium” infection status could be retrospectively determined.
MATERIALS AND METHODS
Trapping of adult midges.
Midges were trapped during peak activity seasons (2011 and 2012) using suction UV light traps (John W. Hock Company, Gainesville, FL, USA). The traps were placed 1.5 to 2 m above the ground on a horse farm (site, Beit-Elazari [31°50′45″N, 34°48′34″E; altitude, 54 m above sea level]). Insects were collected overnight in a plastic container. The live insects were then anesthetized using CO2. Culicoides midges were separated from other trapped insects and gently placed into screened cardboard cups. Additionally, one swarm of C. imicola males was collected by sweep net on a dairy cow farm (site, Netzer Sereni [31°55′20″N, 34°49′21″E; altitude, 74 m above sea level]). The males were preserved in absolute ethanol at 4°C until they were analyzed.
Morphological and molecular identification of midges.
Initial identification of the dominant species, C. imicola and members of the Culicoides schultzei species group (difficult to separate morphologically), was initially based on the wing spot pattern and other diagnostic morphological traits (www.culicoides.net). In parallel, molecular identification of individual midges was carried out by internal transcribed spacer 1 (ITS-1) ribosomal DNA (rDNA) PCR, as previously described (29). The midges were further morphologically categorized according to their parity (oviposition) status, which in this genus varies with abdominal pigmentation (30). Parous females have a darker, burgundy-red pigment and have completed at least one gonotrophic cycle (blood fed and laid eggs), while nulliparous females have a lighter abdominal color and have not yet blood fed or laid eggs. The parity status thus provides a relative estimate of the female's age and reproductive success.
Examination of midge survival and “Candidatus Cardinium” infection status.
A total of 438 C. imicola females were analyzed for life span in parallel with “Candidatus Cardinium” infection status, 418 of which were categorized by parity status (i.e., parous or nulliparous). Earlier studies had shown that “Candidatus Cardinium” infection varied geographically, with lower infection prevalence in hotter and drier desert regions of Israel (7). To investigate possible interactions of “Candidatus Cardinium” infection and heat or humidity stress, therefore, midges were kept in cardboard cups held as follows: (i) optimal conditions (25°C with 50% humidity; fed 10% glucose) (n = 129), (ii) starvation (25°C with 50% humidity) (n = 209), and (iii) heat stress (35°C with either 25% [n = 31], 55% [n = 49], or 75% [n = 20] humidity; fed 10% glucose). The midges were inspected daily, and mortality was noted. Dead midges were removed and kept in absolute ethanol at 4°C until their identity and infection status were assessed individually as described above. Individual midges were subjected to DNA extraction and specific “Candidatus Cardinium” PCR detection as described by Morag et al. (7). Under optimal conditions and under starvation, experiments were repeated three times. Heat stress experiments were analyzed individually according to the specific humidity conditions.
Antibiotic treatment.
In order to evaluate the effect of antibiotic treatment on field populations, we conducted a preliminary experiment in which 75 midges were fed with 10% glucose containing 50 μg/ml rifampin for 48 h, as previously described (31). Mortality was checked daily, and once dead, midges were analyzed for “Candidatus Cardinium” infection using PCR. Subsequently, in order to compare life spans between antibiotic-treated and untreated midges, an additional experiment was conducted as follows. Midges from a field-trapped population were separated randomly into two groups under brief CO2 anesthesia. One of the groups was treated with antibiotic as before, and the other group was an untreated control group. The midges were kept under optimal conditions (25°C; 50% relative humidity [RH]) with access to glucose. A total of 385 midges in two replicates were analyzed for life span, comparing the two groups tested: treated with antibiotic (n = 248) versus untreated (n = 137).
Measurement of Culicoides wing length.
Measurement of wing length was performed on a total of 100 C. imicola midges (70 females and 30 males), all trapped during the fall season (September-October 2011). Wings of individual midges (right and left) were removed at the base and then photographed under a binocular microscope using an AxioCam (Zeiss, Germany) camera. Measurements were then performed from the basal arculus to the tip of the wing using the Axiovision program. The average length of both wings was calculated and compared with infection status.
Statistical analysis.
In order to contrast “Candidatus Cardinium”-infected with uninfected populations and to examine differences under specific conditions, their survival distributions were compared using Kaplan-Meier plots, and statistical significance was examined by the log rank Mantel-Cox test. To eliminate acclimation stress as a factor, midges that died on the first day of the experiment were excluded from the survival analysis. In the case of repetitive experiments (i.e., blocks of the same experiment conducted under specific conditions but at different times), experiments were initially analyzed individually and then pooled for comprehensive statistical analyses under each specific condition. The Cox regression model was used to simultaneously test the effects of two experimental factors—the main effect (either “Candidatus Cardinium” infection or antibiotic treatment) and the “block” effect—as well as the interaction between the two factors.
In addition, in order to examine separately the association of survival with “Candidatus Cardinium” infection and parity status (parous or nulliparous females), further survival analysis was performed after stratifying the data for parity.
For comparisons of average wing lengths between populations, a t test for equality of means was used following Levene's test to ensure variances were comparable. Frequency analysis of “Candidatus Cardinium” prevalence in different parity categories was performed using the Pearson chi-square test. All analyses were performed using SPSS 19.0 (SPSS, Inc., Chicago, IL, USA), with a P value of <0.05 indicating statistically significant differences.
RESULTS AND DISCUSSION
Survivorship and parity status.
This study design, which used field-collected insects, reflected initial variation in the ages of the insects, and this uncontrolled source of variation could influence the results. Nevertheless, we found no evidence of a “Candidatus Cardinium” effect on adult survival of field populations of C. imicola subsequently held in the laboratory compared to midges without “Candidatus Cardinium” infection. This applied to midges under all tested conditions: optimal (n = 65; P = 0.465), starvation (n = 81; P = 0.213), and heat stress according to specific humidity conditions (25%, n = 20, P = 0.446; 55%, n = 49, P = 0.834; 75%, n = 31, P = 0.557). Cox regression model testing of the block effect showed that overall survival in one of the three blocks differed from that in the other two (P < 0.001), which could result from different predetermined field conditions on that collection day. However, in this model, even considering the block effect, the effect of “Candidatus Cardinium” on survival and the interaction between the block effect and the “Candidatus Cardinium” effect remained not significant under both conditions tested, optimal and starvation (“Candidatus Cardinium,” P = 0.842 and P = 0.621; interaction, P = 0.269 and P = 0.661), supporting previous analyses.
The prevalence of “Candidatus Cardinium” did not differ between parous midges (50.98%; n = 255) and nulliparous midges (49.69%; n = 163) (Pearson chi-square test; P = 0.797). This indicates that the results are not biased by females' age and suggests “Candidatus Cardinium” did not affect the relative abilities of females to survive to become parous. After stratifying the data for parity, there consistently was no difference in survival of midges with different “Candidatus Cardinium” status and under all tested conditions: optimal (parous, n = 33, P = 0.245; nulliparous, n = 32, P = 0.745), starvation (parous, n = 32, P = 0.255; nulliparous, n = 18, P = 0.387), and heat (parous, n = 19, P = 0.776; nulliparous, n = 31, P = 0.69).
In a previous study, we examined the association of various environmental factors that are relevant either to the immature or to the adult stage and found that LST is strongly associated with “Candidatus Cardinium” prevalence (7). Since LST may be especially relevant to immature stages, we suggested that the symbiont infection may have an influence during early developmental stages of the host. Difficulties in obtaining, identifying, and maintaining eggs and larvae of field-collected Culicoides species limited our initial experiments to adults. However, exploring the relevance of “Candidatus Cardinium” infection status to immature Culicoides fitness is a logical and necessary step in full evaluation of the symbiont's possible role in the host biology.
Antibiotic treatment.
While our results are limited to the effect of “Candidatus Cardinium” on its Culicoides host, it is possible that coinfection with other symbionts within the midge that were previously detected using denaturing gradient gel electrophoresis (DGGE) (7) and clone libraries (unpublished data) also may influence the observed results, as was shown for coinfection by “Candidatus Cardinium” and Wolbachia (1).
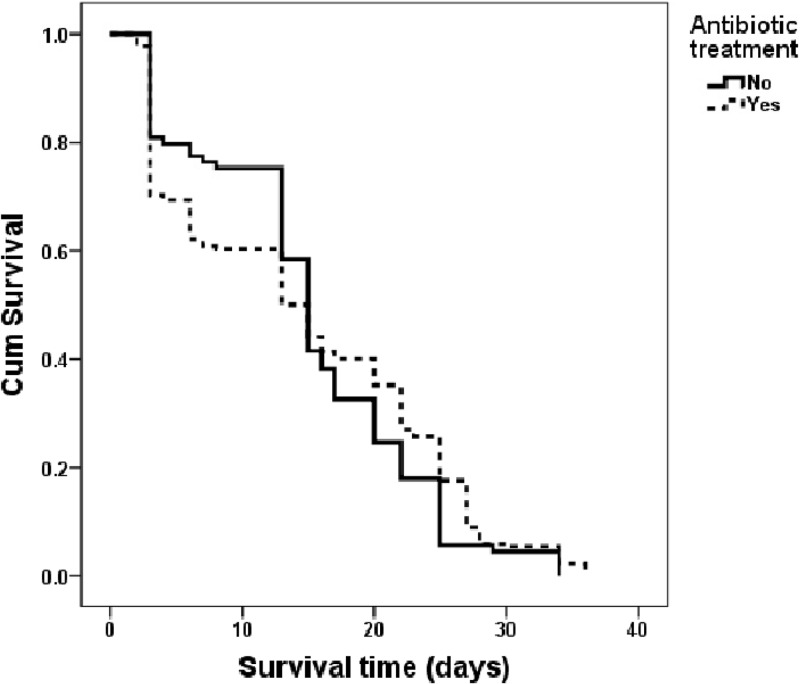
Thus, we conducted a set of experiments on Culicoides populations treated with antibiotics, considering that antibiotic treatment would probably affect other bacteria, as well. A preliminary study, in which midges were fed with antibiotic, as previously performed for other hosts (31), resulted in curing of the symbiont. This was determined because “Candidatus Cardinium” DNA could be detected by PCR only until day 7 after antibiotic treatment (data not shown). Thereafter, survival of midges treated with antibiotics did not differ from that of untreated midges (n = 311; log rank Mantel-Cox test, P = 0.338) (Fig. 1). Further supporting that conclusion is the Cox regression model, which revealed no effect of antibiotics on survival (P = 0.714) and no block effect (P = 0.785).
Fig 1.
Kaplan-Meier survival curves of field-collected C. imicola comparing midges treated with antibiotic to untreated midges. The midges were held under optimal conditions (25°C; 50% humidity) and fed with 10% sugar water. A total of 311 midges were analyzed (log rank Mantel-Cox test; P = 0.338). Cum, cumulative.
In aggregate, these findings support our conclusion that “Candidatus Cardinium” does not have an overt effect on the survival of adult C. imicola midges.
Culicoides body size.
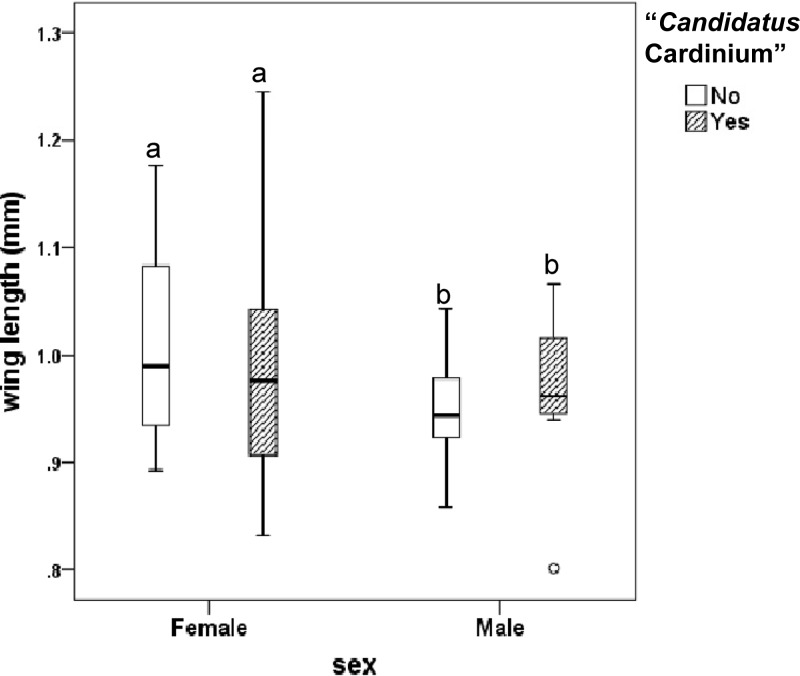
Beyond survival, in Culicoides spp., wing length is a commonly used metric reflecting both body mass and fecundity (25, 26). Many Culicoides species are characterized by typical male swarms (32), and such swarms likely comprise a competitive arena for mating of many important vector species (Diptera) (26). The average wing lengths of field populations did not differ between midges infected with “Candidatus Cardinium” (0. 985 mm; standard deviation [SD] = 0.093 mm) and uninfected midges (1.011 mm; SD = 0.09) in females (P = 0.292; n = 70) or in males (infected, 0.964 mm, SD = 0.078; not infected, 0.949 mm, SD = 0.045) (P = 0.514; n = 30) (Fig. 2). This finding suggests that “Candidatus Cardinium” probably does not affect midge size or perhaps also has no impact on the male sexual flying behavior of Culicoides midges tested, although those aspects need further testing. Regardless of “Candidatus Cardinium” infection, we confirmed a difference (P = 0.008; t test) between wing lengths of females (average, 0.993 mm; SD = 0.092) and males (average, 0.953 mm; SD = 0.055), although the males were not from the same population and their size thus should be compared only with caution to that of the females.
Fig 2.
Box plots presenting wing lengths of field-collected C. imicola males (n = 30) and females (n = 70) with different “Candidatus Cardinium” infection status. The bottom and top of the boxes are first and third quartiles, respectively. The whiskers represent minimum and maximum length. The median line is shown in each box plot. Different letters above the box plots indicate significant differences (t test for equality of means; Levene's test for equal variances).
Conclusions.
The comprehensive role of “Candidatus Cardinium” in Culicoides species fitness requires more study. In view of our previous data (7), we suggest that “Candidatus Cardinium” effects may be more relevant to immature stages and therefore not evident in adult stages. Alternately, considering the intermediate prevalence of “Candidatus Cardinium,” the symbiont may have weak reproductive manipulation impacts (e.g., limited CI inducement), effects on other fitness parameters, or size/survival effects too subtle to be seen in the present experiments using field-collected material. Using field material, as was done here, does have advantages in terms of realism; these specimens were naturally infected (or not) and had experienced many of the rigors of field existence before collection and testing. Further research using laboratory-reared populations might allow better resolution, including mating experiments, generating different populations of midges, and experimenting with curing and infection of the symbiont.
ACKNOWLEDGMENT
This research was supported by research grant no. IS-4380-11C from BARD, the United States-Israel Binational Agricultural Research and Development Fund, to Y.G. and B.A.M.
Footnotes
Published ahead of print 2 August 2013
REFERENCES
- 1.White JA, Kelly SE, Cockburn SN, Perlman SJ, Hunter MS. 2011. Endosymbiont costs and benefits in a parasitoid infected with both Wolbachia and Cardinium. Heredity 106:585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zchori-Fein E, Perlman JS, Kelly SE, Katzir N, Hunter MS. 2004. Characterization of a ‘Bacteroidetes' symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of ‘Candidatus Cardinium hertigii'. Int. J. Syst. Evol. Microbiol. 54:961–968 [DOI] [PubMed] [Google Scholar]
- 3.Weeks AR, Marec F, Breeuwer JA. 2001. A mite species that consists entirely of haploid females. Science 292:2479–2482 [DOI] [PubMed] [Google Scholar]
- 4.Hunter MS, Perlman SJ, Kelly SE. 2003. A Bacteroidetes-group bacterial symbiont induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc. Biol. Sci. 270:2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotoh T, Noda H, Ito S. 2007. Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98:13–20 [DOI] [PubMed] [Google Scholar]
- 6.Nakamura Y, Kawai S, Yukuhiro F, Ito S, Gotoh T, Kisimoto R, Yanase T, Matsumoto Y, Kageyama D, Noda H. 2009. Prevalence of Cardinium bacteria in planthoppers and spider mites and taxonomic revision of “Candidatus Cardinium hertigii” based on detection of a new Cardinium group from biting midges. Appl. Environ. Microbiol. 75:6757–6763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morag N, Klement E, Saroya Y, Lensky I, Gottlieb Y. 2012. Prevalence of the symbiont Cardinium in Culicoides (Diptera: Ceratopogonidae) vector species is associated with land surface temperature. FASEB J. 26:4025–4034 [DOI] [PubMed] [Google Scholar]
- 8.Duron O, Hurst GD, Hornett EA, Josling JA, Engelstadter J. 2008. High incidence of the maternally inherited bacterium Cardinium in spiders. Mol. Ecol. 17:1427–1437 [DOI] [PubMed] [Google Scholar]
- 9.Murdock CC, Paaijmans KP, Cox-Foster D, Read AF, Thomas MB. 2012. Rethinking vector immunology: the role of environmental temperature in shaping resistance. Nat. Rev. Microbiol. 10:869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weeks A, Stouthamer R. 2004. Increased fecundity associated with infection by a Cytophaga-like intracellular bacterium in the predatory mite, Metaseiulus occidentalis. Proc. Biol. Sci. 271:S193–S195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J-J, Dong P, Xiao L-S, Dou W. 2008. Effects of removal of Cardinium infection on fitness of the stored-product pest Liposcelis bostrychophila (Psocoptera: Liposcelididae). J. Econ. Entomol. 101:1711–1717 [DOI] [PubMed] [Google Scholar]
- 12.Mellor PS, Boorman J, Baylis M. 2000. Culicoides biting midges: their role as arbovirus vectors. Annu. Rev. Entomol. 45:307–340 [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen LD, Kristensen B, Kirkeby C, Rasmussen TB, Belsham GJ, Bødker R, Bøtner A. 2012. Culicoides as vectors of Schmallenberg virus. Emerg. Infect. Dis. 18:1204–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beer M, Conraths FJ, van der Poel WH. 2013. ‘Schmallenberg virus'-a novel orthobunyavirus emerging in Europe. Epidemiol. Infect. 141:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kedmi M, Van Straten M, Ezra E, Galon N, Klement E. 2010. Assessment of the productivity effects associated with epizootic hemorrhagic disease in dairy herds. J. Dairy Sci. 93:2486–2495 [DOI] [PubMed] [Google Scholar]
- 16.Velthuis AG, Saatkamp HW, Mourits MC, de Koeijer AA, Elbers AR. 2010. Financial consequences of the Dutch bluetongue serotype 8 epidemics of 2006 and 2007. Prev. Vet. Med. 93:294–304 [DOI] [PubMed] [Google Scholar]
- 17.Pais R, Lohs C, Wu Y, Wang J, Aksoy S. 2008. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl. Environ. Microbiol. 74:5965–5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, Chiel E, Duckworth VE, Dennehy TJ, Zchori-Fein E, Hunter MS. 2011. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332:254–256 [DOI] [PubMed] [Google Scholar]
- 19.Fry A, Palmer M, Rand D. 2004. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 93:379–389 [DOI] [PubMed] [Google Scholar]
- 20.Zindel R, Gottlieb Y, Aebi A. 2011. Arthropod symbioses: a neglected parameter in pest and disease-control programmes. J. Appl. Ecol. 48:864–872 [Google Scholar]
- 21.Reisen W. 2002. Epidemiology of vector-borne diseases., p 15–27 In Mullen G, Durden L. (ed), Medical and Veterinary Entomology. Elsevier, NY [Google Scholar]
- 22.Yuval B. 2006. Mating systems of blood-feeding flies. Annu. Rev. Entomol. 51:413–440 [DOI] [PubMed] [Google Scholar]
- 23.Koenraadt CJM. 2008. Pupal dimensions as predictors of adult size in fitness studies of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 45:331–336 [DOI] [PubMed] [Google Scholar]
- 24.Navarro-Campos C, Martinez-Ferrer MT, Campos JM, Fibla JM, Alcaide J, Bargues L, Marzal C, Garcia-Marí F. 2011. The influence of host fruit and temperature on the body size of adult Ceratitis capitata (Diptera: Tephritidae) under laboratory and field conditions. Environ. Entomol. 40:931–938 [DOI] [PubMed] [Google Scholar]
- 25.Linley JR, Hinds MJ. 1976. Seasonal changes in size, female fecundity and male potency in Culicoides melleus (Diptera: Ceratopogonidae). J. Med. Entomol. 13:151–156 [DOI] [PubMed] [Google Scholar]
- 26.Akey DH, Potter HW, Jones RH. 1978. Effects of rearing temperature and larval density on longevity, size and fecundity in the biting gnat Culicoides variipennis. Ann. Entomol. Soc. Amer. 71:411–418 [Google Scholar]
- 27.Hunt GJ, Mullens BA, Tabachnick WJ. 1999. Colonization and maintenance of species of Culicoides, p 33–55 In Mahmood F, Maramarosch K. (ed), Maintenance of animal/human and plant pathogen vectors. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, India. [Google Scholar]
- 28.Reference deleted.
- 29.Morag N, Saroya Y, Braverman Y, Klement E, Gottlieb Y. 2012. Molecular identification, phylogenetic status, and geographic distribution of Culicoides oxystoma (Diptera: Ceratopogonidae) in Israel. PLoS One 7:e33610. 10.1371/journal.pone.0033610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyce AL. 1969. The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. Aust. J. Entomol. 8:11–15 [Google Scholar]
- 31.Zchori-Fein E, Perlman JS. 2004. Distribution of the bacterial symbiont Cardinium in arthropods. Mol. Ecol. 13:2009–2016 [DOI] [PubMed] [Google Scholar]
- 32.Blackwell A, Mordue AJ, Young MR, Mordue W. 1992. The swarming behavior of the Scottish biting midge, Culicoides impunctatus (Diptera, Ceratopogonidae). Ecol. Entomol. 17:319–325 [Google Scholar]